Abstract
This review summarizes earlier studies of long-term heat acclimation (LHA) in rats. Since thermoregulatory changes of LHA are stable and sustained, persisting functional and morphological changes are expected to occur in the thermoregulatory centers. Heat exposure increases the number of newborn cells in the ependymal layer of the third ventricle. With time, these newborn cells migrate into the hypothalamic parenchyma and differentiate to immature or mature neurons, some of which integrate into hypothalamic neuralne tworks. The generation of new functional neurons in the hypothalamus may be an important mechanism of LHA.
Keywords: aging, BrdU, ependymal layer of the third ventricle, heat exposure, heat tolerance, neural network, neuronal progenitor cell, thermoregulation
Abbreviations
- APC
adenomatosis polyposis coli
- BrdU
bromodeoxyuridine
- CTb
cholera toxin b-subunit
- CN
control rats
- Dcx
doublecortin
- GFAP
glial fibrillary acidic protein
- HE
heat-exposed rats
- LHA
long-term heat acclimation
- NeuN
neuronal N
- POA/AH
preoptic area of anterior hypothalamus
- Ta
ambient temperature.
Introduction
Animals can adapt to changes in environments to alleviate external harmful stimuli caused by the novel atmosphere. For temperature acclimation, repeated exposure to thermal stress has been known to provide heat acclimation to improve heat tolerance.1-2 Such thermoregulatory advantage of heat-acclimated subjects is attributable to both peripheral efficient thermoeffector responses to a given level of central thermoregulatory drive and to changes in a gain of controller in the thermoregulatory centers.3 For the peripheral mechanism, there are numerous studies which show functional and morphological changes of thermoeffectors.4-7 For instance, heat acclimation enhanced vascular compliance in rats4,7 and increased the density of arteriovenous anastomoses in acral parts of the body,8 which result in a good capability for keeping nonevaporative heat loss at a high level in the heat. For the central mechanism of heat acclimation, several studies have been performed in the anterior hypothalamus from different aspects, regarding gene expression profiles,9-10 changes in the ratio of thermosensitive to insensitive neurons11 and morphological changes in synaptic structures, e.g. number, thickness, curvature and complexity.12 Their results clearly show thermal stimulation-induced neuronal plasticity in the thermoregulatory centers and suggest a possible contribution of neuronal modifications to the achievement of heat acclimation. However, the central mechanism of heat acclimation has not been fully investigated.
The heat acclimation process has been known to present 2 different appearances, namely short-term and long-term heat acclimation, depending on the length of the term of heat exposure.13-14 Briefly, thermoregulatory changes of short-term heat acclimation can be established within a few days of heat exposure and are lost rapidly after the end of the thermal stimuli,15 while those of long-term heat acclimation are stable and sustained.13 Thus, especially in long-term heat acclimation, persisting functional and/or morphological changes may be expected in the thermoregulatory centers.
For decades, it has been shown that neuronal progenitor cells in the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus proliferate, migrate and differentiate into neurons even in adult animals.16,17 The newly-generated neurons then have a significant role in acquisition and maintenance of brain function.18,19 In addition to these brain regions, recent reports have demonstrated neurogenesis in the hypothalamic area where thermoregulatory centers exist.20-22 In adult rat brain, neuronal progenitor cells exist in the ependymal layer of the third ventricle. In response to various stimuli, the progenitor cells proliferate, and the newborn cells migrate into the hypothalamic parenchyma to differentiate into neurons or glial cells.21,22 The new neurons could be functionally integrated into neuronal networks by forming synapses and producing neuropeptides.22 The preoptic area of anterior hypothalamus (POA/AH) is known to be the dominant thermoregulatory center and the other hypothalamic regions are also involved in maintaining afferent and efferent neuronal pathways contributing to the monitoring of core and skin temperatures and to controlling peripheral thermoeffectors.23 Thus, neurogenesis and associated reconstructions of neuronal networks in the hypothalamic area might have a crucial role in modulating thermoregulatory function following thermal adaptation.
On the basis of these reports, we hypothesized that repeated heat exposure may newly generate hypothalamic neurons which would then be integrated in neuronal networks, contributing to establishing characteristic functional changes in thermoregulatory system of heat-acclimated animals. In this mini-review, we introduce a new possible central mechanism for long-term heat acclimation, i.e. heat exposure-induced newborn neurons in the hypothalamic area may play a physiological role in improving heat tolerance in adult rats.
Heat exposure schedule and general procedure
The pattern of changes in thermoregulatory function due to heat acclimation depends on the manner of heat exposure.24,25 Thus, in this review, the pattern of heat exposure and general method of our previous studies are briefly described. Rats (male Wistar, 5 weeks of age) were simply subjected to a constant ambient temperature (Ta) of 32.0 ± 0.2°C (HE) for 6∼53 days, while control rats (CN) were continuously kept at 24.0 ± 0.1°C. Bromodeoxyuridine (BrdU), a most widely used marker, was injected into the rats' abdominal cavity daily for 5 consecutive days after starting heat exposure to identify newly born cells in the brain. On the 6th (HE6), 13th (HE13), 23rd (HE23), 33rd (HE33), 43rd (HE43) and 53rd (HE53) day of the heat exposure period, the brain slices were used for immunohistochemical analyses.
Heat exposure-induced proliferation of neuronal progenitor cells in the hypothalamus
In the hypothalamic area, BrdU of the brain samples was immunohistochemically stained and BrdU positive (BrdU+) cells were counted. In both CN and HE, BrdU+ cells were detectable in the hypothalamic area on all days examined. However, the total number of BrdU+ cells in HE was significantly greater than that of CN by about 2 fold.26,27 The results strongly suggest that moderate heat exposure promotes proliferation of the neuronal progenitor cells within 5 d after starting heat exposure. Representative photo samples of BrdU+ cells in the hypothalamic area of HE are shown in Figure 1 (the photo samples are prepared using our previous data already published in a paper26). In HE6, a large number of the BrdU+ cells are detected in the ependymal layer of the third ventricle, while in the other HE subgroups (HE13-HE53), BrdU+ cells were broadly expressed in the parenchyma of the hypothalamus.26 The observations indicate that heat exposure-induced newborn cells in the ependymal layer of the third ventricle migrated into the hypothalamic parenchyma after 6 d of heat exposure. The process is obviously consistent with that described by Xu et al.22 In addition, the enhanced proliferation of progenitor cells by heat exposure seems to be persistent for at least 25 days, since the number of BrdU+ cells in HE was clearly increased when BrdU was injected into rats for 5 consecutive days between 11–15 d and 21–25 d after the onset of heat exposure.26 However, the proliferation of progenitor cells may not be induced after around 31 d of heat exposure, because the number of BrdU+ cells was not affected when BrdU was injected between 31–35 d after the onset of heat exposure.
Figure 1.
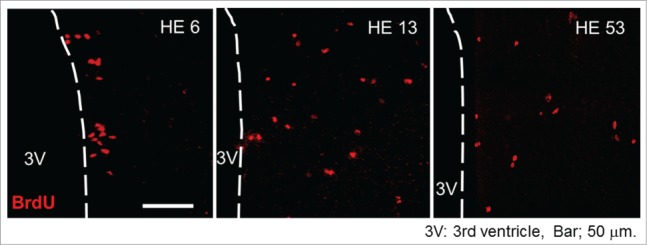
Progenitor cell proliferation and migration in the hypothalamus Representative BrdU-labeled (red) sections of the hypothalamus inspected by laser-scanning confocal microscopy. HE6, HE13 and HE53 show samples on the 6th, 13th and 53rd day of heat exposure, respectively. 3V, third ventricle; scale bar, 50 µm. The photo samples are prepared using our previous data already published in a paper.26
Heat exposure elevates core body temperature. A high temperature physically facilitates biological reactions due to temperature coefficient (Q10) effect and then might accelerate cell proliferation. As described, some brain regions show a vigorous proliferative potency. The subventricular zone of the lateral ventricle gives neuronal precursors that migrate to the olfactory bulb through the rostral migratory stream, and the subgranular zone supplies the granular layer of the hippocampal dentate gyrus with new neurons.16 However, heat exposure had a minimum influence on cell proliferations in both the subventricular zone of the lateral ventricle and subgranular zone.26,27 Thus, a simple rise in tissue temperature may not contribute to proliferation of neuronal progenitor cells. The influence of heat exposure on vigorous proliferation of progenitor cells appears to be limited in the hypothalamic area.
When apoptotic cells in HE6, HE13 and HE23, and corresponding CNs were counted, the numbers of apoptotic cells of HE did not differ from those of CN.26 Since newborn cells were largely increased in HE, the observation suggest that newborn cells hardly die for at least 23 d after commencing heat exposure.
Differentiation of newborn cells in the hypothalamus of heat-exposed rats
To determine whether heat exposure affects the proportion of newborn cells developing a cell type and a neuronal phenotype, brain sections were double-stained with BrdU and various cell markers, i.e., mature neuron (neuronal N, NeuN), immature neruron (doublecortin, Dcx), oligodendrocyte (adenomatosis polyposis coli, APC) and astrocyte (glial fibrillary acidic protein, GFAP) markers.
In HE6, HE13, HE23 and HE33, the percentages of the number of BrdU and NeuN double positive (BrdU+/NeuN+) cells, i.e. newly-bone mature neurons, to the number of BrdU+ cells in the hypothalamus were below 4%. Interestingly, the percentage increased abruptly to 26–34% in HE43 and HE53.26 The observations certify that the proliferated cells in the hypothalamus due to heat stimuli differentiate into mature neurons when the term of heat exposure is more than 33 d (refer to Fig. 2). As described, the process of heat acclimation shows 2 different features, i.e., short-term and long-term heat acclimation, depending on term of heat exposure.14 In rats, conversion from short-term to long-term heat acclimation may occur, at a rough estimate, after 4 weeks of continuous exposure to moderate heat.28 The critical period for establishing long-term heat acclimation appears to be comparable with the period when neurogenesis in the hypothalamic area is energetically promoted (more than 33 days). In adult brain, the time needed for differentiation of proliferated cells from neuronal progenitor cells into nature neurons has been estimated as 2–8 weeks.19,29,30 The specific period for neurogenesis in the hypothalamus in heat-acclimated rats is obviously within the range.
Figure 2.
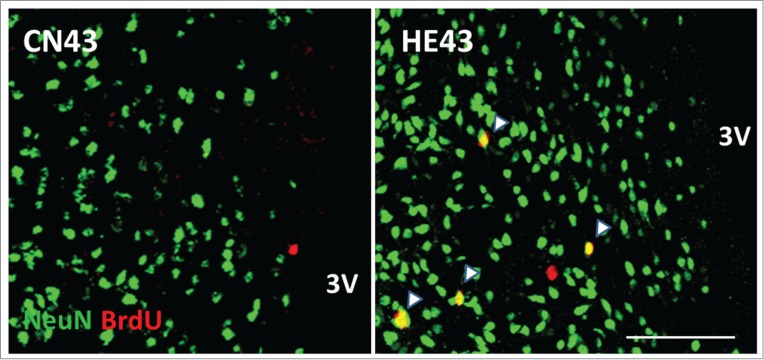
Newborn neurons in the hypothalamic area Representative BrdU (red) and NeuN (green) double-labeled sections of the hypothalamus inspected by laser-scanning confocal microscopy. HE43 and CN43 show samples on the 43rd day of heat exposure and of control, respectively. Yellow dots (shown as arrows) indicate BrdU and NeuN double-positive cells and therefore newborn neurons. 3V, third ventricle; scale bar, 100 µm. The photo samples are prepared using our previous data already published in a paper.26
One might think that heat exposure-induced newborn cells could be automatically differentiated into neurons without any temperature stimuli. When rats were exposed to heat for only 6 d to facilitate hypothalamic progenitor cell proliferation and then kept at a control temperature (24°C), the number of BrdU+/NeuN+ cells was significantly reduced on 53 day after starting heat exposure. Thus, constant heat exposure may be required for promoting differentiation of newborn cells into mature neuron in the rat hypothalamus.
In HE53, a large number of BrdU+ cells (nearly 30%) in the hypothalamus were expressing Dcx, while a small number of hypothalamic newborn cells were stained with APC (4%) and BrdU+ cells rarely expressed GFAP, suggesting that a majority of hypothalamic newborn cells induced by heat exposure takes on a neuronal fate (Fig. 3). In addition, immunohistochemical analysis showed that approximately 15% of newborn cells in the hypothalamus of HE43 were stained by glutamic acid decarboxylase 67, a GABAergic neuron marker, or glutamate transporter, a glutamatergic neuron marker (personal communication). Although further detailed studies are warranted, the type of newborn neurons seems to be not inconsistent with neurons controlling thermoregulatory function in the hypothalamic area.31
Figure 3.
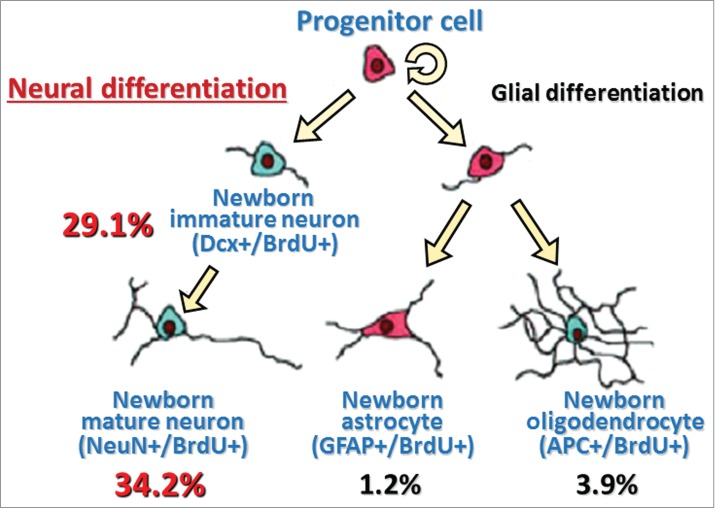
A model with summary for differentiation of heat exposure-induced newborn cells in the hypothalamus Values are percentages of respective differentiated cell number to total newborn cell number (values are obtained from our previous data already published in a paper26). Dcx+/BrdU+, double-labeled with immature neuron marker (doublecortin, Dcx) and BrdU; NeuN+/BrdU+, double-labeled with mature neuron marker (neuronal N, NeuN) and BrdU; GFAP+/BrdU+, double-labeled with astrocyte marker (glial fibrillary acidic protein, GFAP) and BrdU; APC+/BrdU+, double-labeled with oligodendrocyte marker (adenomatosis polyposis coli, APC) and BrdU.
Physiological significance of hypothalamic neurogenesis in long-term heat acclimation
Recent studies have suggested that newborn neurons generated from the adult hypothalamic progenitor cells can be integrated into neural networks by forming synapses and functionally be working in,32 e.g., a feeding control system.30,33 When a part of BrdU+ cells in the hypothalamus were double-labeled with markers of synaptic vesicle membrane proteins,34 a part of BrdU+ cells were surrounded by the markers.26 Thus, heat exposure-generated newborn cells are able to establish synaptic connections with existing neurons of the hypothalamus.
There are numbers of studies showing the anatomical localization of thermo-sensitive and/or thermo-regulative neurons in the hypothalamus.23,35-37 The POA/AH is well known to be the most important region in the neuronal circuit for thermoregulation.23,38,39 Other areas, such as the ventromedial, dorsomedial or paraventricular nuclei and the posterior and lateral hypothalamic areas, are also known to participate in thermoregulation.40-42 According to the estimation in HE53, 23.2% of BrdU+/NeuN+ cells were located in the POA/AH, while 11.3%, 16.9%, and 4.7%, were detected in the the ventromedial, dorsomedial and paraventricular nuclei, respectively. Additionally, 17.3% of BrdU+/NeuN+ cells were found in the posterior hypothalamic area, and the double-positive cells were hardly seen in the lateral hypothalamic area.26 Therefore, in rats with long-term heat acclimation, the primary region where neurogenesis occurred was the POA/AH. This observation is again suggestive of an involvement of newborn neurons in heat acclimation-induced changes in thermoregulatory function.
As described, heat acclimation improves heat tolerance by altering various physiological functions. For instance, when heat-acclimated animals are exposed to acute heat stress, the magnitude of rises in their core temperature is smaller than that of non-acclimated animals. In our study where cytosine arabinoside, a mitosis inhibitor, was continuously infused intracerebroventricularly into rats during heat exposure, progenitor cell proliferation (and hence neurogenesis) due to heat stress was clearly abolished. In such rats, heat tolerance, estimated by a rise in core temperature in the heat, was not improved.43 The fact indicates a good possibility that hypothalamic neurogenesis is essential for achieving heat tolerance in heat-acclimated animals.
Taken together, it is supposed that enhancement of neuronal progenitor cell proliferation followed by neuronal differentiation and incorporation of newborn neurons in the neural network especially in the POA/AH are physiological crucial factors for establishing long-term heat acclimation in rats. Further detailed studies are needed to verify the hypothesis.
Aging and heat-exposure induced neurogenesis
The influence of aging on decremental neurogenesis has been shown in several brain regions.44-46 In human studies, the ability to control body temperature in response to acute heating has been known to diminish with advancing age, e.g. due to aging-dependent decreases in sweating capacity.47,48 Thus, we investigated how aging affect establishment of long-term heat acclimation in rats. Male rats, aged 5 weeks (Young), 10–11 months (Adult), or 22–25 months (Old) old, were subjected to heat for 40–50 d In consistent with the previous study, the number of BrdU+/NeuN+ cells in the hypothalamus was increased due to heat exposure, and the number was the highest in the POA/AH among the hypothalamic regions examined in Young.27 However, this was not the case in Adult and Old. After the heat exposure schedule, the ability of heat tolerance was estimated. During acute heat load, the amounts of increases in core temperature of Young, Adult and Old were significantly smaller than those of their respective controls, indicating that aged rats are still capable of acclimating to heat. However, the magnitude of rises in core temperature became greater with advancing age. It therefore seems that in rats, aging interferes with heat exposure-induced hypothalamic neurogenesis and then hinders the improvement of heat tolerance associated with long-term heat acclimation.
Summary and conclusions
In rats, constant exposure to moderate heat facilitates proliferation of neuronal progenitor cells existing in the ependymal layer of the third ventricle. The proliferation initiates within the first 5 d of heat exposure period and may persist for the following 20 d However, the events may end around 30 day after commencing heat exposure. The newborn cells seem to migrate into the hypothalamic parenchyma and dominantly differentiate into immature and mature neurons mainly in the POA/AH, the main part of thermoregulatory centers. Interestingly, differentiation into mature neurons is drastically augmented after 33 d of heat exposure, the days comparable with the term needed for attaining long-term heat acclimation in rats. After 43–53 day heat exposure, some newborn neurons appeared to establish synaptic connections with pre-existing neurons in the hypothalamic area and to be integrated in a neural network in the hypothalamus. Disturbance of heat-exposure induced neurogenesis, due to intracerebroventricular infusion of a chemical agent or physiological aging, interferes with improvement of heat tolerance, which is characteristically observed in animals with long-term heat acclimation. Changes of thermoregulatory function in long-term heat-acclimated rats may be at least in part attributable to generations of neurons in the hypothalamus.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Eynan M, Knubuvetz T, Meiri U, Navon G, Gerstenblith G, Bromberg Z, Hasin Y, Horowitz M. Heat acclimation-induced elevated glycogen, glycolysis, and low thyroxine improve heart ischemic tolerance. J Appl Physiol 2002; 93:2095-104; PMID:12391086; http://dx.doi.org/ 10.1152/japplphysiol.00304.2002 [DOI] [PubMed] [Google Scholar]
- 2.Shein NA, Doron H, Horowitz M, Trembovler V, Alexandrovich AG, Shohami E. Altered cytokine expression and sustained hypothermia following traumatic brain injury in heat acclimated mice. Brain Res 2007; 1185:313-20; PMID:17963735; http://dx.doi.org/ 10.1016/j.brainres.2007.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol A Mol Integr Physiol 2002; 131:475-83; PMID:11867273; http://dx.doi.org/ 10.1016/S1095-6433(01)00500-1 [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Sugimoto E, Okuno T, Morimoto T. Changes in blood volume and vascular compliance during body heating in rats. Pflugers Arch 1988; 412:354-8; PMID:3174391; http://dx.doi.org/ 10.1007/BF01907551 [DOI] [PubMed] [Google Scholar]
- 5.Saha SK, Ohno T, Tsuchiya K, Kuroshima A. Adaptive modification of membrane phospholipid fatty acid composition and metabolic thermosuppression of brown adipose tissue in heat-acclimated rats. Int J Biometeorol 2000; 43:163-8; PMID:10789917; http://dx.doi.org/ 10.1007/s004840050003 [DOI] [PubMed] [Google Scholar]
- 6.Maruyama M, Hara T, Hashimoto M, Koga M, Shido O. Alterations of calf venous and arterial compliance following acclimation to heat administered at a fixed daily time in humans. Int J Biometeorol 2006; 50:269-74; PMID:16450115; http://dx.doi.org/ 10.1007/s00484-005-0023-6 [DOI] [PubMed] [Google Scholar]
- 7.Li GH, Katakura M, Maruyama M, Enhkjargal B, Matsuzaki K, Hashimoto M, Shido O. Changes of noradrenaline-induced contractility and gene expression in aorta of rats acclimated to heat in two different modes. Eur J Appl Physiol 2008; 104:29-40; PMID:18512069; http://dx.doi.org/ 10.1007/s00421-008-0772-0 [DOI] [PubMed] [Google Scholar]
- 8.Demicka A, Caputa M. Effect of warm rearing on the development of thermolytic effectors in rats. J Therm Biol 1993; 18:257-62; http://dx.doi.org/ 10.1016/0306-4565(93)90011-H [DOI] [Google Scholar]
- 9.Labunskay G, Meiri N. R-Ras3/(M-Ras) is involved in thermal adaptation in the critical period of thermal control establishment. J Neurobiol 2006; 66:56-70; PMID:16215997; http://dx.doi.org/ 10.1002/neu.20191 [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer H, Eli-Berchoer L, Horowitz M. Acclimatory-phase specificity of gene expression during the course of heat acclimation and superimposed hypohydration in the rat hypothalamus. J Appl Physiol 2006; 100:1992-2003; PMID:16469936; http://dx.doi.org/ 10.1152/japplphysiol.00850.2005 [DOI] [PubMed] [Google Scholar]
- 11.Pierau FK, Schenda J, Konrad M, Sann H. Possible implications of the plasticity of temperature sensitive neurons in the hypothalamus ion In: Thermal Balance in Health and Disease: Recent Basic Research and Clinical Progress, (Advances in Pharmacological Sciences; ). Basel: Birkhäuser Basel, 1994; 31-36. [Google Scholar]
- 12.Armstrong LE, Stoppani J. Central nervous system control of heat acclimation adaptations: an emerging paradigm, Rev Neurosci 2002; 13: 271-85; PMID:12405229; http://dx.doi.org/ 10.1515/REVNEURO.2002.13.3.271 [DOI] [PubMed] [Google Scholar]
- 13.Horowitz M, Meiri U. Thermoregulatory activity in the rat: effects of hypohydration, hypovolemia and hypertonicity and their interaction with short term heat acclimation. Comp Biochem Physiol 1985; 82:577-82; http://dx.doi.org/ 10.1016/0300-9629(85)90436-0 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M, Kaspler P, Simon E, Gerstberger R. Heat acclimation and hypohydration: involvement of central angiotensin II receptors in thermoregulation. Am J Physiol 1999; 277:47-55 [DOI] [PubMed] [Google Scholar]
- 15.Sakurada S, Shido O, Sugimoto N, Fujikake K, Nagasaka T. Changes in hypothalamic temperature of rats after daily exposure to heat at a fixed time. Pflügers Arch 1994; 429:291-3; PMID:7892117; http://dx.doi.org/ 10.1007/BF00374326 [DOI] [PubMed] [Google Scholar]
- 16.Gage FH. Mammalian neural stem cells. Science 2000; 287:1433-8; PMID:10688783; http://dx.doi.org/ 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- 17.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature 2000; 405:951-5; PMID:10879536; http://dx.doi.org/ 10.1038/35016083 [DOI] [PubMed] [Google Scholar]
- 18.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 2006; 7:179-93; PMID:16495940; http://dx.doi.org/ 10.1038/nrn1867 [DOI] [PubMed] [Google Scholar]
- 19.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 2014; 94: 991-1026; PMID:25287858; http://dx.doi.org/ 10.1152/physrev.00004.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 2001; 21:6706-17; PMID:11517260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markakis EA, Palmer TD, Randolph-Moore L, Rakic P, Gage FH. Novel neuronal phenotypes from neural progenitor cells. J Neurosci 2004; 24:2886-97; PMID:15044527; http://dx.doi.org/ 10.1523/JNEUROSCI.4161-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 2005; 192:251-64; PMID:15755543; http://dx.doi.org/ 10.1016/j.expneurol.2004.12.021 [DOI] [PubMed] [Google Scholar]
- 23.Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci 2000; 85:18-25; PMID:11189023; http://dx.doi.org/ 10.1016/S1566-0702(00)00216-2 [DOI] [PubMed] [Google Scholar]
- 24.Shido O, Sakurada S, Nagasaka T. Effect of heat acclimation on diurnal changes in body temperature and locomotor activity in rats. J Physiol 1991; 433: 59-71; PMID:1841960; http://dx.doi.org/ 10.1113/jphysiol.1991.sp018414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shido O, Yoneda Y, Nagasaka T. Changes in body temperature of rats acclimated to heat with different acclimation schedules. J Appl Physiol 1989; 67:2154-7; PMID:2600044 [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki K, Katakura M, Hara T, Li G, Hashimoto M, Shido O. Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflugers Arch 2009; 458:661-73; PMID:19252922; http://dx.doi.org/ 10.1007/s00424-009-0654-2 [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki K, Katakura M, Inoue T, Hara T, Hashimoto M, Shido O. Aging attenuates acquired heat tolerance and hypothalamic neurogenesis in rats. J Comp Neurol 2015; 523:1190-201. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp Biochem Physiol A Mol Integr Physiol 2002; 131:475-83; PMID:11867273; http://dx.doi.org/ 10.1016/S1095-6433(01)00500-1 [DOI] [PubMed] [Google Scholar]
- 29.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008. February;18(1):108-15.; http://dx.doi.org/ 10.1016/j.conb.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ming G, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005; 28:223-50; PMID:16022595; http://dx.doi.org/ 10.1146/annurev.neuro.28.051804.101459 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 2011; 301:R1207-28; PMID:21900642; http://dx.doi.org/ 10.1152/ajpregu.00109.2011 [DOI] [PubMed] [Google Scholar]
- 32.Robins SC, Trudel E, Rotondi O, Liu X, Djogo T, Kryzskaya D, Bourque CW, Kokoeva MV. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One 2013; 8:e78236; PMID:24205170; http://dx.doi.org/ 10.1371/journal.pone.0078236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 2005; 310:679-83; PMID:16254185; http://dx.doi.org/ 10.1126/science.1115360 [DOI] [PubMed] [Google Scholar]
- 34.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol 1999; 406:449-60; PMID:10205022; http://dx.doi.org/ 10.1002/(SICI)1096-9861(19990419)406:4%3c449::AID-CNE3%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 35.Bratincsák A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience 2004; 127:385-97; PMID:Can't; http://dx.doi.org/ 10.1016/j.neuroscience.2004.05.016 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience 2005; 133:1039-46; PMID:15927405; http://dx.doi.org/ 10.1016/j.neuroscience.2005.03.044 [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 2008; 11:62-71; PMID:18084288; http://dx.doi.org/ 10.1038/nn2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama T. Thermosensitive neurons in the brain. Jpn J Physiol 1985; 35:375-89; PMID:2997523; http://dx.doi.org/ 10.2170/jjphysiol.35.375 [DOI] [PubMed] [Google Scholar]
- 39.Kanosue K, Yanase-Fujiwara M, Hosono T. Hypothalamic network for thermoregulatory vasomotor control. Am J Physiol Regul Integr Comp Physiol 1994; 267:R283-8 [DOI] [PubMed] [Google Scholar]
- 40.Thornhill JA, Halvorson I. Electrical stimulation of the posterior and ventromedial hypothalamic nuclei causes specific activation of shivering and nonshivering thermogenesis. Can J Physiol Pharmacol 1994; 72, 89-96; PMID:8012903; http://dx.doi.org/ 10.1139/y94-014 [DOI] [PubMed] [Google Scholar]
- 41.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci 2001; 21:4864-74; PMID:11425913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 2003; 460:303-26; PMID:12692852; http://dx.doi.org/ 10.1002/cne.10643 [DOI] [PubMed] [Google Scholar]
- 43.Matsuzaki K, Katakura M, Hara T, Hashimoto M, Shido O. Improvement of heat tolerance by hypothalamic neurogenesis in long-term heat-acclimated rats. J Physiol Sci 63 suppl 2013; 1:S31 [Google Scholar]
- 44.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging 2008; 29:129-47; PMID:17092610; http://dx.doi.org/ 10.1016/j.neurobiolaging.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 1996; 16:2027-33; PMID:8604047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci 2010; 12:569-79; http://dx.doi.org/ 10.1016/j.tins.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. J Appl Physiol 1993; 75:2162-7; PMID:8307874 [DOI] [PubMed] [Google Scholar]
- 48.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 1998; 84: 1323-32; PMID:9516200 [DOI] [PubMed] [Google Scholar]


