Abstract
Alzheimer's disease (AD), the most common dementia in the elderly, is characterized by cognitive impairment and severe autonomic symptoms such as disturbance in core body temperature (Tc), which may be predictors or early events in AD onset. Inclusions of phosphorylated Tau (p-Tau) are a hallmark of AD and other neurodegenerative disorders called “Tauopathies.” Animal and human studies show that anesthesia augments p-Tau levels through reduction of Tc, with implications for AD. Additionally, hypothermia impairs memory and cognitive function. The molecular networks related to Tc that are associated with AD remain poorly characterized. Under physiological conditions, Tau binds microtubules, promoting their assembly and stability. The dynamically regulated Tau-microtubule interaction plays an important role in structural remodeling of the cytoskeleton, having important functions in neuronal plasticity and memory in the hippocampus. Hypothermia-induced increases in p-Tau levels are significant, with an 80% increase for each degree Celsius below normothermic conditions. Although the effects of temperature on Tau phosphorylation are evident, its effects on p-Tau degradation remain poorly understoodWe review information concerning the mechanisms of Tau regulation of neuron plasticity via its effects on microtubule dynamics, with focus on pathways regulating the abundance of phosphorylated Tau species. We highlight the effects of temperature on molecular mechanisms influencing the development of Tau-related diseases. Specifically, we argue that cold might preferentially affects central nervous system structures that are highly reliant upon plasticity, such as the hippocampus, and that the effect of cold on Tau phosphorylation may constitute a pathology-initiating trigger leading to neurodegeneration.
Keywords: Alzheimer's disease, brain plasticity, hippocampus, hypothermia, memory and degradation, microtubule dynamics, temperature, tauopathy
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid β
- BAG2
BCL2-associated athanogene 2
- P-Tau
Phosphorylated tau
- SVZ
Subventricular zone
- Tc
Core body temperature
- 3R
3 microtubule binding domain
- 4R
4 microtubule binding domain.
Introduction
Alzheimer's disease (AD), the most common form of dementia, is a disease associated with chronic progressive neurodegeneration, with short-term memory loss as one of the earliest clinical symptoms, followed by escalating cognitive decline and social dependence,1 and eventually ending in death.2 AD has reached epidemic proportions, representing a major economic, medical and social burden. It afflicts approximately 26 million people worldwide and is expected to increase to more than a 100 million in the next 35 years. The number of AD cases rise with advancing age and increase substantially after 65 years of age.1,3 Four million new cases of dementia are diagnosed each year, and approximately 70% of these cases are attributed to AD.4 The presence of inclusions of phosphorylated Tau (p-Tau) is one of the main hallmarks of AD and many other neurodegenerative disorders classified as “tauopathy,” which include Frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17), Pick's disease, corticobasal degeneration and progressive supranuclear palsy; tauopathies are reviewed in further detail by Sergeant and colleagues.5 These inclusions are highly abundant in specific areas of AD patient brains, and the hippocampus is one of the earliest sites affected.6 The reasons for the susceptibility of the hippocampus to the accumulation of phosphorylated Tau as one of the first-affected brain regions in ADs are not presently understood, and the relationship between short-term memory, plasticity and regulation of microtubule dynamics by Tau in this region may be key to furthering our understanding of AD and its progression.
Because the majority of AD cases (99%) are likely due to environmental factors,7-9 AD is considered to be a multifactorial disorder. While the incidence of AD increases dramatically with age, the mechanisms underlying the link between age and the development of AD remain unclear.9 Whittington and Colleagues.9 suggest body temperature as an important risk factor which favors Tau hyperphosphorylation and aggregation. This possibility ought not be overlooked. Interestingly, animal and human studies link the effects of cold exposure to increases in Tau phosphorylation, raises the possibility of an association between age-dependent deficits in temperature homeostasis and Tau dysregulation in AD and other dementias.9-11 Additionally, AD patients frequently exhibit an increase in Tc amplitude and acrophase, the cause of which remains to be conclusively determined.12-21 Pre-clinical and clinical studies have indicated that anesthesia also induces an increase in p-Tau levels through a reduction in Tc, with implications for AD genesis and/or progression.22-28
In this review we focus our attention on the transcriptional and post-translational mechanisms of Tau regulation of microtubule dynamics, and the importance of this regulation to regions of the brain with high neuron plasticity. We highlight the effects of temperature on molecular mechanisms influencing the abundance of phosphorylated Tau in the brain, and how they might influence the development of Tau-related diseases. Specifically, we argue that cold may preferentially affect central nervous system structures that are highly reliant upon plasticity, such as the hippocampus, and that the effect of cold on Tau phosphorylation may constitute a pathology-initiating trigger leading to neurodegeneration.
Plaques and Tangles – Cause or Consequence?
Histopathologically, AD is characterized 2 features: extracellular amyloid plaques composed of amyloid β (Aβ) peptide, and intracellular neurofibrillary tangles (NFTs) of hyperphosphorylated Tau protein. The presence of Aβ peptide in AD brain was initially regarded as a primary cause of brain dysfunction, although subsequent studies suggest that the presence of Aβ may be a consequence of AD-initiating events, rather than the cause of AD itself.29-31 This issue is still under investigation and the “amyloid cascade” hypothesis that has emerged and instructed much of the research on Alzheimer's disease for more over 25 years 32,33 should be carefully reevaluated in addressing the primary cause of dementia. Several amyloid-independent mechanisms have been proposed to lead to AD,34 and plaques of amyloid β accumulate in aged individuals without AD pathology. These observations suggest that Aβ peptide is unlikely to be the sole participant in the development of AD. Accordingly, therapies targeting the accumulation of amyloid β plaques via clearance of amyloid β have proved largely unsuccessful, suggesting that Aβ plaques might not be as detrimental to neurons as once thought, but may instead represent a neuroprotective response,30 furthering the skepticism surrounding the attribution of AD solely to amyloid β.
The severity of dementia in AD has been found to correlate with the number of NFTs, while there was no correlation between AD severity and plaque burden.35 Furthermore, the presence of mutations in Tau that give rise to FTDP-17.36 suggests that Tau dysfunction independently of amyloid β is sufficient to cause neuronal dysfunction leading to dementia and neuronal death. Further, Aβ toxicity is at least partly dependent on Tau expression,37 and Tau knockout rescues premature mortality in amyloid precursor protein transgenic mice.38
The microtubule-associated protein (MAP) Tau is encoded by the MAPT gene.39 Under physiological conditions, Tau binds to microtubules, promotes their assembly and stability.40 (Fig. 1). The Tau-microtubule interaction is a dynamic process that plays an important role in the structural remodeling of the cytoskeleton during neuronal plasticity, with functions in neurite elongation, synaptic and spine formation and memory.
Figure 1.
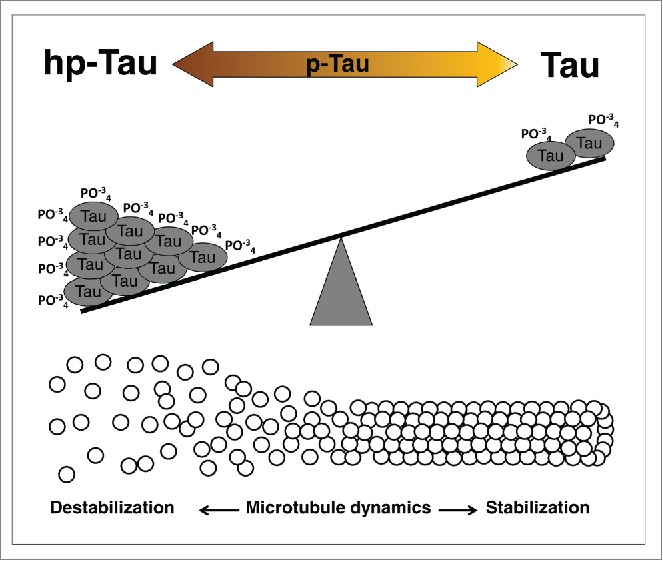
Tau phosphorylated levels changes microtubules dynamics. The molecule Tau, a microtubule-associated protein (MAP), is a phosphoprotein (p-Tau) and its function are directly associated with its phosphorylation levels. The balance between high phosphorylated Tau (hp-Tau), and low phosphorylated Tau (Tau) promotes changes in microtubules dynamics leading to destabilization or stabilization, respectively. Brain of Alzheimer's patients has approximately 4-fold higher levels of Tau phosphorylation as compared to normal brain. This increase in hp-Tau may be a result from a dysregulation of Tau kinase/phosphatase system or from a failure in the degradation machinery.
The dynamic regulation of Tau binding to microtubules is achieved by 3 distinct mechanisms. The first is via alternative splicing, which gives rise to 6 Tau isoforms that differ based on the presence of either 3 (3R) or 4 (4R) C-terminal microtubule binding domains.41-43 While only 3R Tau is expressed in fetal brain, the relative abundance of 4R to 3R Tau in adult brains is approximately equal, with 4R and 3R existing in an approximate ratio of one-to-one. Increases in the ratio of 4R to 3R Tau have been described in several tauopathies.44 The 3 microtubule binding domains of 3R Tau confer a relatively lower affinity for microtubules binding, which is permissive of higher cytoskeletal plasticity and augmented cellular transport.45 The adult hippocampus expresses higher levels of 3R Tau than does the cortex.46 and the expression of a neonatal Tau isoform, 0N/3R Tau, persists during adult neurogenesis in the subgranular cell layer of the hippocampus, suggesting that microtubule dynamics are differently regulated in regions of the brain where regeneration and plasticity are essential to function.45,47-51 A schematic representation of 3R/4R Tau isoform, plasticity and pTau is shown in Figure 2. This, and the finding that the hippocampus is one of the first brain areas affected by AD,6 raises the possibility that a more-dynamic, less-avidly binding Tau population preferentially predisposes the neurons in this region to AD pathology.
Figure 2.
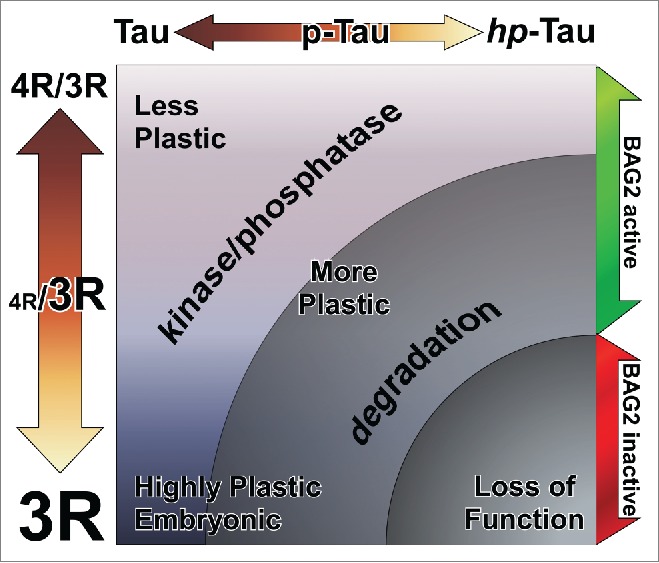
The plasticity of neurons is regulated by Tau-microtubule binding affinity. More-plastic neurons have a greater proportion of 3R Tau in relation to 4R, with an extreme being highly-plastic developing and immature neurons—embryonic neural precursors express only 3R Tau while mature neurons have an approximately equal proportion of 3R and 4R Tau. A further axis of Tau-microtubule binding affinity is Tau protein phosphorylation, with Tau phosphorylation correlating negatively to microtubule binding affinity. The abundance of phosphorylated Tau is dynamically regulated by a balance between Tau kinase and phosphatase activity, but also by direct degradation of phosphorylated Tau protein via BAG2. A failure in BAG2 is permissive of the accumulation of phosphorylated and hyperphosphorylated Tau species which, when combined with a higher proportion of 3R Tau isoforms, as is the case in more-plastic brain regions like the hippocampus, results in a collapse of dynamic microtubule regulation and a loss of neuron functionality.
The second mechanism of regulation of Tau function is via post-translational modification. Tau function is regulated by covalent modification of several modifying molecules at a multitude of sites, within and outside the Tau microtubule binding domains, suggesting a complex regimen of combinatorial control at the single molecule and population levels.52 The degree of Tau phosphorylation correlates negatively to its microtubule binding affinity (Fig. 1), and represents an important mode of fine-tuning microtubule stabilizing and destabilizing dynamics;53 reviewed in.54 Tau has more than 79 phosphorylation sites,2 and various kinases (e.g. GSK-3B, cdk5, MAPK/ERK, CaMKII, JNK, c-Jun and AKT/PKB) are documented to phosphorylate Tau in a site-specific and context-dependent manner. The degree of Tau protein phosphorylation is further regulated by the activity of phosphatases (e.g., PP2A, PP2B and PP1), which reduce the cellular population of phosphorylated Tau protein without affecting the total Tau protein population.55,56
A third mechanism of Tau regulation is the degradation of Tau protein. In AD and other taupathies, the presence of ubiquitin in Tau inclusions.57 suggests a defect in ubiquitin-mediated Tau protein degradation,58 being that most polyubiquitinated proteins are destined for degradation by the proteasome,59,60 and between 80% and 90% of intracellular protein degradation is undertaken by the proteasome.61 The Tau degradation machinery, or “Tau triage system,” consists of the association of the E3 ubiquitin ligase CHIP (carboxyl terminus of Hsp70-interacting protein) with heat shock proteins (Hsp) and others chaperones that direct ubiquitinated Tau toward the 26S proteasome.59,62 Ubiquitin-proteasome pathways were reviewed in detail by Ciechanover.63 The degradation of Tau by the 26S proteasome is ineffective, and results in accumulation of ubiquitinated hyperphosphorylated Tau protein. The co-chaperone BAG2 (Bcl2-associated athanogene 2) interferes with CHIP-Hsp interaction, inhibiting the ubiquitination of Tau, and conveying Tau toward ubiquitin-independent degradation by the 20S proteasome.64
Neuroplasticity – A Liability in the Face of Tau Dysregulation?
The hippocampus lies under the medial temporal lobe and its functions are associated with learning and memory.65 which depend on structural changes such as long-term potentiation and synaptic remodeling.66,67 Further, the hippocampus is one of 2 sites of neurogenesis within the adult brain, and hippocampal neurogenesis within the dentate gyrus (DG) has an important role in plasticity and memory.68,69 An age-dependent decline in neurogenesis in this region may be related to the cognitive impairment associated with normal aging and exacerbated in diseases such as AD.70,71 The renewal of cells within the hippocampus via neurogenesis is important to the maintenance of adult hippocampal function, such as learning and memory consolidation. A decrease in the proliferative neurogenic cell population of DG has been shown to impair hippocampus-dependent tasks.72 Conversely, stimulation of neurogenesis improves spatial memory, which is a specialized domain of hippocampus function.69
The importance of regulation of Tau phosphorylation to AD becomes clear when considering that brain from AD patients has approximately 4-fold higher levels of Tau phosphorylation than normal.73 As discussed, the high levels of phosphorylated Tau protein detected in AD may result from a dysregulation of Tau kinase and/or phosphatase activity, or from a failure to regulate levels of phosphorylated Tau via degradation.74 The contribution of lower-affinity 3R Tau species to increased microtubule dynamics in the hippocampus, coupled to the accumulation of microtubule-destabilizing phosphorylation of Tau suggests a mechanism to explain the unique susceptibility of the hippocampus in the early stages of AD (Fig. 2). Thus, the innate plasticity of hippocampal neurons may represent a vulnerability in the context of the challenge posed by accumulation of phosphorylated Tau, enabling a catastrophic loss of neuron function that spreads to other affected brain regions.
In addition to the hippocampus, the subventricular zone (SVZ) is home to a large population of mitotically dividing neural precursors. Dynamic regulation of the microtubule cytoskeleton is important to the migration and differentiation of neural precursors, and not surprisingly the SVZ expresses high amounts of 3R Tau isoforms.45 Levels of Tau phosphorylation also increase in the SVZ with age, and are higher in the SVZ than in other regions of the brain.75 The SVZ of AD patients also has significantly less proliferating precursor cells than normal,76 which further exacerbates age-dependent cognitive decline due to a failure of homeostatic regeneration by differentiating neuroblasts in target brain regions. In addition to the reduced SVZ neurogenic potential that accompanies normal aging, a dysregulation of Tau phosphorylation within SVZ precursor neurons may dramatically affect the capacity of the 3R Tau-enriched neural precursor population to successfully migrate and differentiate, and thus further contribute to AD pathology(Fig. 2).
Temperature, Tau and Alzheimer's Disease
Recently, a metabolic hypothesis of Tauopathy etiology has emerged to explain the close link between associated risk factors and diseases. Whittington and colleagues suggest 5 risk factors: aging, hypothermia, diabetes mellitus, starvation and anesthesia—each of which encourage the accumulation of hyperphosphorylated Tau.9 Several studies have shown that the Tc of healthy humans over 60 years of age is lower than in young adult. The average Tc of individuals above the age of 60 is approximately 0.4°C lower compared to healthy adults (20-60 year old).77-80 Interestingly, older healthy humans have a greater risk of hypothermia when exposed to environmental cold, as the incidence of morbidity is higher among the aged than in younger adults exposed to extended periods of cold.78-80 These changes may be due to an age-related reduction of peripheral vasoconstriction and reduced metabolic heat production, along with other factors.81
Beyond the cognitive impairment in AD, patients also suffer from non-cognitive behavioral symptoms, which include autonomic dysfunction, agitation, hyperactivity, anxiety, weight loss, depression and disturbed circadian rhythms and sleep.82-85 AD patients also exhibit an increase in Tc amplitude and acrophase, the causes of which remain poorly understood.12-21 Interestingly, using 3xTgAD mice, a transgenic model for AD, Knight and colleagues demonstrate age-dependent changes in Tc,86 reflecting the increased Tc observed in AD patients.15 These thermoregulatory dysfunctions in 3xTgAD mice were shown to be one of the earliest changes that appeared, even before significant AD-related neuropathology, strongly indicating that these symptoms might be a predictor or even an early event in AD onset, rather than merely a consequence of the disease.
Hypothermia induces an increase in p-Tau levels (Fig. 3). For each degree Celsius below normothermic conditions, an 80% increase in Tau phosphorylation is observed.87 Interestingly, cold-induced Tau phosphorylation also occurs in hibernating.88 and non-hibernating animals.9 The effect of cold on the p-Tau fraction was observed using in vivo and in vitro models with different thermic conditions varying from between 3°C – 10°C below 37°C, which is considered the normothermic condition for most homeothermic animals.26,89-92 (Fig. 3). This suggests a link between age-dependent deficits in temperature homeostasis and Tau dysregulation in AD and other dementias.9-11 If the p-Tau/temperature described above is linear, a relative drop of 0.4 degree Celsius in body temperature in an aged healthy human relative to younger adults might represent an approximate 30% increase in p-Tau in aged brain compared to young adult brain. This possibility ought not be overlooked, as it may render the aged brain more vulnerable to neurodegenerative disorders, and in particular those related to Tau dysfunction. It is also tempting to speculate that the elevated Tc observed in AD patients.12-21 may represent a compensatory mechanism to counteract the increase in p-Tau levels and prevent impaired cognition. Interestingly, it has been shown that rats with intracerebroventricular Aβ peptide infusion select higher ambient temperature during night time and an attenuated acquired heat tolerance compared to control animals after long-term heat exposure.93 Because there is evidence that Aβ is a consequence of AD, rather than the cause of the disease itself (see “Plaques and Tangles – Cause or Consequence?” above), we believe that together these data corroborate the above hypothesis of hyperthermia being a protective body response during AD pathogenesis. In light of these, the notion that lowered body temperature may prolong life span, as well discussed by Flouris and Piantoni,94 might be cautiously used, as it might not be completely true for taupathologies related disorders. A better understanding of temperature regulation in physiologic and pathologic conditions during aging needs further investigation.
Figure 3.
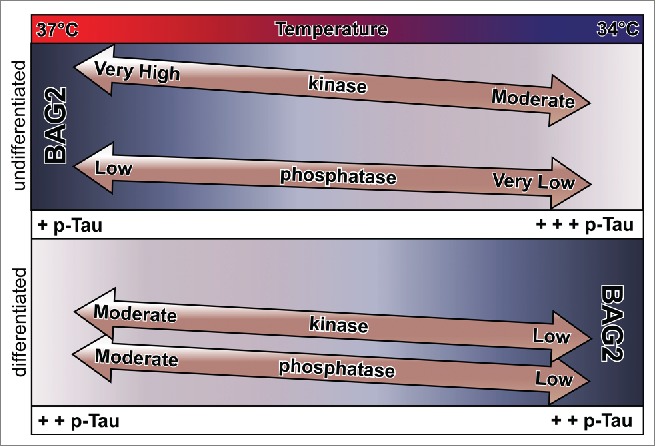
Regulation of Tau phosphorylation levels by temperature and differentiation. Cold induces a decrease in BAG2 expression in undifferentiated cells, while the opposite is true in differentiated cells. Interestingly, while the decrease in BAG2 in undifferentiated cells results in an increase in levels of p-Tau (+p-Tau to +++pTau), the increase in BAG2 in differentiated cells has no change on p-Tau levels (++p-Tau to ++pTau). On cold condition, differentiation leads to a decrease in phosphorylated Tau (+++p-Tau to ++p-Tau), coincident with an increase in Tau phosphatase activity (very low to low) and a decrease in Tau kinase activity (moderate to low). Cold-induced inhibition of kinase and phosphatase activity results in a higher kinase-to-phosphatase activity in undifferentiated cells to differentiated cells. Cold treated undifferentiated cells have an increase in Tau phosphorylation due to a higher kinase activity and due to inhibition of BAG2 expression. In differentiated cells cold-attenuated kinase/phosphatase activity has no effect of p-Tau levels, while BAG2, while present, may be dependent on other temperature sensitive kinases.
Animal and human studies have indicated that anesthesia induces an increase in p-Tau levels through a reduction in Tc: intravenous (chloral hydrate and sodium pentobarbital) and inhalation anesthetics (isoflurane) promote pronounced hyperphosphorylation of Tau at several epitopes which were reversed by the restoration of Tc. This observation raises an important clinical question regarding the impact of anesthesia on p-Tau levels. Does it contribute to AD progression or genesis? Are aged patients more vulnerable to anesthesia? Additionally, hypothermia also promotes memory disruption and impairment of cognitive function,95 raising the possibility that hypothermia induced by anesthesia may account, to some degree, for the progression of impaired learning and memory and cognitive deterioration in the elderly and AD after surgery.96 Interestingly, experiments with isoflurane and dimethyl sulfoxide have been shown to induce Tau hyperphosphorylation in animals that developed hypothermia.22,23 Additionally, reversible Tau phosphorylation in the hippocampus is observed after one hour in animals acutely subjected to cold conditions, followed by a second peak of Tau phosphorylation at 6 hours.91
By using anesthesia-induced hypothermia, La Freche and colleagues.24 showed that 1 hour of cold exposure in mice induced an increase in p-Tau in the brain that was completely restored after 24 hours. However, by repeating the same procedure in subsequent months they observed that the cold effect on p-Tau was no longer transient. After 5 months performing the same experiment in the same animal, p-Tau was shown to be dramatically increased in the hippocampus for 30 days. In other studies, an increase in insoluble Tau and p-Tau were also found weeks to months after isoflurane anesthesia in mouse models of AD and Tauopathy.25,28 In humans, Tang and colleagues found increased Tau e p-Tau in cerebrospinal fluid of patients 2 days after anesthesia.27 Palotas and colleagues.97 found an increase in Tau 6 months after surgical anesthesia.97 Those results suggested that the link between cold and p-Tau fraction are not merely transitory and may have serious implications for mental health. Thus, the impact of temperature as a risk factor for AD should not be overlooked.
As described above, accumulation of hyperphosphorylated Tau present in Tauopathies may be due to a dysfunction of Tau-associated kinases and/or phosphatases,98,99 or even a failure in the Tau degradation process.74 The intracellular pathways associated with cold-induced Tau hyperphosphorylation were initially ascribed to a dysfunction of kinase and/or phosphatase systems.9,26,89 More recent studies also describe a kinase/phosphatase-independent pathway.22,23,92 which raises the possibility of a dysfunction in the proteasome degradation system under conditions of anesthesia-induced hypothermia and an increased p-Tau fraction. Indeed, a recent study from our group suggested that, in addition to a dysfunction of kinase/phosphatase activity, cold-induced Tau phosphorylation may be a consequence of a temperature-sensitive dysregulation of Tau protein turnover.90 In this study, our hypothesis was that cold inhibits proteasomal machinery, resulting in an accumulation of p-Tau (Fig. 3). Careful analysis of these data.90 reveals interesting differences in the ways in which Tau phosphorylation levels are regulated in a temperature- and differentiation-dependent context. Firstly, cold induces a decrease in BAG2 expression in undifferentiated cells. BAG2 degrades p-Tau under normal temperature conditions.64 This decrease in BAG2 expression is accompanied by an increase in p-Tau and in the ratio pTau/total Tau levels (Fig. 3). Overexpression of BAG2 in cold-exposed undifferentiated SH-SY5Y rescued the increased p-Tau levels, indicating that the increase in p-Tau/total Tau in cold-exposed undifferentiated cells is due to cold-induced inhibition of BAG2 expression. BAG2 is repressed by NF-κB (Nuclear factor-kappa B) signaling in undifferentiated SH-SY5Y cells.100 Interestingly, cold induces an increase in BAG2 expression in differentiated cells, yet this increase is not accompanied by a decrease in Tau phosphorylation levels suggesting that BAG2 is regulated in a differentiation-dependent context (Fig. 3). This difference in behavior between differentiated and undifferentiated cells on cold exposure is telling when considered from the perspective of developmental changes in Tau phosphorylation and kinase and phosphatase activity.101 During differentiation, there is a broad decrease in phosphorylated Tau epitopes, which coincides with the expression of higher-molecular weight 4R Tau isoforms. This interesting juxtaposition represents a trade-off in terms of regulation of Tau regulation of microtubule plasticity, with more-plastic less-differentiated cells relying more heavily on microtubule regulation via Tau phosphorylation, while less-plastic more-differentiated cells rely less heavily on Tau phosphorylation and more on an increased ratio of 4R/3R Tau isoforms (Fig. 2). It is interesting to note that this shift is accompanied by a significant increase in Tau phosphatase activity. Thus, less mature cells have higher Tau kinase and lower Tau phosphatase activity while mature cells have lower Tau kinase activity and higher Tau phosphatase activity (Fig. 3). This is noteworthy because the cold-induced inhibition of kinase and phosphatase activity will still leave undifferentiated cells with a relatively higher kinase-to-phosphatase activity compared to differentiated cells. Thus in cold treated differentiated cells, a higher kinase/phosphatase activity will result in an increase in Tau phosphorylation due to a higher overall kinase activity (Fig. 3). In cold treated differentiated cells the attenuated kinase-to-phosphatase activity is further dampened by cold-inactivation.87 It might be that the activity of BAG2 is also dependent upon temperature sensitive kinases like p38 and ERK1/2.102 which would render it inactive under cold conditions. Thus, the regulation of levels of phosphorylated Tau in more-plastic, less-differentiated neurons is more dependent upon degradation by BAG2, than in more-differentiated less-plastic neurons (Fig. 3).
Repression of BAG2 by cold-sensitive pathways in undifferentiated cells may be a causal factor in the accumulation of cytotoxic p-Tau protein via restriction of BAG2-mediated clearance of cellular p-Tau. This mechanism would be especially important in brain structures relying on high plasticity and the presence of undifferentiated neuronal population such as hippocampus, a highly plastic system with undifferentiated cells in comparison to other brain areas. Although cold or anesthesia induced increase in p-Tau through BAG2 in brain structures is not yet described, we speculate that BAG2 levels are differently regulated in hippocampus as compared to other brains areas. In addition, hippocampus might be more vulnerable to cold exposure since a tightly coupled system related to BAG2/Tau is functional.
Conclusion and Future Perspectives
The molecular scenario of Alzheimer's disease genesis and progression is being investigated for decades and is still a puzzle to be solved. Temperature changes are being investigated as a new risk factor for AD having strong influence on microtubule dynamics through Tau function. Anesthesia also promotes changes in the Tc. Tau has an important role in structural remodeling of cytoskeleton during neuronal plasticity including neurite elongation, synapse and spine formation and memory. All these mechanisms play a critical role in the hippocampus, a brain structure responsible for recent memory acquisition that is strongly affected in AD. The high phosphorylated Tau levels in undifferentiated cells present in hippocampus raise several important questions regarding the accumulation of cytotoxic p-Tau protein and AD genesis and progression. Is the hippocampus a vulnerable brain area for the effects of cold on toxic Tau? If so, is BAG2 involved in this vulnerability? These questions may represent an open field that needs further investigation. Because AD is likely the result of both genetic and environmental factors, large-scale epidemiological studies have been unable to satisfactorily evaluate the contribution of geographical (and thus climatological) factors, in particular cold weather averages and seasonal extremes, on the prevalence and progression of AD while controlling for heritable factors, which in the case of sporadic AD remain unknown. Thus, recent advances in the molecular regulation of Tau pathology may compensate for the current lack of epidemiological data regarding the effects of temperature, and may eventually yield important clues to understanding the pathological trigger leading to neurodegeneration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Financial support: CNPq – 449102/2014-9 and Fapesp – 2009/11446-4 to DCC, Fapesp – 2015/02991-0 to M.C.A.
References
- 1.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2 (8): 1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 2000; 33:95-130; PMID:10967355; http://dx.doi.org/ 10.1016/S0165-0173(00)00019-9 [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 2007; 3:186-91; PMID:19595937; http://dx.doi.org/ 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 4.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol 2011; 7:137-52; PMID:21304480; http://dx.doi.org/ 10.1038/nrneurol.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sergeant N, Delacourte A, Buee L. Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta 2005; 1739:179-97; PMID:15615637; http://dx.doi.org/ 10.1016/j.bbadis.2004.06.020 [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol 1993; 33:403-8; PMID:8307060; http://dx.doi.org/ 10.1159/000116984 [DOI] [PubMed] [Google Scholar]
- 7.Bufill E, Bartes A, Moral A, Casadevall T, Codinachs M, Zapater E, Carles Rovira J, Roura P, Oliva R, Blesa R. [Genetic and environmental factors that may influence in the senile form of Alzheimer's disease: nested case control studies]. Neurologia 2009; 24:108-12; PMID:19322689 [PubMed] [Google Scholar]
- 8.Harman D. Alzheimer's disease: role of aging in pathogenesis. Ann N Y Acad Sci 2002; 959:384-95; discussion 463-5; PMID:11976212; http://dx.doi.org/ 10.1111/j.1749-6632.2002.tb02109.x [DOI] [PubMed] [Google Scholar]
- 9.Whittington RA, Papon MA, Chouinard F, Planel E. Hypothermia and Alzheimer's disease neuropathogenic pathways. Curr Alzheimer Res 2010; 7:717-25; PMID:20678067; http://dx.doi.org/ 10.2174/156720510793611646 [DOI] [PubMed] [Google Scholar]
- 10.Collins KJ, Exton-Smith AN, Dore C. Urban hypothermia: preferred temperature and thermal perception in old age. Br Med J (Clin Res Ed) 1981; 282:175-7; PMID:6779937; http://dx.doi.org/ 10.1136/bmj.282.6259.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue Y, Nakao M, Araki T, Ueda H. Thermoregulatory responses of young and older men to cold exposure. Eur J Appl Physiol Occup Physiol 1992; 65:492-8; PMID:1483436; http://dx.doi.org/ 10.1007/BF00602354 [DOI] [PubMed] [Google Scholar]
- 12.Harper DG, Stopa EG, McKee AC, Satlin A, Fish D, Volicer L. Dementia severity and Lewy bodies affect circadian rhythms in Alzheimer disease. Neurobiol Aging 2004; 25:771-81; PMID:15165702; http://dx.doi.org/ 10.1016/j.neurobiolaging.2003.04.009 [DOI] [PubMed] [Google Scholar]
- 13.Harper DG, Stopa EG, McKee AC, Satlin A, Harlan PC, Goldstein R, Volicer L. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry 2001; 58:353-60; PMID:11296096; http://dx.doi.org/ 10.1001/archpsyc.58.4.353 [DOI] [PubMed] [Google Scholar]
- 14.Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry 2005; 13:359-68; PMID:15879584; http://dx.doi.org/ 10.1097/00019442-200505000-00004 [DOI] [PubMed] [Google Scholar]
- 15.Klegeris A, Schulzer M, Harper DG, McGeer PL. Increase in core body temperature of Alzheimer's disease patients as a possible indicator of chronic neuroinflammation: a meta-analysis. Gerontology 2007; 53:7-11; PMID:16940734; http://dx.doi.org/ 10.1159/000095386 [DOI] [PubMed] [Google Scholar]
- 16.Mishima K, Okawa M, Satoh K, Shimizu T, Hozumi S, Hishikawa Y. Different manifestations of circadian rhythms in senile dementia of Alzheimer's type and multi-infarct dementia. Neurobiol Aging 1997; 18:105-9; PMID:8983038; http://dx.doi.org/ 10.1016/S0197-4580(96)00167-4 [DOI] [PubMed] [Google Scholar]
- 17.Okawa M, Mishima K, Hishikawa Y, Hozumi S. Rest-activity and body-temperature rhythm disorders in elderly patients with dementia–senile dementia of Alzheimer's type and multi-infarct dementia. Rinsho Shinkeigaku 1995; 35:18-23; PMID:7781209 [PubMed] [Google Scholar]
- 18.Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep 1991; 14:478-85; PMID:1798879 [DOI] [PubMed] [Google Scholar]
- 19.Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer's disease. Neurobiol Aging 1995; 16:765-71; PMID:8532109; http://dx.doi.org/ 10.1016/0197-4580(95)00059-N [DOI] [PubMed] [Google Scholar]
- 20.Touiton Y, Reinberg A, Bogdan A, Auzéby A, Beck H, Touiton C. Age-related changes in both circadian and seasonal rhythms of rectal temperature with special reference to senile dementia of Alzheimer type. Gerontology 1986; 32:110-8; PMID:3710170; http://dx.doi.org/ 10.1159/000212774 [DOI] [PubMed] [Google Scholar]
- 21.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer's disease. Am J Psychiatry 2001; 158:704-11; PMID:11329390; http://dx.doi.org/ 10.1176/appi.ajp.158.5.704 [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Wu X, Xu Z, Zhang Y, Xie Z. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Abeta generation. PLoS One 2012; 7:e39386; PMID:22745746; http://dx.doi.org/ 10.1371/journal.pone.0039386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien C, Marcouiller F, Bretteville A, El Khoury NB, Baillargeon J, Hebert SS, Planel E. Dimethyl sulfoxide induces both direct and indirect tau hyperphosphorylation. PLoS One 2012; 7:e40020; PMID:22768202; http://dx.doi.org/ 10.1371/journal.pone.0040020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Freche H, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N, Buee-Scherrer V, Lebuffe G, Blum D, et al.. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology 2012; 116:779-87; PMID:22343471; http://dx.doi.org/ 10.1097/ALN.0b013e31824be8c7 [DOI] [PubMed] [Google Scholar]
- 25.Planel E, Bretteville A, Liu L, Virag L, Du AL, Yu WH, Dickson DW, Whittington RA, Duff KE. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J 2009; 23:2595-604; PMID:19279139; http://dx.doi.org/ 10.1096/fj.08-122424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter JB, et al.. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci 2007; 27:3090-7; PMID:17376970; http://dx.doi.org/ 10.1523/JNEUROSCI.4854-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiol 2011; 115:727-32; PMID:21857497; http://dx.doi.org/ 10.1097/ALN.0b013e31822e9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang JX, Mardini F, Caltagarone BM, Garrity ST, Li RQ, Bianchi SL, Gomes O, Laferla FM, Eckenhoff RG, Eckenhoff MF. Anesthesia in presymptomatic Alzheimer's disease: a study using the triple-transgenic mouse model. Alzheimers Dement 2011; 7:521-31 e1; PMID:21745760; http://dx.doi.org/ 10.1016/j.jalz.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer's disease. Alzheimers Dement 2014; 10:372-80; PMID:24589433; http://dx.doi.org/ 10.1016/j.jalz.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 30.Lee HG, Zhu X, Castellani RJ, Nunomura A, Perry G, Smith MA. Amyloid-β in Alzheimer disease: the null versus the alternate hypotheses. J Pharmacol Exp Ther 2007; 321:823-9; PMID:17229880; http://dx.doi.org/ 10.1124/jpet.106.114009 [DOI] [PubMed] [Google Scholar]
- 31.Struble RG, Ala T, Patrylo PR, Brewer GJ, Yan XX. Is brain amyloid production a cause or a result of dementia of the Alzheimer's type? J Alzheimers Dis 2010; 22:393-9; PMID:20847431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy J. Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 2006; 9:151-3; PMID:16914853 [DOI] [PubMed] [Google Scholar]
- 33.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 2011; 10:698-712; PMID:21852788; http://dx.doi.org/ 10.1038/nrd3505 [DOI] [PubMed] [Google Scholar]
- 34.Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. J Neurosci 2010; 30:14946-54; PMID:21068297; http://dx.doi.org/ 10.1523/JNEUROSCI.4305-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurol 1992; 42:631-9; PMID:1549228; http://dx.doi.org/ 10.1212/WNL.42.3.631 [DOI] [PubMed] [Google Scholar]
- 36.Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta 2005; 1739:240-50; PMID:15615642; http://dx.doi.org/ 10.1016/j.bbadis.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 37.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β -amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A 2002; 99:6364-9; PMID:11959919; http://dx.doi.org/ 10.1073/pnas.092136199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science 2007; 316:750-4; PMID:17478722; http://dx.doi.org/ 10.1126/science.1141736 [DOI] [PubMed] [Google Scholar]
- 39.Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res 1986; 387:271-80; PMID:3103857; http://dx.doi.org/ 10.1016/0169-328X(86)90033-1 [DOI] [PubMed] [Google Scholar]
- 40.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 1975; 72:1858-62; PMID:1057175; http://dx.doi.org/ 10.1073/pnas.72.5.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci 1993; 16:460-5; PMID:7507619; http://dx.doi.org/ 10.1016/0166-2236(93)90078-Z [DOI] [PubMed] [Google Scholar]
- 42.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 1989; 3:519-26; PMID:2484340; http://dx.doi.org/ 10.1016/0896-6273(89)90210-9 [DOI] [PubMed] [Google Scholar]
- 43.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 1989; 8:393-9; PMID:2498079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Townsend K, Goldberg TE, Davies P, Conejero-Goldberg C. MAPT isoforms: differential transcriptional profiles related to 3R and 4R splice variants. J Alzheimers Dis 2010; 22:1313-29; PMID:20930284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bullmann T, Hartig W, Holzer M, Arendt T. Expression of the embryonal isoform (0N/3R) of the microtubule-associated protein tau in the adult rat central nervous system. J Comp Neurol 2010; 518:2538-53; PMID:20503426 [DOI] [PubMed] [Google Scholar]
- 46.McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D'Souza I. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol 2008; 511:788-803; PMID:18925637; http://dx.doi.org/ 10.1002/cne.21867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bullmann T, de Silva R, Holzer M, Mori H, Arendt T. Expression of embryonic tau protein isoforms persist during adult neurogenesis in the hippocampus. Hippocampus 2007; 17:98-102; PMID:17183532; http://dx.doi.org/ 10.1002/hipo.20255 [DOI] [PubMed] [Google Scholar]
- 48.Bullmann T, Holzer M, Mori H, Arendt T. Pattern of tau isoforms expression during development in vivo. Int J Dev Neurosci 2009; 27:591-7; PMID:19540327; http://dx.doi.org/ 10.1016/j.ijdevneu.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 49.Fuster-Matanzo A, de Barreda EG, Dawson HN, Vitek MP, Avila J, Hernandez F. Function of tau protein in adult newborn neurons. FEBS Lett 2009; 583:3063-8; PMID:19695252; http://dx.doi.org/ 10.1016/j.febslet.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 50.Fuster-Matanzo A, Llorens-Martin M, Jurado-Arjona J, Avila J, Hernandez F. Tau protein and adult hippocampal neurogenesis. Front Neurosci 2012; 6:104; PMID:22787440; http://dx.doi.org/ 10.3389/fnins.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Yao XQ, Zhang Y, Cao FY, Wang Q, Wang JZ. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus 2009; 20:1339-49; http://dx.doi.org/ 10.1002/hipo.20712 [DOI] [PubMed] [Google Scholar]
- 52.Morris M, Knudsen GM, Maeda S, Trinidad JC, Ioanoviciu A, Burlingame AL, Mucke L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat Neurosci 2015; 18:1183-9; PMID:26192747; http://dx.doi.org/ 10.1038/nn.4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cleveland D, Hwo S, Kirschner M. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 1977; Oct 25:207-25; http://dx.doi.org/ 10.1016/0022-2836(77)90213-3 [DOI] [PubMed] [Google Scholar]
- 54.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron 2011; 70:410-26; PMID:21555069; http://dx.doi.org/ 10.1016/j.neuron.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goedert M, Cohen ES, Jakes R, Cohen P. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer's disease [corrected]. FEBS Lett 1992; 312:95-9; PMID:1330687; http://dx.doi.org/ 10.1016/0014-5793(92)81418-L [DOI] [PubMed] [Google Scholar]
- 56.Wang JZ, Grundke-Iqbal I, Iqbal K. Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Brain Res Mol Brain Res 1996; 38:200-8; PMID:8793108; http://dx.doi.org/ 10.1016/0169-328X(95)00316-K [DOI] [PubMed] [Google Scholar]
- 57.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Titani K, Ihara Y. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron 1993; 10:1151-60; PMID:8391280; http://dx.doi.org/ 10.1016/0896-6273(93)90063-W [DOI] [PubMed] [Google Scholar]
- 58.Iqbal K, Grundke-Iqbal I. Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer's disease. Mol Neurobiol 1991; 5:399-410; PMID:1726645; http://dx.doi.org/ 10.1007/BF02935561 [DOI] [PubMed] [Google Scholar]
- 59.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, et al.. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 2004; 13:703-14; PMID:14962978; http://dx.doi.org/ 10.1093/hmg/ddh083 [DOI] [PubMed] [Google Scholar]
- 60.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem 2004; 279:17957-62; PMID:14963027; http://dx.doi.org/ 10.1074/jbc.M400351200 [DOI] [PubMed] [Google Scholar]
- 61.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol 1998; 8:397-403; PMID:9789328; http://dx.doi.org/ 10.1016/S0962-8924(98)01346-4 [DOI] [PubMed] [Google Scholar]
- 62.Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem 2004; 279:4869-76; PMID:14612456; http://dx.doi.org/ 10.1074/jbc.M305838200 [DOI] [PubMed] [Google Scholar]
- 63.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J 1998; 17:7151-60; PMID:9857172; http://dx.doi.org/ 10.1093/emboj/17.24.7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone BAG2 sweeps paired helical filament- insoluble tau from the microtubule. J Neurosci 2009; 29:2151-61; PMID:19228967; http://dx.doi.org/ 10.1523/JNEUROSCI.4660-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 1992; 99:195-231; PMID:1594723; http://dx.doi.org/ 10.1037/0033-295X.99.2.195 [DOI] [PubMed] [Google Scholar]
- 66.Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol 1973; 232:357-74; PMID:4727085; http://dx.doi.org/ 10.1113/jphysiol.1973.sp010274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004; 429:761-6; PMID:15190253; http://dx.doi.org/ 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011; 472:466-70; PMID:21460835; http://dx.doi.org/ 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 2011; 31:13469-84; PMID:21940440; http://dx.doi.org/ 10.1523/JNEUROSCI.3100-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al.. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009; 325:210-3; PMID:19590004; http://dx.doi.org/ 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci 2010; 33:569-79; PMID:20961627; http://dx.doi.org/ 10.1016/j.tins.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001; 410:372-6; PMID:11268214; http://dx.doi.org/ 10.1038/35066584 [DOI] [PubMed] [Google Scholar]
- 73.Khatoon S, Grundke-Iqbal I, Iqbal K. Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Letters 1994; 351:80-4; PMID:8076698; http://dx.doi.org/ 10.1016/0014-5793(94)00829-9 [DOI] [PubMed] [Google Scholar]
- 74.Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochemical J 2006; 400:511-20; PMID:16939415; http://dx.doi.org/ 10.1042/BJ20060463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Cao FY, Wang Q, Wang JZ. Relationship of adult neurogenesis with tau phosphorylation and GSK-3beta activity in subventricular zone. Neurochem Res 2011; 36:288-96; PMID:21061060; http://dx.doi.org/ 10.1007/s11064-010-0316-y [DOI] [PubMed] [Google Scholar]
- 76.Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S, Ballard C. Altered neurogenesis in Alzheimer's disease. J Psychosom Res 2006; 61:311-6; PMID:16938507; http://dx.doi.org/ 10.1016/j.jpsychores.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 77.Blatteis CM. Age-dependent changes in temperature regulation - a mini review. Gerontology 2011; 58:289-95; PMID:22085834; http://dx.doi.org/ 10.1159/000333148 [DOI] [PubMed] [Google Scholar]
- 78.Kelly G. Body temperature variability (Part 1): a review of the history of body temperature and its variability due to site selection, biological rhythms, fitness, and aging. Altern Med Rev 2006; 11:278-93; PMID:17176167 [PubMed] [Google Scholar]
- 79.Lu SH, Leasure AR, Dai YT. A systematic review of body temperature variations in older people. J Clin Nurs 2010; 19:4-16; PMID:19886869; http://dx.doi.org/ 10.1111/j.1365-2702.2009.02945.x [DOI] [PubMed] [Google Scholar]
- 80.Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J Gerontol A Biol Sci Med Sci 2011; 66:487-92; PMID:21324956; http://dx.doi.org/ 10.1093/gerona/glr001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol (1985) 2003; 95:2598-603; PMID:14600165; http://dx.doi.org/ 10.1152/japplphysiol.00202.2003 [DOI] [PubMed] [Google Scholar]
- 82.Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr Opin Neurol 2002; 15:445-50; PMID:12151841; http://dx.doi.org/ 10.1097/00019052-200208000-00007 [DOI] [PubMed] [Google Scholar]
- 83.Bombois S, Derambure P, Pasquier F, Monaca C. Sleep disorders in aging and dementia. J Nutr Health Aging 2010; 14:212-7; PMID:20191256; http://dx.doi.org/ 10.1007/s12603-010-0052-7 [DOI] [PubMed] [Google Scholar]
- 84.Gillette Guyonnet S, Abellan Van Kan G, Alix E, Andrieu S, Belmin J, Berrut G, Bonnefoy M, Brocker P, Constans T, Ferry M, et al.. IANA (International Academy on Nutrition and Aging) Expert Group: weight loss and Alzheimer's disease. J Nutr Health Aging 2007; 11:38-48; PMID:17315079 [PubMed] [Google Scholar]
- 85.Klaffke S, Staedt J. Sundowning and circadian rhythm disorders in dementia. Acta Neurol Belg 2006; 106:168-75; PMID:17323834 [PubMed] [Google Scholar]
- 86.Knight EM, Brown TM, Gumusgoz S, Smith JC, Waters EJ, Allan SM, Lawrence CB. Age-related changes in core body temperature and activity in triple-transgenic Alzheimer's disease (3xTgAD) mice. Dis Model Mech 2013; 6:160-70; PMID:22864021; http://dx.doi.org/ 10.1242/dmm.010173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Planel E, Miyasaka T, Launey T, Chui DH, Tanemura K, Sato S, Murayama O, Ishiguro K, Tatebayashi Y, Takashima A. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease. J Neurosci 2004; 24:2401-11; PMID:15014115 http://dx.doi.org/ 10.1523/JNEUROSCI.5561-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stieler JT, Bullmann T, Kohl F, Toien O, Bruckner MK, Hartig W, Barnes BM, Arendt T. The physiological link between metabolic rate depression and tau phosphorylation in mammalian hibernation. PLoS One 2011; 6:e14530; PMID:21267079; http://dx.doi.org/ 10.1371/journal.pone.0014530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bretteville A, Marcouiller F, Julien C, El Khoury NB, Petry FR, Poitras I, Mouginot D, Levesque G, Hebert SS, Planel E. Hypothermia-induced hyperphosphorylation: a new model to study tau kinase inhibitors. Sci Rep 2012; 2:480; PMID:22761989; http://dx.doi.org/ 10.1038/srep00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Paula CA, Santiago FE, de Oliveira AS, Oliveira FA, Almeida MC, Carrettiero DC. The Co-chaperone BAG2 Mediates Cold-Induced Accumulation of Phosphorylated Tau in SH-SY5Y Cells. Cell Mol Neurobiol 2015; Epub ahead of print; http://dx.doi.org/ 10.1007/s10571-015-0239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng Q, Cheng B, Yang R, Sun FY, Zhu CQ. Dynamic changes of phosphorylated tau in mouse hippocampus after cold water stress. Neurosci Lett 2005; 388:13-6; PMID:16005567; http://dx.doi.org/ 10.1016/j.neulet.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 92.Maurin H, Lechat B, Borghgraef P, Devijver H, Jaworski T, Van Leuven F. Terminal hypothermic Tau.P301L mice have increased Tau phosphorylation independently of glycogen synthase kinase 3alpha/β. Eur J Neurosci 2014; 40:2442-53; PMID:24754737; http://dx.doi.org/ 10.1111/ejn.12595 [DOI] [PubMed] [Google Scholar]
- 93.Matsuzakia K, Katakuraa M, Sugimotoab N, Haraa T, Hashimotoa M, Shidoa O. β-amyloid infusion into lateral ventricle alters behavioral thermoregulation and attenuates acquired heat tolerance in rats. Temperature 2015; 2(3): 418-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flouris AD, Piantoni C. Links between thermoregulation and aging in endotherms and ectotherms. Temperature 2015; 2:73-85; http://dx.doi.org/ 10.4161/23328940.2014.989793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ancelin ML, de Roquefeuil G, Ledesert B, Bonnel F, Cheminal JC, Ritchie K. Exposure to anaesthetic agents, cognitive functioning and depressive symptomatology in the elderly. Br J Psychiatry 2001; 178:360-6; PMID:11282816; http://dx.doi.org/ 10.1192/bjp.178.4.360 [DOI] [PubMed] [Google Scholar]
- 96.Eckenhoff RG, Planel E. Postoperative cognitive decline: where art tau? Anesthesiol 2012; 116:751-2; PMID:22343470; http://dx.doi.org/ 10.1097/ALN.0b013e31824be8e1 [DOI] [PubMed] [Google Scholar]
- 97.Palotas A, Reis HJ, Bogats G, Babik B, Racsmany M, Engvau L, Kecskemeti E, Juhasz A, Vieira LB, Teixeira AL, et al.. Coronary artery bypass surgery provokes Alzheimer's disease-like changes in the cerebrospinal fluid. J Alzheimers Dis 2010; 21:1153-64; PMID:21504113 [DOI] [PubMed] [Google Scholar]
- 98.Drewes G, Lichtenberg-Kraag B, Doring F, Mandelkow EM, Biernat J, Goris J, Doree M, Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J 1992; 11:2131-8; PMID:1376245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sontag E, Nunbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White CL, 3rd, Mumby MC, Bloom GS. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem 1999; 274:25490-8; PMID:10464280; http://dx.doi.org/ 10.1074/jbc.274.36.25490 [DOI] [PubMed] [Google Scholar]
- 100.Santiago FE, Almeida MC, Carrettiero DC. BAG2 Is Repressed by NF-kappaB Signaling, and Its Overexpression Is Sufficient to Shift Abeta from Neurotrophic to Neurotoxic in Undifferentiated SH-SY5Y Neuroblastoma. J Mol Neurosci 2015; 57:83-9; PMID:25985852; http://dx.doi.org/ 10.1007/s12031-015-0579-5 [DOI] [PubMed] [Google Scholar]
- 101.Yu Y, Run X, Liang Z, Li Y, Liu F, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Developmental regulation of tau phosphorylation, tau kinases, and tau phosphatases. J Neurochem 2009; 108:1480-94; PMID:19183272; http://dx.doi.org/ 10.1111/j.1471-4159.2009.05882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Zhang A, Lu S, Pan X, Jia D, Yu W, Jiang Y, Li X, Wang X, Zhang J, et al.. Adenosine 5'-monophosphate-induced hypothermia inhibits the activation of ERK1/2, JNK, p38 and NF-kappaB in endotoxemic rats. Int Immunopharmacol 2015; 23:205-10; http://dx.doi.org/ 10.1016/j.intimp.2014.09.002 [DOI] [PubMed] [Google Scholar]


