Abstract
The toad, Rhinella schneideri, is a large-bodied anuran amphibian with a broad distribution over South America. R. schneideri is known to be active at night during the warm/rainy months and goes into estivation during the dry/cold months; however, there is no data on the range of body temperatures (Tb) experienced by this toad in the field, and how environmental factors, thermoregulatory behaviors or activity influence them. By using implantable temperature dataloggers, we provide an examination of Tb variation during an entire year under a seminatural setting (emulating its natural habitat) monitored with thermosensors. We also used data on preferred Tb, allowing us to express the effectiveness of thermoregulation quantitatively. Paralleling its cycle of activity, R. schneideri exhibited differences in its daily and seasonal profile of Tb variation. During the active season, toads spent daytime hours in shelters and, therefore, did not explore microhabitats with higher thermal quality, such as open areas in the sun. At nighttime, the thermal suitability of microhabitats shifted as exposed microhabitats experienced greater temperature drops than the more insulated shelter. As toads became active at night, they were driven to the more exposed areas and, as a result, thermoregulatory effectiveness decreased. Our results, therefore, indicate that, during the active season, a compromise between thermoregulation and nocturnal activity may be at play. During the estivation period, R. schneideri spent the entire day cycle inside the shelter. As toads did not engage in nocturnal activity in those areas with low thermal quality, the overall effectiveness of thermoregulation was, indeed, elevated. In conclusion, we showed that daily and seasonal variation in Tb of an anuran species is highly associated with their respective pattern of activity and may involve important physiological and ecological compromises.
Keywords: body temperature, operative temperature, season, summer, thermal preference, winter
Abbreviations
- db
accuracy of thermoregulation
- de
thermal quality of habitat
- E
effectiveness of thermoregulation
- Tb
body temperature
- Te
operative environmental temperature
- Tset
preferred body temperature selected in a thermal gradient
Introduction
Body temperature (Tb) affects behavioral and physiological functions of animals, such as locomotion, foraging and growth.1 The maintenance of Tb within narrow and controlled ranges can optimize these functions and, ultimately, affect fitness. In ectotherms, behavioral thermoregulation is a common strategy for buffering temporal and spatial variations in environmental temperature. Thermoregulatory behaviors include shuttling between microhabitats, basking, regulating activity time and adjusting posture.2-4
Anuran amphibians have been shown to thermoregulate by behavioral means in natural environments.3 Behavioral thermoregulation is also observed in laboratory conditions, where anurans show less variation in preferred Tb in thermal gradients5-8 than displayed in the field.8,9 Most anurans, however, offer little or no resistance to evaporative water loss.9-12 It is known that thermoregulatory adjustments need to accommodate the potentially conflicting compromises related to water balance13 and the prominent tendency of amphibians to lose water is thought to prevent a more efficient regulation of Tb. As a consequence, amphibians, and particularly terrestrial anurans, may have their activities limited, daily and seasonally, by the availability of adequate thermal microhabitats that allow for thermoregulation without serious consequences to water balance.14-16
The toad, Rhinella schneideri, is a terrestrial large-bodied amphibian (Anura, Bufonidae) with a broad distribution over South America (Argentina, Bolivia, Brazil, Paraguay, and Uruguay), inhabiting open areas at Chaco, Cerrado (a savannah-like habitat), and Atlantic Forest biomes.17,18 This toad is also easily found in open and urban areas.18 During the dry and cold season in Southeastern Brazil, R. schneideri estivates buried in shallow burrows excavated in the soil or under rocks, or fallen logs. In the laboratory, during estivation season, this species shows reductions in metabolic and heart rates,5,19 independent of Tb; furthermore, it selects lower preferred Tb in a thermal gradient during winter.5
Due to its large body size and abundance, R. schneideri has been subjected to a number of physiological studies, including some devoted to aspects of its thermal biology. 5,6,19-24 However, we still lack some basic and important information about the thermal biology of this species. In fact, we still do not know the range of Tb's experienced by this toad in the field and how environmental factors and thermoregulatory behaviors determine it, or even how Tb might be related to changes in activity. Herein, we aimed to fill this gap by providing an examination of Tb, recorded over a year, in a group of toads maintained under seminatural conditions within their original area of occurrence. In doing so, we adopted the protocol described by Hertz et al.25 to examine daily and seasonal variations in Tband environmental temperatures, which, combined with preferred Tb of this species in a thermal gradient, allowed us to calculate indexes that evaluate the accuracy and effectiveness of thermoregulation.
Materials and methods
Animals
Rhinella schneideri26 toads (formerly known as Bufo paracnemis and Chaunus schneideri27,28) of both sexes, weighing 335–590 g, were captured under the permit issued by the “Instituto Brasileiro do Meio Ambiente e dos Recursos Renováveis” – IBAMA (Animal License nº 025/2005). Toads were fed beef liver twice a week up to 2 d before surgery for implantation of temperature dataloggers (see below). Throughout the entire duration of the experiment (~1 year), toads were frequently inspected for their general appearance, presence of ectoparasites, position inside the arena and any other noticeable occurrence.
Location and experimental arena
The experiment was carried out in the municipality of Rio Claro, São Paulo state, southeastern Brazil (22°24′53.648″S, 47°33′54.501″W at 626 m a.s.l.). This location is included within the geoclimatic domain of the Tropical Atlantic.29 The climate is mesothermic, with dry winters and rainy hot summers, according to the Köppen-Geiger's system.30 During the experiments, rainfall and relative humidity (RH) data were collected daily by the local meteorological station (CEAPLA, UNESP). Autumn months (March-June) were characterized by 186.8 mm of rainfall (mean 2.00 mm/day), whereas winter months (June-September) had 120.3 mm of rainfall (mean 1.3 mm/day), spring months (September-December) had 389.4 mm of rainfall (mean 4.3 mm/day) and summer months (December-March) had 361.0 mm of rainfall (mean 4.2 mm/day). Maximum, minimum and mean RH (mean ± SEM) were, respectively, 99 ± 0.1, 46 ± 1.4 and 73 ± 0.8 % during autumn, 96 ± 0.4, 39 ± 1.3 and 66 ± 1% during winter, 97 ± 0.3, 49 ± 1.9 and 69 ± 1.4% during spring and 97 ± 0.1, 45 ± 1.3 and 67 ± 1.2% during summer. Minimum (p < 0.001; F(3,314)= 7.7) and mean (p < 0.001; F(3,314)= 8.33) RH were lower during winter and maximal RH was greater during autumn (p < 0.001; F(3,314)= 18.3).
The experimental arena (Fig. 1) consisted of an outdoor 5 × 10 m enclosure located next to the Laboratory of Herpetology and Animal Physiology at São Paulo State University. This arena was fenced in by a 0.9 m high cement wall, topped by a metal net fence dome about 3 m high that covered the enclosure entirely. Inside the enclosure, half buried in the soil, there were 2 concrete dens (2.7 × 1.0 × 0.37 m) to serve as shelters (number 1, Fig. 1). The substrate was planted with grass (Paspalum notatum) and the pen was enriched with logs, trees (Dypsis lutescens, Eugenia uniflora, Schefflera arboricola) and other plants (Philodendron bipinnatifidum). The animals had permanent access to water (number 2, Fig. 1).
Figure 1.
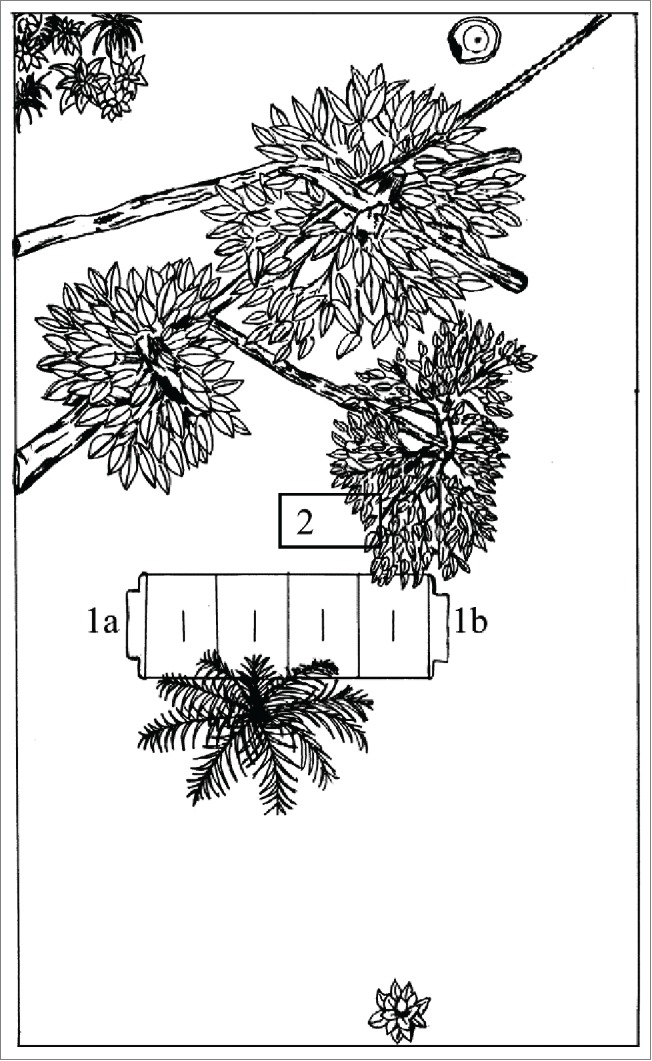
Schema of the superior view of the experimental arena where toads expended a year implanted with temperature dataloggers. Numbers indicate the shelter (1a and 1b are the 2 entrances to the den) and the water access (2). Shade (under trees and other plants) and open areas can be seen here. See text for details.
Intra-abdominal implantation of dataloggers
Animals were anesthetized by submergence in an aqueous 0.3% solution of 3-aminobenzoic acid ethyl ester (MS-222, Sigma, St. Louis, USA) and a temperature datalogger (TidbiT, Onset Computer Corporation, Bourne, MA) was implanted in the abdominal cavity. To this end, each animal was positioned in lateral decubitus, and an incision was made in the skin and muscles of the left side of the abdomen just large enough to insert the datalogger into the coelomic cavity. After suturing the incision, animals were treated with enrofloxacin (Baytril®, Bayer, 3 mg/kg s.c.), a broad-spectrum fluoroquinolone, and an analgesic (Flunixina Meglumina, Banamine®; Schering-Plough, 1.0 mg/kg s.c.), and allowed to recover from anesthesia. These procedures lasted approximately 20 minutes. Animals were allowed to recover for at least one week from surgery before being placed in the experimental arena.
Temperature recordings in the experimental arena
Body temperature of 7 toads was automatically measured by the temperature dataloggers implanted in the abdominal cavity. Dataloggers were calibrated, before and after the experiment, against a mercury thermometer (Labortherm-N, 75 mm) at 5 different temperatures. Before being implanted, the dataloggers were programmed (BoxCar® Pro 4.0 for Windows, Onset Computer Corporation) to collect and store the temperature data every 16 min for a period of 12 months, starting 30 d after implantation.
The same dataloggers were used to sample the temperature of representative thermal microhabitats within the experimental arena: i) in a permanently shaded spot; ii) inside the shelter; iii) in the water bowl provided (25 × 54 × 88.5 cm); and iv) in an exposed open area. The dataloggers registered temperatures every 16 min between February of 2005 and January of 2006. Data from all 4 seasons were grouped by daytime (0600–1800) and nighttime (1800–0600) and means were calculated every 32 min.
In order to validate the environmental temperature readings collected by the dataloggers as representative of operative temperatures (Te) during the experimental period, we simultaneously recorded temperature using a “naked” datalogger, along with a second datalogger that was implanted inside the body cavity of freshly dead toads.31 Both the “naked” dataloggers and the dead toads with implanted dataloggers were placed in each of the 4 microhabitats (2 toads of similar size to the experimental animals were used in each: shaded, open, shelter and water) under the same conditions prevalent during the experiment. Recordings lasted for 2 to 3 d each and were repeated under variable climatic conditions. Dead toads were replaced after 48 h, at most, to avoid any thermal effect of putrefaction. We found no significant differences in the heat exchange rates between the 2 conditions (naked datalogger vs within-carcass) for the temperatures recorded inside the shelter and water. Therefore, the raw data, as recorded by the naked dataloggers, were accepted as representative of Te's for these microhabitats. For the permanently shaded and open area microhabitats, however, there was a significant difference between the heat exchange rates for the naked datalogger and the toad carcass (p < 0.001). In these cases, we found that Te could be confidently predicted from a linear combination between time of day and ambient temperature (taken from the naked datalogger) (Multiple Linear Regression: Rsqr = 0.904, p < 0.001); thus, allowing us to convert all our ambient temperature readings (from the naked datalogger) for the entire duration of the experiment to Te.
Thermal preference in a thermal gradient chamber
The range of preferred Tb's (Tset), was measured in a thermal gradient, where thermoregulatory costs and constraints are null.25 The experiments in the thermal gradient during summer and winter presented here were performed by our research group for other purposes (unpublished data) and studies.5,6 Briefly, the thermal gradient consisted of a chamber (1.00 m long, 0.15 m high and 0.20 m wide) with an aluminum floor. One end of the floor was cooled to 10–11°C by a copper pad connected to a refrigerated water bath, and the other end was heated to 38–40°C by another bath or an electrical resistor. Petri dishes filled with tap water (from an artesian well), placed throughout the chamber, provided access to water in all temperature ranges. Each animal, equipped with a temperature probe secured 3 cm into the colon through the cloaca with skin sutures, was placed in the center of the thermal gradient. The thermistor output was continuously displayed on a chart recorder. After an exploratory period of about 5–12 h, the preferred Tb was recorded continuously and computed each h for 5 (winter) or 10 h (summer). Measurements were taken between 9:00 a.m. and 5:00 pm, and we assumed no difference in Tset between night and day. This assumption was based on the fact that no significant change in preferred Tb was observed in a 24 h period in the same species19 by us or by others in a closely-related toad, Rhinella marina.32,33
Individual Tset was estimated from the central 50% of recorded temperatures selected in the thermal gradient (25% quartile representing the lower bound and 75% quartile representing the upper bound of Tset). The range of Tset for the species was calculated by the average of individual lower and upper bounds of Tset, according to Hertz et al.25
Thermoregulatory indexes
Once Tb, Te and Tset were obtained, the protocol described by Hertz et al.25 was used to estimate the accuracy of thermoregulation (db), thermal quality of the habitat (de), and effectiveness of thermoregulation (E). Indexes were calculated for day and night for all 4 seasons. Summer and winter Tset were assumed for calculating indexes during spring and summer and during autumn and winter, respectively.
The accuracy of thermoregulation is indexed by , which represents the extent to which Tb's overlap Tset.24 The db from individual readings were interpreted using the following criteria: if the Tb reading fell within Tset, db was zero; if Tb fell below the lower bound of Tset, db was the difference between the lower bound of Tset and Tb; if Tb fell above the upper bound of Tset, db was the difference between Tb and the upper bound of Tset. db's were averaged for day and night, during autumn, winter, spring and summer. High indicates low accuracy of thermoregulation, since individuals often experience Tb's out of the range of Tset. that approaches zero represents a species that experiences Tb's within the ranges of Tset, and has, therefore, a high accuracy of thermoregulation.
The thermal quality of the habitat, from the organism's perspective, is represented by , and is calculated analogously to .25 Environments in which is zero offer Te's that are exclusively within the range of Tset and, therefore, have a high thermal quality; the higher the , the lower the thermal quality of the environment.
and were combined to calculate the index E, which represents the effectiveness of thermoregulation of the species. The index E is calculated as 1 – (/). An E value that approaches 1 indicates that animals thermoregulate carefully, and Tb deviations from preferred range (Tset) are much smaller than those of Te, while a value that approach 0 indicates that animals do not thermoregulate and their deviations from Tset are similar to those of Te. A negative value of E indicates that animals, for some reason, do not use microhabitats that offer temperatures within the range of Tset. In order to compare E values from different seasons and time of day, confidence intervals of E were calculated by bootstrap resampling from the distribution of Te and Tb, as described by Hertz et al.25
Statistical analyses
All the results are reported as mean ± SE. Operative temperature (Te) data were reduced to means every 32 minutes, which were used in all analyses; the existence of differences in Te among seasons was tested by one-way ANOVA. Within each season, a 2-way ANOVA was performed to test if Te differed between time of day and among microhabitats. The Tb data were averaged for daytime and nighttime in each season for each toad (n = 7) and a 2-way ANOVA was used to test the possible differences between time of day and among seasons. For each individual tested in the thermal gradient, the lower and upper bounds of Tset (25 and 75% quartiles, respectively) were calculated and compared between summer (n = 14) and winter (n = 11) by t test. Comparisons of and were performed by 2-way ANOVA (factors: season and time of day). In all the analyses, when a significant effect was found, a Tukey test was performed to compare the means. A significant difference was accepted when p < 0.05.
Results
Operative temperatures (Te)
Te in the 4 representative microhabitats in the experimental arena (shaded area, shelter, water and open area) differed among seasons, being, as expected, higher during summer and lower during winter (one-way ANOVA, p < 0.001; F(3,65036)= 5015).
Within each season, Te was influenced by time of day and microhabitat (Tables 1 and 2). The Tukey test revealed that, in general, daytime temperatures within the shelter, shaded area and water were lower than nighttime temperatures (p < 0.05). In the open area, on the other hand, daytime temperatures were higher than nighttime temperatures (p < 0.05). The temperatures between microhabitats also varied, especially at night, where temperatures in the open area dropped considerably compared to the other microhabitats (p < 0.05).
Table 1.
Body temperature (Tb), environmental temperature (Te), deviation of Tb () and of Te () from Tset, and E, calculated for daytime (0600–1800) and nighttime (1800–0600), during the 4 seasons
| |
|
autumn |
|
winter |
|
spring |
|
summer |
|---|---|---|---|---|---|---|---|---|
| day | night | day | night | day | night | day | night | |
| Tb | 20.5 ± 0.3 | 20.6 ± 0.2 | 18.1 ± 0.2 | 18.8 ± 0.2 | 21.6 ± 0.1 | 21.0 ± 0.2 | 23.2 ± 0.2 | 22.5 ± 0.2 |
| Te | Shelter | |||||||
| 21.1 ± 0.1 | 21.5 ± 0.1 | 18.2 ± 0.04 | 18.7 ± 0.04 | 21.8 ± 0.03 | 22.1 ± 0.03 | 24.3 ± 0.03 | 23.9 ± 0.3 | |
| Shaded area | ||||||||
| 20.7 ± 0.1 | 21.1 ± 0.1 | 18.2 ± 0.1 | 19.0 ± 0.1 | 22.0 ± 0.1 | 22.1 ± 0.1 | 23.7 ± 0.04 | 22.8 ± 0.1 | |
| Water | ||||||||
| 19.9 ± 0.1 | 20.5 ± 0.1 | 16.8 ± 0.1 | 17.9 ± 0.1 | 20.7 ± 0.1 | 21.3 ± 0.1 | 23.3 ± 0.1 | 22.6 ± 0.1 | |
| Open area | ||||||||
| 20.7 ± 0.1 | 15.1 ± 0.1 | 19.8 ± 0.1 | 13.9 ± 0.1 | 21.9 ± 0.1 | 16.3 ± 0.1 | 17.2 ± 0.1 | 22.6 ± 0.1 | |
| 3.5 ± 0.02 | 3.4 ± 0.02 | 5.9 ± 0.02 | 5.2 ± 0.02 | 4.5 ± 0.02 | 5.0 ± 0.02 | 3.1 ± 0.02 | 3.5 ± 0.02 | |
| 3.9 ± 0.03 | 4.6 ± 0.04 | 6.0 ± 0.04 | 6.6 ± 0.03 | 4.6 ± 0.03 | 5.6 ± 0.03 | 3.3 ± 0.03 | 4.0 ± 0.04 | |
| 95% confidence interval of E | 0.09 – 0.12 | 0.26 – 0.28 | 0.00 – 0.03 | 0.20 – 0.22 | 0.01 – 0.05 | 0.09 – 0.11 | 0.05 – 0.09 | 0.11 – 0.15 |
Table 2.
Results of the 2-way ANOVAs testing for the effects of time of day and microhabitat on environmental temperatures (Te) during summer, autumn, winter and spring
| Predictor | F | df | P |
|---|---|---|---|
| autumn | |||
| time of day | 425.5 | 1 | <0.001 |
| microhabitat | 894.5 | 3 | <0.001 |
| time of day × microhabitat | 898.4 | 3 | <0.001 |
| winter | |||
| time of day | 297.5 | 1 | <0.001 |
| microhabitat | 285.2 | 3 | <0.001 |
| time of day × microhabitat | 1086.9 | 3 | <0.001 |
| spring | |||
| time of day | 730.0 | 1 | <0.001 |
| microhabitat | 1027.1 | 3 | <0.001 |
| time of day × microhabitat | 1217.9 | 3 | <0.001 |
| summer | |||
| time of day | 388 | 1 | <0.001 |
| microhabitat | 1845 | 3 | <0.001 |
| time of day × microhabitat | 1258 | 3 | <0.001 |
Hourly mean temperatures were calculated by microhabitat for each season (Fig. 2). Year-round, the shelter presented the lowest daily temperature amplitude, and the open area, the highest. The lowest mean temperature reached in the open area was 12.7, 11.2, 14.4 and 13.9°C for autumn, winter, summer and spring, respectively. These temperatures are at least 3.7°C lower than the minimal temperature reached by other microhabitats, and as much as 6°C higher during summer. At the other end, the highest hourly mean temperature was also observed in the open area: 25.4, 25.1, 26.3 and 25.8°C for autumn, winter, summer and spring, respectively. The difference between the maximal temperature in the open area and the maximal temperature in the other microhabitats was lowest during summer. During nighttime, the shelter presented the highest hourly mean temperatures.
Figure 2.
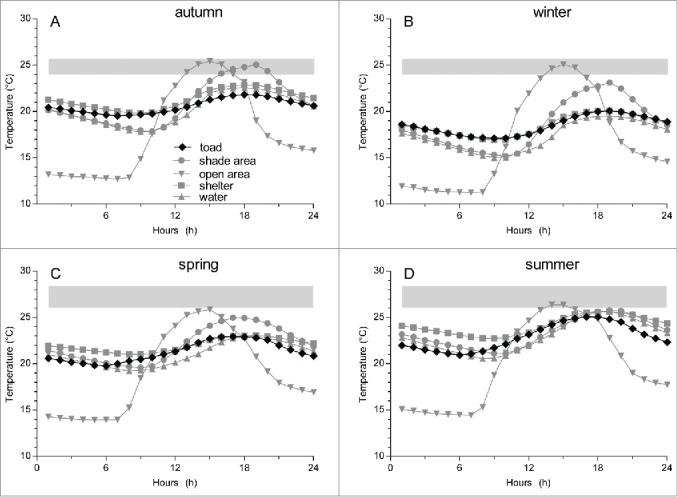
Hourly means of environmental temperatures (Te) at 4 representative microhabitats and body temperatures (Tb) of toads, Rhinella schneideri, during autumn, winter, spring and summer. The shaded area represents the ranges of preferred Tb (Tset) calculated in the laboratory during the summer and winter seasons.
Body temperatures (Tb) in the arena
Body temperature changed throughout the year (2-way ANOVA, p < 0.001, F(3,48) = 153.9; interaction factor: p = 0.006; F(3,48) = 4.71). Daytime Tb differed among all seasons, and was higher during summer and lower during winter. Nighttime Tb was also higher during summer and lower during winter, but did not differ between spring and autumn (Tukey test; p < 0.05).
Hourly mean Tb's averaged across all individuals are plotted in Figure 2. The difference between the highest and the lowest hourly mean temperatures was greatest during summer (4.1°C) and lowest during winter (2.9°C).
Thermal preference in the thermal gradient (Tset)
The lower bound of Tset did not differ between summer and winter (26.0 ± 0.6 for summer; 24.0 ± 1.1 for winter; p = 0.1). In contrast, the upper bound of Tset was higher during summer (28.4 ± 0.7) compared to winter (25.8 ± 1.0) (p = 0.03).
Comparisons between Te, Tb and Tset
All hourly means of Te fell below the upper bounds of Tset while all hourly mean Tb's fell below the lower bound of Tset (Fig. 2). Hourly mean Tb's closely matched the Te's from the shelter during winter. During summer, on the other hand, mean Tb's matched the temperature of the shelter only for some phase of day. During nighttime, mean Tb's dropped and matched the temperatures of the open area (Fig. 2).
More than 80% of Te and Tb readings during all seasons fell outside Tset (Figs. 3 and 4). The proportions of Te and Tb readings falling inside and outside Tset are shown in Figures 3 and 4. A greater proportion of Te readings fell within Tset during summer (11.4% for daytime and 9.2% for nighttime) and autumn (11.1% for daytime and 8.5% for nighttime) compared to winter (4.9% for daytime and 1.3% for nighttime) and spring (5.9% for daytime and 1.6% for nighttime) (Fig. 3). The proportion of Tb readings that fell within Tset was also greater during summer (5.1% for daytime and 3.1% for nighttime) and autumn (8.9% for daytime and 8.8% for nighttime) compared to winter (0.4% for daytime and 0.6% for nighttime) and spring (1.5% for daytime and 0.5% for nighttime) (Fig. 4).
Figure 3.
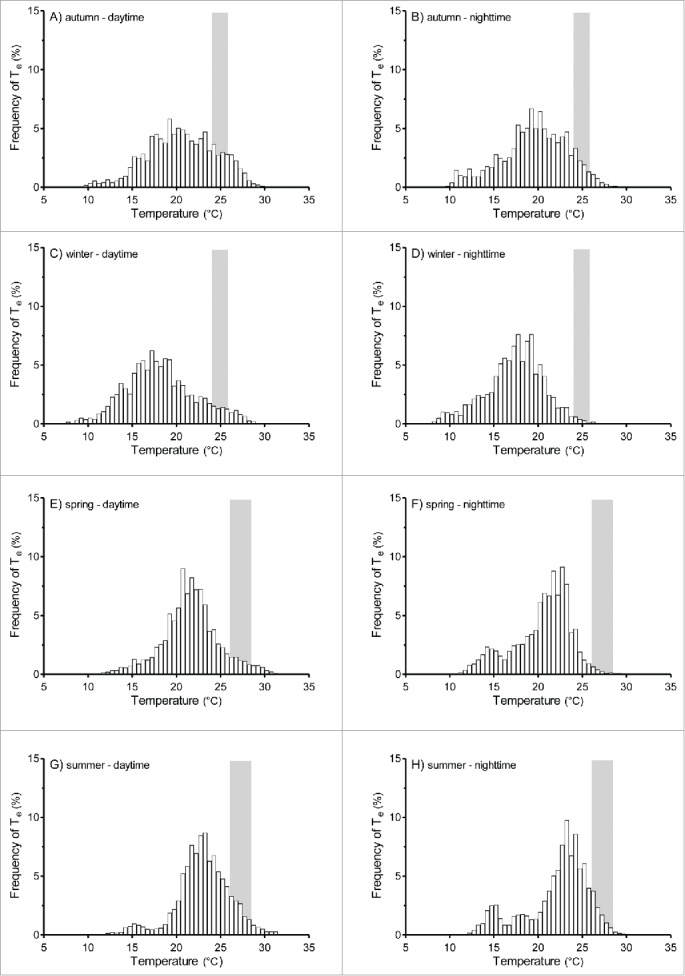
Frequency distribution of environmental temperatures (Te) registered during the 4 seasons at daytime and nighttime. Daytime: 0600–1800; nighttime: 1800–0600. The shaded area represents the ranges of preferred Tb (Tset) calculated in the laboratory during the summer and winter seasons.
Figure 4.
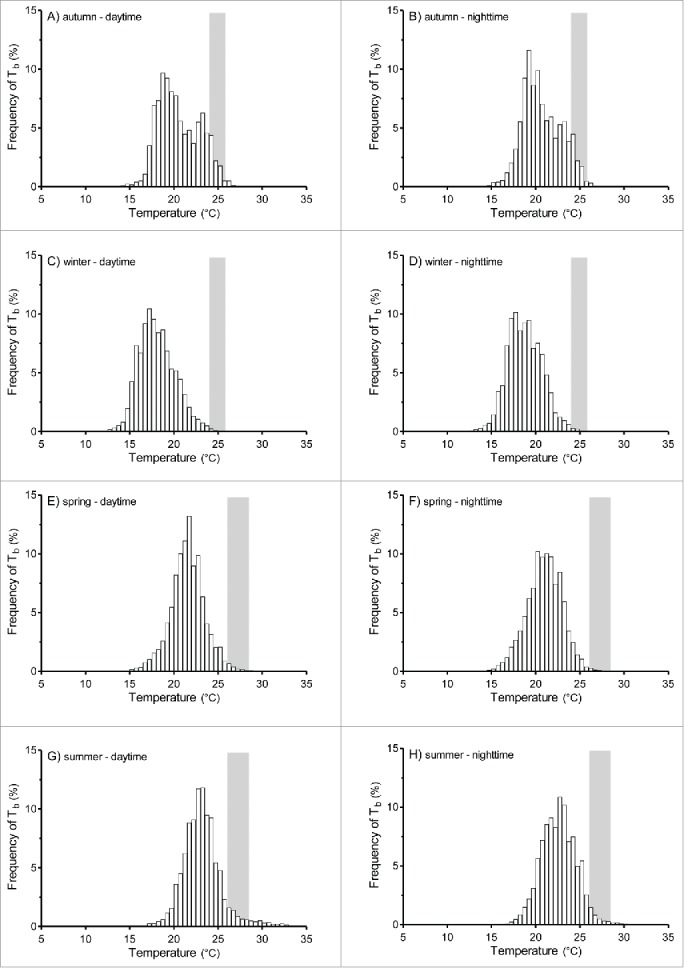
Frequency distribution of body temperatures (Tb) registered during all seasons at daytime and nighttime. Daytime: 0600–1800; nighttime: 1800–0600. The shaded area represents the ranges of preferred Tb (Tset) calculated in the laboratory during the summer and winter seasons.
Thermoregulatory indexes
Within each season and were calculated for daytime and nighttime (Table 1). The thermal quality of the environment, represented by de, was influenced by season (p < 0.001, F(3,65032)= 2052) and time of day (p < 0.001, F(1,65032)= 880; interaction factor: p < 0.001, F(3,65032)= 9.1). Environmental temperatures were closer to Tset (higher thermal quality) during summer and, within each season, during daytime. The accuracy of thermoregulation, represented by db, was also influenced by season (p < 0.001; F(3,104838)= 7746) and time of day (p < 0.001; F(1,104838)= 11; interaction factor: p < 0.001; F(3,104838)= 27). Toads kept Tb closer to Tset during summer and autumn.
All values of E were positive and did not exceed 0.28, indicating only moderate thermoregulation (Table 1). During all seasons, E was higher during nighttime compared to daytime. Considering nighttime E, since toads are nocturnal, E indicates a higher degree of thermoregulation during winter and autumn compared to summer and spring.
Discussion
Our seminatural experimental conditions tried to emulate, as much as possible, the same conditions that R. schneideri is bound to be exposed to in nature. In terms of climatic variables, animals were subjected to same natural weather conditions that free-ranging animals (that do occur in the region) were also exposed. In terms of microhabitat availability, we tried to provide landscape elements that allowed for plenty of thermoregulatory opportunities. No other interventions, other than occasional food supplementation and visual inspection, were introduced during the experiments. Thus, we are confident that if any captivity-related bias was present in our data, its effect is most probably negligible. In agreement with that, we did not observe unnatural behaviors of toads in terms of daily and seasonal activity. Animals were active during the early hours after sundown during the summer and spring and were inactive during day and night during the winter when they spend their time in the provided shelter or, more rarely, under shallow burrows in the soil covered with plant debris.
Our data show seasonal and daily variation in environmental temperatures, as well as variation between microhabitats tested. In general, the shelter presented stable temperatures throughout the entire daily cycle (i.e., day and night) and, as a result, the nighttime temperatures of the shelter were closer to Tset compared to other microhabitats. The seasonal cycle of activity of the toads5,34 was clearly reflected in our body temperature data. For example, during winter when the toads became inactive and retreated into the shelter, their Tb was practically indistinguishable from the Te's registered for this microhabitat. Thus, the thermal inertia of the shelter is likely to have provided the toads with a stable range of temperatures suitable to endure the estivation period. Indeed, selection of the shelter as the estivation site buffered the Tb of toads from the extreme variation prevalent in the other microhabitats during this season (see Fig. 2). During spring and summer, Tb's closely matched the temperature of the shelter only during daytime. At night, when toads were active, Tb's dropped further from Tset and matched the temperatures of the open area, indicating that the toads, as they become active, leave the shelters to explore other microhabitats, even though these microhabitats have lower thermal quality compared to the shelter. Thus, at night, E values during summer and spring are closer to zero compared to winter and autumn, reflecting the engagement of the toads in activities in a low thermal quality environment. During winter, on the other hand, despite being inactive, the choice of microhabitat in which to permanently retreat to for the season allows for Tb to be closer to Tset compared to summer and spring, resulting in higher E values.
The low thermoregulatory index of the active toads illustrates clearly the potential compromises between body temperature regulation and other ecologically relevant activities.35 Indeed, the lower Tb's, in relation to the range of Te's, were the result of the toads leaving the shaded/sheltered area and exploring other microhabitats where lower air temperatures prevailed. Although it is difficult to ascertain the exact nature of such movements, foraging and reproduction seem to be the most likely candidates. In fact, the beginning of spring is the reproductive period for this species,36,37 and during spring and summer nights, it was possible to observe insects (possible prey) inside the arena. Finally, another possible explanation for the mismatch between activity Tb and Tset may be that Tset is a conservative condition, rather than an adaptation to the current thermal environment.1 Such an idea, in the specific case of the Rhinella group, remains to be tested.
The reduction in activity during the cold and dry season by tropical terrestrial toads seems often related to the control of water loss, rather than the avoidance of extreme temperatures.15 Rhinella schneideri estivates during winter without forming a cocoon and, in the laboratory, downregulates its heart rate and metabolic rate,5,19 which may help to save energy and reduce respiratory water loss. In the present study, although winter presented slightly lower RH, rainfall was more than 2 times lower than spring and summer, which may reflect the actual reduction of the levels of humidity within the micro-habitats explored by the toads. Thus, spending all the winter inside the shelter may be a strategy to avoid water loss, as microhabitat selection becomes an effective way to minimize evaporation 14,31,38 in toads due to the high permeability of their skin.39,40 In this context, our results discussed above indicate that the seasonal decrease in the thermal quality of habitats may also be an important component influencing the onset of estivation in this species.
Our study provides a detailed account of seasonal and daily variation in Tb of an abundant and widespread anuran of the Neotropics under conditions quite similar to their natural habitats. As far as we know, no other study has provided this kind of information for any other anuran species, including those from all other temperate or tropical areas. This lack of information is unfortunate, both in terms of preventing a more refined ecological contextualization for physiological effects of temperature under experimental conditions, as well as the potential effects of temperature change and its possible consequences to the conservation of amphibians under a global climate change scenario. In terms of procedure, we demonstrated that it is viable to apply the current procedures for thermoregulatory quantification25 under a more controlled experimental situation. In the specific case of R. schneideri, the adoption of such an approach revealed that nocturnal activity may be an important constraint to Tb regulation in anurans, even though such activity is largely limited to seasons with a higher thermal quality. On the other hand, during seasons characterized by an overall lower thermal quality (winter/autumn), the onset of inactivity (estivation), combined with microhabitat selection, may allow for a more accurate (although largely passive) maintenance of Tb.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Jacopo Mortola and Dr Guilherme Gomes for gently helping us with some mathematical corrections and Dr. Augusto S. Abe for the use of space in the laboratory.
Funding
The study was supported by Conselho Nacional de Ciência e Tecnologia (CNPq; 150016/2003–4, KCB; 302045/2012–0, DVA) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2013/04190–9; DVA), Brazil.
References
- 1.Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. J Therm Biol 2002; 27:249-68; http://dx.doi.org/ 10.1016/S0306-4565(01)00094-8 [DOI] [Google Scholar]
- 2.Cowles RB, Bogert CM. A preliminary study of the thermal requirements of desert reptiles. Bull Am Mus Nat Hist 1944; 83:261-94 [Google Scholar]
- 3.Lillywhite HB. Behavioral thermoregulation in the bullfrog, Rana catesbeiana. Copeia 1970; 1970:158- 68; http://dx.doi.org/ 10.2307/1441983 [DOI] [Google Scholar]
- 4.Dreisig H. Control of body temperature in shuttling ectotherms. J Therm Biol 1984; 9:229-33; http://dx.doi.org/ 10.1016/0306-4565(84)90001-9 [DOI] [Google Scholar]
- 5.Bícego-Nahas KC, Gargaglioni LH, Branco LG. Seasonal changes in the preferred body temperature, cardiovascular, and respiratory responses to hypoxia in the toad, Bufo paracnemis. J Exp Zool 2001; 289:359-65; http://dx.doi.org/ 10.1002/jez.1017 [DOI] [PubMed] [Google Scholar]
- 6.Guerra AR, Gargaglioni LH, Noronha-De-Souza CR, Abe AS, Branco LG, Bícego KC. Role of central nitric oxide in behavioral thermoregulation of toads during hypoxia. Physiol Behav 2008; 95:101-7; PMID:18558414; http://dx.doi.org/ 10.1016/j.physbeh.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Sherman E, Baldwin L, Fernandez G, Deurell E. Fever and thermal tolerance in the toad Bufo marinus. J Therm Biol 1991; 16:297-301; http://dx.doi.org/ 10.1016/0306-4565(91)90021-S [DOI] [Google Scholar]
- 8.Smits AW. Activity patterns and thermal biology of the toad Bufo boreas halophilus. Copeia; 1984:689-96; http://dx.doi.org/ 10.2307/1445150 [DOI] [Google Scholar]
- 9.Carey C. Factors affecting body temperatures of toads. Oecologia (Berl.) 1978; 35:197-219; http://dx.doi.org/ 10.1007/BF00344732 [DOI] [PubMed] [Google Scholar]
- 10.Prates I, Navas CA. Cutaneous resistance to evaporative water loss in Brazilian Rhinella (Anura: Bufonidae) from contrasting environments. Copeia 2009; 2009; 618-22; http://dx.doi.org/ 10.1643/CP-08-128 [DOI] [Google Scholar]
- 11.Wygoda ML. Low cutaneous evaporative water loss in arboreal frogs. Physiol Zool 1984; 57:329-37 [Google Scholar]
- 12.Young JE, Christian KA, Donnellan S, Tracy CR, Parry D. Comparative analysis of cutaneous evaporative water loss in frogs demonstrates correlation with ecological habits. Physiol Biochem Zool 2005; 78:847-56; PMID:16052451; http://dx.doi.org/ 10.1086/432152 [DOI] [PubMed] [Google Scholar]
- 13.Scarpellini CS, Bícego KC, Tattersall GJ. Thermoregulatory consequences of salt loading in the lizard Pogona vitticeps. J Exp Biol 2015; 218:1166-74; PMID:25714566; http://dx.doi.org/ 10.1242/jeb.116723 [DOI] [PubMed] [Google Scholar]
- 14.Schwarzkopf L, Alford RA. Desiccation and shelter-site use in a tropical amphibian: comparing toads with physical models. Functional Ecology 1996; 10:193-200; http://dx.doi.org/ 10.2307/2389843 [DOI] [Google Scholar]
- 15.Seebacher F, Alford RA. Movement and microhabitat use of a terrestrial amphibian (Bufo marinus) on a tropical island: seasonal variation and environmental correlates. J Herpetol 1999; 33:208-14; http://dx.doi.org/ 10.2307/1565716 [DOI] [Google Scholar]
- 16.Seebacher F, Alford RA. Shelter microhabitats determine body temperature and dehydration rates of a terrestrial amphibian (Bufo marinus). J Herpetol 2002; 36:69-75; http://dx.doi.org/ 10.1670/0022-1511(2002)036%5b0069:SMDBTA%5d2.0.CO;2 [DOI] [Google Scholar]
- 17.Haddad CFB, Toledo LF, Prado CPA, Loebmann D, Gasparini JL, Sazima I. Guia dos Anfíbios da Mata Atlântica - Diversidade e Biologia. São Paulo: Editora Anolis Books, 1rst ed 2013. [Google Scholar]
- 18.AmphibiaWeb : Information on amphibian biology and conservation; web application. 2015. Berkeley, California: AmphibiaWeb; Available:http://amphibiaweb.org/. (Accessed: May 28, 2015) [Google Scholar]
- 19.Glass ML, Fernandes MS, Soncini R, Glass H, Wasser JS. Effects of dry season dormancy on oxygen uptake, heart rate, and blood pressures in the toad, Bufo paracnemis. J Exp Zool 1997; 279:330-6; PMID:9360314; http://dx.doi.org/ 10.1002/(SICI)1097-010X(19971101)279:4%3c330::AID-JEZ2%3e3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- 20.Bicego KC, Branco LG. Discrete electrolytic lesion of the preoptic area prevents LPS-induced behavioral fever in toads. J Exp Biol 2002; 205:3513-8; PMID:12364403 [DOI] [PubMed] [Google Scholar]
- 21.Bícego-Nahas KC, Steiner AA, Cárnio EC, Antunes-Rodrigues J, Branco LG. Antipyretic effect of arginine vasotocin in toads. Am J Physiol 2000; 278:R1408-14 [DOI] [PubMed] [Google Scholar]
- 22.Bícego KC, Steiner AA, Antunes-Rodrigues J, Branco LG. Indomethacin impairs LPS-induced behavioral fever in toads. J Appl Physiol 2002; 93:512-6; http://dx.doi.org/ 10.1152/japplphysiol.00121.2002 [DOI] [PubMed] [Google Scholar]
- 23.Branco LG, Glass ML, Wang T, Hoffmann A. Temperature and central chemoreceptor drive to ventilation in toad (Bufo paracnemis). Respir Physiol 1993; 93:337-46; PMID:8235132; http://dx.doi.org/ 10.1016/0034-5687(93)90079-P [DOI] [PubMed] [Google Scholar]
- 24.Zena LA, Gargaglioni LH, Bícego KC. Temperature effects on baroreflex control of heart rate in the toad, Rhinella schneideri. Comp Biochem Physiol Part A, Mol Int Physiol 2015; 179:81-8; http://dx.doi.org/ 10.1016/j.cbpa.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 25.Hertz PE, Huey RB, Stevenson RD. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am Nat 1993; 142:796-818; PMID:19425957; http://dx.doi.org/ 10.1086/285573 [DOI] [PubMed] [Google Scholar]
- 26.Werner F, Herpetologische Nova Zool Anz 1894; 17, 410-15 [Google Scholar]
- 27.Chaparro JC, Pramuk JB, Gluesenkamp AG. A new species of arboreal Rhinella (Anura: Bufonidae) from cloud forest of southeastern Peru. Herpetologica 2007: 63:203-12; http://dx.doi.org/ 10.1655/0018-0831(2007)63%5b203:ANSOAR%5d2.0.CO;2 [DOI] [Google Scholar]
- 28.Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, Sá RO, Channing A, Wilkinson M, Donnellan SC, et al.. The Amphibian Tree of Life. Bull Am Mus Nat Hist 2006. 297:1-291; http://dx.doi.org/ 10.1206/0003-0090(2006)297%5b0001:TATOL%5d2.0.CO;2 [DOI] [Google Scholar]
- 29.Ab'Saber AN. Os domínios morfoclimáticos na América do Sul: primeira aproximação. Geomorfologia 1977; 53:1-23 [Google Scholar]
- 30.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 2007; 11:1633-44; http://dx.doi.org/ 10.5194/hess-11-1633-2007 [DOI] [Google Scholar]
- 31.Seebacher F, Alford RA. Shelter microhabitats determine body temperature and dehydration rates of a terrestrial amphibian (Bufo marinus). J Herpetol 2002: 36:69-75; http://dx.doi.org/ 10.1670/0022-1511(2002)036%5b0069:SMDBTA%5d2.0.CO;2 [DOI] [Google Scholar]
- 32.Mullens DP, Hutchison VH. Diel, seasonal, postprandial and food-deprived thermoregulatory behavior in tropical toads (Bufo marinus). J Therm Biol 1992; 17:63-7; http://dx.doi.org/ 10.1016/0306-4565(92)90021-7 [DOI] [Google Scholar]
- 33.Sievert LM. Thermoregulatory behaviour in the toads Bufo marinus and Bufo cognatus. J Therm Biol 1991; 16:309-12; http://dx.doi.org/ 10.1016/0306-4565(91)90023-U [DOI] [Google Scholar]
- 34.Vasconcellos MM, Colli GL. Factors Affecting the Population Dynamics of Two Toads (Anura: Bufonidae) in a Seasonal Neotropical Savanna. Copeia 2009; 2:266-76; http://dx.doi.org/ 10.1643/CE-07-099 [DOI] [Google Scholar]
- 35.Huey RB, Slatkin M. Cost and Benefits of Lizard Thermoregulation. Q Rev Biol 1976; 51:363-84; PMID:981504; http://dx.doi.org/ 10.1086/409470 [DOI] [PubMed] [Google Scholar]
- 36.Moreira G, Barreto L. Seasonal variation in nocturnal calling activity of a savanna anuran community in central Brazil. Amphibia–Reptilia 1997; 18:49-57; http://dx.doi.org/ 10.1163/156853897X00305 [DOI] [Google Scholar]
- 37.Rossa-Feres DC, Jim J. Distribuição sazonal em comunidades de anfíbios anuros na região de Botucatu, São Paulo. Revista Brasileira de Biologia 1994; 54:323-34 [Google Scholar]
- 38.Zug GR, Zug PB. The marine toad, Bufo marinus: a natural history resume of native populations. Smithsonian contributions to zoology 1979; 284 [Google Scholar]
- 39.Tracy CR. A Model of the Dynamic Exchanges of Water and Energy between a Terrestrial Amphibian and Its Environment. Ecological Monograhs 1976; 46:293-326; http://dx.doi.org/ 10.2307/1942256 [DOI] [Google Scholar]
- 40.Wygoda M. Adaptive Control of Water Loss Resistance in an Arboreal Frog. Herpetologica 1988; 44:251-7 [Google Scholar]


