Abstract
The Transient Receptor Potential Ankyrin 1 ion channel is heat-sensitive in invertebrate and ancestral vertebrates, cold-sensitive in rodents, and temperature-insensitive in primates. This remarkable divergence in temperature sensitivity is in contrast to its role in sensing electrophilic compounds, which is conserved during animal evolution.
Keywords: activation, cold-sensitive, evolution, heat-sensitive, TRPA1, temperature-insensitive
Abbreviation: TRPA1, Transient Receptor Potential Ankryin 1 ion channel; TRPM8, Transient Receptor Potential Melastatin 8 ion channel; TRPV1, Transient Receptor Potential Vanilloid 1 ion channel; AITC, allyl isothiocyanate
The Transient Receptor Potential Ankyrin 1 (TRPA1) is a non-selective cation channel that belongs to the superfamily of transient receptor potential ion channels. In 2003, Story et al reported that mouse TRPA1 is activated by noxious cold with a temperature threshold of 17°C.1 Since TRPA1 is expressed in a subset of sensory neurons; and its activation leads to Ca2+ signaling and neuronal excitability, TRPA1 was proposed to be a receptor for noxious cold. This initial finding has generated tremendous interest. Several labs confirmed cold activation of TRPA1, particularly with convincing data from single channel recordings.2 However, other labs disputed the cold activation, or concluded that cold activation is mediated indirectly by intracellular Ca2+ 3,4. Efforts to address this discrepancy were hindered by technical difficulty and a wide variations of experimental conditions, such as different expression systems (Chinese Hamster Ovary cells, Human embryonic kidney 293 cells, Xenopous oocytes), different methodologies (Two electrode voltage clamp, whole cell, single channel recordings, Ca2+ imaging), different ionic conditions (with and without Ca2+), and different clones (mouse and human).
In a recent paper, Chen et al. attributed the previous controversy on TRPA1 cold sensitivity to species difference. By using Ca2+ assay, whole-cell and single channel recordings, TRPA1 channels from 4 mammalian species (rat, mouse, rhesus monkey and human) were characterized in parallel.5 It was found that cold activates rat and mouse TRPA1, but has no effect on human or rhesus monkey TRPA1 under the same conditions. Furthermore, none of the 4 channels respond to heat stimulation. Beside rodents and primates, TRPA1 is present in many other species including fruit fly, mosquitos, snake, frog, lizard and chicken. Surprisingly, TRPA1 from these species are activated by heat.6,7 Thus, TRPA1 exhibits a spectrum of temperature sensitivity, including heat-activation, cold-activation and temperature-insensitive.
The thermal divergence suggests that TRPA1 may evolve to perform distinct functions to suit the need of respective species. Ectotherms such as fruit fly, frog and lizard lack internal capability of thermal regulation. They rely on heat-seeking (basking in the sun) or heat-escaping behaviors (hiding in the shade) to maintain body temperature. The heat-sensitive TRPA1 may generate a sensory input to fulfill these behavior strategies. Indeed, larvae of fruit fly chooses to move away from the heated portion of a thermal gradient, and this ability is lost when TRPA1 expression is reduced by RNA interference.7 In other species, TRPA1 may also serve more aggressive purpose, such as infrared detection of prey by snakes.6 For small endotherms such as rodents, cold is a major life-threatening challenge. Although TRPA1 does not drive cold-defense thermoeffectors,8 there is a possible role of TRPA1 in detecting noxious cold, or triggering cold avoidance and habitat selection (e.g., hiding in a barrel). On the other hand, the highly evolved primates mostly live in warm climates (monkey) or are skillful in manipulating the surrounding temperature (human), therefore temperature sensitivity of TRPA1 is not required.
The gain or loss of thermal sensitivity of TRPA1 may coincide with the emergence of other thermal sensitive ion channels such as TRPV1 (Transient Receptor Potential Vanilloid 1 ion channel) and TRPM8 (Transient Receptor Potential Melastatin 8 ion channel). TRPV1 first appeared in ancestral vertebrates, and unlike TRPA1, maintains its heat-activation across species. TRPM8 is a major sensor of cool temperature. The establishment of these mechanisms may allow TRPA1 to diverge from temperature sensing and focus on another core function, sensing of noxious, reactive electrophiles (e.g., allyl isothiocyanate, or AITC, from wasabi). Mosquitos and humans are separated by hundreds of million years of evolution, and their TRPA1 channels are only 35% identical at amino acid level. Despite the low sequence homology, both channels are activated by AITC and mediate AITC-induced nocifensive behavior in respective species. TRPA1 may also perform similar function in many other species such as fruit fly, frog, lizard, chicken, rat, mouse and monkey. Therefore, in contrast to the divergence in thermal sensing, the role of TRPA1 in electrophile sensing is conserved during animal evolution.
Electrophiles activate TRPA1 thorough a well characterized mechanism, namely, covalent modification of cysteine residues localized in the N-terminus of channel protein. However, how temperature opens TRPA1 is not well understood. Through comparison of human and rodent channel sequences, a residue in the transmembrane domain 5 was found to be critical (Glycine 878 in rodents and Valine 875 in primates), as the Glycine 878 to Valine mutation abolishes cold activation of rodent channels (Fig. 1).5 The Glycine residue exists in the cold-sensitive rodent channels, whereas a Valine residue is conserved in heat-insensitive human and monkey, as well as in heat-activated channels such as rattle snake TRPA1. Strikingly, the same residues also underlie the effect of menthol, a chemical ligand that activates primate channel but block rodent channel (i.e., opposite to cold). Therefore cold and menthol may elicit opposite conformational changes through the same residues. Although more work is needed, the convergence of 2 distinct stimuli through the same residues indicates their essential role in channel gating.
Figure 1.
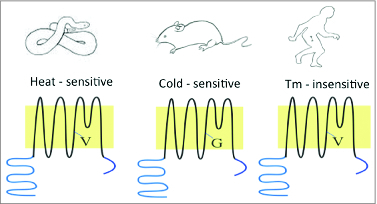
The divergent thermal sensitivity of TRPA1 channels. TRPA1 is heat-sensitive in invertebrate and ancestral vertebrate (e.g., rattle snake), cold-sensitive in rodents (e.g., rat) and temperature-insensitive in primates (e.g., human). A Glycine (G) residue is critical for cold-activation of rodent TRPA1, whereas an equivalent Valine (V) residue is preserved in other species.
In summary, TRPA1 exhibits remarkable evolutionary divergence and conservation: its thermal sensitivity varies from heat-activation, cold-activation to temperature-insensitive; whereas its role in electrophilic detection is conserved. Characterization of TRPA1 channels from additional species will provide more insights on the molecular evolution and structure-function of TRPA1. Currently TRPA1 has been pursued as an important therapeutic target for treating pain and respiratory diseases. Species difference should be an important consideration in TRPA1 drug discovery efforts.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Story GM, et al.. Cell 2003; 112:819-29; PMID:12654248; http://dx.doi.org/ 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- 2.Sawada Y, et al.. Brain Res 2007; 1160:39-46; PMID:17588549; http://dx.doi.org/ 10.1016/j.brainres.2007.05.047 [DOI] [PubMed] [Google Scholar]
- 3.Jordt SE, et al.. Nature 2004; 427:260-5; PMID:14712238; http://dx.doi.org/ 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- 4.Zurborg S, et al.. Nat Neurosci 2007; 10:277-9; PMID:17259981; http://dx.doi.org/ 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, et al.. Nat Commun 2013; 4:2501; PMID:24071625; http://dx.doi.org/ 10.1038/ncomms3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laursen WJ, et al.. Temperature 2015; 2:214-26; http://dx.doi.org/ 10.1080/23328940.2014.1000702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada FN, et al.. Nature 2008; 454:217-20; PMID:18548007; http://dx.doi.org/ 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Oliveira C, et al.. J Neurosci 2014; 34:4445-52; PMID:24671991; http://dx.doi.org/ 10.1523/JNEUROSCI.5387-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]


