Abstract
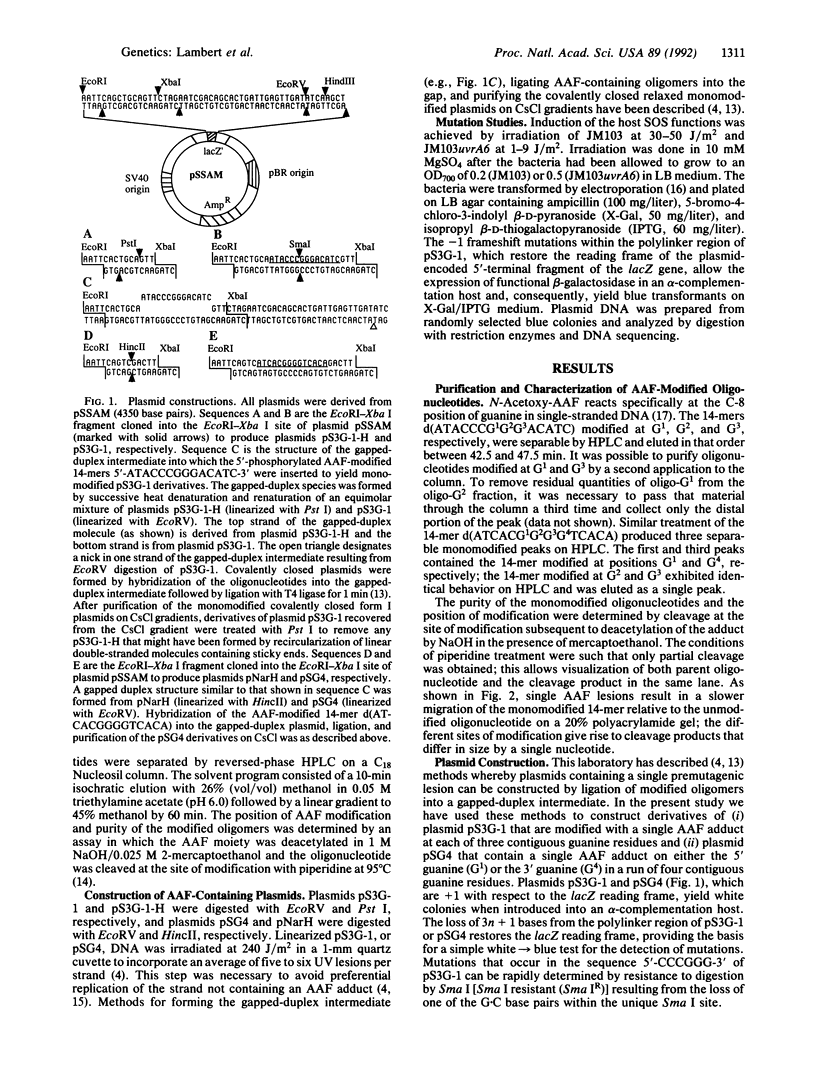
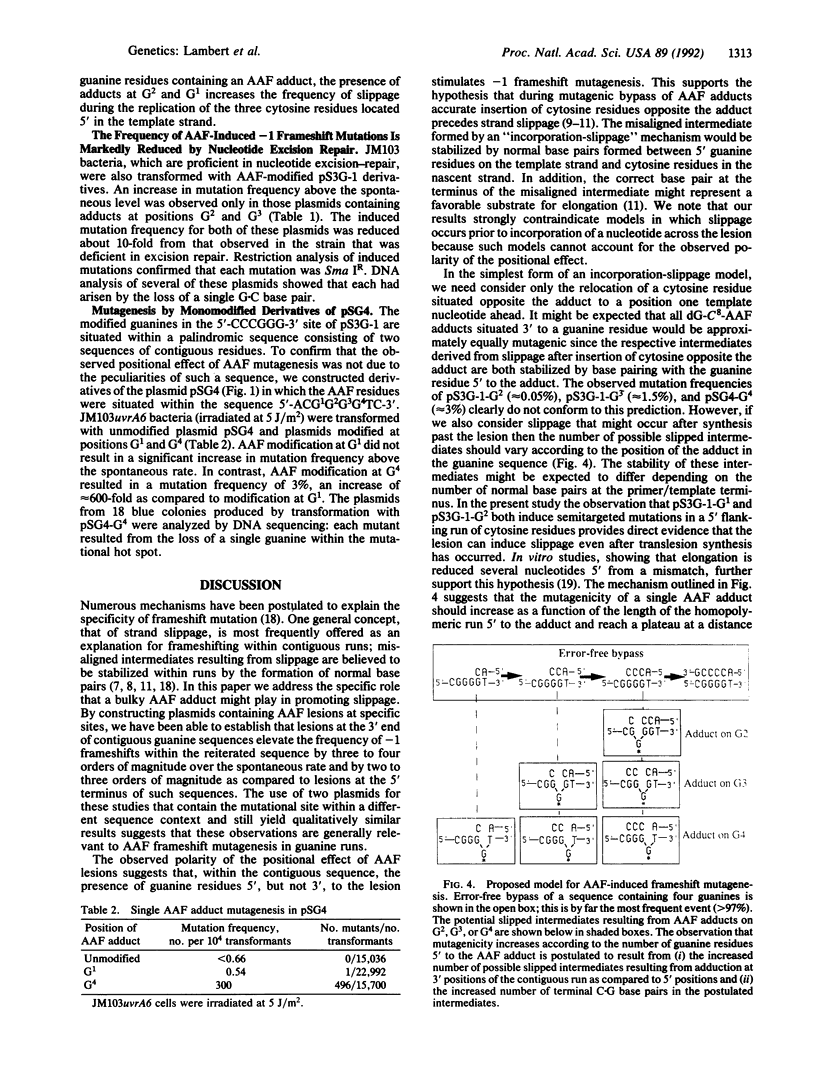
We have constructed plasmids pS3G-1 and pSG4 that contain single acetylaminofluorene adducts within contiguous runs of three (5'-CCCG1G2G3-3') and four (5'-CG1GGG4T-3') guanine residues, respectively. In Escherichia coli, the frequency of induced -1 frameshift mutations was strongly dependent on the position of modification: pS3G-G3 was approximately 100-fold and 10-fold more mutagenic than pS3G-G1 and pS3G-G2, respectively; pSG4-G4 was approximately 600-fold more mutagenic than pSG4-G1. Mutagenesis was SOS-dependent and was markedly reduced in bacteria that were proficient in nucleotide excision repair as compared to a repair-deficient uvrA6 mutant. DNA sequencing showed that -1 frameshift events in pS3G-1 consisted of either targeted mutations (greater than 90% of induced mutations) within the guanine sequence or semitargeted mutations (greater than 10%) in the 5' flanking repetitive cytosine sequence. Semitargeted events, which were observed when acetylaminofluorene modification was at G1 and G2, show that a lesion can reduce the fidelity of replication at positions 5' to its location on the template strand. No semitargeted frameshifts were observed in plasmid pSG4, which lacks a repetitive sequence 5' to the adduct. Our results are consistent with a model for frameshift mutagenesis in which the acetylaminofluorene adduct (i) allows accurate incorporation of cytosine opposite the bulky lesion during DNA synthesis and (ii) impedes elongation of primer/template termini formed opposite the adduct or 5' to the adduct on the template strand, providing increased opportunity for the formation of slipped frameshift intermediates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bebenek K., Kunkel T. A. Frameshift errors initiated by nucleotide misincorporation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4946–4950. doi: 10.1073/pnas.87.13.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belguise-Valladier P., Fuchs R. P. Strong sequence-dependent polymorphism in adduct-induced DNA structure: analysis of single N-2-acetylaminofluorene residues bound within the NarI mutation hot spot. Biochemistry. 1991 Oct 22;30(42):10091–10100. doi: 10.1021/bi00106a005. [DOI] [PubMed] [Google Scholar]
- Bichara M., Fuchs R. P. DNA binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli. A correlation between the conformation of the premutagenic lesion and the mutation specificity. J Mol Biol. 1985 Jun 5;183(3):341–351. doi: 10.1016/0022-2836(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Burnouf D., Koehl P., Fuchs R. P. Single adduct mutagenesis: strong effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4147–4151. doi: 10.1073/pnas.86.11.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans F. E., Miller D. W., Beland F. A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis. 1980;1(11):955–959. doi: 10.1093/carcin/1.11.955. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Janel-Bintz R., Maenhaut-Michel G., Takahashi M., Fuchs R. P. Roles of recA mutant allele (recA495) in frameshift mutagenesis. Biochimie. 1991 Apr;73(4):491–495. doi: 10.1016/0300-9084(91)90117-j. [DOI] [PubMed] [Google Scholar]
- Kalnik M. W., Norman D. G., Li B. F., Swann P. F., Patel D. J. Conformational transitions in thymidine bulge-containing deoxytridecanucleotide duplexes. Role of flanking sequence and temperature in modulating the equilibrium between looped out and stacked thymidine bulge states. J Biol Chem. 1990 Jan 15;265(2):636–647. [PubMed] [Google Scholar]
- Kalnik M. W., Norman D. G., Swann P. F., Patel D. J. Conformation of adenosine bulge-containing deoxytridecanucleotide duplexes in solution. Extra adenosine stacks into duplex independent of flanking sequence and temperature. J Biol Chem. 1989 Mar 5;264(7):3702–3712. [PubMed] [Google Scholar]
- Koehl P., Burnouf D., Fuchs R. P. Construction of plasmids containing a unique acetylaminofluorene adduct located within a mutation hot spot. A new probe for frameshift mutagenesis. J Mol Biol. 1989 May 20;207(2):355–364. doi: 10.1016/0022-2836(89)90259-3. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Maenhaut-Michel G., Fuchs R. P. Specific strand loss in N-2-acetylaminofluorene-modified DNA. J Mol Biol. 1987 Feb 20;193(4):651–659. doi: 10.1016/0022-2836(87)90348-2. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Lambert I. B., Gordon A. J., Bryant D. W., Glickman B. W., McCalla D. R. The action of 1-nitroso-8-nitropyrene in Escherichia coli: DNA adduct formation and mutational consequences in the absence of nucleotide excision-repair. Carcinogenesis. 1991 May;12(5):879–884. doi: 10.1093/carcin/12.5.879. [DOI] [PubMed] [Google Scholar]
- Miller M., Harrison R. W., Wlodawer A., Appella E., Sussman J. L. Crystal structure of 15-mer DNA duplex containing unpaired bases. Nature. 1988 Jul 7;334(6177):85–86. doi: 10.1038/334085a0. [DOI] [PubMed] [Google Scholar]
- Morden K. M., Gunn B. M., Maskos K. NMR studies of a deoxyribodecanucleotide containing an extrahelical thymidine surrounded by an oligo(dA).oligo(dT) tract. Biochemistry. 1990 Sep 18;29(37):8835–8845. doi: 10.1021/bi00489a047. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Reckmann B., Grosse F., Krauss G. The elongation of mismatched primers by DNA polymerase alpha from calf thymus. Nucleic Acids Res. 1983 Oct 25;11(20):7251–7260. doi: 10.1093/nar/11.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley L. S. Frameshift mutation: determinants of specificity. Annu Rev Genet. 1990;24:189–213. doi: 10.1146/annurev.ge.24.120190.001201. [DOI] [PubMed] [Google Scholar]
- Roy S., Sklenar V., Appella E., Cohen J. S. Conformational perturbation due to an extra adenosine in a self-complementary oligodeoxynucleotide duplex. Biopolymers. 1987 Dec;26(12):2041–2052. doi: 10.1002/bip.360261206. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Koffel-Schwartz N., Fuchs R. P. N-acetoxy-N-acetyl-2-aminofluorene-induced mutagenesis in the lacI gene of Escherichia coli. Carcinogenesis. 1990 Jul;11(7):1087–1095. doi: 10.1093/carcin/11.7.1087. [DOI] [PubMed] [Google Scholar]
- Singer B., Chavez F., Goodman M. F., Essigmann J. M., Dosanjh M. K. Effect of 3' flanking neighbors on kinetics of pairing of dCTP or dTTP opposite O6-methylguanine in a defined primed oligonucleotide when Escherichia coli DNA polymerase I is used. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8271–8274. doi: 10.1073/pnas.86.21.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985 Apr;109(4):633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson S. A., Crothers D. M. Proton nuclear magnetic resonance studies on bulge-containing DNA oligonucleotides from a mutational hot-spot sequence. Biochemistry. 1987 Feb 10;26(3):904–912. doi: 10.1021/bi00377a035. [DOI] [PubMed] [Google Scholar]





