Abstract
In the past, studies on stress responses and sleep/wake regulation were performed separately. The discovery of orexin (hypocretin) in 1998, however, dramatically changed the course of research and new findings regarding its role in these complex processes provided a better insight into their interactions and intricacies. Orexin-containing neuronal activity has been found to be minimal during sleep. It increases during the waking period and further increases during the active waking period, which includes stress responses and exploratory behaviors. Autonomic regulation of the body, which includes body temperature, blood flow, and ventilation, is also activated along with the change in vigilance states. Our recent findings suggest that orexin neurons act as a conductor of orchestration for vigilance states, behaviors, and autonomic functions. Body temperature regulation by orexin neurons seems to be mediated by one of its cotransmitters while cardiovascular and respiratory regulation are mediated by orexin itself.
Keywords: autonomic nervous system, breathing regulation, cardiovascular control, fight-or-flight, pain, stress, sleep, thermoregulation
Abbreviations
- AP5
D-(−)-2-amino-5-phosphonopentanoic acid
- BAT
brown adipose tissue
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DHA
dorsal hypothalamic area
- DMH
dorsomedial hypothalamus
- GABA
γ-aminobutylic acid
- LHA
lateral hypothalamic area
- ORX-AB
orexin-neuron-ablated mice
- ORX-KO
orexin-knockout mice
- PeF
perifornical area
- PGE2
prostaglandin E2.
Introduction
Research on neural mechanisms of state-dependent adjustments of central autonomic regulation has been sparse, despite the importance of this event from the perspective of quality of life. In addition to calm and resting states, our daily life involves many perturbations that induce active conditions such as locomotion, eating, and communication. During such active periods, cardiovascular, respiratory, and body temperature regulation needs to be adjusted according to situational demands, which differ from those during resting states, by modulating or resetting homeostatic points.1 One of the neural substrates regulating such adjustment, at least in the context of defensive behavior, appears to be located in the dorsal hypothalamus because stimulation of this area elicits perfectly coordinated behavioral “rage” associated with its specific autonomic responses. This response was termed the “defense response” and the area was termed the “defense area” of the hypothalamus.2
Several neurotransmitters have been proposed to be involved in the modulation of the efferent pathways of defense responses against stressors. For example, the activation of serotonin-1A receptors in the medullary raphe reduces cardiovascular changes,3 and the inhibition of serotonin-3 receptors in the nucleus tractus solitarius prevents the inhibition of baroreflex bradycardia during the defense response.4 Microinjections of adenosine into the rostral ventrolateral medulla augment the increase in blood pressure induced by electrical stimulation of the hypothalamic defense area.5 The pros and cons of glutamate participation in the cardiovascular component of the defense response have been a topic of debate.6,7 However, there is no report on the molecular basis of the defense response and of the mechanism underlying the multifaceted nature of simultaneous and coordinated changes in the cardiovascular, respiratory, sensory, thermal, and behavioral parameters. Localization of orexin-containing cell bodies in the perifornical area (PeF) and dorsomedial hypothalamus (DMH) (Fig. 1), which overlap the “defense area,” prompted us to investigate the possible role of orexin in the defense response against stressors.
Figure 1.
Distribution of orexin neurons in the brain. (A) Schematic drawing of a sagittal section of a rodent brain showing the restricted distribution of orexin-containing neuronal cell bodies (circles) in the hypothalamus and the wide distribution of their axons (arrows) throughout the brain. The vertical line indicates the plane in B. Adapted from Nambu et al. © Elsevier. Reproduced by permission of Elsevier. Permission to reuse must be obtained from the rightsholder. (B) A coronal section through the hypothalamus. The rectangle denotes the area examined in C. DMH, dorsomedial hypothalamus; f, fornix; LHA, lateral hypothalamic area; mt, mammillothalamic tract; PeF, perifornical area. (C) The distribution of the orexin-like immunoreactivity (circles) in a representative mouse brain. Bar, 200 µm in C. Adapted from Zhang et al.71 © Tomoyuki Kuwaki. Reproduced by permission of Tomoyuki Kuwaki. Permission to reuse must be obtained from the rightsholder.
Orexin Neurons
Orexin (hypocretin)
Orexins (orexin-A and orexin-B), also known as hypocretins (hypocretin 1 and hypocretin 2, respectively), are hypothalamic neuropeptides.8,9 They are cleaved from a common precursor molecule, prepro-orexin (130 residues), to form orexin-A (33 amino acids) and orexin-B (28 amino acids).9,10 Although orexins were first described as hypothalamic neuropeptides that influenced appetite and consciousness, it was later found that orexins also modulate reward processes,11 pain processing,12,13 and autonomic regulation of the cardiovascular,14-16 respiratory,17,18 and neuroendocrine systems.19
Coexisting transmitter/modulators
Orexin neurons contain not only orexin, but also other putative neurotransmitter/modulator candidates such as glutamate,20-22 dynorphin,23 galanin,24 and nitric oxide.25 Dynorphin and glutamate may act synergistically with orexin to promote wakefulness.26 However, the precise role(s) of the substances that are colocalized with orexin are largely unknown.
Anatomy of orexin-containing neurons
Orexin-containing cell bodies are restricted to the lateral hypothalamic area (LHA), the PeF, and the DMH (Fig. 1). Their distribution is rostrocaudally restricted within 1 mm in mice.27 Conversely, orexin-containing nerve terminals and receptors are widely distributed in the hypothalamus, thalamus, cerebral cortex, circumventricular organs, brain stem, cerebellum, and spinal cord, suggesting that the orexin neurons have widespread connections with other regions in the brain.28-30 Specifically, the cardiorespiratory and thermoregulatory-related areas that receive orexinergic innervation are the nucleus tractus solitarius, the pre-Bötzinger complex, the periaqueductal gray, the rostral ventrolateral medulla, the intermediolateral cell column of the spinal cord, and the retrotrapezoid, hypoglossal, medullary raphe, parabrachial/Kölliker-Fuse, and phrenic nuclei (Fig. 2).14, 17, 30-42 Approximately 50% of the hypothalamic neurons that innervate both the sympathetic efferent nuclei and either the motor or the medial prefrontal cortex, which is implicated in mental stress, show orexin-like immunoreactivity.43,44
Figure 2.
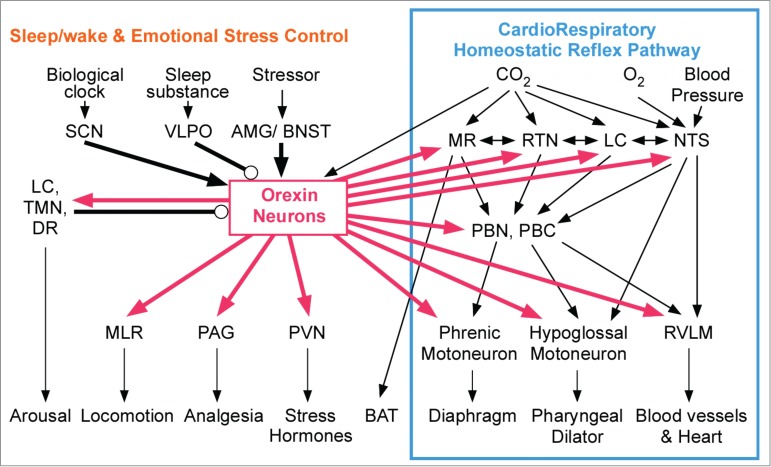
Pivotal role of orexin neurons in linking state-dependent behavioral regulatory systems and internal autonomic homeostatic reflex pathways. Among the known connections from/to orexin neurons in the hypothalamus, the thick lines indicate the selected brain nuclei that are relevant to our study. Many nuclei that are located at both input (MR, RTN, LC, NTS) and output (cardiorespiratory and thermogenic motor neurons) interfaces in the internal homeostatic reflex pathway receive projections from the orexin neurons (right half). Simultaneously, orexinergic connections are engaged in sleep/wake regulation and emotional stress-induced behavioral changes (left half). Thus, orexin can modulate internal autonomic homeostasis in a state-dependent and feedforward manner. Arrows indicate a probable excitatory connection, and circles indicate an inhibitory connection. Connections shown with thin lines are either direct or indirect. Abbreviations: AMG, amygdala; BAT, brown adipose tissue; BNST, bed nucleus of the stria terminalis; DR, dorsal raphe; LC, locus coeruleus; MLR, medullary locomotor region; MR, medullary raphe; NTS, nucleus tractus solitarius; PAG, periaqueductal gray; PBC, pre-Bötzinger complex; PBN, parabrachial nucleus; PVN, paraventricular nucleus; RTN, retrotrapezoid nucleus; RVLM, rostral ventrolateral medulla where sympathetic cardiovascular premotor neurons are located; SCN, suprachiasmatic nucleus; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic nucleus. Adapted from Kuwaki and Zhang.41 © Elsevier. Reproduced by permission of Elsevier. Permission to reuse must be obtained from the rightsholder.
Moreover, orexin neurons receive inputs from the sites that regulate the circadian clock, the sleep/wake cycle, and emotional stress, including brain regions such as the ventrolateral preoptic area, the locus coeruleus, the dorsal raphe, the amygdala, the bed nucleus of stria terminalis, and the suprachiasmatic and tuberomammillary nuclei (Fig. 2).41,45-49 Numerous neurons in the amygdala, a putative center for biological value judgments,50 were retrogradely labeled after a cholera toxin B subunit was injected into the PeF48 and by the transsynaptic transport of tetanus toxin that was expressed by the orexin promoter.47
These anatomic features establish the basis for the hypothesis of the contribution of orexin to the link between the regulatory systems of consciousness (sleep/wake cycle or emotional stress) and unconscious homeostatic reflexes (Fig. 2).41,51,52
Orexin and arousal
Orexins, which play a key role in the stabilization of wakefulness, are thought to be arousal-promoting peptides.53 Diminished orexin function can result in a specific clinical syndrome, namely, narcolepsy, in animals and humans.54,55 Orexin neurons show the following state-dependent activity:56-60 orexin neuron activity increases just before waking, remains high during wakefulness, and increases considerably during exercise and/or heightened alertness (Fig. 3).
Figure 3.
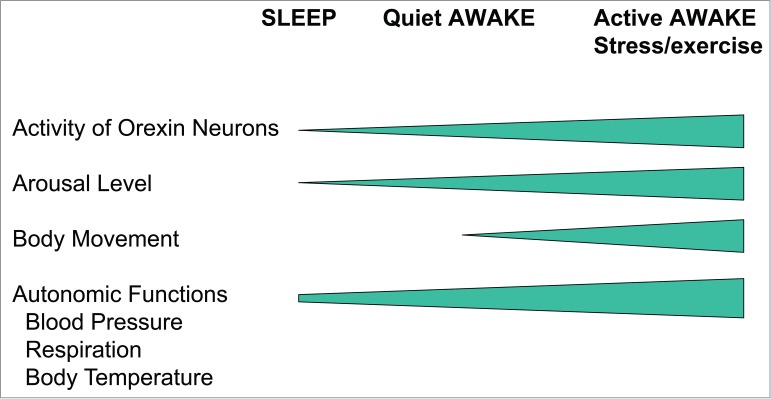
Relationship between the animal's vigilance state and activity of orexin neurons or autonomic functions. Clear resemblance between the orexin neuronal activity and the autonomic functions imply a causative relationship or at least an interrelationship between the two.
Autonomic activities and resultant body temperature and cardiorespiratory functions also increase along with the vigilance state (Fig. 3). Such clear resemblance between the orexin neuronal activity and the autonomic functions imply causative relationship or at least interrelationship between the 2.
Orexin-deficient mice
At present, there are 2 genetically engineered mice models of orexin deficiency (Fig. 4) that are used to study the possible roles of intrinsic orexin in physiological functions, including sleep/wake regulation. One is the prepro-orexin knockout (ORX-KO) mouse that was developed by a conventional knockout technique54 and the other is the orexin neuron-ablated (ORX-AB) mouse.61 The latter was developed using a transgenic technique that introduced a truncated Machado-Joseph disease gene product (ataxin-3) with an expanded polyglutamine stretch that was under the control of the orexin promoter. In these orexin/ataxin-3-transgenic mice, orexin-containing neurons selectively degenerate postnatally, and a loss of 99% or more of the neurons occurs by the age of 4 months.61 An ablation model animal is available in rats in addition to mice62 because transgenic but not homologous recombination techniques are easily applicable to rats. Orexinergic neurons contain not only orexin, but also other neuropeptides or modulatory factors (see section IIC). In addition to orexin, these substances also disappear in the ‘orexin neurons' of ORX-AB mice. More recently, a conditional ablation mouse model has made available using a tetracycline response element.63 In this model, expression of a neurotoxin, namely diphtheria toxin subunit-A, in orexin neurons can be initiated by omitting doxycycline, a derivative of tetracycline, from the food when it suits the researcher's purpose. After a period of 2 weeks, 95% of the orexin neurons are ablated by expression of the diphtheria toxin.
Figure 4.

Orexin deficient mice used in our study. Prepro-orexin knockout mice lack neuropeptide orexin but neurons and co-transmitters are preserved. Orexin neuron ablated mice lack orexin neurons. Therefore, not only orexin but also co-transmitters are eliminated.
Behavior-Associated Changes in Autonomic Functions
Emotional stress
Animals cope with stressors utilizing 2 strategies.64 An active coping strategy (fight-or-flight) is evoked if the stress is predictable, controllable, or escapable. A passive coping strategy (immobility or decreased responsiveness to the environment) is evoked if the stress is inescapable. The active strategy is associated with sympathoexcitation (hypertension, tachycardia, thermogenesis), whereas the passive strategy is associated with sympathoinhibition and/or parasympathetic activation (hypotension, bradycardia). The passive strategy also helps to facilitate recovery and healing. The active strategy is called the “fight-or-flight” response from a behavioral point of view or the “defense response” from an autonomic point of view. The passive strategy is sometimes called “paradoxical fear” or “playing dead.” Parts of the neural substrates that mediate active vs passive emotional coping have been identified within the brainstem.65,66
Sleep and wake
It is well known that blood pressure, respiration, and body temperature fluctuate with a ˜24-hour rhythm (circadian rhythm) with nadirs occurring during nighttime in humans. In sharp contrast to humans, mice and rats sleep for a short duration (an episode of sleep lasts for 10–30 min) many times during both daytime and nighttime. These animals are called nocturnal because their total wake time is longer during the nighttime than the daytime and not because they are continuously awake during the nighttime as humans are during the daytime. In these fragmented sleepers, blood pressure, respiration, and body temperature decrease while they are sleeping regardless of the time of day. Therefore, the state of vigilance is a strong determinant of these autonomic parameters. Although these phenomena are well known, their underlying mechanisms remain to be elucidated.
Lessons from Orexin-Deficient Mice
Taking into account the background mentioned above, we hypothesized that orexin-containing neurons in the hypothalamus may be the missing link between arousal/active stress-coping behaviors and the associated bodily changes that are mainly governed by the autonomic nervous system. In fact, stressors activate orexin-containing neurons.12,67-73 Anatomical (Figs. 1, 2) and physiological (Fig. 3) evidence supports our hypothesis. In order to test the hypothesis, we used ORX-KO and ORX-AB mice and examined their basal autonomic functions and responses to stressful stimuli. We measured not only body temperature but also blood pressure, heart rate, respiration, and pain behavior because the defense response is characterized by the multifaceted nature of the bodily responses.
Basal parameters
In both ORX-KO and ORX-AB mice basal blood pressure was significantly lower by about 20 mmHg as compared to their wild-type littermates in either anesthetized or conscious condition in our experiments.51,52, 74,75 It should be noted here that a similar baseline hypotension was reported in ORX-AB rats,62 while another group reported no difference in blood pressure between ORX-AB and wild type mice.76 We do not currently know the exact reason for the differences but possible differences in environmental stresses may explain the discrepancy because orexin neurons are deeply involved in stress-induced hypertension (see below). Recently, another group reported that high blood pressure in the spontaneously hypertensive rats could be normalized by treatment with an orexin receptor antagonist.77 This is supported by a recent finding that showed an increase in the number of orexin neurons in spontaneously hypertensive rats.78 Administration of orexin into normal mice and rats increased blood pressure.15,18, 31,79-81 Therefore, the activity of orexin neurons seems to be related to the determination of basal blood pressure, at least when it is exaggerated.
In contrast, heart rate and cardiac contractile parameters determined by echocardiography did not differ between orexin-deficient mice and wild-type mice.74,75 Basal ventilation (respiratory frequency and tidal volume)82 and basal body temperature71 were not significantly altered in ORX-KO or ORX-AB mice. Average body temperature as 24 hrs mesor was 35.5±0.1°C in wild type mice, 35.8 ± 0.2°C in ORX-KO mice, and 35.5±0.3°C in ORX-AB mice. However, change in body temperature associated with spontaneous exercise was significantly smaller in ORX-KO (0.7 ± 0.1°C, n = 8 ) and in ORX-AB (0.8 ± 0.0°C, n = 14 ) than that in wild-type mice (1.1 ± 0.1°C, n = 8, p < 0.05).83 Possible differences in the intensity of spontaneous exercise did not explain the variation because change in heart rate associated with spontaneous exercise was significantly greater in ORX-KO (487 ± 13 bpm) and in ORX-AB (478 ± 7 bpm) than in wild-type mice (437 ± 9 bpm, p < 0.01).
A similar abnormality in the magnitude of state-dependent body temperature change was observed when the animals fell asleep. The drop in body temperature during sleep in ORX-KO mice was smaller than in wild-type mice.84 The same was true in humans. Narcolepsy patients showed higher body temperature during sleep because their temperature drop was smaller than the control subjects but not because their body temperature was generally high.85 Taken together, orexin neurons may not be involved in the determination of basal body temperature when the animals are awake and at rest but do contribute to a change of body temperature associated with spontaneous movement and sleep even when there is no stimulation to the animals.
Cardiorespiratory responses during stress
To date, 3 lines of evidence support our hypothesis of the contribution of orexin to the defense response. First, the stimulation of the PeF with the GABA-A receptor antagonist, bicuculline, resulted in an attenuated defense response in urethane-anesthetized ORX-KO and ORX-AB mice. Increases in arterial blood pressure, heart rate, respiratory frequency, and the ß-band power of electroencephalogram measurements (an index of cortical arousal) were smaller and/or shorter in ORX-KO mice than in their wild-type littermates.74 Similarly, increased blood pressure, heart rate, and respiratory minute volume and vascular dilatation in the skeletal muscle were attenuated in ORX-AB mice.75
Secondly, the suppression of the baroreceptor reflex during the defense response was attenuated in ORX-AB mice, whereas characteristics of the baroreceptor reflex (gain and slope) at rest were normal in these mice.75 During the defense response, the baroreceptor reflex is suppressed or reset to a higher-pressure range in order to allow a higher blood pressure than in resting conditions.1,86 The suppression of the baroreflex is mediated by the DMH-medullary link.4 Orexin appeared to contribute to the suppression of the baroreflex during defense responses, but not to the baroreflex during resting conditions. A pharmacological study that used an orexin receptor antagonist supports this notion.87
Third, an attenuation of the defense response in the ORX-KO and ORX-AB mice was also observed in the mice during natural stimulation in unanesthetized and freely moving conditions. We tested the defense response in conscious animals using the resident-intruder test or the air-jet stress paradigm in order to rule out the possibility that the observed differences between the orexin-deficient mice and their wild-type littermates resulted from differences in anesthetic susceptibilities. As expected, the emotional stressor-induced increases in blood pressure, heart rate, and locomotor activity were smaller in orexin-deficient mice (ORX-KO and ORX-AB) than in their wild-type littermates.74,75
Stress-induced analgesia
Cardiorespiratory response is not the sole characteristic of the defense response. The defense response is characterized by a coordinated change in cardiovascular, respiratory, sensory, and motor functions. One of the multifaceted features of the defense response, stress-induced analgesia, was examined. In wild-type mice, foot shock induces long-lasting analgesia, as evidenced by increases in tail-flick latency from noxious hot water. Although ORX-KO mice showed moderate analgesia, the effect was significantly smaller than that shown by their wild-type littermates.12
Stress-induced hyperthermia
Because cardiorespiratory responses during stress (see section IVB) were equally attenuated in ORX-KO and ORX-AB mice and stress-induced analgesia was attenuated in ORX-KO mice (section IVC above), we concluded at this point that orexin was the main contributor to these responses, and colocalized transmitter/modulator candidates had only a minor role, if any. In line with this notion, we hypothesized that stress-induced hyperthermia would also be influenced by orexin. On the contrary, we found that ORX-AB mice, but not ORX-KO mice, had blunted handling stress-induced hyperthermia (Fig. 5A).71 The brown adipose tissue (BAT), which is a major thermogenic organ in rodents, did not respond to handling stress (Fig. 5B), although it did respond to direct pharmacologic stimulation (Fig. 5C). These abnormalities in ORX-AB mice were not observed in ORX-KO mice, in which the orexin peptide is deficient but the neurons are preserved. A similar abnormality in stress-induced thermogenesis has recently been reported in a rat model of orexin neuron ablation.88 Therefore, the integrity (orexin and other coexisting neurotransmitter/modulators) of the orexin neurons is indispensable for the complete expression of multiple facets of the fight-or-flight response.41,51, 52,89
Figure 5.

(A) Effect of repeated handling stress on the rectal temperature of mice of 3 genotypes. The temperature measurement (insertion of thermistor probe into the animal's rectum) itself was used as a stressor and repeatedly applied at 10-min intervals for 2 hours. Data are presented as mean ± SEM of orexin knockout mice (ORX-KO, n = 37), orexin neuron-ablated mice (ORX-AB, n = 32), and their corresponding wild-type littermates (WT, n = 43). (B) Expression of uncoupling protein (UCP)-1 in the brown adipose tissue (BAT) of stressed or naïve mice. BAT was dissected from stressed mice (4 rectal temperature measurements at 10 min intervals) and from naïve (unstressed) mice of the same genotypes. The total RNA extracted from the BAT and cDNA was reverse transcribed. Expression of mRNA for UCP-1, a major protein responsible for thermogenesis in the BAT, was determined by quantitative real-time PCR in triplicate and normalized with β-actin mRNA. Data are presented as mean ± SEM of 7 to 9 animals. Note that handling stress increased the expression of UCP-1 in wild type and ORX-KO mice but not in ORX-AB mice. (C) BAT function test. The change of BAT temperature in response to a β3-agonist, CL316243, was examined in chloralose- (75 mg/kg, ip) and urethane- (750 mg/kg, ip) anesthetized mice. The animals received intraperitoneal injections of saline (dashed line) or CL316243 (1 mg/kg, solid line). Data are presented as mean ± SEM of the peak values during the observation period of 120 minutes (n = 5 for each group). These results indicate that blunted stress-induced hyperthermia in ORX-AB was caused by the loss of orexin neurons and abnormal BAT regulation and not due to an abnormality of BAT, per se. Adapted from Zhang et al.71 © Tomoyuki Kuwaki. Reproduced by permission of Tomoyuki Kuwaki. Permission to reuse must be obtained from the rightsholder.
Next, we asked whether the observed abnormality in ORX-AB mice was restricted to handling stress-induced hyperthermia or whether it could be generalized to other types of stressors because different stressors activate different brain regions.90 For this purpose, we used 2 forms of thermogenic perturbations including environmental cold exposure and brain PGE2 injections that mimic inflammatory fever.91 Again we found that ORX-AB mice, but not ORX-KO mice, exhibited a blunted PGE2-induced fever (Fig. 6) and intolerance to cold (5°C) exposure (Fig. 7). In addition, body temperature decrease in response to isoflurane anesthesia was greater in ORX-AB mice than that in the wild-type mice.92 These findings were similar to the results previously obtained with handling stress-induced thermogenesis.
Figure 6.

Effect of microinjections of PGE2 into the medial preoptic area on body temperature and the electromyogram in the mice of the 4 genotypes. In chloralose (80 mg·kg−1)/urethane (800 mg·kg−1)-anaesthetised mice, ACSF (20 nL) and PGE2 (1 mg·mL−1 in ACSF) were sequentially microinjected into the medial preoptic area (MPO) (C, D). Time-related changes in brown adipose tissue (BAT) temperature and nuchal EMG (a measure of shivering) are shown in A and B, respectively, and the changes expressed as the area under the curve above the baseline in them for 70 min are summarized in A' and B', respectively. Data are presented as mean ± SEM of orexin-knockout mice (ORX-KO, n = 6), orexin neuron-ablated mice (ORX-AB, n = 6), and their corresponding wild-type littermates (WTKO, n = 4 and WTAB, n = 5). (D) Summary for injected sites. Left-side shows dye-distribution in WTKO and WTAB mice and right-side shows that of ORX-KO and ORX-AB mice. In the actual experiment, drugs and dye were injected into unilateral MPO. *p < 0.05 compared with baseline values before injection (Bonferroni's post hoc test). n.s., not significant. ac, anterior commissure; MnPO, median preoptic area. Adapted from Takahashi et al.91 © Tomoyuki Kuwaki. Reproduced by permission of Tomoyuki Kuwaki. Permission to reuse must be obtained from the rightsholder.
Figure 7.
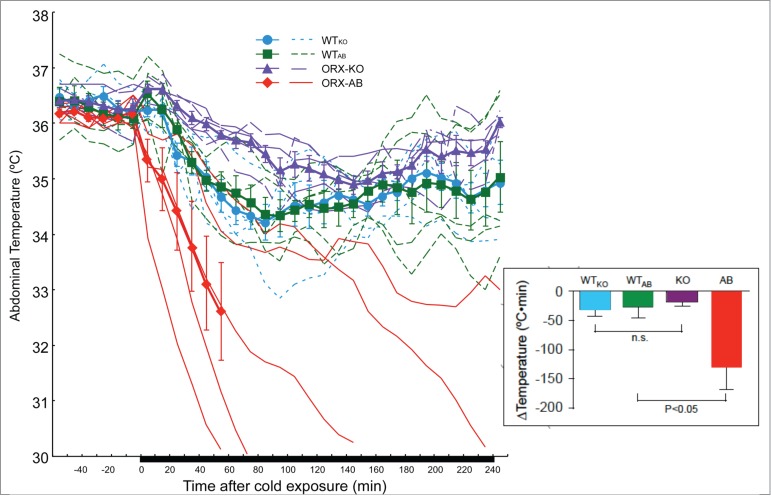
Effect of cold exposure on abdominal temperature in freely moving mice with indwelling telemeters of the 4 genotypes. Mice were exposed to a cold environment (5°C) for 4 h while abdominal temperature was continuously monitored by a telemetric system. When the animal's body temperature decreased below 30°C, cold exposure was terminated and the animal was immediately warmed up with a heating lamp. Time-related changes in abdominal temperature are shown. The thin lines indicate the data from an individual animal, and the thick lines are the mean ± SEM (n = 5 in each group) of orexin-knockout mice (ORX-KO), orexin neuron-ablated mice (ORX-AB), and their corresponding wild-type littermates (WTKO and WTAB). Note that only 1 mouse out of 5 ORX-AB mice tolerated the 4 h of cold exposure. Insets are the changes in the abdominal temperature expressed as the area under the curve (AUC) above the baseline. AUC was calculated for only 60 min during the initial part of the cold exposure because of the endpoint in some ORX-AB mice. *p < 0.05 compared with baseline value before cold exposure (Bonferroni's post hoc test). n.s., not significant. Adapted from Takahashi et al.91 © Tomoyuki Kuwaki. Reproduced by permission of Tomoyuki Kuwaki. Permission to reuse must be obtained from the rightsholder.
As for the co-transmitter candidates in the orexin neurons, we found that glutamate receptor antagonists (d-(−)-2-amino-5-phosphonopentanoic acid, AP-5 and 6-cyano-7-nitroquinoxaline-2,3-dione, CNQX) but not orexin receptor antagonists (SB334867 and OX2 29) successfully inhibited PGE2-induced fever in wild-type mice (Fig. 8).91 These results suggest that orexin neurons are important in general thermogenic processes, and their importance is not restricted to stress-induced thermogenesis. In addition, these results indicate the possible involvement of glutamate in orexin neurons implicated in PGE2-induced fever.
Figure 8.
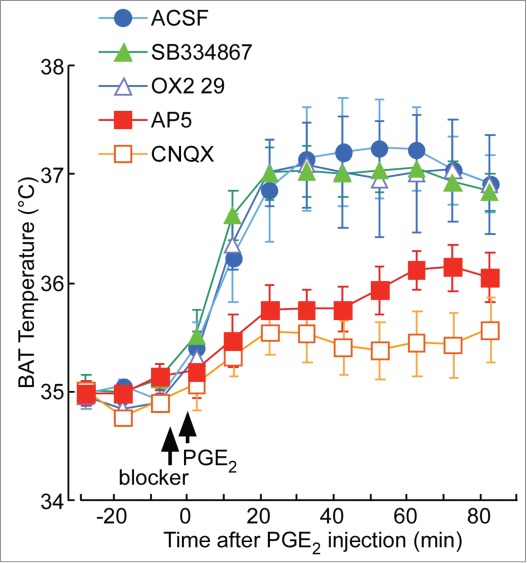
Effect of orexin receptor-antagonists (SB334867 and OX2 29) and glutamate receptor-antagonists [d-(−)-2-amino-5-phosphonopentanoic acid (AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)] on PGE2-induced fever. In chloralose (80 mg·kg−1)/urethane (800 mg·kg−1)-anaesthetised and artificially ventilated C57BL/6 mice, an orexin receptor antagonist, a glutamate receptor antagonist, or ACSF was administered into the lateral ventricle in a volume of 2 µL at the time indicated by the arrow. After 5 min, PGE2 (1 mg·mL−1, 2 µL) was injected into the same ventricle. Time-related changes in brown adipose tissue (BAT) temperature are shown. Tested drugs were the following: an orexin receptor-1-specific antagonist, SB334867 (1 mM, n = 6); an orexin receptor-2-specific antagonist, OX2 29 (100 mM, n = 5); a NMDA-selective glutamate receptor antagonist, AP5 (10 mM, n = 8); an AMPA/kainate-selective glutamate receptor antagonist, CNQX (10 mM, n = 6); and ACSF (n = 7). Only 1 drug was tested in each animal. Data are presented as mean ± SEM. Note that the glutamate receptor antagonists, but not the orexin receptor antagonists, successfully inhibited the PGE2-induced fever and shivering. Adapted from Takahashi et al.91 © Tomoyuki Kuwaki. Reproduced by permission of Tomoyuki Kuwaki. Permission to reuse must be obtained from the rightsholder.
Relevant Data from Normal Rodents and Narcoleptic Humans
Exogenous administration of orexin, co-transmitter candidates, and their antagonists
The exogenous administration of orexin induces both hyperthermia93,94 and hypothermia.95 Another report showed that orexin injections in the medullary raphe increased blood pressure and heart rate but not BAT thermogenesis.96 Administration of an orexin-A receptor antagonist, SB334867, induced either hyperthemia94,97 or had no effect (our result, see Fig. 8). Therefore, no consensus has been reached about the possible role of orexin peptides in thermoregulation. During the course of our experimentation, we found that PGE2-induced BAT thermogenesis was normal in ORX-KO mice but PGE2-induced shivering was blunted in this mutant (Fig. 6). Therefore, we think that orexin alone may play a role in some form(s) of the thermogenesis, but the main neurotransmitter that is important for thermogenesis is a co-transmitter in the orexin neurons, which is most likely glutamate.
Microinjection of glutamate receptor antagonists into the raphe pallidus inhibited the activation of BAT sympathetic nerves that were evoked by stimulation of DMH/DHA,98 by PGE2,99 and by cold exposure.100 Microinjection of a non-selective glutamate receptor antagonist, kynurenate, into the DMH/DHA inhibited BAT sympathetic activation evoked by PGE2 into the preoptic area.101 Conversely, dynorphin,102 nitric oxide,103 and galanin104 within the brain have been suggested as thermolytic. Therefore, glutamate is the most probable transmitter for the thermogenesis associated with activation of orexin neurons.
Functional neuroanatomy
We observed that numerous neurons with orexin-like immunoreactivity expressed c-Fos, a marker of neuronal activation, after foot shock stress,12,69 handling stress,71 PGE2-injection, and cold exposure.91 Moreover, disinhibition of the amygdala, a putative center for biological value judgments,50 induced significantly larger numbers of orexin-positive neurons that expressed c-Fos in the PeF/DMH (58.2 ± 6.4%) than did the vehicle (18.2 ± 4.4%).49
Transneuronal retrograde transport of a pseudorabies virus from the BAT, a key structure in nonshivering thermogenesis, identified the caudal raphe neurons as a site of orexinergic innervation32 and orexin-containing neurons in the hypothalamus.105 Retrograde transport of cholera toxin-B subunit from the caudal raphe nucleus identified hypothalamic neurons that contained orexin and neurons that did not contain orexin but were innervated by orexin (Fig. 9).91 The abovementioned neuroanatomical data supports our hypothesis of the state-dependent modulation of central thermogenic regulation by orexin neurons.
Figure 9.
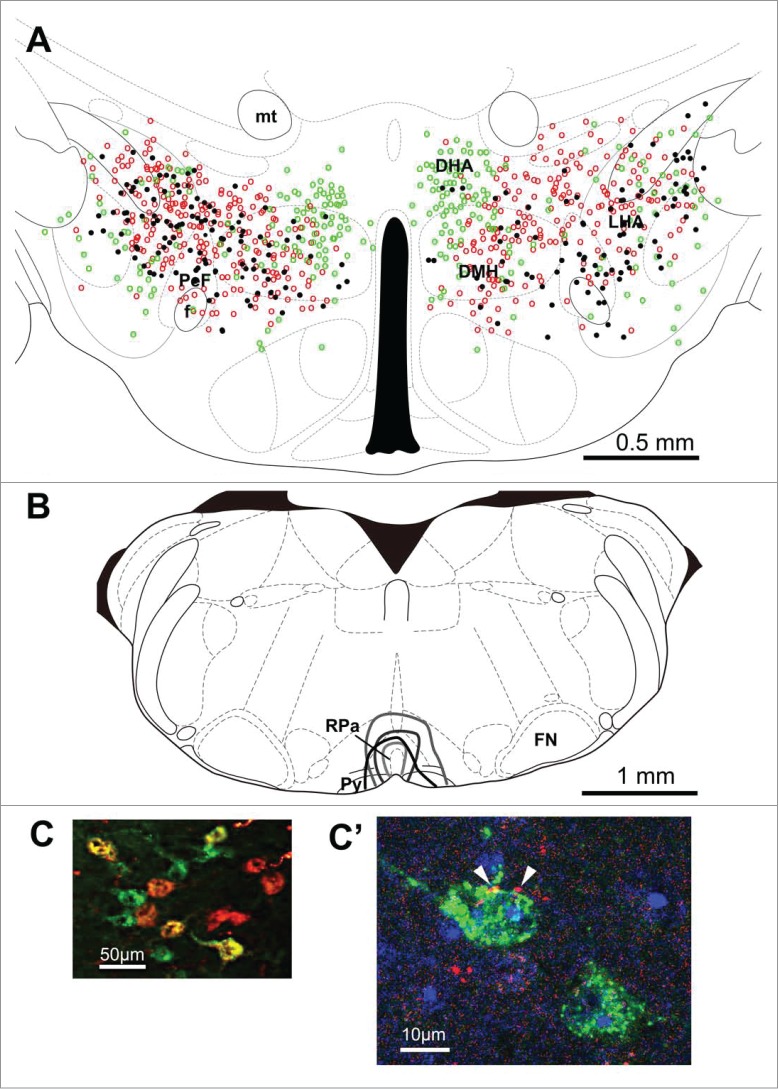
Distribution of retrogradely labeled neurons in the hypothalamus following cholera toxin b subunit injection into the rostral raphe pallidus. Schematic diagram in (A) shows locations of cholera toxin subunit b (CTb)-containing (green circles), orexin-immunoreactive (red circles), and double-labeled (black dots) neurons following CTb injection into the rostral raphe pallidus (RPa) (B) of wild-type mice. Results in 4 animals (1 typical specimen per animal) were plotted on an atlas drawing. (C) shows representative photograph. (C') shows an example of orexin-immunoreactive nerve terminals (arrow) attaching to the CTb-positive cell found in the dorsal hypothalamic area (DHA). DMH, dorsomedial hypothalamus; f, fornix; FN, facial nucleus; LHA, lateral hypothalamic area; mt, mammillary tract; PeF, perifornical area; Py, pyramidal tract. Adapted from Takahashi et al.91 © Tomoyuki Kuwaki. Reproduced by permission of Tomoyuki Kuwaki. Permission to reuse must be obtained from the rightsholder.
Recently, Dr. Nakamura's group showed that activation of DMH neurons but not of PeF/LH neurons including orexin neurons resulted in activation of the medullary raphe presympathetic neurons and successive BAT thermogenesis.106 Together with our observation, we could conclude that orexin neurons in the DMH but not in PeF/LH contribute to stress-induced thermogenesis. However, their method is unable to specifically stimulate only orexin neurons and is activating other neurons as well. In particular, neurons containing melanin-concentrating hormone intermingle in the LH with orexin neurons and activation of these neurons leads to hypotension, hypothermia, and sleep.107-109 Activation of both orexin neurons and melanin-concentrating hormone neurons may cancel their individual effects. Considering that our conclusion was based on orexin neuron-specific loss-of-function experiments (namely knockout, ablation, and antagonism), further studies using orexin neuron-specific gain-of-function experiments such as optogenetic stimulation of genetically defined neurons are needed.
Human narcolepsy
There are only a few reports describing autonomic regulation in narcoleptic patients. Sachs and Kaijser110 reported that unmedicated narcoleptic patients showed attenuated autonomic reflexes (changes in blood pressure and heart rate) in a handgrip test and Valsalva maneuver, but not in face immersion or orthostatic tests. Because some, but not all, reflexes were disturbed, they proposed that peripheral nerves were intact and that the defect was localized to the central nervous system. As for body temperature regulation, the amplitude of circadian rhythmicity of body temperature was small and this was because the nocturnal drop was small.85 Plasma concentration of the stress hormone adrenocorticosterone was lower in the patients than in the controls,111 indicating low stress sensitivity in the patients.
Concluding Remarks
Orexin neurons do not seem to individually regulate cardiovascular, respiratory, and body temperature systems but orchestrate them in a context-dependent manner (Fig. 10). Although vigilance state-dependent responses and emotional stress-dependent responses may appear to be independent, we assume that the common features of these responses are state-dependent and feedforward adjustments of central ventilatory and autonomic regulation in order to fit it to the situational demands that are associated with behavioral and metabolic changes. An animal's arousal state or alertness is minimal during sleep, increases during quiet wakefulness, and further increases during active wakefulness and involves a number of activities such as exercise, food seeking, stress, or panic (Fig. 3). The level of this activation of arousal by orexin neurons is found to be greater in the dark (active period of the circadian cycle) than in the light (inactive period) in nocturnal mice. Ostensibly, independent autonomic regulation (associated with sleep/wake changes and with stress responses) seems to be a different facet of a single and continuous control system in which orexin neurons play an important role. In line with this notion, orexin has recently been shown to play a key role in the cardiovascular and behavioral responses that are associated with panic attacks in both animal models and humans.112
Figure 10.
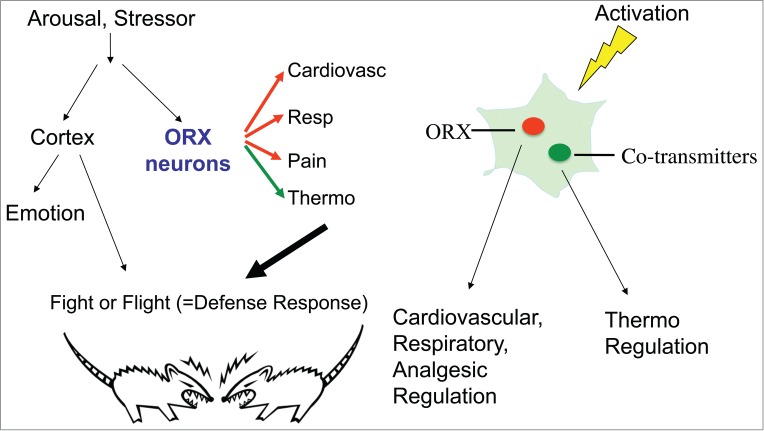
Hypothetical diagram showing the key role of orexin neurons in the fight-or-flight response. When animals confront a stressor, orexin neurons mediate cardiovascular, respiratory, and analgesic (Pain) responses by using orexin as a transmitter. At the same time, orexin neurons regulate body temperature (Thermo) by using cotransmitters other than orexin. The probable candidate is glutamate. These responses are collectively called the “defense response” which prepares the body for the fight-or-flight behavior. Adapted from Kuwaki.51 © Elsevier. Reproduced by permission of Elsevier. Permission to reuse must be obtained from the rightsholder.
It is currently unknown why both orexin and its co-transmitter candidate(s) are required for the orchestration of state-dependent adjustment of central autonomic regulation. However, cotransmission allows for more complex communication between neurons. Diversity in the synaptic control of cardiovascular, respiratory, and thermoregulatory neurons appears to be necessary for animals to adapt themselves to the constantly changing situations and behavioral states (Fig. 10). The orexin and co-transmitter system is likely to function as one of the essential modulators for the orchestration of the circuits that control autonomic functions and behavior.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
About the Author
Tomoyuki Kuwaki is a Professor in Physiology at the Kagoshima University Graduate School of Medical and Dental Sciences (Japan). He is an editorial board member for the journals Journal of Physiological Sciences and Scientific Reports. His main research interest is brainmechanisms of stress coping and relaxation of stress-induced autonomic malfunctions.

Funding
Part of the work was supported by a Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
About the Author
Tomoyuki Kuwaki is a Professor in Physiology at the Kagoshima University Graduate School of Medical and Dental Sciences (Japan). He is an editorial board member for the journals Journal of Physiological Sciences and Scientific Reports. His main research interest is brain mechanisms of stress coping and relaxation of stress-induced autonomic malfunctions.

References
- 1.Kumada M, Terui N, Kuwaki T. Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Prog Neurobiol 1990; 35:331-61; PMID:2263735; http://dx.doi.org/ 10.1016/0301-0082(90)90036-G [DOI] [PubMed] [Google Scholar]
- 2.Hess WR. The Diencephalon: Autonomic and Extrapyramidal Functions. Grune & Stratton, New York, 1954 [Google Scholar]
- 3.Nalivaiko E, Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in the medullary raphe reduces cardiovascular changes elicited by acute psychological and inflammatory stresses in rabbits. Am J Physiol Regul Integr Comp Physiol 2005; 289:R596-R604; PMID:15802554; http://dx.doi.org/ 10.1152/ajpregu.00845.2004 [DOI] [PubMed] [Google Scholar]
- 4.Sévoz-Couche C, Comet MA, Hamon M, Laguzzi R. Role of nucleus tractus solitarius 5-HT3 receptors in the defense reaction-induced inhibition of the aortic baroreflex in rats. J Neurophysiol 2003; 90:2521-30; http://dx.doi.org/ 10.1152/jn.00275.2003 [DOI] [PubMed] [Google Scholar]
- 5.Thomas T, Spyer KM. The role of adenosine receptors in the rostral ventrolateral medulla in the cardiovascular response to defence area stimulation in the rat. Exp Physiol 1996; 81:67-77; PMID:8869140; http://dx.doi.org/ 10.1113/expphysiol.1996.sp003919 [DOI] [PubMed] [Google Scholar]
- 6.Sun MK, Guyenet PG. Hypothalamic glutamatergic input to medullary sympathoexcitatory neurons in rats. Am J Physiol 1986; 251:R798-R810; PMID:3766781 [DOI] [PubMed] [Google Scholar]
- 7.Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses Am J Physiol 1994; 267:H1549-56; PMID:7943401 [DOI] [PubMed] [Google Scholar]
- 8.de Lecea L, Kilduff T, Peyron C, Gao X, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett Fn, et al.. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 1998; 95:322-7; PMID:9419374; http://dx.doi.org/ 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, et al.. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92:573-85; PMID:9491897; http://dx.doi.org/ 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 10.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 2001; 24:429-58; PMID:11283317; http://dx.doi.org/ 10.1146/annurev.neuro.24.1.429 [DOI] [PubMed] [Google Scholar]
- 11.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci 2006; 29:571-7; PMID:16904760; http://dx.doi.org/ 10.1016/j.tins.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 12.Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport 2005; 16:5-8; PMID:15618879; http://dx.doi.org/ 10.1097/00001756-200501190-00002 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Nozaki-Taguchi N, Chiba T. Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol 2002; 137:170-6; PMID:12208773; http://dx.doi.org/ 10.1038/sj.bjp.0704851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dun NJ, Le Dun S, Chen CT, Hwang LL, Kwok EH, Chang JK. Orexins: a role in medullary sympathetic outflow. Regul Pept 2000; 96:65-70; PMID:11102654; http://dx.doi.org/ 10.1016/S0167-0115(00)00202-0 [DOI] [PubMed] [Google Scholar]
- 15.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol 1999; 277:R1780-5; PMID:10600926 [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Shimoyama M, Fukuda Y, Kuwaki T. Multiple components of the defense response depend on orexin: Evidence from orexin knockout mice and orexin neuron-ablated mice. Autonom Neurosci: Basic Clin 2006; 126–127:139-45; PMID:16574499 [DOI] [PubMed] [Google Scholar]
- 17.Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 2005; 98:1387-95; PMID:15557013; http://dx.doi.org/ 10.1152/japplphysiol.00914.2004 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett 2005; 385:131-6; PMID:15941620; http://dx.doi.org/ 10.1016/j.neulet.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 19.Jászberényi M, Bujdosó E, Pataki I, Telegdy G. Effects of orexins on the hypothalamic-pituitary-adrenal system. J Neuroendocrinol 2000; 12:1174-8; http://dx.doi.org/ 10.1046/j.1365-2826.2000.00572.x [DOI] [PubMed] [Google Scholar]
- 20.Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 2001; 12:435-40; PMID:11209963; http://dx.doi.org/ 10.1097/00001756-200102120-00048 [DOI] [PubMed] [Google Scholar]
- 21.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol 2003; 465:593-603; PMID:12975818; http://dx.doi.org/ 10.1002/cne.10860 [DOI] [PubMed] [Google Scholar]
- 22.Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin A and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neurosci 2003; 119:1033-44; http://dx.doi.org/ 10.1016/S0306-4522(03)00238-0 [DOI] [PubMed] [Google Scholar]
- 23.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, et al.. Orexin (hypocretin) neurons contain dynorphin. J Neurosci 2001; 21:RC168(1-6); PMID:11567079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol 1999; 11:653-63; PMID:10447804; http://dx.doi.org/ 10.1046/j.1365-2826.1999.00378.x [DOI] [PubMed] [Google Scholar]
- 25.Cheng SB, Kuchiiwa S, Gao HZ, Kuchiiwa T, Nakagawa S. Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci Res 2003; 46:53-62; PMID:12725912; http://dx.doi.org/ 10.1016/S0168-0102(03)00026-9 [DOI] [PubMed] [Google Scholar]
- 26.Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurons. Acta Physiol 2010; 198:223-35; http://dx.doi.org/ 10.1111/j.1748-1716.2009.02036.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunanaga J, Deng B-S, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol 2009; 166:184-6; PMID:19442935; http://dx.doi.org/ 10.1016/j.resp.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 28.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res 1999; 827:243-60; PMID:10320718; http://dx.doi.org/ 10.1016/S0006-8993(99)01336-0 [DOI] [PubMed] [Google Scholar]
- 29.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, et al.. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 1998; 402:442-59; PMID:9862320; http://dx.doi.org/ 10.1002/(SICI)1096-9861(19981228)402:4%3c442::AID-CNE2%3e3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 30.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 2001; 435:6-25; PMID:11370008; http://dx.doi.org/ 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- 31.Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 2001; 281:R1801-7; PMID:11705764 [DOI] [PubMed] [Google Scholar]
- 32.Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol 2005; 123:147-56; PMID:15742197; http://dx.doi.org/ 10.1007/s00418-005-0761-x [DOI] [PubMed] [Google Scholar]
- 33.Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, Mendelowitz D. Hypocretin-1 (orexin-a) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther 2005; 314:1322-7; PMID:15947034; http://dx.doi.org/ 10.1124/jpet.105.086421 [DOI] [PubMed] [Google Scholar]
- 34.Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res 2001; 903:257-62; PMID:11382413; http://dx.doi.org/ 10.1016/S0006-8993(01)02318-6 [DOI] [PubMed] [Google Scholar]
- 35.Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience 2003; 122:541-50; PMID:14614918; http://dx.doi.org/ 10.1016/j.neuroscience.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 36.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 1998; 18:9996-10015; PMID:9822755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 2006; 499:64-89; PMID:16958085; http://dx.doi.org/ 10.1002/cne.21105 [DOI] [PubMed] [Google Scholar]
- 38.Smith BN, Davis SF, Van Den Pol AN, Xu W. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience 2002; 115:707-14; PMID:12435409; http://dx.doi.org/ 10.1016/S0306-4522(02)00488-8 [DOI] [PubMed] [Google Scholar]
- 39.Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. Neuroreport 2002; 13:433-6; PMID:11930155; http://dx.doi.org/ 10.1097/00001756-200203250-00014 [DOI] [PubMed] [Google Scholar]
- 40.Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol 2008; 164:204-12; PMID:18455970; http://dx.doi.org/ 10.1016/j.resp.2008.03.011 [DOI] [PubMed] [Google Scholar]
- 41.Kuwaki T, Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respir Physiol Neurobiol 2010; 174:43-54; PMID:20416404; http://dx.doi.org/ 10.1016/j.resp.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 42.Nisimaru N, Mittal C, Shirai Y, Sooksawate T, Anandaraj P, Hashikawa T, Nagao S, Arata A, Sakurai T, Yamamoto M, et al.. Orexin-neuromodulated cerebellar circuit controls redistribution of arterial blood flows for defense behavior in rabbits. Proc Natl Acad Sci USA 2013; 110:14124-31; PMID:23912185; http://dx.doi.org/ 10.1073/pnas.1312804110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience 2003; 118:853-66; PMID:12710992; http://dx.doi.org/ 10.1016/S0306-4522(02)00997-1 [DOI] [PubMed] [Google Scholar]
- 44.Krout KE, Mettenleiter TC, Karpitskiy V, Nguyen XV, Loewy AD. CNS neurons with links to both mood-related cortex and sympathetic nervous system. Brain Res 2005; 1050:199-202; PMID:15975562; http://dx.doi.org/ 10.1016/j.brainres.2005.04.090 [DOI] [PubMed] [Google Scholar]
- 45.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005; 437:1257-63; PMID:16251950; http://dx.doi.org/ 10.1038/nature04284 [DOI] [PubMed] [Google Scholar]
- 46.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 2007; 8:171-81; PMID:17299454; http://dx.doi.org/ 10.1038/nrn2092 [DOI] [PubMed] [Google Scholar]
- 47.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, et al.. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 2005; 46:297-308; PMID:15848807; http://dx.doi.org/ 10.1016/j.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 48.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol 2006; 494:845-61; PMID:16374809; http://dx.doi.org/ 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Zhang N, Sakurai T, Kuwaki T. Orexin neurons in the hypothalamus mediate cardiorespiratory responses induced by disinhibition of the amygdala and bed nucleus of the stria terminalis. Brain Res 2009; 1262:25-37; PMID:19368849; http://dx.doi.org/ 10.1016/j.brainres.2009.01.022 [DOI] [PubMed] [Google Scholar]
- 50.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 1997; 20:517-23; PMID:9364666; http://dx.doi.org/ 10.1016/S0166-2236(97)01125-9 [DOI] [PubMed] [Google Scholar]
- 51.Kuwaki T. Orexin links emotional stress to autonomic functions. Autonom Neurosci 2011; 161:20-7; PMID:20813590; http://dx.doi.org/ 10.1016/j.autneu.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 52.Kuwaki T, Zhang W. Orexin Neurons and Emotional Stress. In Sleep Hormones (Vitamins and Hormones vol 89). Academic Press, New York, 2012; 135-58. PMID: 22640612 [DOI] [PubMed] [Google Scholar]
- 53.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci 2009; 29:10939-49; PMID:19726652; http://dx.doi.org/ 10.1523/JNEUROSCI.1205-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell TE, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al.. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999; 98:437-51; PMID:10481909; http://dx.doi.org/ 10.1016/S0092-8674(00)81973-X [DOI] [PubMed] [Google Scholar]
- 55.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000; 27:469-74; PMID:11055430; http://dx.doi.org/ 10.1016/S0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M, Hassani O, Jones B. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci 2005; 25:6716-20; PMID:16014733; http://dx.doi.org/ 10.1523/JNEUROSCI.1887-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mileykovskiy B, Kiyashchenko L, Siegel J. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 2005; 46:787-98; PMID:15924864; http://dx.doi.org/ 10.1016/j.neuron.2005.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake–sleep states in the mouse. Neuroscience 2008; 153:860-70; PMID:18424001; http://dx.doi.org/ 10.1016/j.neuroscience.2008.02.058 [DOI] [PubMed] [Google Scholar]
- 59.Sakurai T. The role of orexin in motivated behaviors. Nature Rev Neurosci 2014; 15:719-31; http://dx.doi.org/ 10.1038/nrn3837 [DOI] [PubMed] [Google Scholar]
- 60.Mahler S, Moorman D, Smith R, James M, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 2014; 17:1298-303; PMID:25254979; http://dx.doi.org/ 10.1038/nn.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, et al.. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001; 30:345-54; PMID:11394998; http://dx.doi.org/ 10.1016/S0896-6273(01)00293-8 [DOI] [PubMed] [Google Scholar]
- 62.Schwimmer H, Stauss HM, Abboud F, Nishino S, Mignot E, Zeitzer JM. Effects of sleep on the cardiovascular and thermoregulatory systems: a possible role for hypocretins. J Appl Physiol 2010; 109:1053-63; PMID:20705949; http://dx.doi.org/ 10.1152/japplphysiol.00516.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabuchi S, Tsunematsu T, Black S, Tominaga M, Maruyama M, Takagi K, Minokoshi Y, Sakurai T, Kilduff T, Yamanaka A. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci 2014; 34:6495-509; PMID:24806676; http://dx.doi.org/ 10.1523/JNEUROSCI.0073-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korte S, Koolhaas J, Wingfield J, McEwen B. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev 2005; 29:3-38; PMID:15652252; http://dx.doi.org/ 10.1016/j.neubiorev.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 65.Nosaka S. Modifications of arterial baroreflexes: obligatory roles in cardiovascular regulation in stress and poststress recovery. Jpn J Physiol 1996; 46:271-88; PMID:8988438; http://dx.doi.org/ 10.2170/jjphysiol.46.271 [DOI] [PubMed] [Google Scholar]
- 66.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 2000; 53:95-104; PMID:11033213; http://dx.doi.org/ 10.1016/S0361-9230(00)00313-0 [DOI] [PubMed] [Google Scholar]
- 67.Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience 2003; 121:201-17; PMID:12946712; http://dx.doi.org/ 10.1016/S0306-4522(03)00334-8 [DOI] [PubMed] [Google Scholar]
- 68.Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun 2000; 270:318-23; PMID:10733946; http://dx.doi.org/ 10.1006/bbrc.2000.2412 [DOI] [PubMed] [Google Scholar]
- 69.Kuwaki T, Zhang W, Nakamura A. State-dependent adjustment of the central autonomic regulation: Role of orexin in emotional behavior and sleep/wake cycle In Central Mechanisms of Cardiovascular Regulation. (ed. Kubo T, Kuwaki T) Research Signport, Kerala (India: ), 2007; 57-73 [Google Scholar]
- 70.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci 2004; 24:11439-48; PMID:15601950; http://dx.doi.org/ 10.1523/JNEUROSCI.3459-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol 2010; 588:4117-29; PMID:20807795; http://dx.doi.org/ 10.1113/jphysiol.2010.195099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport 2002; 13:1351-3; PMID:12151801; http://dx.doi.org/ 10.1097/00001756-200207190-00027 [DOI] [PubMed] [Google Scholar]
- 73.Furlong TM, Vianna DML, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 2009; 30:1603-14; PMID:19811530; http://dx.doi.org/ 10.1111/j.1460-9568.2009.06952.x [DOI] [PubMed] [Google Scholar]
- 74.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 2003; 285:R581-93; PMID:12750151; http://dx.doi.org/ 10.1152/ajpregu.00671.2002 [DOI] [PubMed] [Google Scholar]
- 75.Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am J Physiol Regul Integr Comp Physiol 2006; 290:R1654-63; PMID:16410401; http://dx.doi.org/ 10.1152/ajpregu.00704.2005 [DOI] [PubMed] [Google Scholar]
- 76.Bastianini S, Silvani A, Berteotti C, Elghozi J-L, Franzini C, Lenzi P, Martire VL, Zoccoli G. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep 2011; 34:213-8; PMID:21286242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li A, Hindmarch C, Nattie E, Paton J. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol 2013; 591:4237-48; PMID:23671161; http://dx.doi.org/ 10.1113/jphysiol.2013.256271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clifford L, Dampney BW, Carrive P. Spontaneously hypertensive rats have more orexin neurons in their medial hypothalamus than normotensive rats. Exp Physiol 2015; 100:388-98; PMID:25640802; http://dx.doi.org/ 10.1113/expphysiol.2014.084137 [DOI] [PubMed] [Google Scholar]
- 79.Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 2000; 278:R692-7; PMID:10712290 [DOI] [PubMed] [Google Scholar]
- 80.Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 2002; 104:75-81; PMID:11830280; http://dx.doi.org/ 10.1016/S0167-0115(01)00351-2 [DOI] [PubMed] [Google Scholar]
- 81.Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res 1999; 831:248-53; PMID:10412003; http://dx.doi.org/ 10.1016/S0006-8993(99)01457-2 [DOI] [PubMed] [Google Scholar]
- 82.Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol 2007; 102:241-8; PMID:16959906; http://dx.doi.org/ 10.1152/japplphysiol.00679.2006 [DOI] [PubMed] [Google Scholar]
- 83.Miyata K, Ootsuka Y, Kuwaki T. Ultradian rhythm is coordinated by brain system with involvement of orexin neuron system. J Physiol Sci 2012; 62:S215 [Google Scholar]
- 84.Mochizuki T, Klerman E, Sakurai T, Scammell T. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 2006; 291:R533-40; PMID:16556901; http://dx.doi.org/ 10.1152/ajpregu.00887.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mosko S, Holowach J, Sassin J. The 24-hour rhythm of core temperature in narcolepsy. Sleep 1983; 6:137-46; PMID:6878983 [DOI] [PubMed] [Google Scholar]
- 86.McDowall LM, Horiuchi J, Killinger S, Dampney RAL. Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am J Physiol Regul Integr Comp Physiol 2006; 290:R1020-6; PMID:16284085; http://dx.doi.org/ 10.1152/ajpregu.00541.2005 [DOI] [PubMed] [Google Scholar]
- 87.Iigaya K, Horiuchi J, McDowall LM, Lam AC, Sediqi Y, Polson JW, Carrive P, Dampney RA. Blockade of orexin receptors with Almorexant reduces cardiorespiratory responses evoked from the hypothalamus but not baro- or chemoreceptor reflex responses. Am J Physiol Regul Integr Comp Physiol 2012; 303:R1011-22; PMID:23019212; http://dx.doi.org/ 10.1152/ajpregu.00263.2012 [DOI] [PubMed] [Google Scholar]
- 88.Mohammed M, Ootsuka Y, Yanagisawa M, Blessing W. Reduced brown adipose tissue thermogenesis during environmental interactions in transgenic rats with ataxin-3-mediated ablation of hypothalamic orexin neurons. Am J Physiol Regul Integr Comp Physiol 2014; 307:R978-89; PMID:25324552; http://dx.doi.org/ 10.1152/ajpregu.00260.2014 [DOI] [PubMed] [Google Scholar]
- 89.Kuwaki T. A key role of orexin (hypocretin) neurons in the fight-or-flight response. Physiology News 2011; 83:15-7 [Google Scholar]
- 90.Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat Neurosci Res 1997; 29:183-207; PMID:9436645; http://dx.doi.org/ 10.1016/S0168-0102(97)00095-3 [DOI] [PubMed] [Google Scholar]
- 91.Takahashi Y, Zhang W, Sameshima K, Kuroki C, Matsumoto A, Sunanaga J, Kono Y, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J Physiol 2013; 591:5623-43; PMID:23959674; http://dx.doi.org/ 10.1113/jphysiol.2013.261271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuroki C, Takahashi Y, Ootsuka Y, Kanmura Y, Kuwaki T. The impact of hypothermia on emergence from isoflurane anesthesia in orexin neuron-ablated mice. Anesthesia and Analgesia 2013; 116:1001-5; PMID:23477964; http://dx.doi.org/ 10.1213/ANE.0b013e31828842f0 [DOI] [PubMed] [Google Scholar]
- 93.Monda M, Viggiano A, Luca VD. Paradoxical effect of orexin A: hypophagia induced by hyperthermia. Brain Res 2003; 961:220-8; PMID:12531489; http://dx.doi.org/ 10.1016/S0006-8993(02)03953-7 [DOI] [PubMed] [Google Scholar]
- 94.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci 2011; 31:15944-55; PMID:22049437; http://dx.doi.org/ 10.1523/JNEUROSCI.3909-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Székely M, Pétervári E, Balaskó M, Hernádi I, Uzsoki B. Effects of orexins on energy balance and thermoregulation. Regul Pept 2002; 104:47-53; http://dx.doi.org/ 10.1016/S0167-0115(01)00348-2 [DOI] [PubMed] [Google Scholar]
- 96.Luong LNL, Carrive P. Orexin microinjection in the medullary raphe increases heart rate and arterial pressure but does not reduce tail skin blood flow in the awake rat. Neurosci 2012; 202:209-17; http://dx.doi.org/ 10.1016/j.neuroscience.2011.11.073 [DOI] [PubMed] [Google Scholar]
- 97.Verty ANA, Allen AM, Oldfield BJ. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology 2010; 151:4236-46; PMID:20685882; http://dx.doi.org/ 10.1210/en.2009-1235 [DOI] [PubMed] [Google Scholar]
- 98.Cao W-H, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacol 2006; 51:426-37; http://dx.doi.org/ 10.1016/j.neuropharm.2006.03.031 [DOI] [PubMed] [Google Scholar]
- 99.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neurosci 2003; 122:5-15; http://dx.doi.org/ 10.1016/S0306-4522(03)00527-X [DOI] [PubMed] [Google Scholar]
- 100.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2007; 292:R127-36; PMID:16931649; http://dx.doi.org/ 10.1152/ajpregu.00427.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2004; 286:R320-5; PMID:14563660; http://dx.doi.org/ 10.1152/ajpregu.00515.2003 [DOI] [PubMed] [Google Scholar]
- 102.Handler CM, Piliero TC, Geller EB, Adler MW. Effect of ambient temperature on the ability of mu-, kappa- and delta-selective opioid agonists to modulate thermoregulatory mechanisms in the rat. J Pharmacol Exp Ther 1994; 268:847-55; PMID:8113997 [PubMed] [Google Scholar]
- 103.Steiner AA, Antunes-Rodrigues J, McCann SM, Branco LG. Antipyretic role of the NO-cGMP pathway in the anteroventral preoptic region of the rat brain. Am J Physiol Regul Integr Comp Physiol 2002; 282:R584-93; PMID:11792670; http://dx.doi.org/ 10.1152/ajpregu.00391.2001 [DOI] [PubMed] [Google Scholar]
- 104.Lyudyno V, Krasnova I, Smirnova M, Klimenko V. Antipyretic effect of neuropeptide galanin in endotoxin-induced fever. Bull Exp Biol Med 2001; 1:60-3; http://dx.doi.org/ 10.1023/A:1017538814753 [DOI] [PubMed] [Google Scholar]
- 105.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 2002; 110:515-26; PMID:11906790; http://dx.doi.org/ 10.1016/S0306-4522(01)00555-3 [DOI] [PubMed] [Google Scholar]
- 106.Kataoka N, Hioki H, Kaneko T, Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 2014; 20:1-13; PMID:24988453; http://dx.doi.org/ 10.1016/j.cmet.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 107.Takase K, Kikuchi K, Tsuneoka Y, Oda S, Kuroda M, Funato H. Meta-analysis of melanin-concentrating hormone signaling-deficient mice on behavioral and metabolic phenotypes. Plos one 2014; 9:e99961; PMID:24924345; http://dx.doi.org/ 10.1371/journal.pone.0099961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glick M, Segal-Lieberman G, Cohen R, Kronfeld-Schor N. Chronic MCH infusion causes a decrease in energy expenditure and body temperature, and an increase in serum IGF-1 levels in mice. Endocrine 2009; 36:479-85; PMID:19859841; http://dx.doi.org/ 10.1007/s12020-009-9252-5 [DOI] [PubMed] [Google Scholar]
- 109.Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka K, Hasuwa H, Kilduff T, Terao A, Yamanaka A. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci 2014; 34:6896-909; PMID:24828644; http://dx.doi.org/ 10.1523/JNEUROSCI.5344-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sachs C, Kaijser L. Autonomic regulation of cardiopulmonary functions in sleep apnea syndrome and narcolepsy. Sleep 1982; 5:227-38; PMID:6813932 [DOI] [PubMed] [Google Scholar]
- 111.Kok S, Roelfsema F, Overeem S, Lammers G, Strijers R, Frölich M, Meinders A, Pijl H. Dynamics of the pituitary-adrenal ensemble in hypocretin-deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time-keeping by the master pacemaker. J Clin Endocrinol Metab 2002; 87:5085-91; PMID:12414876; http://dx.doi.org/ 10.1210/jc.2002-020638 [DOI] [PubMed] [Google Scholar]
- 112.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nature Med 2010; 16:111-5; PMID:20037593; http://dx.doi.org/ 10.1038/nm.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]



