Abstract
Capsaicin, a selective activator of the chemo- and heat-sensitive transient receptor potential (TRP) V1 cation channel, has characteristic feature of causing long-term functional and structural impairment of neural elements supplied by TRPV1/capsaicin receptor. In mammals, systemic application of capsaicin induces complex heat-loss response characteristic for each species and avoidance of warm environment. Capsaicin activates cutaneous warm receptors and polymodal nociceptors but has no effect on cold receptors or mechanoreceptors. In this review, thermoregulatory features of capsaicin-pretreated rodents and TRPV1-mediated neural elements with innocuous heat sensitivity are summarized. Recent data support a novel hypothesis for the role of visceral warmth sensors in monitoring core body temperature. Furthermore, strong evidence suggests that central presynaptic nerve terminals of TRPV1-expressing cutaneous, thoracic and abdominal visceral receptors are activated by innocuous warmth stimuli and capsaicin. These responses are absent in TRPV1 knockout mice. Thermoregulatory disturbance induced by systemic capsaicin pretreatment lasts for months and is characterized by a normal body temperature at cool environment up to a total dose of 150 mg/kg s.c. Upward differential shift of set points for activation vasodilation, other heat-loss effectors and thermopreference develops. Avoidance of warm ambient temperature (35°C, 40°C) is severely impaired but thermopreference at cool ambient temperatures (Tas) are not altered. TRPV1 knockout or knockdown and genetically altered TRPV1, TRPV2 and TRPM8 knockout mice have normal core temperature in thermoneutral or cool environments, but the combined mutant mice have impaired regulation in warm or cold (4°C) environments. Several lines of evidence support that in the preoptic area warmth sensitive neurons are activated and desensitized by capsaicin, but morphological evidence for it is controversial. It is suggested that these neurons have also integrator function. Fever is enhanced in capsaicin-desensitized rats and the inhibition observed after pretreatment with low i.p. doses does not support in the light of their warmth sensitivity the concept that abdominal TRPV1-expressing nerve terminals serve as nonthermal chemosensors for reference signals in thermoregulation.
Keywords: capsaicin, fever, preoptic area, TRPV1, TRPM8, thermoregulation, visceral thermoreceptors, warm receptors
Abbreviations: DRG, dorsal root ganglion (ganglia); EGFP, enhanced green fluorescent protein; LC, locus coeruleus; LPS, lipopolysaccharide; NTS, nucleus of the solitary tract; PG(s), prostaglandin(s); POA, the preoptic area (of the hypothalamus); RTX, resiniferatoxin; (s)EPSC(s), (spontaneous) excitatory postsynaptic current(s); Ta(s), ambient temperature(s); Tr, rectal temperature; TRP, transient receptor potential; Ts, skin temperature; Tt, tail temperature
Introduction
The core body temperature of humans and homoeothermic animals is constant within a narrow range. The nervous control mechanisms with sensors, integrators and effectors for regulation several physiological and behavioral systems operate to control variables for heat loss or heat conservation/production mechanisms. Over a century the thermoregulatory “black box” was challenged not only by testing responsiveness of the body to various Tas but key question was to resolve how the body temperature regulation shifts to a higher level during fever. The constant level and the shift level under modified conditions in regulatory systems are usually operated with a reference signal referred in various thermoregulatory models as “set point”1-3 or “balance point”.4,5 Nevertheless, other conceptual models as variable gain of thermosensory inputs or considering the thermosensors themselves as “comparators” for generation signals at critical elements of thresholds6 were also proposed.
Responsiveness of two groups of counteracting thermosensors as warm sensors which increase their firing rate by warming and cold receptors which are activated by cooling have overlapping thermal sensitivity ranges. Therefore, at various Ta or Tr levels, inputs from the two sets of pathways with opposing activity directions are simultaneously influenced. This balance of coordinated operation of thermoregulatory efferent pathways by cross inhibitory influences is in fact a cardinal common core in various thermoregulatory models.
Capsaicin, the hot principle of chili peppers, has a profound effect on thermoregulation affecting only the warm sensitive side of the regulatory system providing in this way a unique experimental tool. In humans, capsaicin at near threshold range of concentration (from 2 × 10−7 g/ml) elicits warm sensation7,8 and hot or even burning sensation is evoked at higher concentrations over 10−6 g/ml. Important feature of this compound is that it induces selective sensory desensitization in animal experiments, but also on the human skin or tongue where after application in 1% concentration the hot pungent effect of mustard oil or capsaicin is selectively abolished, warm sensation impaired but the difference limen to cold or tactile stimuli remains unaltered.7 In experimental animals like rats, mice and guinea-pigs, it elicits profound hypothermia, nociception but afterwards a long-term unresponsiveness of the sensory receptors takes place to chemonociceptive stimuli and the hypothermic effect of capsaicin is abolished leaving other exteroceptive stimuli fully effective including the cold sensation evoked by menthol.7-9 Subsequent single unit studies supported that noxious heat-sensitive C-polymodal nociceptors and in the skin of rabbit ear a warm receptor were activated and desensitized by capsaicin in contrast to C-cold receptors the temperature responsiveness of which was not influenced by capsaicin.10,11 Several lines of evidence suggested that these selective effects of capsaicin are due to an action on a “capsaicin receptor”12,13 suggested 22 years before it has been cloned by the group of David Julius.14 The receptor is in fact a thermosensor cation channel now named on molecular structural ground as TRP V1 which is activated by several chemical agents, protons, noxious heat and depolarizing electrical stimuli.15,16
TRPV1 has a temperature threshold in the noxious heat range of 43°C as detected on cultured dorsal root ganglia (DRG) neurons or transfected cells which fits well to that of the cutaneous polymodal nociceptors which form the dominant capsaicin-sensitive subgroup of sensory receptors. Nevertheless, the few cutaneous warm receptors picked up in single unit studies were also activated by capsaicin at innocuous warm stimuli.17 Furthermore, phosphorylation the TRPV1 channels by intracellular kinases (PKCε, PKA, PLC) or TrkA shift the thermal threshold of TRPV1 to the warm receptor range.15,18,19 It is particularly interesting that induction of PKCβII in capsaicin-sensitive DRG neurons reduced the activation threshold of TRPV1 to 36.8°C20 providing evidence that TRPV1 could operate not only as a nociceptive transducer for signaling pain sensation but in other neurons or nerve terminals as a plasma membrane transducer molecule for signaling innocuous warm stimuli for thermoregulation. Another possible involvement of TRPV1 receptors in thermoregulation was raised on the ground of its chemosensor function which manifests itself in activation by a large scale of endogenous ligands suitable to serve for thermoregulation as a nonthermal reference signal.4,5,21,22
The aim of the present survey is to summarize the thermoregulatory effects of capsaicin under physiological condition and during fever to draw conclusions about the possible role of TRPV1 in this respect. Earlier in vivo data on the thermoregulatory effects of capsaicin are compared with recent discoveries including other TRPV1 agonists, gene-modified animals, tracing the gating function of TRPV1 at various levels of the nervous system to shed light on the role of warm sensors and TRPV1 in regulation of body temperature homeostasis. On this ground, a thermoregulatory model is proposed which underlines the important role of visceral capsaicin-sensitive warm sensors in setting the body temperature at a regulated constant level. Earlier thermoregulatory models hardly considered an important input from visceral thermosensors owing to few experimental evidence for monitoring core body temperature outside the central nervous system. Emphasis was made particularly on thermosensors within the preoptic area of the hypothalamus (POA).23-29
Cutaneous thermoreceptors
In the skin, few innocuous thermoreceptive single unit fibers were isolated and among them cold receptors prevail which increase their activity below a temperature of 35°C. Warm receptors increase their firing rates at temperatures above 25°C and in some cases above 35°C.30 Specific warm receptors of human hairy skin have a skin temperature (Ts) threshold about 32°C.31,32 On the scrotal skin of the rat, both selective warm fibers which showed dynamic and static discharges with a threshold of 30°C and peak activity at 42°C33 as well as bimodal mechano-warm sensitive units with only static responsiveness were described beyond cold receptors.34
It is remarkable, however, that recording from afferent units of the sural and plantar nerves supplying the skin of the rat foot out of 55 A-delta units and 120 C-units no warm receptor was found in contrast to the cold receptors (5% of the units) and large number of noxious heat-sensitive nociceptors which formed the largest group.35 On the rabbit ear, out of 96 single units only two C-afferents were cold fibers and one was bimodal C-warm fiber.11 Close arterial injection of 20 μg capsaicin evoked discharges in the warm unit and after higher doses desensitized it to thermal stimuli while capsaicin induced neither activation nor desensitization of the cold fibers to cooling responsiveness. On the burn-induced blister base on the cat's hindpaw, capsaicin (5 × 10−4 g/ml) activated all three C-polymodal nociceptors and one C-warm receptor tested.36 In several single unit studies on sensory nerves of the rat, monkey and humans, only in one study was shown on the human skin which reported that capsaicin activated warm receptors37 beyond the selective activation of all or almost all polymodal nociceptors. They formed the overwhelming majority of units within the thin fiber afferents (C-and A-delta).17
Recently, another approach was taken by testing the central terminals of sensory neurons with thermal stimuli or capsaicin. In a slice, preparation of trigeminal superficial dorsal horn of the rat warming the bath at physiological temperature ranges enhanced the spontaneous excitatory postsynaptic currents (sEPSC) on neurons of the nucleus caudalis trigemini. At a bath temperature of 36°C, the frequencies of sEPSCs were quite high in most neurons. This group of neurons responded with enhancement of sEPSC frequencies to increments of bath temperatures in 30–36°C ranges indicative for thermosensitive presynaptic nerve terminals. Neurons with low sEPSC spontaneous activity (<10Hz) showed minor thermal responsiveness. Activation of TRPV1 channels with 100 nM capsaicin increased miniature EPSC frequency in 70% of the first group of neurons but some low sEPSC group neurons were also activated.38 Tetrodotoxin did not alter the responses of thermosensitive high sEPSC to capsaicin or temperature indicating a direct activation on the presynaptic nerve terminals while in the mild thermal sensitivity of low sEPSC responsive group of neurons was eliminated by tetrodotoxin. Histochemistry showed that neurons stained with TRPV1 antibody both among the high and low temperature sensitive types of neurons which indicate involvement of other thermosensitive channels in a group of neurons responding to warming stimuli.38 In vivo in the cat, microiontophoretically applied capsaicin to the trigeminal nucleus caudalis excited most of the neurons but microinjection to the cerebellum evoked no activation.39
Beyond the TRPV1 channel, other thermo-TRP channels activated by innocuous warm temperature ranges particularly TRPV3 with temperature threshold of 30–33°C can be considered.15 Furthermore, background potassium leak channels (TRAAK and TREK-1) which have been identified in capsaicin-insensitive sensory neurons40 could also play important roles in triggering thermal signals in warm receptors. Furthermore, voltage-gated K+ channel β 2 (KVβ2)41 which interacts with TRPV1 in DRG neurons should also be tested to decide its role in warm sensory input from thermoreceptors during thermoregulation.
It is nevertheless important to underline two facts (1) capsaicin according to the present state of knowledge is a highly selective compound gating only the TRPV1 channel but not other TRP channels (for ref, see 15). (2) High doses of capsaicin induce long-term loss of function of capsaicin-sensitive TRPV1-expressing sensory neurons and particularly their peripheral and central processes. Thus, it is not restricted only to the TRPV1 channel. The affected neural structures are impaired in function and their responsiveness to all types of stimuli are also diminished or eliminated (sensory desensitization) both in vitro and particularly under in vivo conditions.13,17,42,43 In respect of thermoregulation, the issue of colocalization of TRPV1 with other TRP heat-sensitive channels is controversial even if we discount data obtained on rats pretreated with capsaicin in the neonatal age44 where the neurotoxicity of capsaicin in a state of asphyxia destroys a much broader scope of neurons including cold receptors which are spared after adult treatment (for ref see 9, 42). In the rat, independent expression of TRPV1 and TRPM8 mRNA in separate population of neurons of DRG45 as well as different ontogenetic development of cold neural circuitry using mouse transgenic TRPM8 line and markers such as TRPV1 were described.46
Visceral thermosensors
Before the TRPV1 era, the role of core body temperature in thermal homeostasis was mainly focused on heating or cooling the POA and other areas of the brain23,30 and the role of visceral thermoreceptors in thermoregulation was analyzed only in few cases mainly in large animals from technical reasons. In the sheep, intra-abdominal heating induced panting and reduction of hypothalamic and core body temperatures. Thermoreceptors were considered to be within the walls of the rumen, intestine and around the mesenteric vessels. Cutting the splanchnic nerves abolished these responses.47 In anesthetized rats, cardiovascular responses evoked by application of fluid at 45°C to mucosa or serosa of gastrointestinal organs were completely inhibited by capsaicin pretreatment but relevance of these results from the point of view of thermoregulation is questionable.48 It has been described over 30 years on 13 splanchnic fibers in the rabbit49 that there are warm-sensitive receptors localized to the dorsal wall of the upper abdomen near to the aorta. Static and dynamic responses of the receptors resembled to that described for cutaneous warm receptors.31 Some units increased firing up to 40°C while others to 46°C from a threshold around 30°C. Recently, warm-sensitive afferent splanchnic C-fiber units were studied in detail in an in vitro mesenteric preparation of the rat. Receptors investigated were usually on or adjacent to blood vessels of the splanchnic vascular distribution but some located on the brown adipose tissue or lymph nodes.50 Slowly adapting warm-sensitive units had in most cases (21/33) background activity at 31–33°C and most of them were mechano-insensitive (72%). Warming during ramp heating elicited increasing firing in the non-noxious range but usually maximum frequencies were reached at noxious ranges (46–49°C). The warm-sensitive units were activated and sensitized to thermal stimuli by low concentration of bradykinin (90 nM), but in this study capsaicin was not applied simply referred to the similarities to another study where warming the abdominal cavity evoked responses evoked by capsaicin-sensitive afferents.48
In respect of the nodose ganglion of the vagal nerve, recent studies revealed that about 80% of these neurons contain mRNA for TRPV1 and in 54% of the cells capsaicin elicited increase in intracellular calcium.51 In cats, three groups of mechano- and acid-insensitive vagal thermoreceptors of lower esophagus and stomach were described which were connected to unmyelinated C-fibers and activated in temperature ranges as cold receptors (10–30°C), warm receptors (39–50°C) and mixed receptors (10–35°C and 40–50°C). These receptors, however, did not have a background below 39°C.52
Thermosensitivity of vagal afferent neurons of the mouse and involvement of TRP channels in the responses were analyzed under in vitro condition.53 Single nodose cell bodies which had been retrogradely labeled from the upper gut was shown to express mRNA by RT-PCR method of TRPV1, TRPV2, TRPV4 and TRPM8 thermoTRP channels but by immunohistochemical methods only TRPV1 and TRPV2 neurons were labeled. The majority of neurons responded to warming from 28°C to 38°C (89.6%) whereas fewer responded to cooling in the range of 28–12°C. Neurons of the latter group were activated by high concentration (100 µM) of icilin a TRPM8 agonist. Influx of Ca2+ was evoked by capsaicin in heat-sensitive neurons and capsaicin enhanced the responsiveness of the warm-sensitive group of neurons to 38–45°C.54
Under in vitro condition, patch clamp recordings were made from the rat vagal jugular and nodosal capsaicin-sensitive neurons innervating the lungs and airways as determined by retrograde labeling from the lungs using fluorescent tracer.53 Temperature-induced inward currents were recorded within 23–41°C temperature ranges. The temperature threshold of warm-sensitive neurons was 34.4 ± 0.4°C (n = 87) and Q10 value in the thermoregulatory relevant temperature range 35–41°C was 29.5 ± 6.4, while below this range (23–30°C) the Q10 value was only 2.84 (Fig. 1). 58% of the temperature-sensitive neurons were activated also by capsaicin (0.3–1 µM). TRPV1 antagonists as AMG 9810 (0.3 µM) or capsazepine (10 µM) reduced the temperature-evoked whole cell inward currents and ruthenium red (3 µM) an effective blocker of TRPV1-4 channels abolished them almost completely indicating a definite but partial involvement of TRPV1 receptors in thermosensitivity of a group of pulmonary vagal sensory receptors.53 Increasing the temperature from normal (36°C) to hyperthermic (∼40.6°C) level in this in vitro pulmonary sensory neuron preparation, inward currents evoked by capsaicin were enhanced (Fig. 1). Similar effects were observed when 2-aminoethoxy diphenyl borate (2-APB) an agonist on TRPV1-3 receptors was used but only at higher temperature ranges.55
Figure 1.
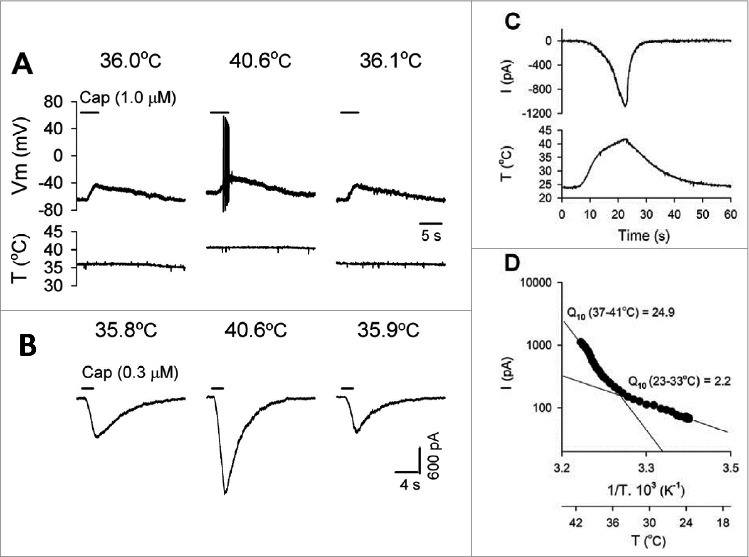
Effect of increasing temperature in vitro on the response of vagal pulmonary sensory neurons to capsaicin (Cap). (A) Experimental records illustrating that both membrane depolarization and number of action potentials evoked by Cap (1 μM, 4 s) were increased in current-clamp mode when the temperature was increased from 36.0 to 40.6°C in a jugular neuron (22.9 pF); the response recovered when the temperature was returned. Vm, membrane potential: T, temperature. (B) Experimental records illustrating that the Cap (0.3 μM, 2 s) – evoked current was increased when the temperature was increased from 35.8 to 40.6°C in a nodose neuron (23 pF) in voltage-clamp mode. (C) Whole cell inward current by increasing temperature and its temperature dependency representative experimental record illustrating that an inward current was evoked in a jugular neuron (37,4 pF) when a temperature ramp of 23–41°C was applied. (D) Arrhenius plot of the data in (C) illustrating two distinct phases of the response to increase in temperature. Q10 values were derived from linear fits of the data in low- and high-temperature ranges. © American Physiological Society. Permission to reuse must be obtained from the rightsholder.
In accordance with these in vitro findings, single unit recordings from the vagal nerve showed that pulmonary C-fiber activity increased drastically (>5 fold) when the intrathoracic temperature was increased from 36°C to 41°C and these fibers were also activated by capsaicin. The threshold of firing started at 39.2°C. Indomethacin failed to prevent the thermosensitive enhancement of capsaicin-induced single unit firings.56 In another study,57 thermal sensitivity of bronchopulmonary receptors with mechanosensitive receptive fields in small bronchioles were in most cases heat responsive (50°C) activated also by capsaicin (68%) or cold sensitive activated by the TRPM8 agonist menthol (15%).
Temperature enhancement of the inhaled air from 28–29°C to 33–34°C in tracheotomized cats enhanced the spontaneous discharge frequency characteristic for bronchopulmonary vagal C-afferent fibers in a group of bronchopulmonary vagal C-fibers. Capsaicin was not tested in this study but these fibers were already described earlier58,59 and were shown to be excited in the dog by capsaicin. Laryngeal chemoreflex in decerebrated piglets was enhanced by elevating body temperature and in animals where the TRPV1 receptors were antagonized by 5′ iodoresiniferatoxin application to the region of nucleus of the solitary tract (NTS) the thermal effect was abolished.60
Andresen's group made a series of seminal works on brainstem slice preparation for analysis the TRPV1-sensitive thermosensitive features of central terminals of visceral vagal afferents. Glutamate release and postsynaptic currents (EPSC) were recorded from the NTS. Two types of EPSC responses to stimulation were described: (1) the rapid synchronous type and (2) the longer-lasting asynchronous EPSC type. The second type of asynchronous EPSC but not the first type operates in a TRPV1-dependent manner.61,62 In other words, the long-lasting asynchronous EPSC and glutamate release were observed only on slices obtained from wild type mice but were absent in preparations from the TRPV1 gene-deleted mice (Fig. 2). Furthermore, EPSCs observed only in TRPV1+ nerve terminals were activated also by innocuous temperature in the 25–38°C range. They were calcium-dependent currents but not inhibited by the Na+ channel blocking agent tetrodotoxin.62 Furthermore, they were attenuated by the TRPV1 antagonist SB366791. Capsaicin (100 nM) substantially increased the rate of spontaneous asynchronous EPSCs but not the synchronous ones. After the capsaicin exposure, the TRPV1-dependent EPSC evoked by monosynaptic activation by high-intensity shocks were abolished. In the NTS, TRPV1 channels of the glutamatergic nerve terminals of unmyelinated afferents were active at normal temperatures and when the tissue temperature was increased between 30°C and 42°C their activity increased steeply.61,62 A review about the possible importance of this vagal C-afferent system in homeostatic control of visceral organs but not in thermoregulation was discussed.63 For further characteristic features of these vagal sensory neurons including suggestion of phenotyping segregation by capsaicin responsiveness are summarized in this and in another paper of this group.64
Figure 2.
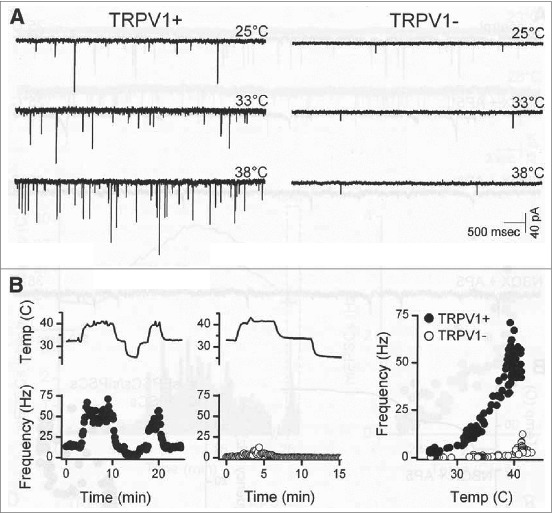
Temperature strongly augmented spontaneous glutamate released from second-order NTS neurons TRPV1+/+ but not from TRPV1−/− mice. (A) Original traces of sEPSCs from representative neurons ( left, TRPV1+/+ ; right, TRPV1−/− ) shown that TRPV1+/+ neurons typically had higher spontaneous EPSC rates than TRPV1−/− neurons at each temperature. (B) Temperature (upper trace) rapidly and reversibly changed spontaneous EPSC rates in a TRPV1+/+ (filled circles), but not in a TRPV1−/− (unfilled circles), neuron. Frequency points are counts > 10 s bins, expressed as average frequency. Note that (A) and (B) are from same neurons. © Society for Neuroscience. Permission to reuse must be obtained from the rightsholder.
Thus, several recent reports provide evidence that TRPV1-expressing capsaicin-sensitive visceral primary afferent neurons, their thoracic or abdominal receptors and central terminals could serve as thermoreceptors within the physiological range of thermoregulation. More efforts are needed, however, to clarify their role how they regulate various effectors and behavioral responses in thermal homeostasis. From this aspect, the following results should be mentioned.
Local warming of the lumbosacral spinal cord within physiological ranges (37–41°C) elicited in dogs panting and vasodilation65 and on spinal cord slices from the rat 54% of neurons from dorsal horn laminae I and II were warm-sensitive and share response characteristics with temperature-sensitive POA neurons.66 In conscious rats, local warming the medulla oblongata within the temperature range of 34–37°C inhibited shivering, while cooling had an opposite effect. Bar pressing behavior for warm airflow within the chamber was inhibited when the spinal cord was warm and enhanced when its temperature was shifted down.67 The effect of capsaicin in these studies however, was not tested. For central pathways for thermoregulation from visceral and spinal afferents see the reviews.27,28,29,68
Regarding the role of abdominal vagal afferents in thermoregulation, several interesting studies using TRPV1 agonists - mainly resiniferatoxin (RTX) for sensory desensitization - and TRPV1 antagonists led to a challenging new concept for thermoregulation. It has been suggested that TRPV1-expressing abdominal receptors being highly sensitive to endogenous ligands serve not as thermoreceptors but as chemosensitive thermoneutral reference signals.4,5,69,70 Evidence for this completely new paradigm seemed at first glance well established but more recent data discussed above indicate that TRPV1-expressing capsaicin-sensitive visceral afferents are activated by warmth stimuli within the physiological range and these afferents supply not only abdominal but also thoracic organs. Results in favor of this theory will be discussed later when thermoregulation of capsaicin-desensitized rats will be outlined.
Heat-loss responses evoked by capsaicin
Capsaicin stimulates warm-sensitive neurons with receptors from the skin and visceral organs but not the cold receptors. Furthermore, certainly the noxious heat-sensitive groups of polymodal nociceptors are the major targets of this compound. Thus, systemic application of capsaicin in mammalian species evokes activation of afferent pathways for heat-loss responses and nociception.8,24,68 Birds are, however, not sensitive to capsaicin.71,72 Complex heat-loss responses (activation of heat dissipating effectors with inhibition of heat gain mechanisms e.g. shivering) have been shown in the rat, mouse, guinea-pig, dog, rabbit, goat and the hibernator animal of golden-mantled ground squirrel.8
In the rat, 0.1–0.2 mg/kg s.c. dose of capsaicin elicits about 1°C fall in body temperature at cool Ta and after 1 mg/kg avoidance of a warm chamber (behavioral thermoregulation).42,73,74 Taking the average change in body temperature during a 6 hours period after the s.c. injection from 1 to 10 mg/kg, the fall in body temperature is dose-dependently increasing but in the dose range of 20–50 mg/kg this effect is significantly smaller indicating a desensitization within this time period. Sign of impaired heat sensitivity is also indicated by the subsequent dose-dependent enhancement of body temperature from 6 to 32 hours' time periods after 2 mg/kg to 50 mg/kg single doses.75 Up to a s.c. given dose of 16 mg/kg capsaicin, the rat's O2 consumption decreased from 104 ± 11 ml/dm2/h to 74 ± 2 ml/dm2/h only in a cool environment but not at thermoneutral (29–30°C) Ta.72 In rats anesthetized with urethane, 5mg/kg s.c. capsaicin elicited cutaneous vasodilatation (increase in tail temperature, Tt) and enhanced the heat production (O2 consumption ) during the decrease of core body temperature. Capsaicin-induced heat-loss was not altered by sympathetic denervation of the intercapsular brown adipose tissue but inhibited the increase in O2 consumption. It has been concluded that capsaicin independently influences networks for heat-loss and heat production76 which is in accordance with the excitatory effect of this pungent agent both on warm sensors and nociceptors, but I think it is not due to simultaneous activation of two counteracting thermoregulatory neural circuitries.
In response to acute capsaicin application, heat-defense thermoregulatory effectors as cutaneous vasodilatation, salivation have been described in several species together with polypnoe in cats and dogs. Behavioral heat-loss responses as body extension, increased cold-seeking behavior, temperature selection, salivation and grooming, increased skin cooling operant behavior were also described. Heat-gain effectors in a cold environment as enhanced metabolic rate, shivering, piloerection in different species were shown to be inhibited by capsaicin application.8,24,77 In addition to experiments on cats, goats and humans, the following data are also worthy to mention.
In cats, 3–10 mg/kg s.c. capsaicin resulted in a fall in body temperature up to 1–3°C accompanied by panting and sweating of the footpad skin as detected by the increase in the pH of the plantar skin.78 In goats, capsaicin evoked panting in thermoneutral environment but not always in the cold79 I.v. injection of capsaicin to dogs evokes shallow fast breathing beyond the classical Bezold–Jarish reflex of apnea, bradycardia and hypotension. In a species like the rat which does not pant during heat exposure capsaicin given i.v. elicits the Bezold–Jarish reflex without fast breathing.58
In humans, gustatory sweating in warm climate in response to hot chili ingestion has been described since a long-time.8 More recently, it has been shown that capsaicin ingestion in a dose of 2 mg/kg one hour prior exposure to a warm environment (38°C) did not alter the person's ability to keep the body temperature and O2 consumption at the level of the controls, but the mean Ts was higher. The authors concluded that capsaicin under these conditions does not alter in humans thermoregulation against overheating of their body in a warm environment.80
Long-Term Disturbance of Thermoregulation after Pretreatment with Capsaicin
After treatment of rats with increasing doses of capsaicin within 2–4 days from 30–50 mg/kg s.c. up to a total dose of 120–300 mg/kg, the animals become insensitive to capsaicin and capsaicin does not induce hypothermia even after several months. Thermoregulation of these “capsaicin-desensitized” animals against heat defense is also impaired.8,24,68,75,81–85 Thus, acute effects of capsaicin are mediated by TRPV1 activation but long-term effects are due to sensory desensitization (“defunctionalization”) of TRPV1-expressing neural structures. A single s.c. dose from 5 mg/kg capsaicin induces already a long-term disturbance in heat defense. Similar irreversible impairment in thermoregulation develops after given the highly potent TRPV1 agonist compound RTX which also desensitize the capsaicin-sensitive neural structures.5,83
Characteristic feature of these pretreated animals is that for about 2 days after the pretreatment75,81,85 the colonic temperature is higher by 1–2°C but remains normal afterwards at Tas of 20–29°C. The degree of desensitization to capsaicin and impaired heat-loss responsiveness is dose dependent.74 The circadian rhythmicity of body temperature in light and dark cycles are preserved.24
In rats pretreated with 50 + 100 mg/kg s.c. capsaicin, testing both the physiological (tail vasodilation) and behavioral (grooming, temperature preference) responses at various Ta ranges led to the conclusion that there is a differential upward shift in the threshold for activation of various heat-loss effector systems.10 In one set of experiments, the rat was in a restraint cage. Core body (rectal) temperature (Tr) and Ts in the chamber where Ta was changed and Ts at the middle of the tail which situated in a constant room temperature (Tt) to detect vasodilatation of the tail were recorded by four thermocouples. Fig. 3A shows records obtained from a control and from a capsaicin-desensitized rat. At thermoneutral Ta of 29°C, there was no significant difference in Tr and Ts between the two groups. The core body temperature of controls and capsaicin-desensitized rats was 38.1 ± 0.2°C(n = 9) and 37.9 ± 0.2°C (n = 15) Ts 32 ± 0.6°C and 31.6 ± 0.4°C respectively. After 40-min heating, the Ta to 35°C the Tr of controls enhanced by 0.9°C while that of the pretreated group by 1.8°C (60–70 days after the pretreatment). Vasodilatation was evoked in all rats, but the difference between the two groups was striking. In the capsaicin-pretreated group, tail vasodilation started later (21.6 ± 1.1 min vs. 16.1 ± 1.8 min) at higher rise in cutaneous threshold temperature for vasodilation (Ts 4.4 ± 0.4°C vs. 2.8 ± 0.5°C) when the enhancement of body temperature was also significantly higher (Tr 1.1 ± 0.2°C vs. 0.4 ± 0.1). Furthermore, as shown on the record of Fig. 3A, from time to time vasoconstrictor episodes interrupted the tail vasodilation while in the control rat the marked vasodilatation ceiling was remarkably abrupt. Consequently within a 15 min period starting from the onset of vasodilatation, Tr rose by 0.8 ± 0.1°C in the pretreated group and only 0.4 ± 0.1°C in the controls (n = 9). All these differences are statistically significant. Arrows on the figure indicate grooming activity which appeared in controls within 5 min around the vasodilatation started while in desensitized rats grooming was either absent or in two rats started much later when Tr already enhanced by 1.7 ± 0.3°C.
Figure 3.
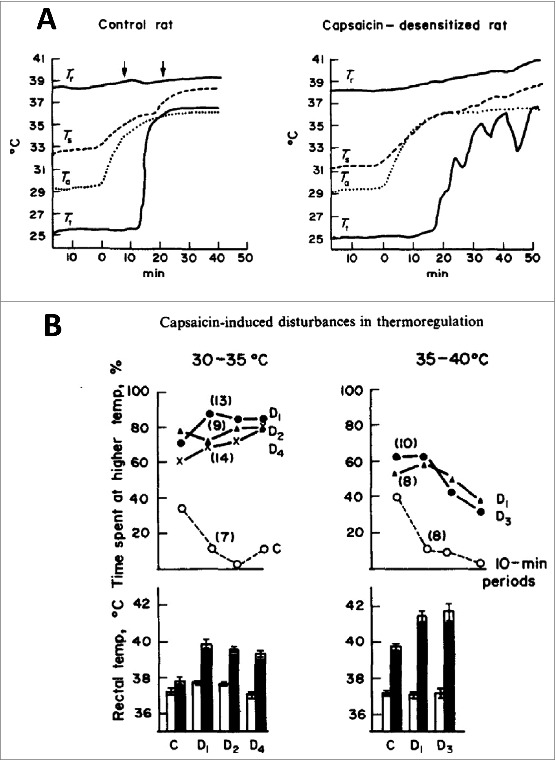
(A) Effect of increasing Ta on Tr, Ts, and Tt of two rats. Arrows indicate grooming activity. Note the oscillations in vasodilation indicating vasoconstrictor phases and steep rise in Tr of the capsaicin-desensitized rat. (B) Thermopreference of rats between two Tas having 5°C difference in warm Ta as indicated (upper row of data). During the 40 min exposure the duration of time spent at the chamber with higher temperature were determined at 10 min intervals as percent of time periods (each dots). Rectal temperature (mean ± SE) before (white colors) and after the experiment. Number of rats tested are indicated in brackets (from ref 13). Capsaicin pretreatment was performed 3–4 (D1), 7–12 (D2), 40–50 (D3) and 90–120 days (D4) before the experiment. © Elsevier. Permission to reuse must be obtained from the rightsholder.
Ambient thermopreference of these rats between two identical chambers (20 cm in height and diameter with a thermoinsulator plastic floor) having 5°C difference in chamber temperatures did not differ in the cool ranges (10 vs. 15°C, 15 vs. 20°C, 20 vs. 25°C) and at all 10 min periods both the capsaicin-pretreated and control rats spent similarly more time in the chamber having higher temperature. Consequently in cool environment Tr of pretreated and control groups of rats before and after 40 min exposure were also identical. Fig. 3B shows, however, the surprising fact that the capsaicin-desensitized rats from 3–4 days to 90–120 days after the pretreatment choose the warmer 35°C Ta and not the 30°C thermoneutral one, although their Tr rose up to 40°C. On the other hand, the controls avoided the moderately hot environment and their Tr increased only slightly. Significant differences were also seen between the preference of Ta and Tr of capsaicin-desensitized and control groups (p < 0.02) in the higher temperature range (35°C vs. 40°C). The higher temperature was not preferred by the capsaicin-pretreated group, but note, that their Tr approached the dangerous level of 42°C. These data clearly showed a differential upward shift of activation various heat-loss effectors. Neglecting the escape from a heat chamber where body temperature rose over 40°C when heat-loss vasodilation apparently is already turned on albeit in a braked manner as can be seen in Fig. 3A is an interesting thermoregulatory state particularly since these animals at room or cool temperatures regulate their body temperature like the controls. These data clearly show that thermoregulation of these rats cannot be explained by a hypothetical set point or set points reference signals by activating coordinated counteracting heat-loss and heat-gain pathways. The loss of cold-seeking behavior was also demonstrated using another experimental setup from where the capsaicin-pretreated rats did not leave the darker warm chamber to a lighter cool area although their colonic temperature reached the dangerous level of 42°C.42,74 It has been concluded that rats pretreated with capsaicin in adult age regulate their body temperature with an irreversible “upward widening of the thermoneutral zone”. Each organized neural circuitry for heat-loss effector systems are triggered at different higher levels of overheated core or shell temperatures (although these rats regulate their body temperature in cool environment like the controls). These data resemble to the “multiple thermostat”3 or multiple “balance point”4 theories, but provide an example for an impairment in thermoregulation only against overheating of the body, with intact regulation against cold which could not been explained by earlier models.
The role and site of action on salivary glands as a source of thermoregulatory impairment in capsaicin-desensitized rats84 was disproved in a series of experiments of Benedek and Obal Fjr and coworkers.24,82,85-89 They observed no difference between enhanced body temperature of rats treated with 50 mg/kg or 300 mg/kg s.c. capsaicin 1 hour after exposure them to 38°C temperature but concluded that after the lower dose mainly the peripheral warm sensors were impaired while after the higher dose the heat-loss response to preoptic heating was also inhibited.85 After the high dose, lower Tr was recorded below 22°C Ta.90 This might indicate, that POA warmth-sensitive neurons subserve integrative function and after high systemic doses when these neurons are also markedly inhibited in function slightly impaired regulation against cold can also be observed.
Peripheral warming of the scrotal and abdominal skin induced excitation of a subgroup of POA neurons and inhibition of another group of neurons. Both after neonatal pretreatment of rats with capsaicin (50 mg/kg s.c.) or in adult-pretreated rats (250 mg/kg s.c.), significantly less percent of neurons responded by excitation while the percent of insensitive neurons and those which were inhibited remained within the nonsignificant range.91 The altered thermoregulation of capsaicin-desensitized rats was suggested to be due to combined desensitization of preoptic warm sensors92,93 and peripheral warm receptors.75,85,90 Preoptic warming of POA induces in rats relaxation and sleep in cats.93 Sleep and body temperature enhancement in capsaicin-desensitized rats at moderate warm (32°C) Ta is accompanied by an increase in time spent in sleep.94 After intracerebral injection of capsaicin, slow wave sleep periods was lower at 27°C by 3°C than that of the controls while the rapid eye movement sleep was maximum at 33°C Ta being higher than that of the controls indicating that rabid eye movement sleep is under capsaicin-sensitive POA inhibitory control.95 In rats, i.p. administration of 1.5 mg/kg capsaicin enhanced the duration of hexobarbital general anesthesia (reappearance of righting reflex). It was a puzzling observation96 that zingerone a 1000 times less potent vanilloid than capsaicin7,8 from ginger produced in response to doses of 200–400 mg/kg i.p. beyond hypothermia also a general anesthesia for over 20 min. This phenomenon was almost absent in rats desensitized with a total dose of 70–200 mg/kg s.c. given capsaicin 3–50 days before the experiment. General anesthesia evoked by some nonvanilloid pungent ketone structures were also markedly inhibited in the capsaicin pretreated rats. Afterwards no further experiments were made in this line since similar desensitization with capsaicin did not inhibit the effect of pentobarbital or inhalational ether anesthesia96 or urethane.73
Thermoregulation of adult rats which were treated with capsaicin as neonates were also studied.97,98 In these pretreated rats, s.c. or i.p. given capsaicin did not induce heat-loss response with skin vasodilatation, but thermoregulatory operant behavior for skin cooling in a warm environment was similar as in the controls. The loss of selective actions of capsaicin-desensitization in neonatal rats and the role of preoptic warm sensors in effects of capsaicin in these rats9,68 will be discussed later.
Thermoregulation of TRPV1 gene-modulated mice
In TRPV1-gene-deleted (TRPV1−/−) mice, all effects including the pronounced hypothermic effect of capsaicin was completely absent but the basal body temperature remained apparently normal.99,100 The daily body temperature rhythm and level corresponded to that of the controls101,102 albeit in one study101 the difference between the daily minima in light and maxima in dark i.e. the amplitude was slightly, but significantly larger in the TRPV1 KO mice than that in the TRPV1+/+ littermate group. The rise in body temperature after radiant heat exposure (36–37°C) was identical in the two groups of animals in contrast to capsaicin-pretreated wild-type mice where it was clearly enhanced like in the capsaicin-pretreated rats.101
Body temperature, circadian rhythm and thermal tolerance to increased (35°C), or decreased (4°C) Tas remained unaltered in another type of TRPV1−/− mice. Fever evoked by the pyrogen lipopolysaccharide (LPS, 100 μg/kg i.p.) was attenuated.99,102 Note, that after systemic capsaicin desensitization where all functions of the TRPV1-expressing neurons of the body are impaired: fever induced by yeast or LPS are markedly enhanced.73,103 Comparison of various effector mechanisms both autonomic and behavioral ones revealed further features of thermoregulation of TRPV−/− mice. Lower O2 consumption (hypometabolism), lower Tt (enhanced vasoconstriction) and higher locomotor activity were observed.104 Furthermore, since the locomotor activity was measured in a thermogradient setting with stainless-steel grid floor suitable to induce profound effect on cutaneous thermosensation, a significantly lower Ta prevalence was observed in TRPV1 KO mice. It has been concluded based mainly on the vasomotor reaction of the tail that TRPV1 KO mice have significantly higher thermoneutral zone (<31.5°C–>33.5°C vs. 30.5–32°C in controls) and the fact that TRPV1 KO mice preferred lower temperature range was attributed to a compensation to dissipate extra heat originated from the increased locomotor activity. Whether it is due to a diminished input from overlapping thermosensitivity of cutaneous warm and cold receptors which might result enhanced locomotion and wider thermoneutral zone or real changes of core body temperature could not be decided. In addition, an age-associated overweight TRPV−/− mice and tonic peripheral TRPV1-mediated thermoregulatory control are emphasized.104 During fasting, an adaptive daytime hypothermia for saving some energy is significantly greater in wild-type than in TRPV1-KO mice.105
Transgenic small hairpin RNA construct TRPV1 (shRNA-knockdown) mice with 92% selective reduction of TRPV1 channels were normothermic and thermopreference to warm Ta range (30 vs. 35°C) was normal similarly as in TRPV1 KO mice except slightly impaired thermopreference was observed in male mice. Tail vasodilation and hypothermia evoked by s.c. RTX injection was markedly inhibited.106
Effect of point mutations16 and chimeric modification of carboxy terminal domain segments between TRPV1 and TRPV3107 could selectively alter the heat sensitivity and desensitizing ability of the channel including shifting the threshold temperature to the non-noxious, thermoregulatory relevant ranges from 43°C to 27°C. This molecular aspect of TRPV1 heat sensitivity is, however, out of the scope of this chapter. Similarly, briefly is mentioned the thermoregulation of mutant mice with a TRPV1-lineage in which Cre recombinase was expressed under the control of TRPV1 (TRPV1-Cre) and of TRPV1-DTA transgenic mice where TRPV1-Cre was crossed with ROSA-stop-DTA line with complete loss of responsiveness to capsaicin.108 The TRPV1-DTA mutant mice with ablation of TRPV1 TRPV2 and TRPM8 channels although showed complete loss of responsiveness to noxious hot and cold stimuli their resting body temperature and general behavior were indistinguishable from those of littermate controls. Nevertheless, in warm (35°C) or cold (4°C) environments these mutant animals were far less able to maintain their body temperature. Taking together these data with the normal thermopreference of mice deficient in both TRPV3 and TRPV4 genes109 indicate the importance of non-TRP channels in thermoregulation at room temperature without thermal stress. This field including defined alternate thermosensor targets need further studies. On the other hand, a definite role of TRPV1-expressing capsaicin-sensitive neurons in defense against overheating of the body with intact thermoregulatory behavior against cooling is well established.
Evidence for an action of capsaicin on the median preoptic area (POA)
Several lines of evidence support the role of warm-sensitive central thermosensors within the POA in the heat-dissipating acute thermoregulatory effects evoked by capsaicin and in the subsequent long-lasting state of impaired counter-regulation against overheating of the body.8,24,68,82,83 The first functional92 and ultrastructural data93 led to the conclusion that both peripheral and central warm sensors are stimulated and desensitized by capsaicin to their natural thermal stimuli.3,82 The following experimental data support a site of action of capsaicin on POA warm sensors.
Preoptic heating in the rat elicits fall in body temperature with vasodilation and inhibition of shivering. Fig. 4A shows in two control rats that diathermic heating of the POA by 1–4°C above the hypothalamic temperature elicits an immediate fall in body temperature in a temperature-dependent and reproducible fashion.92 After systemic capsaicin treatment, these effects were strongly inhibited in a dose-dependent manner. After the lower dose range of 100–160 mg/kg s.c., capsaicin inhibition was observed only for 1–2 days but the effect of POA heating was diminished after a higher total dose of 204–244 mg/kg for over a month. Quantitative data from these two affected groups are shown in Fig. 4B. In these capsaicin-pretreated rats – unlike those which were pretreated with the smaller dose range 5–7 days before – preoptic heating by 1–3°C evoked smaller fall in body temperature and were less prone to inhibit shivering. I made all of the POA experiments during the sixties and summarized the quantitative data later71 (Fig. 4B and C) which pointed to the fact that heating the hypothalamic area by 1–3°C the fall in body temperature was smaller but heating by 4°C to 41–42°C level was not diminished in the capsaicin-desensitized rats. These findings on one hand suggested involvement of capsaicin-sensitive preoptic neurons in the thermoregulatory heat-loss effects during overheating of the body and on the other hand warns to the importance of measurement the exact temperature near to the thermode what have been often overlooked or underestimated by placing the thermocouple at a few millimeter distance from the heating thermode in some earlier studies (discussed this aspect in detail in ref 71). Tail vasodilation to POA heating is also diminished in capsaicin-pretreated (300 mg/kg s.c.) rats for 1–2 months and heat-loss effect of capsaicin microinjection into the POA was abolished.89 On the other hand, in rats pretreated with a 50 mg/kg s.c. dose of capsaicin in the neonatal age neither inhibited the effect of POA heating in adult age,97 nor induced change in the hypothermia induced by capsaicin microinjection into the POA.110 In the latter study, the animals showed impaired counter-regulation in a warm environment therefore the importance of POA in capsaicin effects was challenged. Note, however, that neurotoxic effects of neonatal capsaicin pretreatment is not restricted only to the TRPV1-expressing neurons.9 Furthermore, several secondary events were also described after neonatal pretreatment111 which might result in faster restitution in the central nervous system than in the periphery. Nevertheless, it has been concluded already in the seventies that beyond the POA warm sensors other capsaicin-sensitive structures are also important in the impaired regulation against overheating of the body in capsaicin-pretreated animals.71,74
Microinjection of capsaicin (2–80 nmol) into the POA elicits immediate dose-dependent fall in body temperature with inhibition of shivering.92 Repeated application in the higher dose range elicited desensitization i.e. the fall in body temperature became progressively smaller. These POA-pretretreated/desensitized rats were shown to have impaired thermoregulation in a warm environment for several days and respond with higher enhancement of body temperature than the solvent treated rats to stressful stimuli e.g. pinching the tail.92 It should be underlined that rats pretreated by desensitizing capsaicin doses in the POA or rats after preoptic electrolytic lesions responded to s.c. capsaicin injection with diminished hypothermic response, but it was never abolished74 suggesting a definite but clearly not exclusive role for POA in the capsaicin-induced effects on thermoregulation. In POA-pretreated rats, heat reinforcement bar pressing behavior in cold Ta was also strongly inhibited,24 similarly as in rats after intracerebroventricular (icv) pretreatment.112 Microinjection of capsaicin into the POA increased the activity of units which respond to local warming but had no effect on thermally-insensitive units. Using multibarrel microelectrodes, capsaicin excited 16/27 warm units, inhibited 12/17 cold units and had no effect on 35/60 thermally-insensitive units. Signs of desensitization to repeated capsaicin application was observed also at single unit level of warm-sensitive neurons.113 In rats pretreated in adult age with 50 mg/kg or 300 mg/kg, s.c. given capsaicin preoptic injection of capsaicin failed to induce hypothermic reaction while pretreatment of rats in neonatal age with 50 mg/kg s.c. capsaicin was without any desensitizing effect.110 Taking the other side of the coin in a warm environment rats desensitized with 35–200 μg of capsaicin injected into the POA showed significantly higher hyperthermia 1–19 days after the treatment than that of the solvent treated or untreated ones.73 Furthermore, POA-treated rats started to escape later from a heat chamber of 40–42°C than the controls but after 40 min their body temperature and warm avoidance behavior were not different from that of the controls.78 Note, in those rats which were pretreated by systemic capsaicin doses cold-seeking behavior was lacking. These observations suggest also the importance of extrahypothalamic warm sensors in behavioral thermoregulation.
Figure 4.
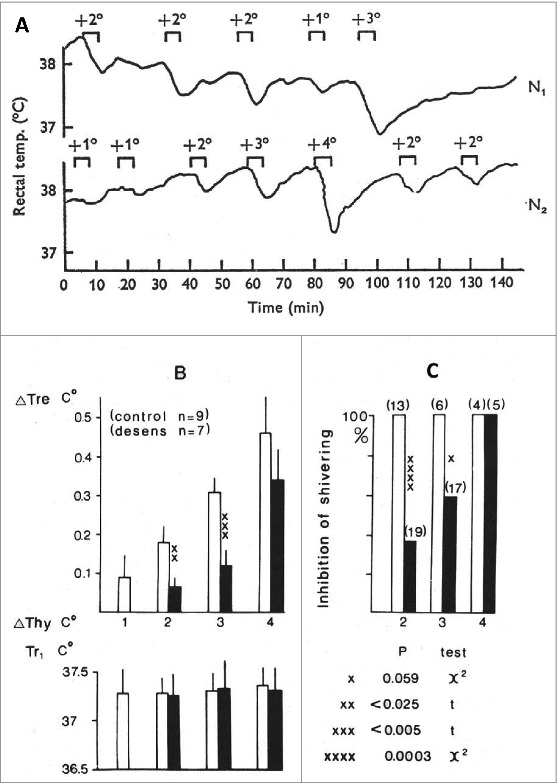
(A) Tr of two controls rats (N1 male 225g, N2 female 235g) in response to diathermy heating of the preoptic area (POA). The increment of POA temperature and duration of heatings are indicated. (B) Fall in body temperature (ΔTre) of rats caused by increasing the hypothalamic temperature (ΔThy, by 1–4°C for 5 min; Ta 20–22°C. Tr1: Tr at the beginning of hypothalamic heating. White (control rats) and black (desensitized rats) columns are averages with standard error of mean. (C) Inhibition of visible shivering during the heating period in percent of trials. Observations were made on four control and five capsaicin-desensitized rats; number of trials indicated in brackets. Statistically significant differences are indicated by crosses. (A) Records from ref. 92, and figures of (B) were calculated also from this series of experiments71). (A) © John Willey and Sons. Permission to reuse must be obtained from the rightsholder. (B) © Elsevier. Permission to reuse must be obtained from the rightsholder.
In the hypothalamus slice preparation, capsaicin evokes glutamate release and enhance EPSCs.114,115 Furthermore, on the medial preoptic nucleus of POA in whole-cell patch-clamp studies not only the frequency of glutamatergic EPSCs but GABA-ergic inhibitory postsynaptic currents were increased by capsaicin in a tetrodotoxin-insensitive manner.116 Microinjection of capsaicin into the rat POA by electrophoretic multibarrel microelectrodes induced activation of warm-sensitive neurons and inhibited the cold-sensitive ones (12/17) while it was ineffective on most of the thermo-insensitive neurons.24,117
Neuronal response of the locus coeruleus (LC) in the rat has been described to be activated by thermal stimuli of both non-noxious and noxious ranges. In capsaicin-desensitized animals, these responses were reduced.118 In whole cell preparation of neurons of slices from LC, miniature EPSCs were evoked by capsaicin (1 μM) which were abolished by capsazepine (10 μM), iodoresiniferatoxin (300 nM) and Ca2+ removal.119 Spontaneous sEPSPs evoked by capsaicin and purinoceptor agonists activated separate receptor systems as evidenced by selective antagonist compounds in LC neurons in a rat brain slice preparation.120 The source and role of these presynaptic neurons, their thermosensitivity and involvement in thermoregulation is, however, not known,121,122 but LC receives afferent innervation also from POA.119
Cutaneous vasodilation and enhanced locomotion can be evoked by intranigral microinjection of capsaicin (100 nmol).123,124 Glutamatergic synaptic transmission to dopaminergic neurons of the substantia nigra are enhanced under in vitro condition by capsaicin (1–10 μM).125 Relevance of these data in thermoregulation have not been raised. In rats, capsaicin microinjection into the medial ventrolateral medulla enhanced the heat production and fever evoked by E coli lipopolysaccharide.126,127 Direct injection of capsaicin (65 nmol) into the dorsal raphe nucleus evokes skin vasodilation and a fall in body temperature128 and this area is also sensitive to local heating24 but the relevance of these results in thermoregulation remained unclear.
Electrophysiological and morphological evidence
It has been established since a long time23 that in the POA a group of neurons increase their firing rate to local heating beyond the major group of temperature-insensitive ones.24,26,67,129 Furthermore, local heating of POA evoked also heat-loss responses.130,131 High Q10 thermosensitive neurons were, however, described in other regions of the brain both in vivo or in vitro132 and heat-loss responses were also evoked by local heating several brain areas122,133 Furthermore, considerable group of warm sensitive POA neurons are also activated in tissue slices by osmotic pressure or glucose.134 Thermosensitive neurons of POA respond also to temperature changes localized to the skin, spinal cord, medulla oblongata or midbrain indicating that they have also an integrative function.24
Nakayama described for the first time, that capsaicin (1 mg/kg) given s.c. to anesthetized rats activated most of the warm sensitive neurons of the POA (17/21) for 40–100 min and inhibited the cold responsive units.25 Similar activation was observed also if capsaicin was injected (5 μg s.c.) to newborn rats.135 In both studies, the excitation started within 5–15 min after the injection and it could be due either to a direct action of capsaicin on these neurons or indirect activation through afferent pathways. Nevertheless, evidence for a direct action of warming on some POA neurons is strong. In a hypothalamic slice preparation from the rat using patch-clamp recording warm-sensitive neurons, temperature-insensitive and cold-sensitive neurons were identified. Warm-sensitive neurons had a threshold around 35–36°C and thermal sensitivity 4.6 spikes/s/°C. Warming above threshold temperatures reversibly induced single channel and spike activities. Single channel currents were clearly identified to heating even in the presence of tetrodotoxin.136 Unfortunately in this study, capsaicin was not tested. Evidence for a direct action of capsaicin was obtained on rats after systemic capsaicin pretreatment since in these rats the percent of thermosensitive neurons was reduced. After a high dose of capsaicin (282 ± 67 mg/kg s.c.), out of 265 units only 10% were warm-sensitive and 3% cold-sensitive while out of 139 units identified in control animals 21% were warm-sensitive and 6.5% cold-sensitive.24,137 Furthermore, in capsaicin-pretreated rats the response of units to repeated heating was diminishing which was not observed on neurons of controls.
Systemic capsaicin pretreatment both in adult and neonatal rats elicits for months pronounced mitochondrial swelling in B-type neurons of trigeminal or DRG without affecting A-type neurons with myelinated fibers or satellite cells (for refs 9, 42, 43). In capsaicin-desensitized rats which were pretreated from few days to several weeks before a milder form of mitochondrial damage in small type neurons situated in the median POA below the anterior commissure was observed by blinded observer in facts well before we described its effect on sensory neurons.93 Recent morphological studies characterized the warm-sensitive neurons of median POA as having their dendrites perpendicular to the third ventricle which displayed EPSP and inhibitory potentials.129 Synaptic structures to cell body of POA neurons with damaged mitochondria from capsaicin-pretreated rats were also commonly seen at the ultrastructural level.
TRPV1 mRNA have been detected in this area,138 but all these data together are still not enough to have a definite identification with all functional, morphological and electrophysiological features, particularly since recently a highly sensitive Cre-induced lineage tracing of TRPV1 (BAC transgenic TRPV1-Cre reporter mice) showed positivity in the projections of trigeminal and vagal afferents but not in the POA. Instead in the caudal hypothalamic area was stained where terminals of capsaicin-sensitive neurons from POA might terminate with higher level of TRPV1 expression.139
Role of capsaicin-sensitive thermosensors in fever and drug development
Fever
Discoveries that after capsaicin treatment warm sensors in the periphery7 and in the POA92 are desensitized to their natural thermal stimuli and that in warm environment capsaicin-pretreated rats could not be regulated against overheating of their body initiated also early studies to test how it influences fever evoked by pyrogens. Febrile response to pyrogens as E. coli LPS or yeast suspension evoked higher rise in body temperature in capsaicin-desensitized rats (100 mg/kg s.c.). Antipyretics as phenylbutazone, acetanilide, aminopyrine or sodium salicylate were fully effective.73 Subsequently, it turned out, that the characteristic biphasic fever evoked by 10 μg/kg LPS in capsaicin-pretreated rats (90 mg/kg s.c.) have similar time course but at a significantly higher level mainly due to an enhanced heat production. In the pretreated rats, the maximum body temperature was 43°C while in the controls about 40.5°C.24,103 The cascade release of proinflammatory cytokines in the rats and in gene-modified mice and particularly the discovery of the molecular mechanism of antipyretic drugs as cyclooxygenase inhibitors focused on the role of prostaglandins (PGs) and their action as the final mediator of the cascade to induce fever in the hypothalamus during infection.140 Milton and Wendlandt reported already in 1971 that icv. PGE1 enhances body temperature with vigorous shivering.141 In capsaicin-desensitized rats (150 mg/kg s.c.), PGE2 microinjection into the POA (25 ng) or into the lateral ventricle (100 ng) elicited similar rise in body temperature as in the control rats74 which might indicate that PGE2 does not act on the capsaicin-sensitive POA warm sensors. More recently, surprising discovery was made by Székely, Balaskó and Romanovsky (1997) who observed that in rats pretreated with small intraperitoneal capsaicin doses LPS fever (10 μg/kg iv) was markedly inhibited while after high subcutaneous injection higher rise in body temperature occurred.142 It was proposed that endogenous pyrogens by releasing endovanilloid TRPV1 agonists from the abdominal organs act on capsaicin-sensitive afferents and if this mechanism is selectively abolished release of PGs in the POA is also prevented in this way. These results were subsequently confirmed and disappearance of the first phase of LPS fever was reported after i.p. injection of 2 + 3 mg/kg capsaicin.143 Furthermore, a more detailed study revealed that 5 mg/kg i.p. capsaicin pretreatment 10 days before the LPS injection (10 μg/kg iv) abolished completely the first phase and part of the second phase. Slightly different effects were observed if instead of capsaicin another TRPV1 agonist RTX was used. After 20 μg/kg i.p. RTX, the first phase was attenuated, but the second and third febrile phases tended to be higher than in the solvent-treated controls similarly after a higher dose (200 μg/kg) when these effects were more pronounced.144 In respect of the role of capsaicin-sensitive abdominal afferents in the first phase of fever involvement of hepatic branches of the vagal nerve21 but not the splanchnic nerves were shown.146 Since after 20 μg/kg i.p. RTX pretreatment abolished the hyperthermia induced by a TRPV1 antagonist AMG0347, the importance of this vagal mechanism was emphasized in the on-target side effect of TRPV1 antagonists. This i.p. RTX pretreatment did not inhibit the capsaicin-induced eye-wiping response, or the pulmonary Bezold–Jarish reflexes to instillation capsaicin into the eye or given intravenously, respectively. Since it had neither an effect on nociception detected on hot plate but abolished the writhing response to i.p. given irritants it has been concluded that this pretreatment desensitizes only the TRPV1-expressing afferents from the abdominal organs, but not from other parts of the body.69 TRPV1 is excited by several endogenous chemical mediators while threshold temperature for opening the TRPV1 channel is in the nociceptive (>43°C) but not in the thermoregulatory range.15,16 Therefore, on the basis of the above results the authors concluded that activated TRPV1 channels by nonthermal chemical stimuli in abdominal viscera tonically inhibit the autonomic cold-defense effectors in thermoregulation.69 Cold-seeking behavior against overheating of the body in a thermopreference paradigm is, however, similarly inhibited by i.p. RTX pretreated rats performed in abdominal denervated (bilateral vagotomy plus splanchnicotomy) and sham-operated animals (Bölcskei et al. unpublished results from our laboratories). Therefore, nonabdominal TRPV1-expressing afferents also seems to play significant role in these experiments. Furthermore, as discussed earlier peripheral and central terminals of capsaicin-sensitive afferents and sensitivity to warmth stimuli could also play an important role in heat defense and TRPV1-targeted drug actions. The advantage of heat avoidance models is that they exclude interventional sites on neural circuitries of various effectors in keeping a stable body temperature. For example after depletion of brain serotonin by parachlorophenylalanine similar hyperthermia was observed in warm Ta as after systemic capsaicin pretreatment but in the former case the impaired operation of the effector systems were compensated by faster cold-seeking behavior74 while in the latter case the opposite effect was found as described earlier.
Capsaicin, thermoregulation and drug development
Cloning the nocisensor TRPV1 capsaicin receptor has opened promising perspectives to introduce a new chapter of analgesic drugs acting on nociceptors. After testing the first drug candidates, their potential hyperthermic on-target side effect appeared as major challenge.69,145,147 TRPV1 is an unorthodox cation channel which in the strict sense is not a ligand-gated channel to convey special chemical message between two cells by a defined chemical ligand and is neither a voltage-gated one under physiological conditions and particularly it is a thermosensor. Point mutations in this membrane protein revealed that mutants with intact thermal responsiveness without vanilloid or proton sensitivity have been already constructed. Thus, TRPV1 operates in a “multisteric” way i.e. various conformational changes of the TRPV1 protein play role in opening the channel by different stimuli. This special feature provides clues for combinatorial chemistry to develop second-generation TRPV1 antagonists with potent analgesic potency but without effect on thermoregulation or heat sensation.16,148 Further possibilities for developing nocisensor blockade analgesics are under way by revealing potential sites on vesicular trafficking, scaffold proteins, TRPV1 phosphorylation by intracellular kinases. Characterizing endogenous ligands in different painful conditions and their selective blocked or to reveal inhibitor mediators released in tissues which could be utilized to produce analgesic effect by acting on capsaicin-sensitive nociceptors are also under investigation. These aspects are, however, not within the scope of this overview and were summarized by the author in another review.9
General overview, comments and a hypothesis
Early reviews on the thermoregulatory effects of capsaicin
The thermoregulatory effects of capsaicin including both the immediate profound heat-loss response evoked by parenteral or preoptic application and the long-term “desensitizing” effect which manifests itself in blockade of thermoregulatory effects of capsaicin-type pungent agents and against overheating of the body with intact body temperature at room temperature, have been analyzed from the fifties of the last century. All these early data including conference abstracts published up to 1980 had been already collected and reviewed by the author in 1982.8 These studies revealed that effects of capsaicin are mediated by stimulation followed by desensitization of central (POA) and peripheral warm sensors. This state after two days of hyperthermia with enhanced metabolic rate was followed by a state of normal basal core temperature in thermoneutral environment when activation of thermoeffectors (increase in metabolic rate, cutaneous vasoconstriction) in cold environment remained unimpaired. In humans, topical desensitization impaired sensation of warmth while difference limen in cold range and threshold concentration of menthol, a TRPM8 agonist which induce cold sensation were not altered.7
All conceptual models of thermoregulation provide operational mechanisms for a coordinated regulation of several effectors in normal condition and in fever. During a feverish state body temperature is shifted to a higher level where heat-loss and heat-gain effectors are switched on and of similarly as in normothermic condition.1-4,30 How could in capsaicin-pretreated/desensitized animals operate their thermoregulatory system around a normothermic level when only input signals from warm sensors are diminished? The question still remained open after this Hungarian discovery and was analyzed further mainly by Japanese groups of Nakayama and Tetsuro Hori by using single unit recordings from the hypothalamus. These results were summarized together with some of further important findings in a thorough review.24 In 1986, full list of papers published in refereed journals on capsaicin from 1978 to 1983 including those on thermoregulation was summarized by Buck and Burks81 Five years later also in the series of Pharmacology Reviews a full overview on actions of capsaicin was written by Peter Holzer.111
Novel theory on the role of capsaicin receptor TRPV1 in thermoregulation
After cloning the receptor of capsaicin TRPV1 which turned out to be a thermosensor cation channel with a temperature threshold around the heat nociceptive (43°C) range but gated also by several irritants and endogenous lipid-type mediators a burst of interest for developing nociceptor blocking analgesics were initiated worldwide in drug industry. From this point of view, thermoregulatory effects of capsaicin appeared as side effect70,147 since some TRPV1 antagonist drug candidates with promising analgesic profile induced hyperthermia or diminished noxious heat sensitivity inducing burn risk which prevented their therapeutic application. On the other hand, identification of TRPV1 introduced powerful experimental tools with advanced techniques (gene-modulated mice, recording EPSC from TRPV1-expressing presynaptic nerve terminals, usage of TRPV1 antagonists, tracing TRPV1-expressing neural structures etc.). They have opened the horizons to reveal new aspects to analyze thermoregulatory mechanism by means of capsaicin.
These data already resulted in a new concept for thermoregulation proposed by Romanovsky and his coworkers.4,5,22,69,70,104 In this review, more recent further data are included and for the action of TRPV1-expressing neurons in thermoregulation another hypothesis is proposed, particularly about the role of visceral TRPV1-expressing receptors.
Summary of thermosensory and thermoregulatory findings on TRPV1 knockout mice provide evidence, that lack of TRPV1 channel by itself induces no major alteration in core body temperature.68,99-101 Beyond mediation of thermal hyperalgesia and noxious pain function, the role of these capsaicin receptors in thermoregulation remained enigmatic. Specific contribution of TRPV1 in temperature regulation as a thermosensor seemed unlikely owing to its high-temperature threshold (>43°C) and involvement of other thermo-TRPV channels (TRPV3, TRPV4) with lower (>32°C) thermal threshold were neither supported.109
TRPV1 channels are, however, highly sensitive to endogenous chemical agents and some striking paradoxical observations made a starting point of raising an alternative theory for thermoregulations in which TRPV1 serves not as a thermosensor but as a chemosensor for tonic inhibition from the abdominal receptors.4,5 In rats desensitized by high systemic doses of capsaicin, fever is markedly enhanced, but surprisingly i.p. desensitization with a small dose of TRPV1 agonist RTX inhibited the fever.142,143 These data suggested that in the abdominal viscera release of endogenous mediators initiate the fever response and chemically excitable abdominal TRPV1-expressing nerve terminals play in this effect the pivotal role.4,5,22,69,70,104 Since after a small i.p. RTX dose the eye-wiping test of nociception and pulmonary Bezold–Jarish reflex were not inhibited, it was assumed that the sensory desensitizing effect is restricted to the abdominal cavity and they serve as tonic inhibition heat gain mechanisms in the thermoregulatory system to counteract overheating of the body.69 Caveat is needed, however, to deduce from these experiments that only the abdominal organs were desensitized. It is a characteristic feature of capsaicin desensitization described for the eye-wiping test that after smaller capsaicin doses which induce incomplete desensitization the first responses are not inhibited and clear blockade appears after repeated applications.9,13,42 Furthermore, slow depolarization of membrane potential evoked by RTX is unsuitable to evoke the Bezold–Jarish reflex in rats in spite of the fact that its action on these sensory receptors was demonstrated by the fact of desensitization induced by the application.149 Thus, without burst of firing on these pulmonary receptors (similarly as evoked by instillation of capsaicin into the eye) is needed to evoke the reflex responses. In contrast during core body temperature regulation tonic frequency of firing but not fast bursts are needed. According to our observations (Bölcskei et al. unpublished), denervation of abdominal viscera does not interfere with the effect of sensory desensitization evoked by small i.p. dose of RTX. Consequently in drug research abolishment of hypethermia in rats pretreated with small i.p. dose of RTX70 is not necessarily prove that this side effect of TRPV1 antagonist can be attributed to an effect on blocking chemosensitivity of abdominal TRPV1-expressing sensory nerve terminals.
Novel capsaicin-sensitive core body warmth sensors
In the light of more recent results and some early observations summarized in this review, the following new informations should be considered to explain the thermoregulatory effects of capsaicin.
TRPV1-expressing capsaicin-sensitive afferents with warm sensor features as increasing their firing rate in innocuous range (e.g. from 35°C to 41°C) have been identified both from the thoracic and abdominal organs. Thus, their threshold is much lower than that observed on transfected cell lines, sensory neurons in culture or on cutaneous polymodal nociceptors. Warm sensors in visceral organs have similar thermosensitivity as the canonical cutaneous warmth receptors, and they have capsaicin sensitivity.
In the nucleus of the NTS on one set of neurons from TRPV1+/+ mice but never in the TRPV1 knockouts glutamatergic, capsaicin-sensitive EPSCs are evoked which are also activated by innocuous temperature stimuli. Thermal activation occurred only on TRPV1+/+ neurons. Thus, both peripheral and central nerve terminals of vagal sensory neurons of abdominal and thoracic organs could serve as thermal signals for thermoregulation. This new scope of visceral warm sensors in thermoregulation was in respect of vagal afferentation hitherto not considered as inputs for thermoregulatory circuitries to trigger effector responses. Further experiments are needed to support their role in thermoregulation and reveal their pathways within the central nervous system.
In respect of the splanchnic nerve, warm receptors were described and their central terminals in the lamina I and II of the spinal cord were identified. Furthermore, works of Eckhard Simon and coworkers revealed65,66 that heating the lumber spinal cord elicits heat-loss responses similarly as POA-heating.
Action of capsaicin on warm sensors of the preoptic anterior hypothalamic regions (POA) is well established albeit some important details remained still open. Table 1 summarizes the evidence for the actions of capsaicin on POA warm sensors.
Table 1.
Evidence for the thermoregulatory function of capsaicin-sensitive warm sensors in the POA
| 1) Microinjection of capsaicin induces dose-dependent immediate coordinated heat-loss response. |
| 2) After repeated microinjections desensitization to the effects of capsaicin occurs. |
| 3) POA-desensitized rats in subsequent days show impaired physiological and behavioral regulation against overheating in warm Ta and fall in body temperature to s.c. capsaicin is inhibited but not abolished. |
| 4) Microiontophoretic application of capsaicin to warm-sensitive POA neurons enhanced their firing rate and inhibited the activity of the cold one. |
| 5) Systemic injection of capsaicin to thermocontrolled POA induces pronounced activation of the warm-sensitive units and inhibition of the cold-sensitive ones. |
| 6) After systemic capsaicin pretreatment a long-term reduction ofa) heat-loss response to POA heating or to capsaicin microinjection into the POA occursb) the proportion of warm or cold units in the POA is reduced by about 50%c) mitochondrial swelling milder than in the dorsat root ganglia in a group of POA neurons below the anterior commissure was observed in electronmicroscopic preparations. |
| 7) After preoptic lesions heat-loss responses evoked by subcutaneous capsaicin injection was diminished, shortened but never abolished. |
| 8) Presence of TRPV1 in the POA region has been demonstrated by mRNA138 or H3RTX-binding assays150 but by Cre-reporter TRPV1 positivity was described not in the POA but in the dorsal part of the hypothalamus.108,139 |
Role of POA warm sensors in thermoregulation
Results of Table 1 with references of 1–7 points have been discussed earlier, but some comments are needed to the last point.
In respect of morphological studies on some small neurons in the median preoptic nucleus, similar ultrastructural changes were observed as in DRG neurons but synapses on them seemed to support their integrative function.93 External heating to 40°C elicited c-fos activation in the median nucleus of the preoptic area and nucleus of dorsomedial hypothalamus.151 In addition, POA heating152 or capsaicin at 36°C but not at 22°C induced c-fos activation in the supraoptic nucleus suitable to produce in this way by a POA-heating-mediated water retention.152,153 mTRPV1 expression.138 H3RTX binding in the POA region150 also supports a site of action of capsaicin in this brain region beyond several other brain regions. The regulatory function of extrahypothalamic TRPV1-expressing neurons is still unknown, particularly since they could not be detected by Cre-reporter TRPV1 signals.108,139 To resolve this apparent conflict of data, low level of TRPV1 in several brain areas which is under the detecting level of Cre-recombinant TRPV1 technique was raised.154
POA thermosensor units in most cases respond also to heating the skin24 and the percent of neurons which are activated by cutaneous heating is markedly less in rats pretreated with high dose of capsaicin (300 mg/kg s.c.) or also after neonatal pretreatment (50 mg/kg s.c.).91 It is surprising, however, that responses of POA warm sensors to local heating is inhibited after this high dose of adult pretreatment but remained intact after neonatal pretreatment.98 These data clearly show on one hand that POA warm sensors have inputs from other thermosensors and in this way could form an integrative function. On the other hand, difference exist in respect of POA function between adult and neonatal pretreated rats in respect of heat sensitivity of POA neurons while in both groups of pretreated rats input signals from cutaneous warm receptors were similarly inhibited.
Fig. 5 shows a schematic representation of potential warmth sensors in thermoregulation based mainly on functional, electrophysiological data related to the capsaicin-sensitive TRPV1-expressing warm-sensitive neurons or nerve terminals. The main new message is that beyond the thermosensors within the brain and particularly in the POA (A), thoracic and abdominal visceral receptors and central presynaptic terminals of capsaicin-sensitive sensory neurons of the vagal and splanchnic nerves also might play an important role to monitor core body temperature (B, C, D). Changes in Ta influence cutaneous warm sensors (E), but their central terminals in the spinal dorsal horn (D) or nucleus tractus trigemini (NTG) (B) are also thermosensitive sites which may contribute also in signaling core body temperature. Out of the extrahypothalamic brain nuclei which may participate in warmth sensation the LC is indicated but some evidence were also reported to other brain areas e.g. in respect of periaqueductal gray matter.127
Figure 5.
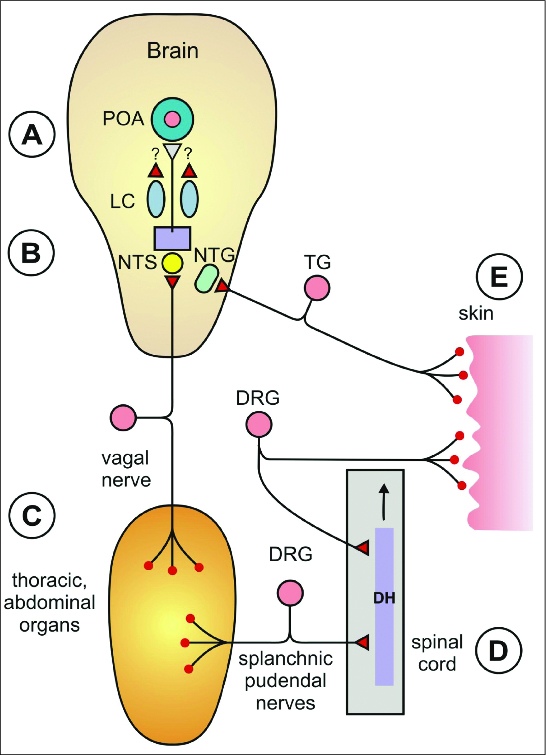
Schematic representation of involvement of TRPV1-expressing capsaicin-sensitive neural elements suitable for signaling as warmth sensors for thermoregulation. (A) Brain with brainstem, POA median preoptic area of the hypothalamus, LC locus coeruleus. (B) NTS nucleus of the solitary tract NTG caudal nucleus of the trigeminal nerve. (C) Thermosensors from thoracic and abdominal organs. (D) Spinal card, DH dorsal horn; (E) skin with warm receptors. TG trigeminal ganglion, DRG dorsal root ganglion. Pink color: TRPV1-expressing neurons, red circles: capsaicin-sensitive warmth sensory receptors, red triangles: capsaicin-sensitive presynaptic nerve terminals sensitive to warmth stimuli.
Role of TRPM8 in thermoregulation
Regarding the potential role of TRPM8 in capsaicin-sensitive neurons the following comments are needed. Capsaicin pretreatment induces loss of all functions of the TRPV1-expressing neural structures including other thermoresponsive channels coexpressed with it.
Recent data using mice engineered to express the enhanced green fluorescent protein (EGFP) from the TRPM8 locus (TRPM8EGFP) resulted in EGFP-positive DRG neurons from homozygote mice.155 These EGFP-positive neurons form a distinct population of neurons from the TRPV1-expressing peptidergic and nonpeptidergic DRG neural populations. TRPM8 and TRPV1 coexpression was found only in 1% of DRG neural population. The peripheral projections of TRPM8-stained neurons form cold spots with unique endings in the superficial layer of the epidermis.155 These cells without functional TRPM8 channels lose their responsiveness to menthol or cold stimuli supporting further evidence that TRPM8 is the major sensor of environmental cold temperatures which at sensory neuron level is not involved with additional responsiveness to warmth stimuli or capsaicin.
Nevertheless, in a group of colonic splanchnic afferents some RT-PCR immunofluorescence of TRPM8 protein was described and single fiber recording from these colonic afferents showed a group of units which were activated by both icilin, a TRPM8 agonist and capsaicin in 3 μM concentration.156 Single unit studies from other visceral organs showed either warm or cold sensitive units but no receptors were found with one exception52 which could increase the firing rate in a U-type form in the innocuous temperature range. Therefore, the classical concept of existence of separate afferentation from cutaneous cold sensors and warmth sensors for triggering distinct neural circuitries got also strong support from recent data of engineering various thermo-TRP proteins in respect of visceral, brain and spinal thermosensors. Note, that the other thermo-TRP channel of TRPA115 which is gated by noxious cold stimuli is coexpressed with TRPV1 in nociceptive neurons but there is no evidence that it plays any role in thermoregulation.157
As it has been mentioned in control animals and on the human skin, all single-unit studies support that cold receptors and warm receptors form different populations and this seems to be valid also to visceral afferent fibers, and preoptic thermosensitive unit responses. Furthermore, capsaicin did not induce stimulation or desensitization of single cold fiber responsiveness to cooling, and on the human tongue capsaicin desensitization impaired warmth sensation but left intact cold sensation and the sensory effect of TRPM8 agonist of menthol.7,9,10
It is remarkable, that about 50% of vagal afferents express TRPV115 and interoceptors together with central terminals of both exteroceptive and interoceptive TRPV1-expressing capsaicin-sensitive neurons are activated by innocuous warmth stimuli. These data form a novel possible input for thermoregulation against overheating of the body. Sensory desensitization of all these neural structures results in a long-term state of one-sided impairment of thermoregulation in which warm-seeking behavior in cold environment remains unaltered. Molecular and system biological repertoire of analysis adjustment of homeothermy in these animals could serve interesting starting points for further research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
The author gives thanks to Brigitta Incze for the thorough typing of the text and to the artistic work of Ágnes Kemény for drawing Figure 5.
Funding
This work was supported by grant KTI_NAP_13-1-2013-0001.
About the Author
János Szolcsányi
is Professor emeritus and the former head of the Department of Pharmacology and Pharmacotherapy University Medical School of Pécs in Hungary. He is the member of the Hungarian Academy of Sciences. He has a life-long interest to study capsaicin-sensitive sensory mechanisms to open new horizons for the pharmacology and physiology of nociceptors and reveal their role in function on warmth sensors in thermoregulation. He described the first evidence for the existence of a pharmacological receptor for capsaicin which was cloned two decades later and denoted now as TRPV1. He revealed the site of action of capsaicin on warmth sensors in the skin and preoptic area of the hypothalamus which after activation are selectively put out of function. The role and operation features of TRPV1 and other receptors on nociceptors paved the way for developing novel groups of analgesic/anti-inflammatory agents pointing to ways of avoiding possible thermoregulatory on-target side effects. Novel sensory-efferent and systemic sensocrine functions of these nociceptive endings and their participation in pathological experimental models and human diseases belong also to his main field of interest.
References
- 1.Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol 1985 2006; 100:1347-54; PMID:16540713; http://dx.doi.org/ 10.1152/japplphysiol.01064.2005 [DOI] [PubMed] [Google Scholar]
- 2.Bligh J. A theoretical consideration of the means whereby the mammalian core temperature is defended at a null zone. J Appl Physiol 2006; 100:1332-7; PMID:16540711; http://dx.doi.org/ 10.1152/japplphysiol.01068.2005 [DOI] [PubMed] [Google Scholar]
- 3.Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science 1978; 201:16-22; PMID:351802; http://dx.doi.org/ 10.1126/science.351802 [DOI] [PubMed] [Google Scholar]
- 4.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 2007; 292:R37-46; PMID:17008453; http://dx.doi.org/ 10.1152/ajpregu.00668.2006 [DOI] [PubMed] [Google Scholar]
- 5.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 2009; 61:228-61; PMID:19749171; http://dx.doi.org/ 10.1124/pr.109.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi S. Temperature-sensitive neurons in the hypothalamus: A new hypothesis that they act as thermostats, not as transducers. Prog Neurobiol 1989; 32:103-35; PMID:2645618; http://dx.doi.org/ 10.1016/0301-0082(89)90012-9 [DOI] [PubMed] [Google Scholar]
- 7.Szolcsányi J. A pharmacological approach to elucidation of the role of different nerve fibers and receptor endings in mediation of pain. J Physiol (Paris) 1977; 73:251-9; PMID:25479918 [PubMed] [Google Scholar]
- 8.Szolcsányi J. Capsaicin-type pungent agents producing pyrexia In: Handbook of Experimental Pharmacology. Milton AS. ed; Springer Verlag, Berlin; 1982; 60:437-78. [Google Scholar]
- 9.Szolcsányi J, Pintér E. Transient receptor potential vanilloid 1 as a therapeutic target in analgesia. Expert Opin Ther Targets 2013; 17:641-567; PMID:23421411; http://dx.doi.org/ 10.1517/14728222.2013.772580 [DOI] [PubMed] [Google Scholar]
- 10.Szolcsányi J. Disturbances of thermoregulation induced by capsaicin. J Thermal Biol 1983; 8:207-12; http://dx.doi.org/ 10.1016/0306-4565(83)90106-7 [DOI] [Google Scholar]
- 11.Szolcsányi J. Capsaicin and nociception. Acta Physiol Hung 1987; 69:323-2; PMID ; PMC 1192532. [PubMed] [Google Scholar]
- 12.Szolcsányi J, Jancsó-Gábor A. Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency. Arzneim Forsch (Drug Res) 1975; 25:1877-81; PMID:1243658 [PubMed] [Google Scholar]
- 13.Szolcsányi J, Jancsó-Gábor A. Sensory effects of capsaicin congeners. II. Importance of chemical structure and pungency in desensitizing activity of capsaicin-type compounds. Arzneim Forsch (Drug Res) 1976; 26:33-7; PMID:947170 [PubMed] [Google Scholar]
- 14.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 15.Nilius B, Szállási A. Transient receptor potentials as drug targets: from science of basic research to the art of medicine. Pharmacol Rev 2014; 66:676-814; PMID:24951385; http://dx.doi.org/ 10.1124/pr.113.008268 [DOI] [PubMed] [Google Scholar]
- 16.Szolcsányi J, Sándor Z. Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol Sci 2012; 33:646-55; PMID:23068431; http://dx.doi.org/ 10.1016/j.tips.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 17.Szolcsányi J. Capsaicin-sensitive sensory nerve terminals with local and systemic efferent functions: facts and scopes of an unorthodox neuroregulatory mechanism. Prog Brain Res 1996; 113: 343-59; PMID:9009744; http://dx.doi.org/ 10.1016/S0079-6123(08)61097-3 [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular pain of heat hyperalgesia. Curr Neuropharmacol 2006; 4:197-206; PMID:18615146; http://dx.doi.org/ 10.2174/157015906778019554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol 2008; 155:1145-62; PMID:18806809; http://dx.doi.org/ 10.1038/bjp.2008.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Hasan R, Zhang X. The basal thermal sensitivity of the TRPV1 ion channel is determined by PKCβII. J Neurosci 2014; 34:8246-58; PMID:24920628; http://dx.doi.org/ 10.1523/JNEUROSCI.0278-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanovsky AA. Thermoregulatory manifestations of systemic inflammation: lessons from vagotomy. Auton Neurosci 2000; 85:39-48; PMID:11189025; http://dx.doi.org/ 10.1016/S1566-0702(00)00218-6 [DOI] [PubMed] [Google Scholar]
- 22.Garami A, Almeida MC, Nucci TB, Hew-Butler T, Soriano RN, Pakai E, Nakamura K, Morrison SF, Romanovsky AA. The TRPV1 channel in normal thermoregulation: what have we learned from experiments using different tools? In: Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and Other Pathological Disorders. Gomtsyan A, Faltynek CR eds; Wiley Press, Hoboken, NJ; 2010; pp.349-402. [Google Scholar]
- 23.Hardy JD, Hellon RF, Sutherland K. Temperature-sensitive neurones in the dog's hypothalamus. J Physiol 1964; 175:242-53; PMID:14241166; http://dx.doi.org/ 10.1113/jphysiol.1964.sp007515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori T. Capsaicin and central control of thermoregulation. Pharmac Ther 1984; 26:389-416; PMID:6085515; http://dx.doi.org/ 10.1016/0163-7258(84)90041-X [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Suzuki M, Ishikawa Y, Nishio A. Effects of capsaicin on hypothalamic thermosensitive neurons in the rat. Neurosci Lett 1978; 7:151-5; PMID:19605104; http://dx.doi.org/ 10.1016/0304-3940(78)90159-3 [DOI] [PubMed] [Google Scholar]
- 26.Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol 1986; 48:639-54; PMID:3010828; http://dx.doi.org/ 10.1146/annurev.ph.48.030186.003231 [DOI] [PubMed] [Google Scholar]
- 27.Morrison Shaun F, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 2011; 16:74-104; PMID:21196160; http://dx.doi.org/ 10.2741/3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Morrison Shaun F. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 2010; 107:8848-53; PMID:20421477; http://dx.doi.org/ 10.1073/pnas.0913358107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura K, Morrison Shaun F. A thermosensory pathway that controls body temperature. Nat Neurosci 2008; 11:62-71; PMID:18084288; http://dx.doi.org/ 10.1038/nn2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hensel H. Neural processes in thermoregulation. Physiol Rev 1973; 53:948-1017; PMID:4355518 [DOI] [PubMed] [Google Scholar]
- 31.Konietzny F, Hensel H. The dynamic response of warm units in human skin nerves. Pflugers Arch 1977; 370: 111-4; PMID:561379; http://dx.doi.org/ 10.1007/BF00707956 [DOI] [PubMed] [Google Scholar]
- 32.Konietzny F, Hensel H. The effect of capsaicin on the response characteristics of human C-polymodal nociceptors. J Therm Biol 1983; 8:213-5; http://dx.doi.org/ 10.1016/0306-4565(83)90107-9 [DOI] [Google Scholar]
- 33.Hellon RF, Hensel H, Schafer K. Thermal receptors in the scrotum of the rat. J Physiol 1975; 248:349-57; PMID:1151787; http://dx.doi.org/ 10.1113/jphysiol.1975.sp010978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierau F-K, Torrey P, Carpenter DO. Afferent nerve fiber activity responding to temperature changes of scrotal skin of the rat. J Neurophysiol 1975; 38:601-12; PMID:1127459 [DOI] [PubMed] [Google Scholar]
- 35.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol 1993; 69:1684-99; PMID:8509832 [DOI] [PubMed] [Google Scholar]
- 36.Foster RW, Ramage AG. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharm 1981; 20:191-8; PMID:7207713; http://dx.doi.org/ 10.1016/0028-3908(81)90203-3 [DOI] [PubMed] [Google Scholar]
- 37.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 1991; 66:212-27; PMID:1919668 [DOI] [PubMed] [Google Scholar]
- 38.Largent-Milnes TM, Hegarty DM, Aicher SA, Andresen MC. Physiological temperatures drive glutamate release onto trigeminal superficial dorsal horn neurons. J Neurophysiol 2014; 111:2222-31; PMID:24598529; http://dx.doi.org/ 10.1152/jn.00912.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salt TE, Hill RG. The effects of microiontophoretically applied capsaicin and substance P on single neurones in the rat and cat brain. Neurosci Lett 1980; 20:329-34; PMID:6160433; http://dx.doi.org/ 10.1016/0304-3940(80)90169-X [DOI] [PubMed] [Google Scholar]
- 40.Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 2009; 28:1308-18; PMID:19279663; http://dx.doi.org/ 10.1038/emboj.2009.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bavassano C, Marvaldi L, Langeslag M, Sarg B, Linder H, Klimaschewski L, Kress M, Ferrer-Montiel A, Knaus HG. Identification of voltage-gated K(+) channel beta 2 (Kvβ2) subunit as a novel interaction partner of the pain transducer Transient Receptor Potential Vanilloid 1 channel (TRPV1). Biochim Biophys Acta 2013; 1833:3166-75; PMID:24036102; http://dx.doi.org/ 10.1016/j.bbamcr.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 42.Szolcsányi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004; 38: 377-84; PMID:15567473; http://dx.doi.org/ 10.1016/j.npep.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 43.Szolcsányi J. Actions of capsaicin on sensory receptors In: Capsaicin in the study of pain. Wood JN. ed; London: Academic Press; 1993; pp 1-26. [Google Scholar]
- 44.Yamashita H, Wang Z, Wang Y, Furuyama T, Kontani Y, Sato Y, Mori N. Impaired basal thermal homeostasis in rats lacking capsaicin-sensitive peripheral small sensory neurons. J Biochem 2008; 143:385-93; PMID:18079164; http://dx.doi.org/ 10.1093/jb/mvm233 [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 2005; 493:596-606; PMID:16304633; http://dx.doi.org/ 10.1002/cne.20794 [DOI] [PubMed] [Google Scholar]
- 46.Takashima Y, Ma L, McKemy DD. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience 2010; 25:828-42; PMID:20580783; http://dx.doi.org/ 10.1016/j.neuroscience.2010.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawson RO, Quick KP. Localization of intra-abdominal thermoreceptors in the ewe. J Physiol 1972; 222:665-7; PMID:5033027; http://dx.doi.org/ 10.1113/jphysiol.1972.sp009820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozsa Z, Mattila J, Jacobson ED. Substance P mediates a gastrointestinal thermoreflex in rats. Gastroenterology 1988; 95:265-76; PMID:2455668 [DOI] [PubMed] [Google Scholar]
- 49.Riedel W. Warm receptors in the dorsal abdominal wall of the rabbit. Pflugers Arch 1976; 361:205-6; PMID:129763; http://dx.doi.org/ 10.1007/BF00583468 [DOI] [PubMed] [Google Scholar]
- 50.Adelson DW, Wei JY, Kruger L. Warm-sensitive afferent splanchnic C-fiber units in vitro. J Neurophysiol 1997; 77:2989-3002; PMID:9212251 [DOI] [PubMed] [Google Scholar]
- 51.Bielefeldt K. Differential effects of capsaicin on rat visceral sensory neurons. Neuroscience 2000; 101:727-36; PMID:11113321; http://dx.doi.org/ 10.1016/S0306-4522(00)00375-4 [DOI] [PubMed] [Google Scholar]
- 52.El Quazzani T, Mei N. Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology 1982; 83:995-1001; PMID:7117811 [PubMed] [Google Scholar]
- 53.Ni D, Gu Q, Hu H-Z, Gao N, Zhu MX, Lee L-Y. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 2006; 291:R541-50; PMID:16513770; http://dx.doi.org/ 10.1152/ajpregu.00016.2006 [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol. 2014; 286:G983-91; PMID:14726308; http://dx.doi.org/ 10.1152/ajpgi.00441.2003 [DOI] [PubMed] [Google Scholar]
- 55.Ni D, Lee L-Y. Effect of increasing temperature on TRPV1-mediated responses in isolated rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 2008; 294:L563-71; PMID:18178674; http://dx.doi.org/ 10.1152/ajplung.00336.2007 [DOI] [PubMed] [Google Scholar]
- 56.Ruan T, Gu Q, Kou YR, Lee Lu Y. Hyperthermia increases sensitivity of pulmonary C-fibre afferents in rats. J Physiol 2005; 565:295-308; PMID:15760937; http://dx.doi.org/ 10.1113/jphysiol.2005.084319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Sun B, Li Q, Luo P, Dong L, Rong W. Sensitivity of bronchopulmonary receptors to cold and heat mediated by transient receptor potential cation channel subtypes in an ex vivo rat lung preparation. Respir Physiol Neurobiol 2011; 177:327-32; PMID:21621010; http://dx.doi.org/ 10.1016/j.resp.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 58.Coleridge JC, Coleridge HM. Afferent vagal C-fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 1984; 99:1-110; PMID:6695127; http://dx.doi.org/ 10.1007/BFb0027715 [DOI] [PubMed] [Google Scholar]
- 59.Green JF, Schmidt ND, Schultz HD, Roberts AM, Coleridge HM, Coleridge JC. Pulmonary C-fibers evoke both apnea and tachypnea of pulmonary chemoreflex. J Appl Physiol Respir Environ Exerc Physiol 1984; 57:562-7; PMID:6469822 [DOI] [PubMed] [Google Scholar]
- 60.Xia L, Bartlett D Jr, Leiter JC. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol 2011; 176:21-31; PMID:21276877; http://dx.doi.org/ 10.1016/j.resp.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS One 2011; 6:e25015; PMID:21949835; http://dx.doi.org/ 10.1371/journal.pone.0025015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci 2010; 30:14470-5; PMID:20980604; http://dx.doi.org/ 10.1523/JNEUROSCI.2557-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andresen MC, Hofmann ME, Fawley JA. The un-silent majority - TRPV1 drives “spontaneous” transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regular Integr Comp Physiol 2012; 303:R1207-16; PMID:23076872; http://dx.doi.org/ 10.1152/ajpregu.00398.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McDougall SJ, Andresen MC. Independent transmission of convergent visceral primary afferents in the solitary tract nucleus. J Neurophysiol 2013; 109:507-17; PMID:23114206; http://dx.doi.org/ 10.1152/jn.00726.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon E. The enigma of deep-body thermosensory specificity. Int J Biometeorol 2000; 44:105-20; PMID:11048999; http://dx.doi.org/ 10.1007/s004840000060 [DOI] [PubMed] [Google Scholar]
- 66.Pehl U, Schmid HA, Simon E. Temperature sensitivity of neurons in slices of the rat spinal cord. J Physiol 1997; 498:483-95; PMID:9032695; http://dx.doi.org/ 10.1113/jphysiol.1997.sp021874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipton JM. Thermosensitivity of medulla oblongata in control of body temperature. Am J Physiol 1973; 224:890-7; PMID:4698806 [DOI] [PubMed] [Google Scholar]
- 68.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 2007; 292:R64-76; PMID:16973931; http://dx.doi.org/ 10.1152/ajpregu.00446.2006 [DOI] [PubMed] [Google Scholar]
- 69.Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ et al.. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 2007; 27:7459-68; PMID:17626206; http://dx.doi.org/ 10.1523/JNEUROSCI.1483-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garami A, Shimansky YP, Pakai E, Oliveira Dl, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci 2010; 30:1435-40; PMID:20107070; http://dx.doi.org/ 10.1523/JNEUROSCI.5150-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierau F-K, Szolcsányi J, Sann H. The effect of capsaicin on afferent nerves and temperature regulation of mammals and birds. J Thermal Biol 1986; 11:95-100; http://dx.doi.org/ 10.1016/0306-4565(86)90026-4 [DOI] [Google Scholar]
- 72.Szolcsányi J, Sann H, Pierau F-K. Nociception in pigeon is not impaired by capsaicin. Pain 1986; 27:247-60; PMID:3797018; http://dx.doi.org/ 10.1016/0304-3959(86)90215-0 [DOI] [PubMed] [Google Scholar]
- 73.Szolcsányi J, Jancsó-Gábor A. Capsaicin and other pungent agents as pharmacological tools in studies on thermoregulation In: The Pharmacology of Thermoregulation Karger. Schönbaum E, Lomax P eds; Karger Press, Basel; 1973; 395-409. [Google Scholar]
- 74.Szolcsányi J, Jancsó-Gábor A. Analysis of the role of warmth detectors by means of capsaicin under different conditions In: Temperature Regulation and Drug Action Karger. Lomax T, Schönbaum E, Jacob J eds; Karger Press, Basel; 1975; 331-8. [Google Scholar]
- 75.Szikszay M, Obál F Jr, Obál F. Dose-response relationship in the thermoregulatory effects of capsaicin. Naunyn Schmiedebergs Arch Pharmacol 1982; 320:97-100; PMID:7121619; http://dx.doi.org/ 10.1007/BF00506307 [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi A, Osaka T, Nama Y, Inou S, Lee TH, Kimura S. Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am J Physiol 1998; 275:R92-8; PMID:9688965 [DOI] [PubMed] [Google Scholar]
- 77.Feketa VV, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Shivering and tachycardic responses to external cooling in mice are substantially suppresses by TRPV1 activation but not by TRPM8 inhibition. Am J Physiol Regul Integr Comp Physiol 2013; 305:R1040-50; PMID:24005250; http://dx.doi.org/ 10.1152/ajpregu.00296.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szolcsányi J. Mechanism of sensory and neuroregulatory functions on the basis of actions of capsaicin and its congeners (in Hungarian) CSc thesis Pécs, University of Pécs Pekár Library of Medicine and Life Sciences 1975; pp 1-277. [Google Scholar]
- 79.Frens J. Effect of capsaicin and pyrogen on thermoregulation in the goat IRCS Med Sci Libr. Compend 1976; 4:176-7 [Google Scholar]
- 80.Nelson AG, Glickman-Weiss E, Day R. The effect of capsaicin on the thermal and metabolic responses of men exposed to 38 degrees C for 120 minutes. Wilderness Environ Med 2000; 11:152-6; PMID:11055559; http://dx.doi.org/ 10.1580/1080-6032(2000)011%5b0152:TEOCOT%5d2.3.CO;2 [DOI] [PubMed] [Google Scholar]
- 81.Jancsó-Gábor A, Szolcsányi J, Jancsó N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol 1970; 206:495-507; PMID:5498502; http://dx.doi.org/ 10.1113/jphysiol.1970.sp009027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buck SH, Burks TF. The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol Rev 1986; 38:179-226; PMID:3534898 [PubMed] [Google Scholar]
- 83.Szállasi A, Blumberg PM. Vanilloid (Capsaicin) receptor and mechanisms. Pharmacological Reviews 1999; 51:159-212; PMID . [PubMed] [Google Scholar]
- 84.Cabanac M, Cormareche-Leydier M, Poirier LJ. The effect of capsaicin on temperature regulation of the rat. Pflugers Arch 1976; 366:217-21; PMID:1033523; http://dx.doi.org/ 10.1007/BF00585881 [DOI] [PubMed] [Google Scholar]
- 85.Obál F Jr, Benedek G, Jancsó-Gábor A, Obál F. Salivary cooling, escape reaction, and heat pain in capsaicin-desensitized rats. Pflugers Arch 1979; 382:249-54; PMID:575415; http://dx.doi.org/ 10.1007/BF00583709 [DOI] [PubMed] [Google Scholar]
- 86.Obál F Jr, Bari F, Benedek G, Obál F. Elevation of thermoregulatory vasodilation threshold in rat after capsaicin treatment. Acta Physiol Acad Sci Hung 1982; 59:203-7; PMID:7168340 [PubMed] [Google Scholar]
- 87.Obál F Jr, Benedek G, Jancsó-Gábor A, Obál F. Tail skin vasodilation and bath test in capsaicin-desensitized rats. Pflugers Arch 1980; 387: 183-8; PMID:7191981; http://dx.doi.org/ 10.1007/BF00584270 [DOI] [PubMed] [Google Scholar]
- 88.Obál F Jr, Hajós M, Benedek G, Obál F, Jancsó-Gábor A. Impaired heat discrimination learning after capsaicin treatment. Physiol Behav 1981; 27:977-81; PMID:7335817; http://dx.doi.org/ 10.1016/0031-9384(81)90357-7 [DOI] [PubMed] [Google Scholar]
- 89.Obál F Jr, Obál F, Benedek G, Jancsó-Gábor A. Central and peripheral impairment of thermoregulation after capsaicin treatment. J Thermal Biol 1983; 8:203-6; http://dx.doi.org/ 10.1016/0306-4565(83)90105-5 [DOI] [Google Scholar]
- 90.Benedek G, Szikszay M, Obál F. Impaired thermoregulation against cold in capsaicin pretreated rats. Pfluegers Arch Eur J Physiol 1983; 399:243-5; PMID:6657468; http://dx.doi.org/ 10.1007/BF00656724 [DOI] [PubMed] [Google Scholar]
- 91.Dux M, Benedek G, Szikszay M. Effects of peripheral heating and systemic calcium injection in the unit activity in the preoptic area of capsaicin-pretreated rats. Acta Physiol Hung 1987; 69:523-6 [Google Scholar]
- 92.Jancsó-Gábor A, Szolcsányi J, Jancsó N. Stimulation and desensitization of the hypothalamic heat sensitive structures by capsaicin in rats. J Physiol 1970; 208:449-59; PMID:5500735; http://dx.doi.org/ 10.1113/jphysiol.1970.sp009130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szolcsányi J, Joó F, Jancsó-Gábor A. Mitochondrial changes in preoptic neurons after capsaicin desensitization of the hypothalamic thermodetectors in rats. Nature 1971; 229:116-7; PMID:4923093; http://dx.doi.org/ 10.1038/229116a0 [DOI] [PubMed] [Google Scholar]
- 94.Benedek G, Obál F Jr, Jancsó-Gábor A, Obál F. Effects of elevated ambient temperatures on the sleep-waking activity of rats with impaired warm reception. Waking Sleeping 1980; 4:87-94; PMID:7395198 [PubMed] [Google Scholar]
- 95.Kumar D, Kumar VM, Mallick HN. Warm sensitive neurons of the preoptic area regulate ambient temperature related changes in sleep in the rat. Indian J Physiol Pharmacol 2011; 55:262-71; PMID:22471234 [PubMed] [Google Scholar]
- 96.Jancsó-Gábor A. Anaesthesia-like condition and/or potentiation of hexobarbital sleep produced by pungent agents in normal and capsaicin-desensitized rats. Acta Physiol Hung 1980; 55: 57-62; PMID:7395532 [PubMed] [Google Scholar]
- 97.Hori T, Tsuzuki S. Thermoregulation in adult rats which have been treated with capsaicin as neonates. Pflugers Arch 1981; 390:219-23; PMID:7196020; http://dx.doi.org/ 10.1007/BF00658265 [DOI] [PubMed] [Google Scholar]
- 98.Dib B. Dissociation between peripheral and central heat loss mechanisms induced by neonatal capsaicin Behav. Neurosci 1983; 97:822-9; PMID:6639750 [DOI] [PubMed] [Google Scholar]
- 99.Caterina MJ, Leffer A, Malmberg AB, Martin WJ, Trafton J, Peters-Zeitz KR, Koltzenburg M, Basbaum AL, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306-13; PMID:10764638; http://dx.doi.org/ 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- 100.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K et al.. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000; 405:183-7; PMID:10821274; http://dx.doi.org/ 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- 101.Szelényi Z, Hummel Z, Szolcsányi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci 2004; 19:1421-4; PMID:15016100; http://dx.doi.org/ 10.1111/j.1460-9568.2004.03221.x [DOI] [PubMed] [Google Scholar]
- 102.Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett 2005; 378:28-33; PMID:15763167; http://dx.doi.org/ 10.1016/j.neulet.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 103.Székely M, Szolcsányi J. Endotoxin fever in capsaicin treated rats. Acta Physiol Hung 1979; 53:469-77; PMID:397719 [PubMed] [Google Scholar]
- 104.Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the TRPV1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 2011; 31:1721-33; PMID:21289181; http://dx.doi.org/ 10.1523/JNEUROSCI.4671-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanizsai P, Garami A, Solymár M, Szolcsányi J, Szelényi Z. Energetics of fasting heterothermia in TRPV1-KO and wild type mice. Physiol Behav 2009; 96:149-54; PMID:18938188; http://dx.doi.org/ 10.1016/j.physbeh.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 106.Tóth DM, Szoke E, Bölcskei K, Kvell K, Bender B, Bosze Z, Szolcsányi J, Sándor Z. Nociception, neurogenic inflammation and thermoregulation in TRPV1 knockdown transgenic mice. Cell Mol Life Sci 2011; 68:2589-601; PMID:21069423; http://dx.doi.org/ 10.1007/s00018-010-0569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joseph J, Wang S, Lee J, Ro JY, Chung M-K. Carboxyl-terminal domain of transient receptor potential vanilloid 1 contains distinct segments differentially involved in capsaicin- and heat-induced desensitization. J Biol Chem 2013; 288:35690-702; PMID:24174527; http://dx.doi.org/ 10.1074/jbc.M113.513374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J 2011; 30:582-93; PMID:21139565; http://dx.doi.org/ 10.1038/emboj.2010.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 2011; 7:37; PMID:21586160; http://dx.doi.org/ 10.1186/1744-8069-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Obál F Jr, Jancsó G, Hajós M, Obál F. Differences in the mechanism of the thermoregulatory impairment induced by capsaicin in newborn and adult rats. Acta Physiol Hung 1987; 69:437-45; PMID:3661220 [PubMed] [Google Scholar]
- 111.Holzer P. Capsaicin: cellular targets, mechanism of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991; 43:143-201; PMID:1852779 [PubMed] [Google Scholar]
- 112.Dib B. Effects of intracerebroventricular capsaicin on thermoregulatory behavior in the rat. Pharmacol Biochem Behav 1982; 16:23-7; PMID:7058212; http://dx.doi.org/ 10.1016/0091-3057(82)90007-7 [DOI] [PubMed] [Google Scholar]
- 113.Hori T, Shibata M, Kiyohara T, Nakashima T, Asami A. Responses of anterior hypothalamic-preoptic thermosensitive neurons to locally applied capsaicin. Neuropharmacology 1988; 27:135-42; PMID:3352871; http://dx.doi.org/ 10.1016/0028-3908(88)90162-1 [DOI] [PubMed] [Google Scholar]
- 114.Sasamura T, Sasaki M, Tohda C, Kuraishi Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. Neuroreport 1998; 9:2045-8; PMID:9674591; http://dx.doi.org/ 10.1097/00001756-199806220-00025 [DOI] [PubMed] [Google Scholar]
- 115.Sasamura T, Kuraishi Y. Peripheral and central actions of capsaicin and VR1 receptor. Jpn J Pharmacol 1999; 80:275-80; PMID:10496326; http://dx.doi.org/ 10.1254/jjp.80.275 [DOI] [PubMed] [Google Scholar]
- 116.Karlsson U, Sundgren-Andersson AK, Johansson S, Krupp JJ. Capsaicin augments synaptic transmission in the rat medial preoptic nucleus. Brain Res 2005; 1043:1-11; PMID:15862512; http://dx.doi.org/ 10.1016/j.brainres.2004.10.064 [DOI] [PubMed] [Google Scholar]
- 117.Hori T, Kiyohara T, Nakashima T, Shibata M, Koga H. Multimodal responses of preoptic and hypothalamic neurons to thermal and nonthermal homeostatic parameters. Can J Physiol Pharmacol 1987; 65:1290-8; PMID:3621078; http://dx.doi.org/ 10.1139/y87-205 [DOI] [PubMed] [Google Scholar]
- 118.Hajós M, Engberg G, Elem M. Reduced responsiveness of locus coeruleus neurons to cutaneous thermal stimuli in capsaicin-treated rats. Neurosci Lett 1986; 70:382-7; PMID:3022199; http://dx.doi.org/ 10.1016/0304-3940(86)90584-7 [DOI] [PubMed] [Google Scholar]
- 119.Marinelli S, Vaughan CW, Christine MJ, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. Physiol 2002; 543:531-40; PMID:12205187; http://dx.doi.org/ 10.1113/jphysiol.2002.022863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khakpay R, Polster D, Köles L, Skorinkin A, Szabo B, Wirkner K, Illes P. Potentiation of the glutamatergic synaptic input to rat locus coeruleus neurons by P2´7 receptors. Purinergic Signal 2010; 6:349-59; PMID:21103218; http://dx.doi.org/ 10.1007/s11302-010-9198-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol 1998; 56:237-67; PMID:9760703; http://dx.doi.org/ 10.1016/S0301-0082(98)00039-2 [DOI] [PubMed] [Google Scholar]
- 122.Steenland HW, Ko SW, Wu L-J, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34; PMID:17092351; http://dx.doi.org/ 10.1186/1744-8069-2-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dawbarn D, Harmar AJ, Pycock CJ. Intranigral injection of capsaicin enhances motor activity and depletes nigral 5-hydroxytryptamine but not substance P. Neuropharmacology 1981; 20:341-6; PMID:6170024; http://dx.doi.org/ 10.1016/0028-3908(81)90006-X [DOI] [PubMed] [Google Scholar]
- 124.Hajós M, Engberg G, Nissbrandt H, Magnusson T, Carlsson A. Capsaicin-sensitive vasodilatatory mechanism in the rat substantia nigra and striatum. J Neural Transm 1988; 74:129-39; PMID:3210010; http://dx.doi.org/ 10.1007/BF01244779 [DOI] [PubMed] [Google Scholar]
- 125.Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, Mercuri NB. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat subtantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci 2003; 23:3136-44; PMID:12716921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osaka T, Lee TH, Kobayashi A, Inoue S, Kimura S. Thermogenesis mediated by a capsaicin-sensitive area in the ventrolateral medulla. Neuroreport 2000; 11:2425-8; PMID:10943697; http://dx.doi.org/ 10.1097/00001756-200008030-00017 [DOI] [PubMed] [Google Scholar]
- 127.Koulchitsky SV. Are the capsaicin-sensitive structures of ventral medulla involved in the temperature response to endotoxin in rats? Neurosci Lett 1998; 244:112-4; PMID:9572598; http://dx.doi.org/ 10.1016/S0304-3940(98)00128-1 [DOI] [PubMed] [Google Scholar]
- 128.Hajós M, Hjorth S, Carlsson A. Injection of capsaicin into the nucleus raphe dorsalis elicits heat loss in the rat. Neurosci Lett 1987; 75:199-204; PMID:2437500; http://dx.doi.org/ 10.1016/0304-3940(87)90297-7 [DOI] [PubMed] [Google Scholar]
- 129.Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature sensitive and insensitive rat hypothalamic neurons. Am J Physiol 2001; 537:521-35; PMID:11731583; http://dx.doi.org/ 10.1111/j.1469-7793.2001.00521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carlisle HJ. Behavioural significance of hypothalamic temperature-sensitive cells. Nature 1966; 209:1324-5; PMID:5956045; http://dx.doi.org/ 10.1038/2091324a0 [DOI] [PubMed] [Google Scholar]
- 131.Carlisle HJ, Laudenslager ML. Observations on the thermoregulatory effects of preoptic warming in rats. Physiol Behav 1979; 23:723-32; PMID:504468; http://dx.doi.org/ 10.1016/0031-9384(79)90166-5 [DOI] [PubMed] [Google Scholar]
- 132.Dean JB, Boulant JA. In vitro localization of thermosensitive neurons in the rat diencephalon. Am J Physiol 1989; 257:R57-64; PMID:2750968 [DOI] [PubMed] [Google Scholar]
- 133.Roberts WW, Mooney RD. Brain areas controlling thermoregulatory grooming, prone extension, locomotion, and tail vasodilatation in rats. J Comp Physiol Psychol 1974; 86:470-80; PMID:4592495; http://dx.doi.org/ 10.1037/h0036147 [DOI] [PubMed] [Google Scholar]
- 134.Silva NL, Boulant JA. Effect of osmotic pressure, and temperature on neurons in preoptic tissue slices. Am J Physiol 1984; 247:R335-45; PMID:6465349 [DOI] [PubMed] [Google Scholar]
- 135.Hori T, Shinohara K. Hypothalamic thermo-responsive neurons in the newborn rat. J Physiol 1979; 294:541-60; PMID:512957; http://dx.doi.org/ 10.1113/jphysiol.1979.sp012945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hori A, Minato K, Kobayashi S. Warming-actived channels of warm-sensitive neurons in rat hypothamic slices. Neurosci Lett 1999; 275:93-6; PMID:10568507; http://dx.doi.org/ 10.1016/S0304-3940(99)00732-6 [DOI] [PubMed] [Google Scholar]
- 137.Hori T. Thermosensitivity of preoptic and anterior hypothalamic neurons in the capsaicin-desensitized rat. Pflugers Arch 1981; 389:297-9; PMID:7195013; http://dx.doi.org/ 10.1007/BF00584793 [DOI] [PubMed] [Google Scholar]
- 138.Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallási A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreacitivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 2000; 97:3655-60; PMID:10725386; http://dx.doi.org/ 10.1073/pnas.97.7.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cavanaugh DJ, Chasler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donell D, Nicoll RA, Julius D, Basbaum A. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 2011; 31:5067-77; PMID:21451044; http://dx.doi.org/ 10.1523/JNEUROSCI.6451-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Narumiya S, Furuyashiki T. Fever, inflammation, pain and beyond: prostanoid receptor research during these 25 years. FASEB Journal 2011; 25:813-8; PMID:21357250; http://dx.doi.org/ 10.1096/fj.11-0302ufm [DOI] [PubMed] [Google Scholar]
- 141.Milton AS, Wendlandt S. Effects on body temperature of prostaglandins of the A, E, and F series on injection into the third ventricle of uranaesthetized cats and rabbits. J Physiol 1971; 218:325-36; PMID:4330929; http://dx.doi.org/ 10.1113/jphysiol.1971.sp009620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Székely M, Balaskó M, Romanovsky AA. Peripheral neural inputs. Their role in fever development. Ann N Y Acad Sci 1997; 813:427-34; PMID:9100916; http://dx.doi.org/ 10.1111/j.1749-6632.1997.tb51728.x [DOI] [PubMed] [Google Scholar]
- 143.Székely M, Balaskó M, Kulchitsky VA, Simons CT, Ivanov Al, Romanovsky AA. Multiple neural mechanism of fever. Auton Neurosci 2000; 85:78-82; PMID:11189030; http://dx.doi.org/ 10.1016/S1566-0702(00)00223-X [DOI] [PubMed] [Google Scholar]
- 144.Dogan MD, Patel S, Rudaya AY, Steiner AA, Székely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Bri J Pharmacol 2004; 143:1023-32; PMID:15492017; http://dx.doi.org/ 10.1038/sj.bjp.0705977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci 2008; 29:550-7; PMID:18805596; http://dx.doi.org/ 10.1016/j.tips.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 146.Dogan MD, Kulchitsky VA, Patel S, Pétervári E, Székely M, PRomanovsky AA,. Bilateral splanchnicotomy does not affect lipopolysaccharide-induces fever in rats. Brain Res 2003; 993:227-9; PMID:14642851; http://dx.doi.org/ 10.1016/j.brainres.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 147.McGaraughty S, Segreti JA, Fryer RM, Brown BS, Faltynek CR, Kym PR. Antagonism of TRPV1 receptors indirectly modulates activity of thermoregulatory neurons in the medial preoptic area of rats. Brain Res 2009; 1268:58-67; PMID:19236852 [DOI] [PubMed] [Google Scholar]
- 148.Voight EA, Gomtsyan AR, Daanen JF, Perner RJ, Schmidt RG, Bayburt EK, DiDomenico S, McDonald HA, Puttfarcken PS, Chen J et al.. Discovery of (R)-1-(7-Chloro-2,2-bis(fluoromethly)chroman-4-yl)-3-(3-methylisoquinolin-5-yl) urea (A-1165442): A temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy. J Med Chem 2014; 57:7412-24; PMID:25100568; http://dx.doi.org/ 10.1021/jm500916t [DOI] [PubMed] [Google Scholar]
- 149.Szolcsányi J, Szállási Á, Szállási Z, Joo F, Blumberg PM. Resiniferation: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther 1990; 255:923-8; PMID:2243359 [PubMed] [Google Scholar]
- 150.Acs G, Palkovics M, Blumberg PM. Specific binding of (3H) resiniferatoxin by human and rat preoptic area, locus ceruleus, medial hypothalamus, reticular formation and ventral thalamus membrane preparations. Life Sci 1996; 59:1899-908; PMID:8950287; http://dx.doi.org/ 10.1016/S0024-3205(96)00537-1 [DOI] [PubMed] [Google Scholar]
- 151.Maruyama M, Nishi M, Konishi M, Takashige Y, Nagashime K, Kiyohara T, Kanosue K. Brain regions expressing Fos during thermoregulatory behavior in rats. Am J Physiol Regul Integr Comp Physiol 2003; 285:R1116-23; PMID:12893652; http://dx.doi.org/ 10.1152/ajpregu.00166.2002 [DOI] [PubMed] [Google Scholar]
- 152.Kyoko Y, Megumi M, Takayoshi H, Kei N, Yutaka F, Ruediger G, Kazuyuki K. Fos expression induced by warming the preoptic area in rats. Brain Research 2002; 933:109-17; PMID:11931855; http://dx.doi.org/ 10.1016/S0006-8993(02)02287-4 [DOI] [PubMed] [Google Scholar]
- 153.Moriya T, Shibasaki R, Kayano T, Takebuchi N, Ichimura M, Kitamura N, Asano A, Hosaka YZ, Forostyak O, Verkhratsky A et al.. Full-length transient receptor potential vanilloid 1 channels mediate calcium signals and possibly contribute to osmoreception in vasopressin neurones in the rat supraoptic nucleus. Cell Calcium 2014; S0143-4160:00185-7; PMID:25479918 [DOI] [PubMed] [Google Scholar]
- 154.Menigoz A, Boudes M. The expression pattern of TRPV1 in brain. J Neurosci 2011; 31:13025-7; PMID:21917785; http://dx.doi.org/ 10.1523/JNEUROSCI.2589-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 2008; 28:566-75; PMID:18199758; http://dx.doi.org/ 10.1523/JNEUROSCI.3976-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw LA, Brierley SM. A novel role for TRPM8 in visceral afferent function. Pain 2011; 152:1459-68; PMID:21489690; http://dx.doi.org/ 10.1016/j.pain.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 157.De Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, Youngblood BD, Wang W, Kort ME, Kym PR et al.. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci 2014; 34:4445-52; PMID:24671991; http://dx.doi.org/ 10.1523/JNEUROSCI.5387-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


