Abstract
We examined whether platelet-activating factor (PAF) and its receptor mediate lipopolysaccharide (LPS)-induced fever and hypothermia in rats. Two highly potent, structurally distinct antagonists of the PAF receptor, CV6209 and WEB2086, were used. At a neutral ambient temperature (Ta) of 30ºC, administration of LPS at a low (10 μg/kg, i.v.) or high (1,000 μg/kg, i.v.) dose resulted in fever. The response to the high dose was turned into hypothermia at a subneutral Ta of 22ºC. Neither LPS-induced fever nor hypothermia was affected by pretreatment with CV6209 (5 mg/kg, i.v.) or WEB2086 (5 mg/kg, i.v.). However, both PAF antagonists were efficacious in blocking the thermoregulatory response caused by PAF (334 pmol/kg/min, 1 h, i.v.), regardless of whether the response was a fever (at 30ºC) or hypothermia (at 22ºC). Additional experiments showed that the thermoregulatory responses to LPS and PAF are also distinct in terms of their mediation by prostaglandins. Neither PAF fever nor PAF hypothermia was affected by pretreatment with the cyclooxygenase-2 inhibitor SC236 (5 mg/kg, i.p.), which is known to abrogate LPS fever. The responses to PAF were also unaffected by pretreatment with the cyclooxygenase-1 inhibitor SC560 (5 mg/kg, i.p.), which is known to attenuate LPS hypothermia. In conclusion, PAF infusion at a picomolar dose causes fever at thermoneutrality but hypothermia in a subthermoneutral environment, both responses being dependent on the PAF receptor and independent of prostaglandins. However, the PAF receptor does not mediate LPS-induced fever or hypothermia, thus challenging the dogma that PAF is an upstream mediator of responses to LPS.
Keywords: Body temperature, cyclooxygenase, endotoxemia, febrile response, LPS, PAF, prostaglandins, systemic inflammation, sepsis, thermoregulation
Abbreviations
- COX
cyclooxygenase
- i.p.
intraperitoneal(ly)
- i.v.
intravenous(ly)
- LPS
lipopolysaccharide
- PAF
platelet-activating factor
- Ta
ambient temperature; Tb, body temperature
Introduction
Platelet-activating factor (PAF) is a highly potent bioactive phospholipid with a diversity of physiological roles, most of which are dependent on the G-protein-coupled PAF receptor.1,2 Particular attention has been paid to the roles of PAF in sepsis and other systemic inflammatory conditions, and anti-PAF therapies to treat these deadly disorders have been proposed.3,4 Much of the knowledge about the roles of PAF in inflammatory disorders derives from the experimental model of lipopolysaccharide (LPS)-induced systemic inflammation. In this model, blood and organ levels of PAF are increased shortly after LPS administration.5–8 Furthermore, many pathophysiological alterations induced by LPS can be mimicked by the administration of PAF at picomolar doses.9–12 Moreover, structurally distinct PAF receptor antagonists block, or at least attenuate, key components of the systemic inflammatory response, including circulatory shock2 and pulmonary edema.13 PAF and its receptor have also been proposed to mediate the thermoregulatory manifestations of the systemic inflammatory response, but evidence in support of this notion is less compelling.
An alteration in deep body temperature (Tb)—either fever or hypothermia—occurs in all septic patients.14,15 Fever and hypothermia also occur in LPS-challenged laboratory rodents, with the development of fever versus hypothermia being a function of the LPS dose and the ambient temperature (Ta). A low dose and a warm environment favor the development of fever; a high dose and a cool environment favor the development of hypothermia (for review, see Romanovsky et al.16). The involvement of the PAF receptor in fever finds support in a single study,12 in which the Ginkgo biloba-derived PAF antagonist, BN52021, attenuated all phases of the polyphasic fever induced by a low dose of LPS in rats kept at a neutral Ta. This compound, however, could exert nonspecific actions, as it is capable of activating PAF acetylhydrolases,17 catabolic enzymes with affinity to a broad spectrum of substrates other than PAF, e.g., oxidized phospholipids.18,19 The participation of the PAF receptor in LPS-induced hypothermia is also supported by a single study.20 Although that study involved a potent and selective PAF antagonist (CV3988), it did not control for fluctuations in Ta, which might have affected the results. To complicate the matter even further, there is evidence against the involvement of the PAF receptor in the Tb responses to LPS, at least when it comes to fever, as LPS fever was only marginally affected by a PAF antagonist in a study in horses21 and not affected at all by another PAF antagonist in a study in humans.22
In view of these contradictions and experimental limitations, we reinvestigated the involvement of the PAF receptor in LPS-induced fever and hypothermia in rats. Two highly potent competitive antagonists of the PAF receptor were tested: CV6209 (a PAF structural analog)23,24 and WEB2086 (a triazolobenzodiazepine derivative).24,25 Both of these compounds also block the PAF receptor by acting as inverse agonists.26 Importantly, neither compound activates PAF acetylhydrolases.27,28. To eliminate another shortcoming of the earlier studies described above, Ta was tightly controlled in our experiments. To avoid stress responses associated with the acute drug administration, LPS was administered via pre-implanted intravenous (i.v.) catheters.
Results
We initially tested the ability of CV6209 or WEB2086 to block PAF-induced Tb responses. Male Wistar rats were pretreated i.v. with CV6209 (5 mg/kg), WEB2086 (5 mg/kg), or the corresponding vehicle at −30 min. Each rat was then infused i.v. with PAF (334 pmol/kg/min) twice: at 0–60 min and 240–300 min. The two infusions were employed to assess the duration of PAF receptor antagonism by CV6209 and WEB2086. The doses of the PAF antagonists chosen have been shown to be efficacious in various in-vivo tests in rats.23,29-31 The dose of PAF and the form of its administration (a complex with albumin) were chosen based on our previous studies12,32 (also see Materials and Methods). At a neutral Ta of 30ºC, vehicle-pretreated rats responded to both infusions of PAF with marked but transient increases in Tb (F12,132 = 13.28, P < 0.001 for the first infusion; F12,156 = 15.62, P < 0.001 for the second infusion); Figure 1. Exposure to a Ta of 22ºC changed the response of the vehicle-pretreated rats from febrile to hypothermic (F12,144 = 58.82, P < 0.001 for the first infusion; F12,144 = 19.80, P < 0.001 for the second infusion), but the transient nature of the responses was preserved (Fig. 2). When the same 2 infusions of PAF were performed following a pretreatment with CV6209, there were no significant changes in Tb, regardless of Ta, meaning that the single pretreatment with CV6209 abolished the Tb responses to both PAF infusions (Figs. 1 and 2). A single pretreatment with WEB2086 was similarly effective at eliminating the febrile responses to both PAF infusions at Ta of 30ºC (Fig. 1). However, WEB2086 was partially effective against the hypothermic responses to both PAF infusions at Ta of 22ºC (Fig. 2): this response was attenuated compared to the response of the vehicle-pretreated controls (F1,12 = 19.97, P < 0.001 for the first infusion; F1,12 = 10.09, P = 0.008 for the second infusion;), but was not eliminated in relation to baseline Tb (F12,72 = 62.40, P < 0.001 for the first infusion; F12,72 = 36.14, P < 0.001 for the second infusion). These data clearly demonstrate that the pretreatment with CV6209 or WEB2086 was efficacious in blocking or attenuating responses to PAF for the entire duration of experiments, i.e., for at least 6 h.
Figure 1.
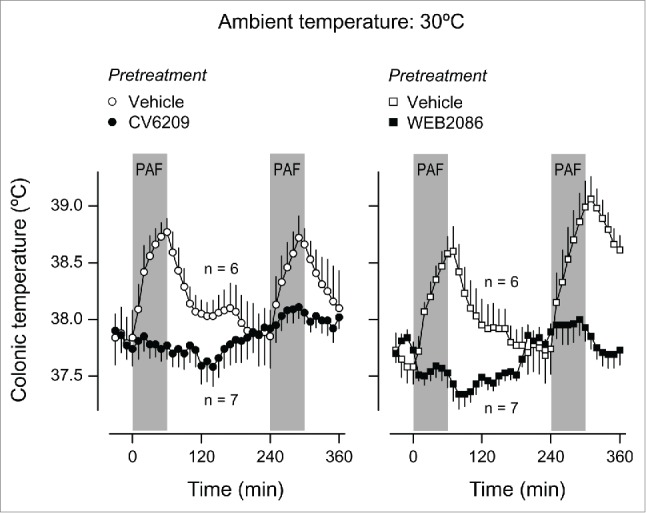
Effect of the i.v. pretreatment with CV6209 (5 mg/kg), WEB2086 (5 mg/kg), or the corresponding vehicle on the Tb responses to PAF at a neutral Ta (30°C). PAF was infused i.v. at a rate of 334 pmol/kg/min for 1 h twice; the two periods of infusion are marked in gray. Data are means ± SEM. The number of animals in each group (n) is indicated.
Figure 2.
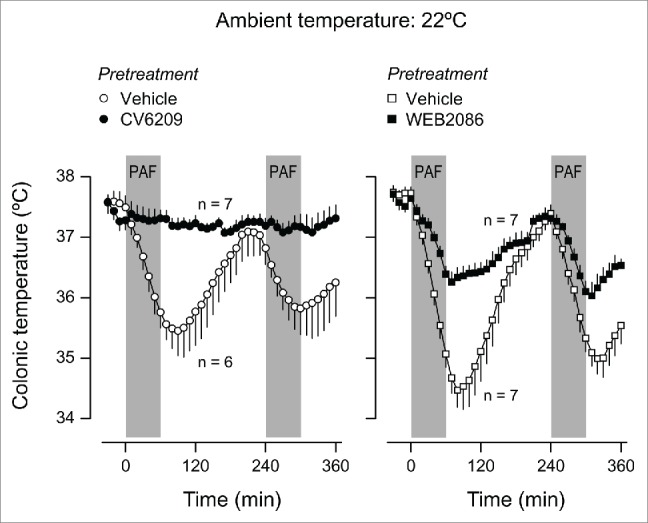
Effect of the i.v. pretreatment with CV6209 (5 mg/kg), WEB2086 (5 mg/kg), or the corresponding vehicle on the Tb responses to PAF at a subneutral Ta (22°C). PAF was infused i.v. at a rate of 334 pmol/kg/min for 1 h twice; the two periods of infusion are marked in gray. Data are means ± SEM. The number of animals in each group (n) is indicated.
The next step was to evaluate how the same pretreatments affected Tb responses to LPS. As shown in Figure 3, fever was the prevailing response to LPS at Ta of 30ºC, regardless of the dose used (10 or 1,000 μg/kg; F80,2640 = 30.53, P < 0.001 for both doses compared to saline). The lower dose elicited 3 well-defined febrile phases with Tb peaks at ∼40, 120, and 300 min. The second and third phases were also present in the response to the higher dose of LPS, whereas the first phase was either small or absent. These fevers developed regardless of whether the rats were pretreated with a vehicle, CV6209, or WEB2086; no statistical differences were revealed between any of the pretreatment groups. When the same experiment was conducted at Ta of 22ºC, hypothermia was the prevailing Tb response to LPS (Fig. 4). The biphasic hypothermic response was significantly different from the response to saline only at the higher LPS dose (F80,2640 = 37.95, P < 0.001). This hypothermic response was unaffected by pretreatment with either CV6209 or WEB2086.
Figure 3.
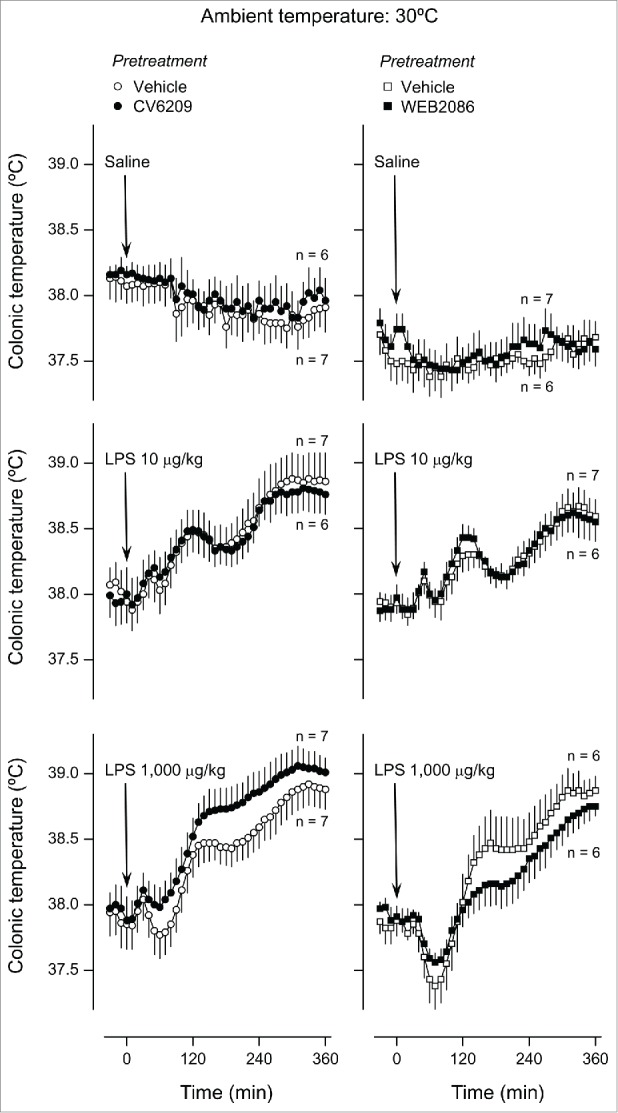
Effect of the i.v. pretreatment with CV6209 (5 mg/kg), WEB2086 (5 mg/kg), or the corresponding vehicle on the Tb responses to LPS at a neutral Ta (30°C). LPS or its vehicle (saline) was administered as an i.v. bolus injection at time zero; LPS doses are indicated. Data are means ± SEM. The number of animals in each group (n) is indicated.
Figure 4.
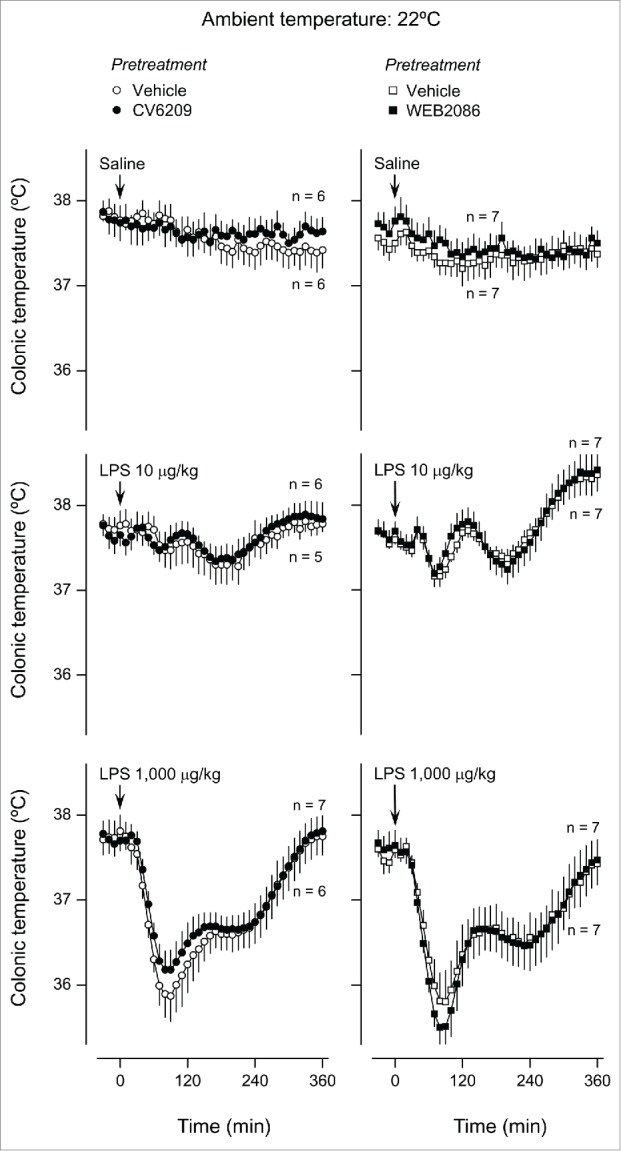
Effect of the i.v. pretreatment with CV6209 (5 mg/kg), WEB2086 (5 mg/kg), or the corresponding vehicle on the Tb responses to LPS at a subneutral Ta (22°C). LPS or its vehicle (saline) was administered as an i.v. bolus injection at time zero; LPS doses are indicated. Data are means ± SEM. The number of animals in each group (n) is indicated.
Owing to the importance of prostaglandins in LPS-induced fever16,33-36 and hypothermia,37–39 the found noninvolvement of the PAF receptor in the responses to LPS raised the possibility that PAF administration could trigger Tb responses in a prostaglandin-independent fashion. To test this possibility, we pharmacologically targeted cyclooxygenase (COX), an important enzyme in prostaglandin biosynthesis. The intraperitoneal (i.p.) pretreatment with the COX-2 inhibitor, SC236, at a dose (5 mg/kg) known to be efficacious in suppressing LPS fever,37,40 exerted no effect on the fever induced by PAF at Ta of 30ºC (Fig. 5). SC236 also failed to attenuate the hypothermia induced by PAF at Ta of 22ºC (Fig. 6). Likewise, pretreatment with the COX-1 inhibitor, SC-560, at a dose (5 mg/kg) known to block LPS hypothermia,33,35 suppressed neither PAF-induced hypothermia (Fig. 6) nor PAF-induced fever (Fig. 5).
Figure 5.
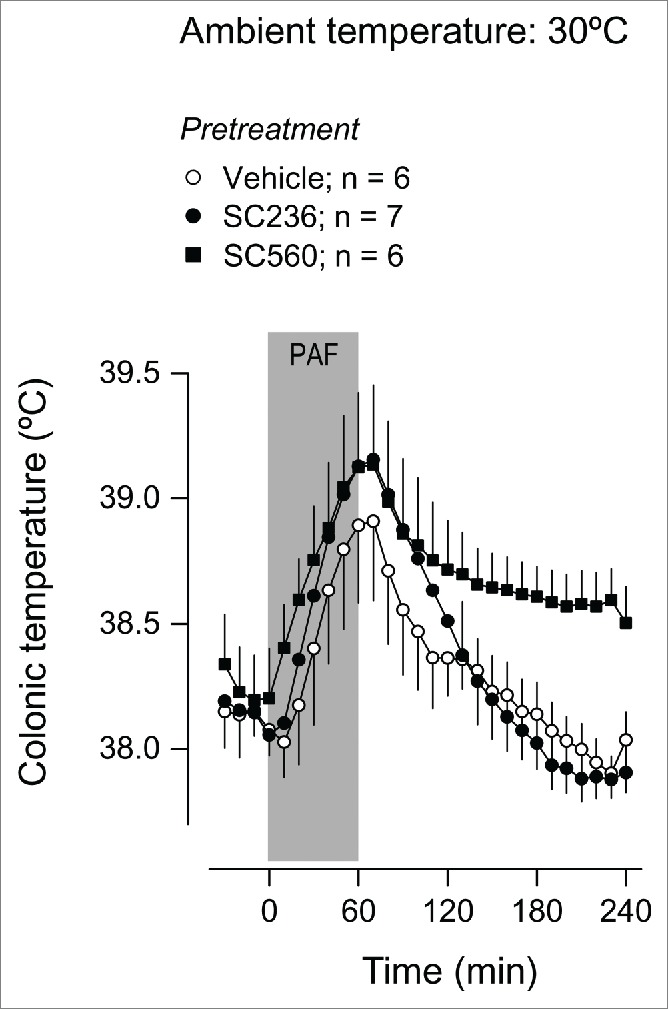
Effect of the i.p. pretreatment with SC236 (5 mg/kg), SC560 (5 mg/kg), or their vehicle on the Tb response to PAF at a neutral Ta (30°C). PAF was infused i.v. at a rate of 334 pmol/kg/min for 1 h; the period of infusion is marked in gray. Data are means ± SEM. The number of animals in each group (n) is indicated.
Figure 6.
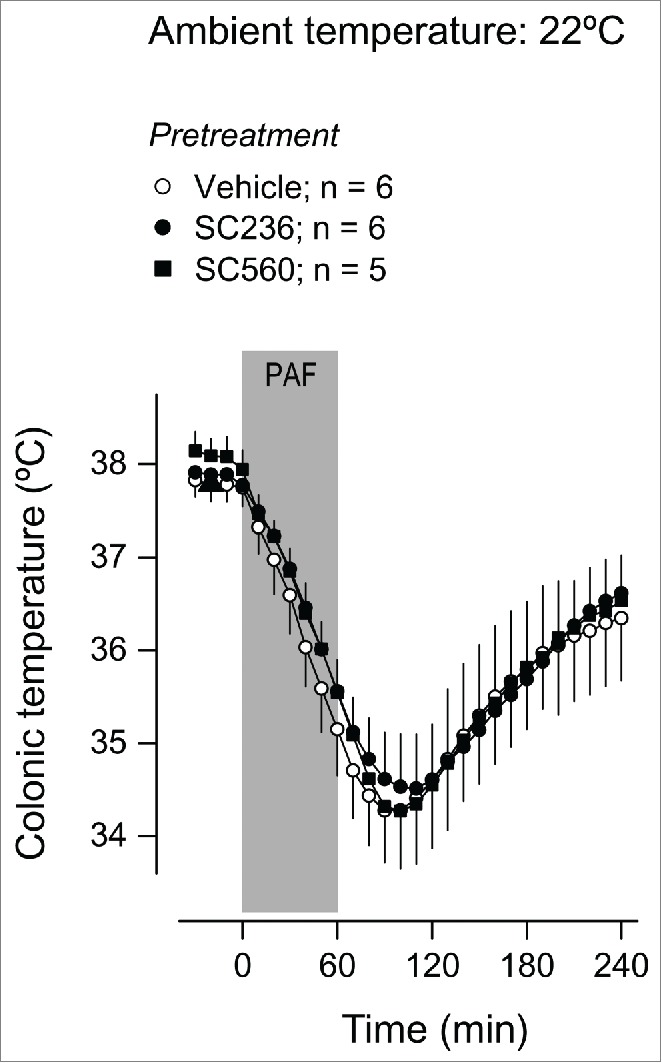
Effect of the i.p. pretreatment with SC236 (5 mg/kg), SC560 (5 mg/kg), or their vehicle on the Tb response to PAF at a subneutral Ta (22°C). PAF was infused i.v. at a rate of 334 pmol/kg/min for 1 h; the period of infusion is marked in gray. Data are means ± SEM. The number of animals in each group (n) is indicated.
Discussion
We conducted a comprehensive study of the effects of PAF receptor blockade on the thermoregulatory responses to LPS, viz., fever and hypothermia. Two potent, structurally unrelated PAF receptor antagonists (CV6209 and WEB2086) were used, and their efficacy in blocking the PAF receptor for the entire duration of experiments was confirmed by studying their effects on Tb responses caused by repeated infusions of PAF. We then studied the effects of these PAF antagonists on a broad spectrum of LPS-induced Tb responses; the febrile and hypothermic components of these responses were revealed to different extents by challenging rats exposed to a neutral or subneutral Ta with a low or high dose of LPS. Last, but not least, the study circumvented 2 methodological pitfalls that frequently interfere with the outcome of thermoregulation experiments: (i) acute stress at the time of the LPS injection was avoided by using preimplanted venous catheters; (ii) fluctuations in Ta were minimized by placing the rats inside an environmental chamber (for a discussion, see Romanovsky et al.41).
The results of this investigation do not support the proposed involvement of the PAF receptor in the Tb responses to LPS, regardless of whether the prevailing response is fever or hypothermia. Although our findings do not exclude a receptor-independent role for PAF in the Tb responses to LPS, it should be considered that we found no indication that PAF could induce a Tb response independently of its receptor. Indeed, all thermoregulatory responses to PAF observed in this study were blocked completely by at least one of the PAF receptor antagonists used (CV6209).
A broader implication of the present findings is that they challenge the notion that PAF occupies a relatively upstream position in the LPS-induced inflammatory cascade, i.e., serves as a common mediator of most, if not all, responses to LPS. Such an upstream position of PAF would agree with the early report that a PAF receptor antagonist (TCV-309) attenuated the surge in plasma tumor necrosis factor-α in endotoxemic chimpanzees.42 However, subsequent studies with PAF receptor antagonists of different classes failed to reproduce this observation in horses and humans.21,22 Later on, other components of the inflammatory response (e.g., neutrophil recruitment43–45 and liver injury46) were also found to occur independently of the PAF receptor. In this context, the noninvolvement of PAF in the Tb responses to LPS is not surprising.
While rejecting a role for PAF in the Tb responses to LPS, the present study confirmed the ability of PAF to affect Tb when administered i.v. at a picomolar dose in rats. As in previous studies,12,32 such a high potency was attained by delivering PAF in the form of a preformed complex with albumin. Ivanov et al.12 have estimated that, in this form, i.v. PAF is at least 170 times more potent at inducing fever than i.v. prostaglandin E2, the most established lipid pyrogen. Such a low dose of PAF is sufficient to induce not only fever at a neutral Ta, but also hypothermia at a subneutral Ta; this is noteworthy because hypothermia is usually a response to stronger inflammatory stimuli.47,48 It is possible that, while being irrelevant to LPS-induced thermoregulatory responses, PAF might be of relevance to thermoregulatory responses in other inflammatory conditions. An example is the prominent role of PAF as a mediator of hypothermia in models of IgG-dependent anaphylaxis.49
Another important finding of the present study is that neither PAF-induced fever nor PAF-induced hypothermia was suppressed by COX inhibition, in spite of PAF's demonstrated ability to induce prostaglandin biosynthesis via the COX-2 pathway in cultured cells of different types.50–52 Therefore, PAF should be added to the list of inflammatory mediators capable of inducing Tb responses independently of COX and prostaglandins. To date, this list (which is based on a relatively small number of studies) includes macrophage inflammatory protein-1 (CCL3 and CCL4),53,54 interleukin-8 (CXCL8),55 and carbon monoxide.56 It should be considered, however, that the relevance of COX-independent fevers is a matter of debate, because both experimental LPS-induced fever57,58 and clinical, infection-associated fevers59,60 are usually quite dependent on COX-2. The relevance of COX-independent hypothermia to systemic inflammation is also unclear and does not agree with studies suggesting that LPS-induced hypothermia is COX-1-dependent.37,39
In conclusion, the present study shows that the PAF receptor does not play a role in LPS-induced fever or hypothermia, thus challenging the notion that PAF is an important upstream mediator in acute systemic inflammation. By the same token, the present study confirms that PAF is a potent pyrogen and cryogen. It also shows that both the febrile and hypothermic effects of PAF are dependent on the PAF receptor and independent of COX. Future studies are necessary to examine whether the pyrogenic and cryogenic properties of PAF are relevant to inflammatory conditions that are different from bacterial LPS-induced systemic inflammation.
Materials and Methods
Animals
Male Wistar rats obtained from Harlan (Indianapolis, IN, USA) were housed in a microisolator caging system with air temperature control (Allentown Caging Equipment, Allentown, NJ, USA). The temperature of the incoming air was maintained at 28ºC. The holding room was on a 12:12 h light-dark cycle (lights on at 07:00 am). Rats had free access to standard chow and tap water. The environment in the cages was enriched with artificial "rat holes" (cylindrical confiners made of stainless steel wire); the same confiners were used in the experiments. In addition to spending time in the confiners voluntarily, the rats were systematically habituated to them (7 daily training sessions, 4 h each). The rats weighed an average of 330 g (240–405 g) at the time of the experiments. Each rat was used in an experiment once and euthanized with sodium pentobarbital (100 mg/kg, i.v.) immediately thereafter. All protocols were approved by the St. Joseph's Hospital and Medical Center Animal Care and Use Committee.
Jugular catheterization
Each rat was anesthetized with ketamine-xylazine-acepromazine (56:6:1 mg/kg, i.p.), treated prophylactically with an antibiotic (enrofloxacin, 5 mg/kg, subcutaneously), and maintained on a heated (37–39ºC) operating board. The left jugular vein was accessed via a longitudinal incision on the ventral surface of the neck. The vein was cleared from connective tissue and ligated, and a silicone catheter (ID 0.5 mm, OD 0.9 mm) filled with heparinized (10 U/ml) saline was inserted toward the superior vena cava. The catheter was secured in place with ligatures. The distal end of the catheter was passed under the skin and exteriorized at the nape. The skin incision was sutured. The rats were allowed to recover from surgery for 5–7 d before being taken in an experiment. During the recovery period, the catheters were flushed with heparinized saline every other day.
Experimental setup
Each rat was placed in a confiner and equipped with a colonic thermocouple, which was inserted 10 cm beyond the anal sphincter and fixed to the base of the tail with adhesive tape. The analog signals from thermocouples were converted to digital by a Digi-Sense multichannel thermometer (Cole-Parmer, Vernon Hills, IL, USA) and sent to a personal computer. Rats in their confiners were kept inside an environmental chamber (model 3940; Forma Scientific, Marietta, OH, USA) for the duration of the experiment. Inside the chamber, Ta was maintained at either 30.0 ± 0.1ºC or 22.0 ± 0.1ºC; relative air humidity was maintained at 50 ± 5%. A saline-filled PE-50 extension of the venous catheter was passed through a wall port to the outside of the chamber, from where drugs were administered with the help of a syringe pump (Stoelting, Wood Dale, IL, USA).
Drugs
PAF (β-Acetyl-γ-O-Alkyl-L-α-phosphatidylcholine, from bovine heart lecithin) and phenol-purified LPS (from E. coli 0111:B4) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The PAF receptor antagonists, CV6209 and WEB2086, were purchased from Enzo Life Sciences (Farmingdale, NY, USA) and Tocris (Minneapolis, MN, USA), respectively. Selective COX inhibitors, SC236 (COX-2) and SC560 (COX-1), were obtained from Cayman Chemical (Ann Arbor, MI, USA). PAF was administered in the form of a 1:1 (molar ratio) complex with fatty acid-free bovine serum albumin (Sigma). To prepare the complex, a saline suspension of PAF (20 nmol/ml) and albumin was sonicated for 3 min and then incubated at 37ºC for 1 h. The resulting preparation was administered as a 60-min i.v. infusion at a rate of 16.7 μl/kg/min. LPS was diluted in saline at a concentration of either 10 or 1,000 μg/ml, and injected i.v. in bolus at a volume of 1 ml/kg. Pretreatment with a PAF receptor antagonist (CV6209 or WEB2086) was performed at 30 min before either the LPS injection or the first infusion of PAF. CV6209 was dissolved in saline to a concentration of 5 mg/ml; it was bolus injected i.v. at a volume of 1 ml/kg. WEB2086 was dissolved in a 1:4 ethanol-saline solution to a concentration of 10 mg/ml; it was administered as a 10-min i.v. infusion at a rate of 50 μl/kg/min. COX-1 and COX-2 inhibitors (SC560 and SC236, respectively) were also administered as pretreatments, but at 90 min before the start of PAF infusion. Both COX inhibitors were dissolved in DMSO to a concentration of 10 mg/ml; they were administered by i.p. injection at a volume of 0.5 ml/kg.
Statistical analyses
All Tb responses were evaluated for the effects of treatments and pretreatments over time by repeated-measures ANOVA followed, as necessary, by the Tukey least significant difference test. The calculations were performed using Statistica Advanced 8.0 (StatSoft, Tulsa, OK, USA), with the level of significance set at P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research has been supported, in part, by grants from the National Institutes of Health (R01NS41233 to AAR), the American Heart Association (AHA 11SDG4880051 to AAS) and the São Paulo Research Foundation (FAPESP 2012/03831–8 to AAS).
References
- 1. Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 2000; 69:419-45; PMID:10966465; http://dx.doi.org/ 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 2. Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev 2000; 80:1669-99; PMID:11015622. [DOI] [PubMed] [Google Scholar]
- 3. Teixeira-da-Cunha MG, Gomes RN, Roehrs N, Bozza FA, Prescott SM, Stafforini D, Zimmerman GA, Bozza PT, Castro-Faria-Neto HC. Bacterial clearance is improved in septic mice by platelet-activating factor-acetylhydrolase (PAF-AH) administration. PLoS One 2013; 8:e74567; PMID:24069320; http://dx.doi.org/ 10.1371/journal.pone.0074567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Xu J, Chen X, Zhang Y, Jiang X, Guo X, Zhao G. Decrease of plasma platelet-activating factor acetylhydrolase activity in lipopolysaccharide induced mongolian gerbil sepsis model. PLoS One 2010; 5:e9190; PMID:20169191; http://dx.doi.org/ 10.1371/journal.pone.0009190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang SW, Feddersen CO, Henson PM, Voelkel NF. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest 1987; 79:1498-509; PMID:3553241; http://dx.doi.org/ 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han SJ, Choi JH, Ko HM, Yang HW, Choi IW, Lee HK, Lee OH, Im SY. Glucocorticoids prevent NF-kB activation by inhibiting the early release of platelet-activating factor in response to lipopolysaccharide. Eur J Immunol 1999; 29:1334-41; PMID:10229101; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199904)29:04%3c1334::AID-IMMU1334%3e3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7. Qu XW, Rozenfeld RA, Huang W, Crawford SE, Gonzalez-Crussi F, Hsueh W. Interaction of platelet-activating factor, spleen and atrial natriuretic peptide in plasma volume regulation during endotoxaemia in rats. J Physiol 1998; 512:227-34; PMID:9729632; http://dx.doi.org/ 10.1111/j.1469-7793.1998.227bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karasawa K, Kato H, Setaka M, Nojima S. Accumulation of platelet-activating factor acetylhydrolase in the peritoneal cavity of guinea pig after endotoxin shock. J Biochem 1994; 116:368-73; PMID:7822256. [DOI] [PubMed] [Google Scholar]
- 9. Bessin P, Bonnet J, Apffel D, Soulard C, Desgroux L, Pelas I, Benveniste J. Acute circulatory collapse caused by platelet-activating factor (PAF-acether) in dogs. Eur J Pharmacol 1983; 86:403-13; PMID:6687572; http://dx.doi.org/ 10.1016/0014-2999(83)90190-5. [DOI] [PubMed] [Google Scholar]
- 10. Doebber TW, Wu MS, Robbins JC, Choy BM, Chang MN, Shen TY. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun 1985; 127:799-808; PMID:2985058; http://dx.doi.org/ 10.1016/S0006-291X(85)80014-0. [DOI] [PubMed] [Google Scholar]
- 11. Clavijo LC, Carter MB, Matheson PJ, Wilson MA, Wead WB, Garrison RN. PAF increases vascular permeability without increasing pulmonary arterial pressure in the rat. J Appl Physiol (1985) 2001; 90:261-8; PMID:11133918. [DOI] [PubMed] [Google Scholar]
- 12. Ivanov AI, Patel S, Kulchitsky VA, Romanovsky AA. Platelet-activating factor: a previously unrecognized mediator of fever. J Physiol 2003; 553:221-8; PMID:14565987; http://dx.doi.org/ 10.1113/jphysiol.2003.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uhlig S, Goggel R, Engel S. Mechanisms of platelet-activating factor (PAF)-mediated responses in the lung. Pharmacol Rep 2005; 57 Suppl:206-21; PMID:16415501. [PubMed] [Google Scholar]
- 14. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369:840-51; PMID:23984731; http://dx.doi.org/ 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 15. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165-228; PMID:23361625; http://dx.doi.org/ 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 2005; 10:2193-216; PMID:15970487; http://dx.doi.org/ 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- 17. Bonin F, Ryan SD, Migahed L, Mo F, Lallier J, Franks DJ, Arai H, Bennett SA. Anti-apoptotic actions of the platelet-activating factor acetylhydrolase I a2 catalytic subunit. J Biol Chem 2004; 279:52425-36; PMID:15456758; http://dx.doi.org/ 10.1074/jbc.M410967200. [DOI] [PubMed] [Google Scholar]
- 18. Karasawa K, Harada A, Satoh N, Inoue K, Setaka M. Plasma platelet activating factor-acetylhydrolase (PAF-AH). Prog Lipid Res 2003; 42:93-114; PMID:12547653; http://dx.doi.org/ 10.1016/S0163-7827(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 19. Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor acetylhydrolases. J Biol Chem 1997; 272:17895-8; PMID:9218411; http://dx.doi.org/ 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 20. Ephgrave K, Kremer T, Broadhurst K, Cullen J. The role of platelet-activating factor in conscious, normotensive endotoxemia. J Surg Res 1997; 68:170-4; PMID:9184676; http://dx.doi.org/ 10.1006/jsre.1997.5009. [DOI] [PubMed] [Google Scholar]
- 21. Carrick JB, Morris DD, Moore JN. Administration of a receptor antagonist for platelet-activating factor during equine endotoxaemia. Equine Vet J 1993; 25:152-7; PMID:8385601; http://dx.doi.org/ 10.1111/j.2042-3306.1993.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 22. Thompson WA, Coyle S, Van Zee K, Oldenburg H, Trousdale R, Rogy M, Felsen D, Moldawer L, Lowry SF. The metabolic effects of platelet-activating factor antagonism in endotoxemic man. Arch Surg 1994; 129:72-9; PMID:8279943; http://dx.doi.org/ 10.1001/archsurg.1994.01420250084011. [DOI] [PubMed] [Google Scholar]
- 23. Terashita Z, Imura Y, Takatani M, Tsushima S, Nishikawa K. CV-6209, a highly potent antagonist of platelet activating factor in vitro and in vivo. J Pharmacol Exp Ther 1987; 242:263-8; PMID:3612533. [PubMed] [Google Scholar]
- 24. Tahraoui L, Floch A, Mondot S, Cavero I. High affinity specific binding sites for tritiated platelet-activating factor in canine platelet membranes: counterparts of platelet-activating factor receptors mediating platelet aggregation. Mol Pharmacol 1988; 34:145-51; PMID:2842653. [PubMed] [Google Scholar]
- 25. Casals-Stenzel J, Muacevic G, Weber KH. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther 1987; 241:974-81; PMID:3598913. [PubMed] [Google Scholar]
- 26. Dupre DJ, Le Gouill C, Rola-Pleszczynski M, Stankova J. Inverse agonist activity of selected ligands of platelet-activating factor receptor. J Pharmacol Exp Ther 2001; 299:358-65; PMID:11561099. [PubMed] [Google Scholar]
- 27. Shimada T, Hirose T, Matsumoto I, Aikawa T. Platelet-activating factor acts on cortisol secretion by perfused guinea-pig adrenals via calcium-/phospholipid-dependent mechanisms. J Endocrinol 2005; 184:381-91; PMID:15684346; http://dx.doi.org/ 10.1677/joe.1.05937. [DOI] [PubMed] [Google Scholar]
- 28. Narahara H, Kawano Y, Nasu K, Yoshimatsu J, Johnston JM, Miyakawa I. Platelet-activating factor inhibits the secretion of platelet-activating factor acetylhydrolase by human decidual macrophages. J Clin Endocrinol Metab 2003; 88:6029-33; PMID:14671207; http://dx.doi.org/ 10.1210/jc.2003-030706. [DOI] [PubMed] [Google Scholar]
- 29. Squadrito F, Ioculano M, Altavilla D, Zingarelli B, Canale P, Campo GM, Saitta A, Calapai G, Bussolino F, Caputi AP. Platelet activating factor interaction with tumor necrosis factor in myocardial ischaemia-reperfusion injury. J Lipid Mediat 1993; 8:53-65; PMID:8257777. [PubMed] [Google Scholar]
- 30. Casals-Stenzel J. Protective effect of WEB 2086, a novel antagonist of platelet activating factor, in endotoxin shock. Eur J Pharmacol 1987; 135:117-22; PMID:3582490; http://dx.doi.org/ 10.1016/0014-2999(87)90602-9. [DOI] [PubMed] [Google Scholar]
- 31. Muacevic G, Heuer HO. Platelet-activating factor antagonists in experimental shock. Arzneimittelforschung 1992; 42:1001-4; PMID:1418068. [PubMed] [Google Scholar]
- 32. Ivanov AI, Steiner AA, Patel S, Rudaya AY, Romanovsky AA. Albumin is not an irreplaceable carrier for amphipathic mediators of thermoregulatory responses to LPS: compensatory role of a1-acid glycoprotein. Am J Physiol Regul Integr Comp Physiol 2005; 288:R872-8; PMID:15576666; http://dx.doi.org/ 10.1152/ajpregu.00514.2004. [DOI] [PubMed] [Google Scholar]
- 33. Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci 2004; 9:2819-26; PMID:15353317; http://dx.doi.org/ 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- 34. Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci 2004; 9:1977-93; PMID:14977603; http://dx.doi.org/ 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- 35. Blatteis CM. Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol Ther 2006; 111:194-223; PMID:16460809; http://dx.doi.org/ 10.1016/j.pharmthera.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 36. Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci 2012; 15:1088-95; PMID:22837039; http://dx.doi.org/ 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. Cyclooxygenase-1 or -2–which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol 2009; 297:R485-94; PMID:19515980; http://dx.doi.org/ 10.1152/ajpregu.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dogan MD, Ataoglu H, Akarsu ES. Effects of selective cyclooxygenase enzyme inhibitors on lipopolysaccharide-induced dual thermoregulatory changes in rats. Brain Res Bull 2002; 57:179-85; PMID:11849824; http://dx.doi.org/ 10.1016/S0361-9230(01)00739-0. [DOI] [PubMed] [Google Scholar]
- 39. Akarsu ES, Mamuk S. Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats. Am J Physiol Regul Integr Comp Physiol 2007; 292:R1846-50; PMID:17272660; http://dx.doi.org/ 10.1152/ajpregu.00786.2006. [DOI] [PubMed] [Google Scholar]
- 40. Zhang YH, Lu J, Elmquist JK, Saper CB. Specific roles of cyclooxygenase-1 and cyclooxygenase-2 in lipopolysaccharide-induced fever and Fos expression in rat brain. J Comp Neurol 2003; 463:3-12; PMID:12811798; http://dx.doi.org/ 10.1002/cne.10743. [DOI] [PubMed] [Google Scholar]
- 41. Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N. Methodology of fever research: why are polyphasic fevers often thought to be biphasic? Am J Physiol 1998; 275:R332-8; PMID:9688996. [DOI] [PubMed] [Google Scholar]
- 42. Kuipers B, van der Poll T, Levi M, van Deventer SJ, ten Cate H, Imai Y, Hack CE, ten Cate JW. Platelet-activating factor antagonist TCV-309 attenuates the induction of the cytokine network in experimental endotoxemia in chimpanzees. J Immunol 1994; 152:2438-46; PMID:8133055. [PubMed] [Google Scholar]
- 43. Arreto CD, Dumarey C, Nahori MA, Vargaftig BB. The LPS-induced neutrophil recruitment into rat air pouches is mediated by TNFa: likely macrophage origin. Mediators Inflamm 1997; 6:335-43; PMID:18472868; http://dx.doi.org/ 10.1080/09629359791479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang SW. Endotoxin-induced pulmonary leukostasis in the rat: role of platelet-activating factor and tumor necrosis factor. J Lab Clin Med 1994; 123:65-72; PMID:8288963. [PubMed] [Google Scholar]
- 45. Olszanecki R, Chlopicki S. Endotoxaemia in rats: role of NO, PAF and TXA2 in pulmonary neutrophil sequestration and hyperlactataemia. J Physiol Pharmacol 1999; 50:443-54; PMID:10574473. [PubMed] [Google Scholar]
- 46. Pearson JM, Bailie MB, Fink GD, Roth RA. Neither platelet activating factor nor leukotrienes are critical mediators of liver injury after lipopolysaccharide administration. Toxicology 1997; 121:181-9; PMID:9231696; http://dx.doi.org/ 10.1016/S0300-483X(97)03660-3. [DOI] [PubMed] [Google Scholar]
- 47. Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci 2006; 23:3359-67; PMID:16820025; http://dx.doi.org/ 10.1111/j.1460-9568.2006.04854.x. [DOI] [PubMed] [Google Scholar]
- 48. Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE 2006; 1:e1; PMID:17183631; http://dx.doi.org/ 10.1371/journal.pone.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol 2002; 109:658-68; PMID:11941316; http://dx.doi.org/ 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 50. Marrache AM, Gobeil F, Jr., Bernier SG, Stankova J, Rola-Pleszczynski M, Choufani S, Bkaily G, Bourdeau A, Sirois MG, Vazquez-Tello A, et al. . Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol 2002; 169:6474-81; PMID:12444157; http://dx.doi.org/ 10.4049/jimmunol.169.11.6474. [DOI] [PubMed] [Google Scholar]
- 51. Teather LA, Wurtman RJ. Cyclooxygenase-2 mediates platelet-activating factor-induced prostaglandin E2 release from rat primary astrocytes. Neurosci Lett 2003; 340:177-80; PMID:12672535; http://dx.doi.org/ 10.1016/S0304-3940(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 52. Koga MM, Bizzarro B, Sa-Nunes A, Rios FJ, Jancar S. Activation of PAF-receptor induces regulatory dendritic cells through PGE2 and IL-10. Prostaglandins Leukot Essent Fatty Acids 2013; 89:319-26; PMID:24120121; http://dx.doi.org/ 10.1016/j.plefa.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 53. Minano FJ, Sancibrian M, Vizcaino M, Paez X, Davatelis G, Fahey T, Sherry B, Cerami A, Myers RD. Macrophage inflammatory protein-1: unique action on the hypothalamus to evoke fever. Brain Res Bull 1990; 24:849-52; PMID:2196977; http://dx.doi.org/ 10.1016/0361-9230(90)90150-X. [DOI] [PubMed] [Google Scholar]
- 54. Davatelis G, Wolpe SD, Sherry B, Dayer JM, Chicheportiche R, Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science 1989; 243:1066-8; PMID:2646711; http://dx.doi.org/ 10.1126/science.2646711. [DOI] [PubMed] [Google Scholar]
- 55. Zampronio AR, Souza GE, Silva CA, Cunha FQ, Ferreira SH. Interleukin-8 induces fever by a prostaglandin-independent mechanism. Am J Physiol 1994; 266:R1670-4; PMID:8203649. [DOI] [PubMed] [Google Scholar]
- 56. Steiner AA, Branco LG. Central CO-heme oxygenase pathway raises body temperature by a prostaglandin-independent way. J Appl Physiol 2000; 88:1607-13; PMID:10797120. [DOI] [PubMed] [Google Scholar]
- 57. Steiner AA, Rudaya AY, Robbins JR, Dragic AS, Langenbach R, Romanovsky AA. Expanding the febrigenic role of cyclooxygenase-2 to the previously overlooked responses. Am J Physiol Regul Integr Comp Physiol 2005; 289:R1253-7; PMID:16081878; http://dx.doi.org/ 10.1152/ajpregu.00371.2005. [DOI] [PubMed] [Google Scholar]
- 58. Li S, Wang Y, Matsumura K, Ballou LR, Morham SG, Blatteis CM. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2-/-, but not in cyclooxygenase-1-/- mice. Brain Res 1999; 825:86-94; PMID:10216176; http://dx.doi.org/ 10.1016/S0006-8993(99)01225-1. [DOI] [PubMed] [Google Scholar]
- 59. Schwartz JI, Chan CC, Mukhopadhyay S, McBride KJ, Jones TM, Adcock S, Moritz C, Hedges J, Grasing K, Dobratz D, et al. . Cyclooxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin Pharmacol Ther 1999; 65:653-60; PMID:10391671; http://dx.doi.org/ 10.1016/S0009-9236(99)90087-5. [DOI] [PubMed] [Google Scholar]
- 60. Kathula SK, Shah K, Polenakovik H, Koduri J. Cyclo-oxygenase II inhibitors in the treatment of neoplastic fever. Support Care Cancer 2003; 11:258-9; PMID:12673465. [DOI] [PubMed] [Google Scholar]


