Abstract
Fever is a complex signal of inflammatory and infectious diseases. It is generally initiated when peripherally produced endogenous pyrogens reach areas that surround the hypothalamus. These peripheral endogenous pyrogens are cytokines that are produced by leukocytes and other cells, the most known of which are interleukin-1β, tumor necrosis factor-α, and interleukin-6. Because of the capacity of these molecules to induce their own synthesis and the synthesis of other cytokines, they can also be synthesized in the central nervous system. However, these pyrogens are not the final mediators of the febrile response. These cytokines can induce the synthesis of cyclooxygenase-2, which produces prostaglandins. These prostanoids alter hypothalamic temperature control, leading to an increase in heat production, the conservation of heat, and ultimately fever. The effect of antipyretics is based on blocking prostaglandin synthesis. In this review, we discuss recent data on the importance of prostaglandins in the febrile response, and we show that some endogenous mediators can still induce the febrile response even when known antipyretics reduce the levels of prostaglandins in the central nervous system. These studies suggest that centrally produced mediators other than prostaglandins participate in the genesis of fever. Among the most studied central mediators of fever are corticotropin-releasing factor, endothelins, chemokines, endogenous opioids, and substance P, which are discussed herein. Additionally, recent evidence suggests that these different pathways of fever induction may be activated during different pathological conditions.
Keywords: chemokine, corticotropin-releasing factor, endogenous opioids, endothelin, fever, prostaglandin, substance P
Abbreviations
- AH/POA
anterior hypothalamus/preoptic area
- BAT
brown adipose tissue
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CINC-1
cytokine-induced neutrophil chemotactic factor-1
- COX-2
cyclooxygenase-2
- CRF
corticotropin-releasing factor
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptor
- EOp
endogenous opioid
- ET
endothelin
- HLI
heat loss index
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- 4-MAA
4-methylaminoantipyrine
- MCP
monocyte chemotactic protein
- MIP
macrophage-inflammatory protein
- mPGES1
microsomal prostaglandin E synthase-1
- NK
neurokinin
- NSAID
nonsteroidal antiinflammatory drug
- OVLT
organun vasculosum laminae terminalis
- nor-BNI
nor-binaltorphimine
- PAF
platelet-activating factor
- PFPF
preformed pyrogenic factor
- PG
prostaglandin
- Poly I:C
polyinosinic-polycytidylic acid
- RANKL
receptor activator of nuclear factor κB ligand
- RANTES
regulated on activation, normal T cells expressed and secreted
- SAL
saline
- SEM
standard error of the mean
- Tb
body temperature
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- Tsv
Tityus serrulatus venom
- Ucn
urocortin
- VMH
ventromedial hypothalamus
Introduction
Endothermic animals have complex mechanisms to maintain body temperature between narrow limits. However, under some conditions (e.g., during infectious diseases), adjustments in body temperature are necessary. The febrile response is a controlled increase in body temperature that is caused by elevation of the hypothalamic set point by endogenous mediators that are produced during such conditions.1,2 Specifically, the preoptic area in the anterior hypothalamus (AH/POA) controls thermoregulatory mechanisms that are responsible for normal temperature maintenance and changes that occur during the febrile response.3,4
The administration of lipopolysaccharide (LPS) from Gram-negative bacteria in laboratory animals is the classic model of fever induction. Therefore, most knowledge of fever-inducing mechanisms is associated with this pathogen-associated molecular pattern. Lipopolysaccharide stimulates Toll-like receptors (TLRs), specifically TLR4, in many cell types, including macrophages,5 adipocytes,6,7 Kupffer cells,8,9 and microglia,10,11 inducing the release of cytokines that deliver messages to the AH/POA to induce fever.1 However, other molecules, such as zymosan, imiquimod, ODN1668, and polyinosinic-polycytidylic acid (Poly I:C), can also stimulate other TLRs. Among the released cytokines that can induce fever, usually called endogenous pyrogens, are interleukin-1β (IL-1β), IL-1α, tumor necrosis factor-α (TNF-α), IL-6, CXCL8/IL-8, CXCL1/cytokine-induced neutrophil chemotactic factor-1 (CINC-1), macrophage-inflammatory protein-1α (MIP-1α), MIP-1β, interferon β (IFN-β), IFN-γ, and receptor activator of nuclear factor κB ligand (RANKL).1,3,12-15 Some of these cytokines, including CXCL8/IL-8, CXCL1/CINC-1, CCL3/MIP-1α, and RANKL, appear to be generated in the central nervous system.12-15 and are discussed herein, whereas others are generated in the periphery. In addition to cytokines, evidence also indicates that platelet-activating factor (PAF), a lipid mediator that is produced during inflammation, can induce fever. Steiner and Romanovsky showed that peripherally infused PAF was able to induce fever in rats.16 Some of these mediators clearly do not participate in the LPS-induced febrile response, such as CCL3.17 and PAF.16 Once these mediators are generated in peripheral sites, several hypotheses have been proposed to explain how they reach the AH/POA to cause fever.18-23 However, the participation of TLRs that are expressed in the central nervous system should also be acknowledged. Compelling evidence suggests the presence of some of these receptors (e.g.,, TLR2, TLR3, TLR4, TLR5, and TLR7) in the central nervous system.24,25 Nakano et al. recently reported an increase in the expression of TLR4 in astrocytes and microglia in circumventricular organs.26 Additionally, using chimeric mice that expressed TLR4 in the central nervous system resident cells versus in haematopoietic cells, the expression of this TLR in the central nervous system resident cells was shown to be necessary for sustaining the expression of inflammatory mediators in the brain.27 Several studies have also shown that microcultures of OVLT cells released TNF-α and IL-6 in response to LPS and Poly I:C.10,11,28 Furthermore, cells from these circumventricular organs respond to these cytokines. Wuchert et al. showed that IL-1β and TNF-α increased calcium transients in cells (i.e., astrocytes and neurons) from the area postrema.29 Neurons from the subfornical organ also responded to IL-1β, with depolarization and an increase in spike frequency.30 Altogether, these studies suggest that these TLR agonists can stimulate the synthesis of cytokines in the central nervous system, particularly in circumventricular organs, and the cytokines that are generated can stimulate these cells. Peripherally or centrally generated cytokines can induce other central mediators near the AH/POA, leading to changes in the firing of neurons that effectively activate heat production and conservation mechanisms, in addition to inhibiting heat loss.
The aim of the present review is to discuss the most known of these mediators that are generated in the central nervous system. We do not focus on cytokines that can be generated in both the periphery and central nervous system. We first discuss the importance of prostaglandins (PGs) in the febrile response that is induced by different exogenous and endogenous pyrogens and how different known antipyretic drugs affect the increase in this mediator in the hypothalamus. We also reexamine the current knowledge of the involvement of other central mediators of fever, such as corticotropin-releasing factor (CRF), endothelins (ETs), chemokines, endogenous opioids (EOps), and substance P.
Prostaglandins
In 1971, Milton and Wendlandt proposed that the antipyretic activity of nonsteroidal antiinflammatory drugs (NSAIDs) was related to their ability to inhibit the synthesis and release of PGs that are induced by endogenous pyrogens, in which the injection of several E-series PGs induced fever in cats and rabbits.31 They also reinforced these conclusions by showing that PGE1, when injected in the third ventricle in cats, induced a dose-related febrile response that was unaffected by acetaminophen. In the same year, Feldberg and Saxena showed that PGE1, when injected intracerebroventricularly, also induced fever in rats and rabbits, and high levels of PG were found in cerebrospinal fluid that was collected from the third ventricle in febrile cats.32 Later, radioimmunoassays were performed during the febrile response, revealing an increase in PGE2 levels in the brains of several species.33-35 Moreover, peripheral IL-1β administration in rats increased PGE2 levels in several hypothalamic areas, particularly in the organun vasculosum laminae terminalis (OVLT), one of the circumventricular organs that lacks a blood-brain barrier and is localized near the AH/POA.36,37
Another arachidonic acid metabolite that is involved in the genesis of the febrile response is PGF2α. This prostanoid also increases body temperature in rats and rabbits when administered intracerebroventricularly.38-40 An increase in the concentration of this PG was also observed in cerebrospinal fluid in rats that received peripheral endotoxin administration.41
However, the precise site of the production of PGs during the febrile response has been a matter of debate for many years. Katsuura et al.42 showed that the endogenous pyrogen IL-1β increased PGE2 levels in cultures of astrocyte cells from rats, suggesting that glial cells could be the source of these eicosanoids during fever. Later, immunoreactive cyclooxygenase-2 (COX-2), the key enzyme that is involved in the production of these eicosanoids, was identified in dendrites and cell bodies of neurons in several brain areas, particularly the paraventricular nucleus of the hypothalamus and AH/POA in naive animals.43 Recently, endothelial cells in the brain were identified as PGE2-producing cells that are critical for the progression of LPS-induced fever. Mice that had selective deletion of COX-2 and microsomal prostaglandin E synthase 1 (mPGES1; a sequential enzyme for PGE2 production after COX-2) in brain endothelial cells.44 and mice that expressed mPGES-1 only in haematopoietic cells did not develop fever in response to this pyrogenic stimulus.45
Since 1990, the OVLT and AH/POA have been considered the hypothalamic sites that are responsible for the production and action of PGs, respectively, during fever.36,37 However, another area that is sensitive to the action of these eicosanoids is the ventromedial hypothalamus (VMH).46 The VMH is an important site for the control of the autonomic nervous system and particularly brown adipose tissue (BAT) thermogenesis.47 Later, several authors confirmed that the effects of PGs on thermogenesis and increase in body temperature depend on the sympathetic activation of heat production in BAT, likely through the central stimulation of sympathetic flow through the solitary tract and VMH.48,49 The concentration of PGE2 in the POA/AH may also be subjected to endogenous antiinflammatory control mechanisms. For example, the febrile response that was induced by intravenous LPS administration in rabbits was reduced by the antiinflammatory cytokine IL-10, although no changes in TNF-α levels were observed, suggesting that IL-10 reduces fever by controlling the production of PGE2.50
However, COX-2 mRNA expression was not found in the POA/HA but rather in blood vessels and leptomeninges that surrounded this area, suggesting that this would be the site that is responsible for PG production during the febrile response.51,52 Later, Cao et al.18,53 showed that systemic IL-1β and TNF-α injections also induced COX-2 mRNA expression in blood vessels in rats.
Eskilsson et al. showed that mPGES-1 is expressed in the mouse brain during non-immune challenge conditions.45 Following immune stimulation, mPGES-1 expression increased only in brain endothelial cells that co-expressed COX-2.45 These results are in agreement with Gaetano et al.,54 who reported increases in COX-2 and mPGES-1 expression in the POA/AH following intravenous LPS injections in mice, whereas no other enzymes that are related to PG synthesis were altered. COX-2 and mPGES-1 knockout mice did not develop a febrile response after LPS injection.44,55,56 In rats, the basal expression of mPGES-1 in the brain is lower than in mice, but its expression increased after LPS or IL-1β injection, and mPGES-1 was co-expressed with COX-2.57,58 Similarly, Rummel et al.59 found that LPS injection into the air pouch increased mPGES-1 mRNA expression in the rat brain, and this induction was suppressed by anti-IL-6 antiserum.
Engstron et al. generated chimeric mice that expressed mPGES-1 in either haematopoietic cells or non-haematopoietic cells.60 Only animals with mPGES-1 expression in non-haematopoietic cells developed fever or an increase in PG concentration in cerebrospinal fluid after LPS administration. Animals with mPGES-1 expression only in haematopoietic cells exhibited elevated PGE2 levels in plasma, but they did not develop fever, suggesting that during fever PGE2 is formed in the brain.60 However, because the febrile response was not completely abolished in this study, the authors suggested that additional sources can synthesize PGs. In fact, Steiner et al. showed that peripherally derived PGE2 is also involved in LPS-induced fever. They constructed mouse chimeras and found that all 3 phases of the typical LPS-induced febrile response depended on TLR4 signaling. The first phase was triggered by TLR4 in haematopoietic cells. The second and third phases depended on TLR4 signaling in both haematopoietic and non-haematopoietic cells.61 These authors subsequently showed that PGE2 synthesis (phosphorylation of cytosolic phospholipase A2 and upregulation of COX-2) occurred in macrophages in the liver and lungs during the onset of fever.62 Moreover, the immune neutralization of plasma PGE2 delayed the initiation of fever.62 Consistent with these studies, Kupffer cell depletion prevented the increase in peripheral PGE2 and rise in body temperature that were induced by LPS in guinea pigs, suggesting that the peripheral generation of this eicosanoid by these cells is also important for fever.63
Recently, Eskilsson et al. generated mice with selective deletion of IL-6 receptor α in several cell types, and only the deletion of this receptor in brain endothelial cells attenuated the LPS-induced febrile response.64 Importantly, this deletion also attenuated the induction of COX-2 in the hypothalamus, suggesting that these cells are responsible for the generation of PGs during fever.64 Corroborating these findings, brain endothelial cells are PGE2-producing cells that are critical for the progression of LPS-induced fever. Mice with selective deletion of COX-2 and mPGES1 in brain endothelial cells.44 and mice that expressed mPGES-1 only in haematopoietic cells did not develop fever in response to this pyrogenic stimulus.45
PGE2 may activate 4 types of G-protein-coupled receptors: EP1, EP2, EP3, and EP4. However, only EP1, EP3, and EP4 have been identified in the AH/POA.65,66 In this region, EP3 receptors are found in many neuron cell bodies and dendrites. Although EP1 and EP4 mRNA expression has also been detected in the AH/POA,65 only EP3 receptor knockout mice did not develop fever in response to PGE2, IL-1β, or LPS.67,68 Moreover, EP3 receptor knockout in neurons in the median POA and medial POA also reduced the febrile response that was induced by PGE2 and LPS.66 The neural circuitry that is activated after EP3 receptor activation to induce fever has been reviewed previously.69,70
Several studies have also shown that selective COX-2 inhibitors act as antipyretics in rats.71-73 A selective COX-2 inhibitor also effectively reduced the LPS-induced febrile response in nonhuman primates, confirming the involvement of this enzyme and PGs in the progression of fever in this species.74
These eicosanoids have also been shown to be involved in the febrile response that is induced by other exogenous stimuli, such as Poly I:C,72 Staphylococcus aureus,73 and complex infectious conditions such as sepsis induced by cecal ligation and puncture.75 The participation of these eicosanoids has also been suggested after stimulation with the TLR7 agonist imiquimod and TLR9 agonist ODN1668, which increased the expression of enzymes that are involved in PGE2 synthesis in the rat hypothalamus.76,77
With regard to endogenous pyrogens, the febrile response that is induced by IL-1β, TNF-α, IFN-γ, IL-6,2,3 ciliary neurotrophic factor,78 IL-11,79 CCL5/RANTES (regulated on activation, normal T cells expressed and secreted),80 CXCL1/CINC-1,14 and CC chemokine ligand 22.81 depends on PG synthesis, in which the febrile response that is induced by these pyrogens is blocked by COX inhibitors. Conversely, the febrile response that is induced by some chemokines, such as CXCL8/IL-8.12 in rats and CCL3/MIP-1α in rabbits and rats, is not reduced by treatment with COX inhibitors, suggesting that these chemokines induce fever through mechanisms that are independent of PG synthesis.82,83 The role of these and other chemokines as central mediators of fever are discussed in more detail below.
We have also shown that low doses of the selective COX-2 inhibitor celecoxib or nonselective COX inhibitor indomethacin blocked the increase in PGE2 concentrations in cerebrospinal fluid after an injection of preformed pyrogenic factor (PFPF) and ET-1, with no antipyretic effect.84,85 These results suggest that there is not always a direct correlation between the febrile response and increases in PGE2 in the central nervous system. Despite the unquestionable evidence of the participation of these prostanoids in fever, other systems and/or central mediators may also contribute to the febrile response.
Corticotropin-Releasing Factor and Urocortin
CRF and urocortins (Ucns) comprise a family of peptides that are mainly involved in the stress response, but they are also important mediators of febrile responses. CRH, mainly synthesized in parvocellular cells in the paraventricular nucleus, is considered the most important hormone that is involved in the release of adrenocorticotropic hormone from the anterior pituitary gland. Once released, CRF acts on 2 types of G-protein-coupled receptors, CRF1 and CRF2.86 CRF binds with high affinity to CRF1 receptors but also has some affinity for CRF2 receptors. Conversely, Ucn1 binds to both receptors with similar affinity, whereas other Ucn family members (Ucn2 and Ucn3) preferentially act on CRF2 receptors.86,87 CRF1 receptors are widely distributed throughout the brain, whereas CRF2 receptors are preferentially expressed in the dorsal raphe, lateral septum, and hypothalamus.88
Early studies indicated that CRF injections in the third ventricle in rabbits reduced the febrile response that was induced by leukocyte pyrogen.89 According to a study by Opp et al.,90 CRF injections in rabbits attenuated the febrile response that was induced by IL-1β, suggesting an antipyretic action of this peptide. However, in the same study, the authors showed that intracerebroventricular injections of CRF increased brain temperature.90 Intracerebroventricular injections of the CRF receptor antagonist α-helical CRF9-41 reduced the febrile response that was induced by an intracerebroventricular injection of IL-1β but not by peripherally administered IL-1β, suggesting that the action of IL-1β is mediated by this CRF only when the cytokine is present in the central nervous system.91 Another rat study showed that intracerebroventricular injections of CRF significantly increased resting oxygen consumption and colonic temperature.92 The increase in body temperature that was induced by CRF injections in the central nervous system in rats was further confirmed in later studies.85,93-95 Subsequently, another study showed that both α-helical CRF9-41 and an antibody against CRF inhibited the increase in body temperature and BAT activity that was induced by an intracerebroventricular injection of IL-1β in rats but not fever that was induced by intracerebroventricular injections of TNF-α or PGE2 in rats.96 Confirming these results, the non-peptide CRF receptor antagonist CP 154,526 also blocked the febrile response that was induced by IL-1β in rats.97 Still unknown, however, is whether these different results are attributable to differential roles of CRF in different species (e.g., rats and rabbits) or whether CRF is involved in the febrile response that is induced only after the generation of IL-1β in the brain because all of the rat studies used this procedure.
The increase in body temperature that is induced by CRF occurs concomitantly with peripheral vasoconstriction, suggesting the activation of heat conservation mechanisms. Furthermore, we showed that the febrile response that was induced by CRF was also reduced by antalarmin, a CRF1 receptor antagonist, suggesting the involvement of this receptor in the febrile response that is induced by this peptide.94
Unexpectedly, CRF does not seem to be involved in the febrile response that is observed in zymosan-induced arthritis in rats,98 although IL-1β is believed to be one of the most important endogenous pyrogens that is involved in the response that is induced by this TLR agonist.98,99 However, Milton et al.100 showed that the febrile response that was induced by Poly I:C in rabbits was reduced by peripheral injections of a CRF antagonist or anti-CRH antibody but not by central injections. Altogether, these results suggest that CRF release is important for the febrile response that is induced by IL-1β and likely other exogenous pyrogens, such as Poly I:C, but still unknown is why some febrile responses that clearly involve the release of IL-1β are not modified by CRF receptor antagonists.
The febrile response that is induced by CRF also does not seem to involve subsequent PG synthesis because it was not blocked by the nonselective COX inhibitors ibuprofen or indomethacin or the COX2 inhibitor celecoxib.94,101 This peptide also does not seem to be released after PGE2 synthesis, in which the febrile response that was induced by an intracerebroventricular injection of PGE2 in rabbits was unaffected by the CRF receptor antagonist α-helical CRF9-41.91
The other member of this peptide family, Ucn1, increases body temperature in rats but not concomitantly with the activation of heat-conservation mechanisms (such as vasoconstriction characterized by a reduction of tail skin temperature in rats).94 Ucn1 induced a hyperthermic response that was characterized by an elevation in tail skin temperature, which seemed to occur independently of the synthesis or release of any mediators.94 Astressin, a CRF2 receptor antagonist, blocked Ucn1-induced hyperthermia.94 In another study, Ucn1 induced hyperthermia, which was blocked by α-helical CRF9-41 and antalarmin but not astressin 2B, suggesting the involvement of specifically CRF1 receptors.102 In contrast, Ucn2- and Ucn3-induced increases in body temperature were not modified by α-helical CRF9-41 or antalarmin, whereas astressin 2B was effective, suggesting that CRF2 receptors are involved in this response.102 The reason for this discrepancy is still unclear.
Endothelins
Endothelins are a family of peptides that consist of 3 isoforms and are potent vasoconstrictors of blood vessels in mammals.103 ET-1 was isolated and sequenced in 1988 from the supernatant of aortic endothelial cells. ET-2 and ET-3 were discovered after isolation of the ET-1 gene.103,104 ET-1 is formed as a pro-peptide (pre-proendothelin-1), which is then cleaved into big-endothelin-1, the circulating functionally inactive form of this peptide.105,106 Conversion enzymes, kinases, and metalloproteases cleave big-endothelin into ET-1, which contains 21 amino acids. The expression of endothelin receptors (ETA and ETB) is regulated in parallel with peptide expression. Both receptors are G-protein-coupled receptors.107,108 ETA receptors have greater affinity for ET-1 and ET-2 and lower affinity for ET-3, whereas ETB receptors have similar affinity for all isoforms.109
In 1998, a study showed that central injections of ET-1 increased body temperature in rats.110 A subsequent study showed that ET-1 injections induced a proper febrile response, in which heat-conservation mechanisms (e.g.,, tail vasoconstriction) were activated after the injections.84 Furthermore, ET-1 injections in the AH/POA but not intravenous injections also induced fever,84,85 suggesting that ET-1 that is synthesized in the brain is important for the febrile response.
Several stimuli, including inflammatory stimuli, can induce the production of ET-1. Such inflammatory stimuli include endotoxins, IL-1β, TNF-α, and transforming growth factor-β.111 ET-1 is primarily produced by endothelial cells, but other cell types (e.g., smooth muscle cells, macrophages, Kupffer cells, and nervous cells) are also potential sources of this peptide.111 During the LPS-induced febrile response, the levels of immunoreactive big-endothelin-1 become undetectable.84 Additionally, the levels of ET-1 in cerebrospinal fluid, which were undetectable in vehicle-treated animals, reached approximately 22 fmol/ml after an injection of LPS.84
The selective ETB receptor antagonist BQ788, when injected in the third ventricle in rats, blocked the LPS-induced febrile response, whereas the ETA receptor antagonist BQ123 was ineffective, suggesting that ET-1 acted on ETB receptors.110 Surprisingly, the febrile response that was induced by central injections of IL-1β and TNF-α (2 of the main cytokines that are involved in the febrile response that is induced by LPS) was unaffected by BQ788 treatment.110 Intracerebroventricular injections of ET-1 increased PGE2 levels in the hypothalamus.85,112 Both the febrile response and the increase in PG levels that were induced by ET-1 in the hypothalamus were reduced by an IL-1 receptor antagonist, suggesting that IL-1β is released after ET-1 to induce fever.85 However, although treatment with the nonselective COX inhibitor indomethacin reduced PG levels, the febrile response that was induced by ET-1 remained unaffected.110, 112 Altogether, these results confirm that ET-1 is one of the central mediators of the febrile response that is induced by LPS. ET-1 appears to act on ETB receptors, activating the febrile response that depends on IL-1β synthesis and occurs independently of PG synthesis.
Although PGs do not appear to be involved in the febrile response that is induced by ET-1, other mediators may participate in this response. For example, the μ opioid receptor-selective antagonist CTAP reversed the febrile response that was induced by ET-1, suggesting that EOps that act on μ-opioid receptors may be released after ET-1 injection to induce fever.95 Other central mediators, such as the chemokine CCL3/MIP-1α (discussed below), also do not appear to be involved downstream of the release of ET-1.17
Recently, Kanashiro et al.98 showed that ET-1, in contrast to LPS, did not participate in the febrile response that was observed in zymosan-induced arthritis. Bastos-Pereira et al.72 showed that ET-1 did not participate in the febrile response that was induced by Poly I:C. Therefore, although ET-1 is an important central mediator of the febrile response that is induced by LPS from Gram-negative bacteria, it does not participate in the febrile response that is induced by any other stimulus.
Chemokines
Chemokines are a family of structurally related proteins (8-15 kDa) that play a pivotal role in inflammation, sharing the ability to activate specific subsets of leukocytes and induce cell migration.113,114 Chemokines have been subdivided into 4 subfamilies by considering the position of either one or 2 conserved cysteine (C) residues that are located near the N-terminus of the protein.114,115 The CC family (β-chemokines), CXC family (α-chemokines), and CX3C family have 4 cysteine residues, with zero, one, and 3 amino acids, respectively, that separate the first 2 cysteine residues. C chemokines have only the second and fourth cysteine residues that are found in other chemokines. The C family includes lymphotactin/XCL1. The CC family includes CCL3/MIP-1α, CCL4/MIP-1β, CCL2/monocyte chemotactic protein (MCP)-1, CCL8/MCP-2, CCL7/MCP-3, CCL5/RANTES,116 and CCL11/eotaxin, among others. The CXC family includes CXCL-8/IL-8, CXCL1/CINC-1, CXCL-2/MIP-2, and CXCL-7/neutrophil activating peptide-2, among others. The CX3C family includes CX3CL1/fraktalkine.117 CC chemokines, such as CCL3/MIP-1α, are attractants of mononuclear cells/macrophages and eosinophils. The C family is selective for lymphocytes, and CX3C chemokines act on diverse cell types, including monocytes, T cells, and natural killer cells.118,119
The role of chemokines in the febrile response was first demonstrated by the ability of MIP-1 (a CC family member) to induce fever in rabbits and rats.82,83 Sherry et al. (1988) showed that MIP-1 had 2 components: MIP-1α and MIP-1β, referred to as CCL3 and CCL4.120 Although structurally similar, CCL3 and CCL4 have different signaling capabilities and different inflammatory activities in vitro and in vivo.121 Both CCL3 and CCL4 induced febrile responses that were insensitive to the action of indomethacin and dexamethasone in rats,83,122,123 suggesting a direct action in the AH/POA, regardless of PG synthesis. CCL3 increased PGE2 in cerebrospinal fluid, which was blocked by indomethacin, but the febrile response remained intact.123 However, CCL3 was also shown to not belong to endogenous mediators that orchestrate the febrile response that is induced by endotoxin. For example, an anti-CCL3 antibody did not alter endotoxin-induced fever but was blocked by the CRF antagonist α-helical CRF9-41.17 Fever that is induced by CCL4 also occurs independently of PG synthesis, but unlike what was found for CCL3, an anti-CCL4 antibody that was injected in the AH/APO reduced LPS-induced fever, indicating that this chemokine is an endogenous mediator of the pyrogenic cascade that occurs during LPS-induced fever in rats.124,125 One possibility is that CCL3 participates in the febrile response that is induced by living infectious agents or pathogen-associated molecular patterns that are different from LPS. For example, fever that was induced by replicating Staphylococcus aureus was inhibited by central injections of anti-CCL3 antibodies (Fig. 1). Interestingly, increases in CCL3 expression after S. aureus infection in the mouse brain and human osteoblasts cultures were reported.126,127
Figure 1.
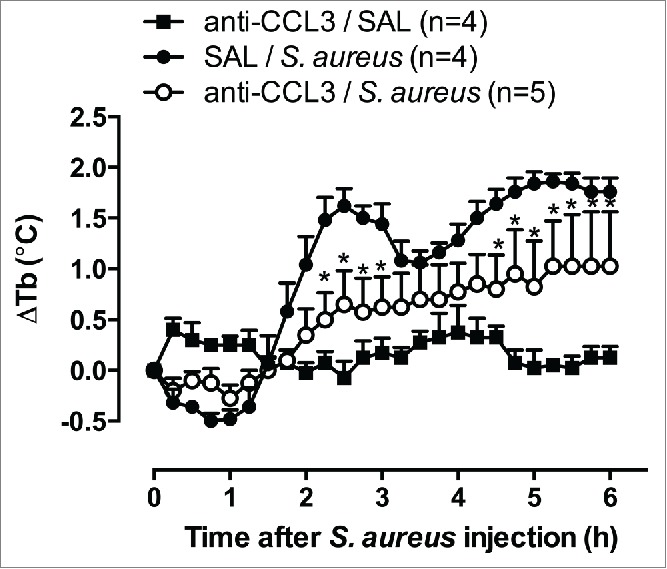
Effect of anti-CCL3 antibody on the febrile response evoked by S. aureus. The anti-CCL3 antibody was injected intracerebroventricularly at a dose of 10 ng, both 15 min and 1 h before an intraperitoneal challenge (time 0 h) with 1010 CFU of S. aureus. Body temperature (Tb) was measured with a battery-operated biotelemetry transmitter in male Wistar rats, weighing 200 g. The data were analyzed using 2-way repeated-measures analysis of variance, followed by the Bonferroni post hoc test. The data are expressed as the mean ± SEM of the change in Tb (ΔTb, °C) for each treatment, and n indicates the number of animals in each group. *p < 0.05, compared with saline (SAL)/S. aureus group. (Martins JM , Soares DM, Souza GEP, unpublished results).
CCL5/RANTES, another member of the CC family, acts on CC chemokine receptor 1 (CCR1), CCR3, and CCR5. CCL5/RANTES is involved in LPS-induced fever in rats.80 Injections of this chemokine in the AH/POA in rats promoted an integrated febrile response that was accompanied by concomitant peripheral vasoconstriction. However, in contrast to CCL3 and CCL4, fever that was induced by CCL5 was accompanied by an increase in PGE2 concentration in cerebrospinal fluid, and both the febrile response and elevation of prostanoid levels in cerebrospinal fluid were prevented by pretreatment with both nonselective and selective cyclooxygenase inhibitors, including indomethacin.80,128 Machado et al..80 found that injections of Met-RANTES, a CCR1 and CCR5 receptors antagonist, inhibited LPS-induced fever indicating that these receptors may also be involved in this response. CCL5 also plays a major role in the febrile response that is induced by S. aureus infection.73
Boddeke et al. showed that cells from the POA that were responsive to CCL5 did not respond to CCL2/MCP-1 and vice versa.129 This implies that CCL2 activates CCR2, whereas CCL5 stimulates CCR1 and/or CCR5 in distinct subpopulations of microglia. In this context, we investigated whether CCL2, similarly to CCL5, is able to induce fever when injected in rats. An intracerebroventricular injection of this chemokine did not alter basal temperature, although it induced the migration of immune cells to the peritoneal cavity in another group of rats after intraperitoneal CCL2 injection (Fig. 2). One speculation is that neither CCR2 nor the CCR2 agonist CCL2 is involved in the central mediation of fever.
Figure 2.
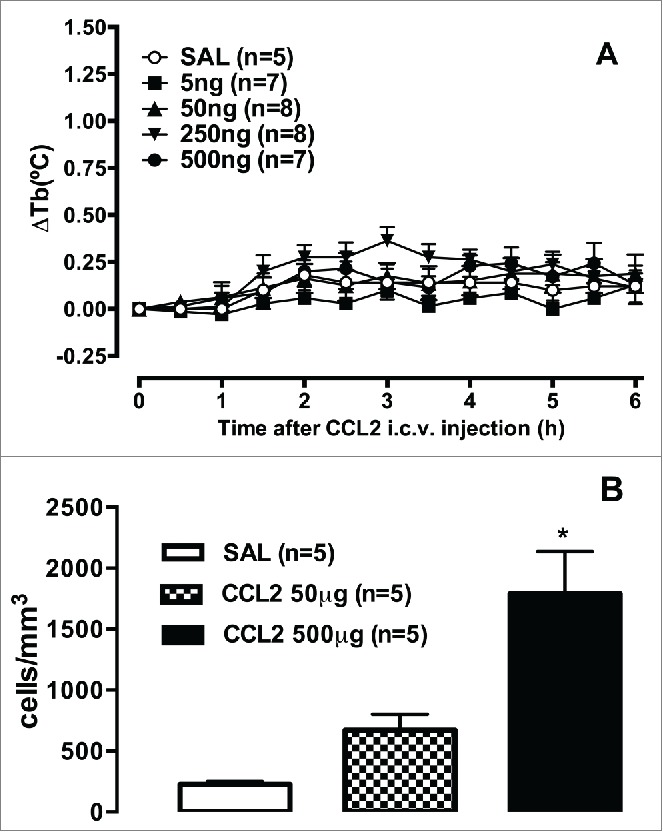
CCL2 does not increase rectal temperature but induces cell migration in rats. (A) Male Wistar rats received an intracerebroventricular injection of 1 μl of saline (SAL) or CCL2 (5, 50, 250, and 500 ng), and body temperature (Tb) was measured by a tele-thermistor probe coupled to a thermometer for 6 h. (B) Male Wistar rats were euthanized 6 h after an intraperitoneal injection of 50 or 500 μg CCL2, and the peritoneal content was collected. Cells from the peritoneal cavity were collected by an injection of 10 ml phosphate-buffered saline. The abdomens were gently massaged, and a blood-free cell suspension was carefully withdrawn with a syringe. The abdominal lavage was placed in plastic tubes, and total cell counts were immediately performed in a Neubauer chamber. The data were analyzed using one way or 2-way repeated-measures analysis of variance, followed by the Bonferroni post hoc test. The data are expressed as the mean ± SEM of the changes in body temperature (A, ΔTb, °C) or number of cells per mm3 (B), and n indicates the number of animals in each group. *p < 0.05, compared with the control group (Soares DM and Souza GEP, unpublished results).
Corroborating these data, Tavares and Miñano.130 showed that microinjections of a neutralizing antibody against CCR5 in the POA did not affect the increase in body temperature that was induced by CCL4. However, pretreatment with the same dose of anti-CCR5 antibody suppressed the febrile response that was induced by CCL5 that was administered in the same region. Thus, these authors demonstrated that hypothalamic CCR5 is functionally involved in fever that is induced by CCL5 but not CCL4. The latter has been suggested to stimulate CCR1. Therefore, although CCL4 and CCL5 are structurally and functionally similar, each has distinct characteristics that enable them to independently regulate specific aspects of the inflammatory response in the host.
Recently, the pyrogenic activity of another member of the CC chemokine family was demonstrated. CCL22 is a selective, high-affinity ligand of CCR4. In 2011, Osborn et al.81 found that an injection of CCL22 in the AH/POA increased core body temperature in mice, and this effect was mediated by the stimulation of BAT thermogenesis also in a PGE2-dependent pathway. In parallel, these authors also found that transcripts that encoded CCL22 and CCR4 were present in the AH/POA. The authors concluded that neurons in the AH/POA express functional CCR4, which in turn respond to CCL22 with an increase in thermogenesis, thus providing an important link between neuroinflammation and thermoregulation.81
Altogether, these studies suggest that the CC chemokine family and its receptors are extensively involved in the genesis of the febrile response, activating both PG-dependent and -independent mechanisms. Chemokines are well known to be promiscuous in the relationship to the receptors they activate. Therefore, the activation of PG-dependent and -independent febrile responses may be related to these receptors. However, unless specific receptor agonists and antagonists are available and tested, this issue will remain unsolved.
Some members of the CXC chemokine family also appear to exert pyrogenic effects. Rothwell et al.131 were the first to demonstrate the ability of CXCL8/IL-8 to induce fever. These authors also showed that the febrile response that was induced by this chemokine was not reduced by the nonselective COX inhibitor ibuprofen. Subsequently, we found that intracerebroventricular CXCL8 injections dose-dependently increased rectal temperature in rats.12 Indomethacin, at doses that reduced fever that was induced by an endotoxin from E. coli or by IL-1β, did not reduce the pyrogenic response to CXCL8, suggesting that the febrile response that is induced by this chemokine is not mediated by PGs in that species. However, differently from rats, indomethacin blocked the febrile response that was induced by central or peripheral administration of CXCL8 in rabbits.132 In humans, both CXCL8 and its 2 receptors, CXC receptor 1 (CXCR1) and CXCR2, are expressed, whereas in rats, no CXCL8 ortholog is expressed, but CXCR2 is found. However, rats express CXCR2 and relevant CXCR2 ligands, such as CXCL1/CINC-1 and CXCL2 (MIP-2), which are functional analogs of CXCL8 with regard to neutrophil recruitment and activation.133 Our group evaluated the pyrogenic activity of CXCL1. CXCL1 injection in the AH/POA induced a febrile response that was effectively blocked by indomethacin and ibuprofen, suggesting that the febrile response that is induced by this chemokine depends on PGE2 generation.14 Supposing that CXCL8 and CXCL1 share the same receptor for signal transduction in rats, one possibility is that these chemokines share the same intracellular pathways upon binding to these receptors, culminating in the synthesis or release of the same array of mediators. This is true with regard to the hyperalgesic effects of both chemokines in rats. Indomethacin does not affect this response, whereas the β-adrenoceptor antagonist atenolol markedly attenuates it.134,135 However, with regard to fever, there is a clear difference between the febrile responses that are induced by CXCL1 and CXCL8.
In rats, CXCR2 has another typical ligand. CXCL2/MIP-2 appears to play an important peripheral role in regulating the mechanisms that initiate fever in both immunocompetent and leukopenic rats.136 In this study, the febrile response that was induced by LPS was accompanied by a pronounced increase in serum CXCL2 concentrations. Systemic administration of anti-rat CXCL2 antibody significantly attenuated the early phase of LPS-induced fever. Unfortunately, there are no data on the involvement of PGs in the febrile response that is induced by CXCL2.
Table 1 summarizes the findings of the studies discussed above and the importance of chemokines as central mediators of fever. Studies that utilized different stimuli to induce the febrile response suggest the key participation of some chemokines among fever mediators in the central nervous system. For example, both mRNA and protein levels of such chemokines as CXCL10, CCL2, CCL5, CCL3, and CXCL1 increased in mouse brains that were infected with West Nile Virus,137 and these animals presented intense fever. Moreover, much evidence suggests a role for chemokines as biomarkers in different fever-related diseases, including Kawasaki disease.138 (i.e., an acute febrile vasculitis) and dengue hemorrhagic fever.139 CXCL12 may also be involved in the febrile response that is induced by the human immunodeficiency virus-1 envelope glycoprotein gp120.140 In Ebola hemorrhagic fever patients, CCL2, CCL3, CXCL1, and CXCL8 levels were all elevated when the prognosis indicated a fatal outcome.141 Much is already known, but further studies are clearly needed to improve the knowledge of the effects of chemokines on fever. Novel information regarding mediation of the febrile response will assist in improving disease diagnosis and providing safer treatments of fever symptoms that are associated with these diseases.
Table 1.
Chemokines and their putative receptors involved in mediation of febrile responses. Summary of the chemokines and the chemokine receptors among mediators involved on the mediation of febrile response. The table shows studies performed with different species, different sites of testing of chemokine receptor agonists or antagonists, if there is involvement or not of PGs, and if the agonist action on brain results in physiological consequences which can be characteristic of integrated febrile response.
| Chemokine | Receptor involved | Species | Site of testing | Involvement of PGs | Physiological consequences | Contribution |
|---|---|---|---|---|---|---|
| CCL3 | CCR1, CCR5 | Rats | Lateral ventricle, AH/POA | No | Fever, HLI decrease | Refs. 17, 123 |
| CCL4 | CCCR1 | AH/POA | No | Fever | Ref. 130 | |
| CCL5 | CCR1, CCR5 | Rats | AH/POA AH/POA | Yes Yes | HLI decrease | Refs. 80, 128 |
| CCL22 | CCR4 | Mice | AH/POA | Yes | ↑BAT activation ↓ respiratory exchange | Ref. 81 |
| CXCL8 | CXCR1, CXCR2 | Rabitts Rats | Intravenous, i.c.v. | Yes No | Fever | Refs. 12, 132 |
| CXCL1 | CXCR2 | Rats | i.c.v., AH/POA | Yes | Ref. 8 | |
| CXCL2 | CXCR2 | Rats | intravenous | — | Initiates LPS fever? | Ref. 14 |
| CXCL10 | Humans | Not investigated | ? | ↑ in periodic fever, swine fever virus, Kawasaki disease, dengue | Refs. 138, 139 | |
| CXCL12 | CXCR4 | Rats | Antagonist at AH/POA ↓HIV fever | ? | Ref. 140 | |
| CCL2 | CCR3 ? CCR4 ? | i.c.v. | — | No fever | Fig. 2, present review. |
Abbreviations: AH/POA, anterior hypothalamus/pre-optic area; BAT, brown adipose tissue; HLI, heat loss index, i.c.v., intracerebroventricular; PG, prostaglandins.
Endogenous opioids
Endogenous opioids act on 3 distinct receptors (μ, κ, and δ),142-144 and they are present in the AH/POA.145 μ-Opioid receptors are also found in the ventromedial POA,145 an area that is also activated during the febrile response that is induced by LPS and PGE2.146,147
The involvement of EOps in thermoregulation and fever has been extensively studied. The activation of μ-opioid receptors by selective agonists produces hyperthermia,148-151 whereas hypothermia is observed after the activation of κ-opioid receptors.151,152 With regard to the role of δ-opioid receptors in thermoregulation, the available information is contradictory.151,153,154 Recent studies have suggested that the activation of these receptors causes hypothermia.155,156 Conversely, EOps do not appear to be involved in maintaining normal body temperature. Central injections of CTAP, a μ-opioid receptor antagonist, or nor-binaltorphimine (nor-BNI), a κ-opioid receptor antagonist, did not modify normal body temperature, at least at low doses.95,157 At higher doses, there seems to be a tonic balance between μ and κ opioid receptors, in which CTAP induces hypothermia and nor-BNI induces hyperthermia, and these effects can be blocked by nor-BNI and CTAP, respectively.157,158
In 2008, Fraga et al.95 showed that intracerebroventricular or intra-hypothalamus injections of the μ-opioid agonist morphine increased body temperature concomitantly with peripheral vasoconstriction, suggesting the activation of heat-conservation mechanisms and indicating that this response is actually a febrile response. Additionally, both nonspecific and μ-opioid receptor-specific agonists increased the activity of hypothalamic cold-sensitive neurons and reduced the activity of warm-sensitive neurons.159,160
Previous studies have shown that EOps are involved in the febrile response that is induced by exogenous pyrogens. During the LPS-induced febrile response, μ-opioid receptors appear to be activated. Naloxone and CTAP, nonspecific and μ-opioid receptor-specific antagonists, respectively, blocked the febrile response.161,162 Additionally, μ-opioid receptor knockout mice did not develop an LPS-induced febrile response.163 These results suggest that μ-opioid receptors are involved in the LPS-induced febrile response. In addition to their role in the central nervous system, peripherally generated opioids may play a role in the febrile response that is induced by LPS in guinea pigs.164
The administration of selective opioid receptor antagonists reduced the febrile response that was induced by TNF-α, IL-6, and CCL4/MIP-1β, but they did not modify the febrile response that was induced by IL-1β in guinea pigs and rats.150,161,162 Other studies showed that IL-1-induced fever was blocked by buprenorphine,165 which has been reported to have high affinity for μ-, κ-, and δ-opioid receptors, with Ki values in the nanomolar range.166 The initial phase of interferon-α-induced fever was also reduced by the nonspecific opioid antagonist naloxone in rats.167 The febrile response that was induced by PGF2α, CRF, and ET-1 but not fever that was induced by PGE2 was also attenuated by μ-opioid receptor blockade.95 Furthermore, indomethacin did not change the febrile response that was induced by morphine, and μ-opioid receptor blockade by CTAP did not modify the increase in PGE2 levels in cerebrospinal fluid or increase in COX-2 expression in the hypothalamus after LPS injection, suggesting that the release of endogenous opioids occurs concomitantly with or after PG synthesis.95
Endogenous opioids are not only involved in the febrile response that is induced by LPS from Gram-negative bacteria; they are also involved in fever that is induced by other exogenous pyrogens of viral origin, such as Poly I:C.72 and glycoprotein gp-120 from human immunodeficiency virus-1.168 To our knowledge, no data are available on the participation of opioids in fever of fungal origin.
Substance P
Substance P is a polypeptide that contains 11 amino acids (Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gli-Leu-Met-NH2) and is more frequently found in primary afferent A fibers, C fibers, and capsaicin-sensitive fibers.169 However, the presence of substance P in the brain has also been demonstrated, including in rats, humans, and monkeys.170 Some rodent studies showed that substance P can also be formed by macrophages, eosinophils, lymphocytes, and dendritic cells, among others.171-173 Its effects are mediated almost exclusively by metabotropic neurokinin-1 (NK1) receptors. NK1 receptors are expressed in several brain structures, including the putamen, caudate nucleus, and hypothalamus, and in the dorsal root ganglion, intestinal intrinsic neurons, macrophages, neutrophils, lymphocytes, and mast cells.172,174-178 Additionally, some in vitro studies have shown that substance P induces the release of cytokines from rodent macrophages and granulocytes, which are key elements of the amplification of inflammatory and febrile responses.179-181 Lipopolysaccharide can also increase NK1 receptor expression in some of these cells.171
There is high expression of substance P receptors in the rat hypothalamus, a region that is critically involved in temperature control and fever responses.182 Substance P and its precursor preprotachykinin A have also been found in the hypothalamus in primates and rats.170,183 Unclear, however, is which cell type is responsible for the formation and release of substance P. Also unclear is where this neuropeptide, once released, acts in the hypothalamus. However, the presence of preprotachykinin A and NK1 receptors in this region is an important observation.
In 1982, Rewerski et al.184 reported that substance P injections in both the hypothalamus and lateral ventricle in rats did not alter body temperature in animals. However, a few years later, other studies showed that substance P actually induces fever and may be involved in the LPS-induced febrile response.185,186 Additionally, an intrapreoptic injection of substance P induced prostaglandin-dependent fever.185 These authors' results concerning the firing rate of warm-sensitive neurons in the anterior hypothalamus in rats did not support their results, since substance P increased the firing rate of warm-sensitive neurons while IL-1β decreased this activity. The discrepancy among these studies may be related to the metabolism of bolus injections of substance P by endopeptidases, including angiotensin-converting enzyme. We also observed variations in the response in animals after substance P injections, and the consistent induction of fever was only observed when the animals were treated with the angiotensin-converting enzyme inhibitor captopril.187
The ability of LPS to induce the release of substance P in the hypothalamus has not been convincingly demonstrated, but LPS has been shown to induce the synthesis and release of substance P both peripherally.188,189 and in the spinal cord.190
Blatteis et al.185 showed that an analog of substance P with broad receptor antagonist activity, when injected in the AH/POA, blocked the febrile response that was induced by LPS in guinea pigs. The participation of substance P in the LPS-induced febrile response was further confirmed by Szelenyi et al.186 In this study, the febrile response that was induced by substance P was observed in restrained female rats that had been maintained at an ambient temperature of 4°C for 3 weeks, which is a condition that is far from normal. The LPS-induced febrile response was blocked by an intracerebroventricular injection of the same substance P receptor antagonist.186 These studies indicate that centrally released substance P may be important for the febrile response. More recently, our group confirmed the participation of substance P and an NK1 receptor antagonist in the LPS-induced febrile response using a more specific antagonist, SR140333. In this study, a peripheral injection of SR140333, which does not cross the blood-brain barrier, did not change the LPS-induced febrile response, whereas intracerebroventricular administration reduced this response.187 However, substance P does not appear to be involved in the febrile response that is induced by IL-1β or CCL-3, 2 important pyrogens that induce fever through PG-dependent and -independent mechanisms, respectively.187 Therefore, substance P might be released after other mediators are released during LPS-induced fever, but further studies are necessary to clarify this issue. Interestingly, in contrast to LPS-induced fever, substance P does not appear to be involved in the febrile response that is induced by the viral mimetic Poly I:C.72
Antipyretics With Mechanisms Unrelated to Cyclooxygenase Inhibition
The studies presented above suggest that during fever, 2 pathways run in parallel. One of the pathways is indomethacin-sensitive and requires peripheral TNF-α, IL-1β, IL-6, and some chemokines (e.g.,, CCL5, CXCL1, and CCL22) that trigger the synthesis of PGE2, PGF2α, and CRF in the brain. The other pathway is PG-independent, instead depending on the activation of a central endothelinergic system via ETB receptors that is positioned downstream of PFPF and CRF or is activated through the release of chemokines, such as CCL3, CCL4, and CXCL8 (Fig. 3). The ability of IL-1β, IL-6, and PGF2α to induce the central release of CRF.85,92 offers the possibility of the integration of both pathways.
Figure 3.
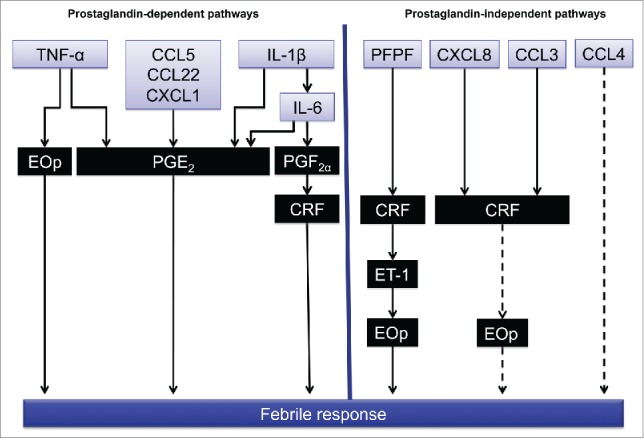
Proposed main central pathways that are activated during fever. The left side shows the prostaglandin-dependent pathways that are activated by TNF-α, CCL5, CXCL1, IL-1β, and IL-6. The right side shows the prostaglandin-independent pathways that are activated by PFPF, CXCL8, CCL3, and CCL4. CRF release may be a key mediator of the integration of both pathways directly or through the release of ET-1 and endogenous opioids (EOp). Dashed arrows represent pathways that have not been thoroughly investigated. Substance P was omitted because its role in these pathways has not yet been elucidated.
Most antipyretics are known to inhibit COX-derived PGE2 to promote its actions, but uncertain is how antipyretics act on the PG-independent pathway to reduce fever that is induced by these mediators. The antipyretic effect of classic NSAIDs, such as aspirin, ibuprofen, indomethacin, and coxibs (COX-2 inhibitors), has been attributed to COX-1 and/or COX-2 inhibition and the subsequent reduction of PG generation.191,192 Nevertheless, the doses of these drugs that are required to block fever that is induced by LPS appear to be higher than those that are necessary to block PG synthesis. This observation seems to support the notion that the antipyretic effects of such drugs are produced through COX-independent mechanisms.71,101 Thus, ibuprofen, aspirin, and salicylate at higher concentrations than those that are required to block COX can inhibit the activation and translocation of transcription factors, such as nuclear factor-κB, and consequently down regulate proinflammatory cytokines.193-195 Ibuprofen was shown to attenuate (i) the recruitment/activation of myeloperoxidase-expressing cells in the liver, (ii) the increase in hepatic COX-2 protein, and (iii) the increase in serum TNF-α in mice that were challenged with the anti-Fas antibody Jo2.196 Moreover, the release of arginine vasopressin from the ventral septal area appears to be involved in the antipyretic mechanism of action of indomethacin and salicylates, in which a V1 receptor antagonist that was injected in the ventral septal area blocked antipyresis that was promoted by these NSAIDs.197-199 In rats, indomethacin antipyresis is accompanied by an increase in arginine vasopressin levels in ventral septal area perfusion fluid.197,199,200 Arginine vasopressin has been reported to induce antipyresis by stimulating septal neurons via V1 receptors rather than by V2 receptors.201 This stimulation is transmitted via septofugal fibers to hypothalamic thermoregulatory structures, leading to the neutralization of neuronal activity that is triggered by endogenous pyrogens.201,202
Our group also found that ibuprofen not only reduced fever that was induced by PGE2-dependent endogenous pyrogens (i.e., IL-1α, TNF-α, and IL-6), similarly to indomethacin, but also reduced fever that was induced by PG-independent endogenous pyrogens (i.e., ET-1 and PFPF). Additionally, similar to indomethacin, antipyresis that was promoted by ibuprofen was antagonized by a V1 receptor antagonist.101 Thus, one possibility is that the effectiveness of ibuprofen in reducing fever that is induced by PFPF, ET-1,101 and CCL3,123 all known PG-independent pyrogens, could be related to arginine vasopressin release.
Diflunisal is an antiinflammatory and analgesic salicylate derivative that is not metabolized to salicylic acid, has less of an effect than aspirin on platelet function in vivo,203,204 and does not cross the human blood-brain barrier (it is not found in cerebrospinal fluid in humans),205 all of which should be related to the absence of antipyretic activity, at least in humans.
Acetaminophen, also called paracetamol, is one of the world's most frequently used drugs to treat fever and pain. Like aspirin and other NSAIDs, acetaminophen exerts analgesic and antipyretic effects through COX-2 inhibition, without affecting mPGES-1 activity or signaling cascades downstream of PGE2.206 Moreover, in contrast to indomethacin and ibuprofen but similarly to dipyrone, acetaminophen does not depend on arginine vasopressin to exert its antipyretic effect.198,199
Also important is the effect of acetaminophen on Tityus serrulatus venom (Tsv)-induced fever. Tsv induces fever in the absence of PG synthesis in the hypothalamus and cerebrospinal fluid207 and appears to depend on kinins (via B1 and B2 receptors), IL-1β, nitric oxide, and vagal neurotransmission, which might change the fire rate of thermoregulatory centers.116 Like dipyrone,207 acetaminophen also abolished PG-independent fever that was induced by ET-1 and Tsv (Fig. 4), suggesting that in addition to blocking COX-2,206 other mechanisms may be involved in its antipyretic effect.
Figure 4.
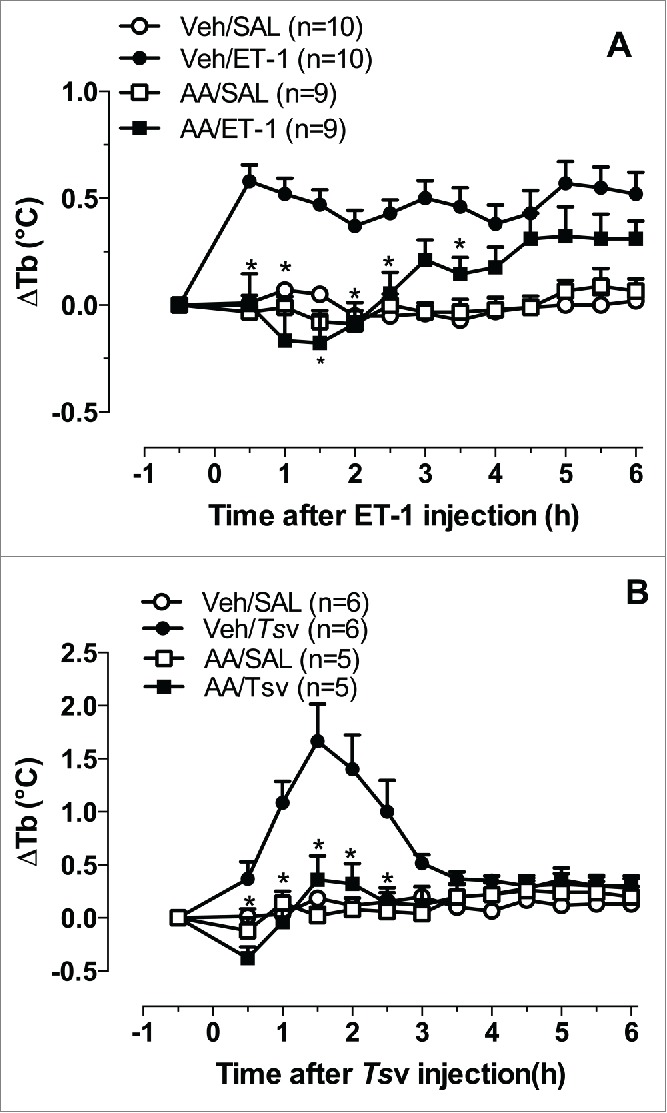
Effects of acetaminophen on the febrile response evoked by ET-1 and Tsv. Acetaminophen (230 mg/kg, p.o.) or vehicle (Veh; 10% ethanol in saline plus 2 µl of Tween 80) was administered 30 min before an intracerebroventricular injection of ET-1 (1 pmol, A) or by an intraperitoneal injection of Tsv (150 µg/kg, B), or the same volume of saline (SAL). Body temperature (Tb) was measured by a tele-thermistor probe coupled to a thermometer for 6 h in male Wistar rats, weighing 200 g. The data were analyzed using 2-way repeated-measures analysis of variance followed by the Bonferroni post hoc test. The data are expressed as the mean ± SEM of the change in for each treatment Tb (ΔTb, °C), and n indicates the number of animals in each group. *p < 0.05, compared with Veh/ET-1 or Veh/Tsv group (De Moraes LA, Melo MCC, Souza GEP, unpublished results).
To state that an antipyretic has additional effects may be questionable because Tsv does not induce or depend on PGE2 synthesis to promote fever. Rats that were treated with dipyrone exhibited a dramatic reduction of PGE2 concentrations in cerebrospinal fluid in response to LPS, with no changes in the hypothalamic content of this prostanoid.112 This finding was related to the low concentration of the main antipyretic metabolite 4-methylaminoantipyrine (4-MAA), after dipyrone treatment in this brain region since animals treated (i.p.) with 4-MAA allowed a double amount of this metabolite in the hypothalamic tissue and a parallel reduction of fever and hypothalamic PGE2 content.207 These findings suggest that an antipyretic drug does not necessarily need to reduce hypothalamic PGE2 to exert its antipyretic effect, supporting the notion that brain endothelial cells are important for PGE2 production during fever.44 or that some antipyretic drugs act outside the brain to block the peripheral synthesis of pyrogenic mediators, including PGE2.61 Another possibility is that the synthesis or effect of other central mediators beyond PGE2 should also be affected by either dipyrone or paracetamol. We are currently performing studies in our laboratory to investigate these issues.
About the Authors
Dr. Glória EP Souza (right photo) published her first study on fever, co-authored with Dr. IR Pelá, in 1992 and since then has been working in the field. She was the PhD supervisor for both Dr. Aleksander R Zampronio (in 1998; left photo) and Dr. Denis M Soares (in 2008; middle photo), who are both still working in the field. Working in 3 different universities in Brazil with collaborations from other countries, the main focus of this group has been to study the mechanisms and mediators that are involved in the febrile response, with a focus on chemokines and other central mediators of fever and the effects of several antipyretic drugs.



Concluding Remarks
The studies reviewed herein reinforce the importance of PG synthesis in the central nervous system to induce fever and clearly show that other central mediators are important in this process. These different pathways are presented in Figure 3. Importantly, different stimuli may activate different pathways. The importance of these pathways may differ under different conditions, such as in fever that is observed after an injection of live E. coli.208 or S. aureus 73 or fever of viral origin.72 Additionally, the ability to generate PGs in the central nervous system is not sufficient to imply the involvement of these mediators in fever. The observations that animals can present a febrile response even when PG levels in the central nervous system are low indicate that other mediators are involved in fever. The febrile response needs to be considered as an integrated response that involves the activation of systems related to heat production and conservation and the inhibition of heat loss. Unknown is whether these central mediators differentially affect these systems. Understanding the ways in which these different mediators act, the cell types or brain regions where these receptors are expressed, and the second messengers that are activated is fundamental to better understand the participation of each mediator in the febrile response, which may consequently facilitate the development of specific treatments for fever of different origins.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests are disclosed.
Funding
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Araucária do Estado do Paraná, Fundação de Amparo a Pesquisa do Estado da Bahia (FAPESB), and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) for financial support.
References
- 1.Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev 1991; 71:93-127; PMID:1986393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res 2001; 34:301-14; PMID:11262580; http://dx.doi.org/ 10.1590/S0100-879X2001000300003 [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA, Cannon JG, Wolff SM. New concepts on the pathogenesis of fever. Rev Infect Dis 1988; 10:168-89; PMID:2451266; http://dx.doi.org/ 10.1093/clinids/10.1.168 [DOI] [PubMed] [Google Scholar]
- 4.Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol 2006; 100:1347-54; PMID:16540713; http://dx.doi.org/ 10.1152/japplphysiol.01064.2005 [DOI] [PubMed] [Google Scholar]
- 5.Gay NJ, Gangloff M, Weber AN. Toll-like receptors as molecular switches. Nat Rev Immunol 2006; 6:693-8; PMID:16917510; http://dx.doi.org/ 10.1038/nri1916 [DOI] [PubMed] [Google Scholar]
- 6.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 2006; 74:443-77; PMID:17027526; http://dx.doi.org/ 10.1016/S0083-6729(06)74018-3 [DOI] [PubMed] [Google Scholar]
- 7.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 2001; 60:349-56; PMID:11681809; http://dx.doi.org/ 10.1079/PNS2001110 [DOI] [PubMed] [Google Scholar]
- 8.Jiang Q, Detolla L, Singh IS, Gatdula L, Fitzgerald B, van Rooijen N, Cross AS, Hasday JD, Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol 1999; 276:R1653-60; PMID:10362744 [DOI] [PubMed] [Google Scholar]
- 9.Budick-Harmelin N, Dudas J, Demuth J, Madar Z, Ramadori G, Tirosh O. Triglycerides potentiate the inflammatory response in rat Kupffer cells. Antioxid Redox Signal 2008; 10:2009-22; PMID:18710323; http://dx.doi.org/ 10.1089/ars.2007.1876 [DOI] [PubMed] [Google Scholar]
- 10.Ott D, Murgott J, Rafalzik S, Wuchert F, Schmalenbeck B, Roth J, Gerstberger R. Neurons and glial cells of the rat organum vasculosum laminae terminalis directly respond to lipopolysaccharide and pyrogenic cytokines. Brain Res 2010; 1363:93-106; PMID:20883673; http://dx.doi.org/ 10.1016/j.brainres.2010.09.083 [DOI] [PubMed] [Google Scholar]
- 11.Wuchert F, Ott D, Murgott J, Rafalzik S, Hitzel N, Roth J, Gerstberger R. Rat area postrema microglial cells act as sensors for the toll-like receptor-4 agonist lipopolysaccharide. J Neuroimmunol 2008; 204:66-74; PMID:18786731; http://dx.doi.org/ 10.1016/j.jneuroim.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 12.Zampronio AR, Souza GE, Silva CA, Cunha FQ, Ferreira SH. Interleukin-8 induces fever by a prostaglandin-independent mechanism. Am J Physiol 1994; 266:R1670-4; PMID:8203649 [DOI] [PubMed] [Google Scholar]
- 13.Minano FJ, Fernandez-Alonso A, Myers RD, Sancibrian M. Hypothalamic interaction between macrophage inflammatory protein-1 α (MIP-1 α) and MIP-1 β in rats: a new level for fever control? J Physiol 1996; 491(Pt 1):209-17; PMID:9011612; http://dx.doi.org/ 10.1113/jphysiol.1996.sp021208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares DM, Machado RR, Yamashiro LH, Melo MC, Souza GE. Cytokine-induced neutrophil chemoattractant (CINC)-1 induces fever by a prostaglandin-dependent mechanism in rats. Brain Res 2008; 1233:79-88; PMID:18694739; http://dx.doi.org/ 10.1016/j.brainres.2008.07.069 [DOI] [PubMed] [Google Scholar]
- 15.Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H, Trichereau J, Paolino M, Qadri F, Plehm R, et al.. Central control of fever and female body temperature by RANKL/RANK. Nature 2009; 462:505-9; PMID:19940926; http://dx.doi.org/ 10.1038/nature08596 [DOI] [PubMed] [Google Scholar]
- 16.Steiner AA, Romanovsky AA. Platelet-activating factor is a potent pyrogen and cryogen, but it does not mediate lipopolysaccharide fever or hypothermia. Temperature 2015; 2:535-42; http://dx.doi.org/ 10.1080/23328940.2015.1030540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares DM, Figueiredo MJ, Martins JM, Machado RR, Kanashiro A, Malvar Ddo C, Pessini AC, Roth J, Souza GE. CCL3/MIP-1 α is not involved in the LPS-induced fever and its pyrogenic activity depends on CRF. Brain Res 2009; 1269:54-60; PMID:19285486; http://dx.doi.org/ 10.1016/j.brainres.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 β: a possible site of prostaglandin synthesis responsible for fever. Brain Res 1996; 733:263-72; PMID:8891309; http://dx.doi.org/ 10.1016/0006-8993(96)00575-6 [DOI] [PubMed] [Google Scholar]
- 19.Blatteis CM, Role of the OVLT in the febrile response to circulating pyrogens. Prog Brain Res 1992; 91:409-12; PMID:1384084; http://dx.doi.org/ 10.1016/S0079-6123(08)62360-2 [DOI] [PubMed] [Google Scholar]
- 20.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 α, murine IL-1 α and murine IL-1 β are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther 1991; 259:988-96; PMID:1762091 [PubMed] [Google Scholar]
- 21.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett 1994; 179:53-6; PMID:7845624; http://dx.doi.org/ 10.1016/0304-3940(94)90933-4 [DOI] [PubMed] [Google Scholar]
- 22.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett 1995; 183:27-31; PMID:7746479; http://dx.doi.org/ 10.1016/0304-3940(94)11105-R [DOI] [PubMed] [Google Scholar]
- 23.Werner MF, Fraga D, Melo MC, Souza GE, Zampronio AR. Importance of the vagus nerve for fever and neutrophil migration induced by intraperitoneal LPS injection. Inflamm Res 2003; 52:291-6; PMID:12861394 [DOI] [PubMed] [Google Scholar]
- 24.Kong Y, Le Y. Toll-like receptors in inflammation of the central nervous system. Int Immunopharmacol 2011; 11:1407-14; PMID:21600311; http://dx.doi.org/ 10.1016/j.intimp.2011.04.025 [DOI] [PubMed] [Google Scholar]
- 25.McCusker RH, Kelley KW. Immune-neural connections: how the immune system's response to infectious agents influences behavior. J Exp Biol 2013; 216:84-98; PMID:23225871; http://dx.doi.org/ 10.1242/jeb.073411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano Y, Furube E, Morita S, Wanaka A, Nakashima T, Miyata S. Astrocytic TLR4 expression and LPS-induced nuclear translocation of STAT3 in the sensory circumventricular organs of adult mouse brain. J Neuroimmunol 2015; 278:144-58; PMID:25595264; http://dx.doi.org/ 10.1016/j.jneuroim.2014.12.013 [DOI] [PubMed] [Google Scholar]
- 27.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 2005; 25:1788-96; PMID:15716415; http://dx.doi.org/ 10.1523/JNEUROSCI.4268-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott D, Wuchert F, Murgott J, Rummel C, Gerstberger R, Roth J. The viral mimetic polyinosinic:polycytidylic acid (poly I:C) induces cellular responses in primary cultures from rat brain sites with an incomplete blood-brain barrier. Neurosci Lett 2012; 530:64-8; PMID:23022505; http://dx.doi.org/ 10.1016/j.neulet.2012.09.038 [DOI] [PubMed] [Google Scholar]
- 29.Wuchert F, Ott D, Rafalzik S, Roth J, Gerstberger R. Tumor necrosis factor-α, interleukin-1beta and nitric oxide induce calcium transients in distinct populations of cells cultured from the rat area postrema. J Neuroimmunol 2009; 206:44-51; PMID:19081643; http://dx.doi.org/ 10.1016/j.jneuroim.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 30.Desson SE, Ferguson AV. Interleukin 1beta modulates rat subfornical organ neurons as a result of activation of a non-selective cationic conductance. J Physiol 2003; 550:113-22; PMID:12879863; http://dx.doi.org/ 10.1113/jphysiol.2003.041210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milton AS, Wendlandt S. Effects on body temperature of prostaglandins of the A, E and F series on injection into the third ventricle of unanaesthetized cats and rabbits. J Physiol 1971; 218:325-36; PMID:4330929; http://dx.doi.org/ 10.1113/jphysiol.1971.sp009620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldberg W, Saxena PN. Fever produced by prostaglandin E1. J Physiol 1971; 217:547-56; PMID:5098081; http://dx.doi.org/ 10.1113/jphysiol.1971.sp009585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coceani F, Lees J, Bishai I. Further evidence implicating prostaglandin E2 in the genesis of pyrogen fever. Am J Physiol 1988; 254:R463-9; PMID:3279826 [DOI] [PubMed] [Google Scholar]
- 34.Watanobe H, Takebe K. Effects of intravenous administration of interleukin-1-β on the release of prostaglandin E2, corticotropin-releasing factor, and arginine vasopressin in several hypothalamic areas of freely moving rats: estimation by push-pull perfusion. Neuroendocrinology 1994; 60:8-15; PMID:8090286; http://dx.doi.org/ 10.1159/000126714 [DOI] [PubMed] [Google Scholar]
- 35.Coceani F, Bishai I, Engelberts D, House RV, Adamson SL. Response of newborn and adult sheep to pyrogens: relation between fever and brain eicosanoid changes. Brain Res 1995; 700:191-204; PMID:8624710; http://dx.doi.org/ 10.1016/0006-8993(95)00946-N [DOI] [PubMed] [Google Scholar]
- 36.Stitt JT. Differential sensitivity in the sites of fever production by prostaglandin E1 within the hypothalamus of the rat. J Physiol 1991; 432:99-110; PMID:1886074; http://dx.doi.org/ 10.1113/jphysiol.1991.sp018378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto A, Murakami N, Sakata Y, Watanabe T, Yamaguchi K. Functional and structural differences in febrile mechanism between rabbits and rats. J Physiol 1990; 427:227-39; PMID:2213598; http://dx.doi.org/ 10.1113/jphysiol.1990.sp018169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morimoto A, Nakamori T, Watanabe T, Ono T, Murakami N. Pattern differences in experimental fevers induced by endotoxin, endogenous pyrogen, and prostaglandins. Am J Physiol 1988; 254:R633-40; PMID:3258478 [DOI] [PubMed] [Google Scholar]
- 39.Rothwell NJ. Central activation of thermogenesis by prostaglandins: dependence on CRF. Horm Metab Res 1990; 22:616-8; PMID:2076859; http://dx.doi.org/ 10.1055/s-2007-1004986 [DOI] [PubMed] [Google Scholar]
- 40.Souza GE, Pela IR, Silva VM, Silva CA, Zampronio AR, Poole S. Role of glucocorticoids in febrile response in rabbits. Ann N Y Acad Sci 1997; 813:327-37; PMID:9100903; http://dx.doi.org/ 10.1111/j.1749-6632.1997.tb51715.x [DOI] [PubMed] [Google Scholar]
- 41.Coelho MM, Luheshi G, Hopkins SJ, Pela IR, Rothwell NJ. Multiple mechanisms mediate antipyretic action of glucocorticoids. Am J Physiol 1995; 269:R527-35; PMID:7573552 [DOI] [PubMed] [Google Scholar]
- 42.Katsuura G, Gottschall PE, Dahl RR, Arimura A. Interleukin-1 β increases prostaglandin E2 in rat astrocyte cultures: modulatory effect of neuropeptides. Endocrinology 1989; 124:3125-7; PMID:2785913; http://dx.doi.org/ 10.1210/endo-124-6-3125 [DOI] [PubMed] [Google Scholar]
- 43.Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 1995; 355:296-315; PMID:7608344; http://dx.doi.org/ 10.1002/cne.903550208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilhelms DB, Kirilov M, Mirrasekhian E, Eskilsson A, Kugelberg UO, Klar C, Ridder DA, Herschman HR, Schwaninger M, Blomqvist A, et al.. Deletion of prostaglandin E2 synthesizing enzymes in brain endothelial cells attenuates inflammatory fever. J Neurosci 2014; 34:11684-90; PMID:25164664; http://dx.doi.org/ 10.1523/JNEUROSCI.1838-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eskilsson A, Tachikawa M, Hosoya K, Blomqvist A. Distribution of microsomal prostaglandin E synthase-1 in the mouse brain. J Comp Neurol 2014; 522:3229-44; PMID:24668417; http://dx.doi.org/ 10.1002/cne.23593 [DOI] [PubMed] [Google Scholar]
- 46.Morimoto A, Murakami N, Nakamori T, Watanabe T. Ventromedial hypothalamus is highly sensitive to prostaglandin E2 for producing fever in rabbits. J Physiol 1988; 397:259-68; PMID:3166062; http://dx.doi.org/ 10.1113/jphysiol.1988.sp016999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature 1981; 289:401-2; PMID:7464907; http://dx.doi.org/ 10.1038/289401a0 [DOI] [PubMed] [Google Scholar]
- 48.Fyda DM, Cooper KE, Veale WL. Nucleus tractus solitarii lesions alter the metabolic and hyperthermic response to central prostaglandin E1 in the rat. J Physiol 1991; 442:337-49; PMID:1798032; http://dx.doi.org/ 10.1113/jphysiol.1991.sp018796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amir S, Schiavetto A. Injection of prostaglandin E2 into the anterior hypothalamic preoptic area activates brown adipose tissue thermogenesis in the rat. Brain Res 1990; 528:138-42; PMID:2245331; http://dx.doi.org/ 10.1016/0006-8993(90)90206-Q [DOI] [PubMed] [Google Scholar]
- 50.Kao CH, Huang WT, Lin MT, Wu WS. Central interleukin-10 attenuated lipopolysaccharide-induced changes in core temperature and hypothalamic glutamate, hydroxyl radicals and prostaglandin-E(2). Eur J Pharmacol 2011; 654:187-93; PMID:21237148; http://dx.doi.org/ 10.1016/j.ejphar.2010.12.038 [DOI] [PubMed] [Google Scholar]
- 51.Cao C, Matsumura K, Yamagata K, Watanabe Y. Induction by lipopolysaccharide of cyclooxygenase-2 mRNA in rat brain; its possible role in the febrile response. Brain Res 1995; 697:187-96; PMID:8593576; http://dx.doi.org/ 10.1016/0006-8993(95)00839-I [DOI] [PubMed] [Google Scholar]
- 52.Cao C, Matsumura K, Yamagata K, Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol 1997; 272:R1712-25; PMID:9227582 [DOI] [PubMed] [Google Scholar]
- 53.Cao C, Matsumura K, Yamagata K, Watanabe Y. Cyclooxygenase-2 is induced in brain blood vessels during fever evoked by peripheral or central administration of tumor necrosis factor. Brain Res Mol Brain Res 1998; 56:45-56; PMID:9602052; http://dx.doi.org/ 10.1016/S0169-328X(98)00025-4 [DOI] [PubMed] [Google Scholar]
- 54.Gaetano L, Watanabe K, Barogi S, Coceani F. Cyclooxygenase-2/microsomal prostaglandin E synthase-1 complex in the preoptic-anterior hypothalamus of the mouse: involvement through fever to intravenous lipopolysaccharide. Acta Physiol (Oxf) 2010; 200:315-24; PMID:20587000; http://dx.doi.org/ 10.1111/j.1748-1716.2010.02157.x [DOI] [PubMed] [Google Scholar]
- 55.Li S, Wang Y, Matsumura K, Ballou LR, Morham SG, Blatteis CM. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2(-/-), but not in cyclooxygenase-1(-/-) mice. Brain Res 1999; 825:86-94; PMID:10216176; http://dx.doi.org/ 10.1016/S0006-8993(99)01225-1 [DOI] [PubMed] [Google Scholar]
- 56.Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 2003; 6:1137-8; PMID:14566340; http://dx.doi.org/ 10.1038/nn1137 [DOI] [PubMed] [Google Scholar]
- 57.Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature 2001; 410:430-1; PMID:11260702; http://dx.doi.org/ 10.1038/35068632 [DOI] [PubMed] [Google Scholar]
- 58.Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci 2001; 21:2669-77; PMID:11306620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rummel C, Matsumura K, Luheshi GN. Circulating IL-6 contributes to peripheral LPS-induced mPGES-1 expression in the rat brain. Brain Res Bull 2011; 86:319-25; PMID:21945087; http://dx.doi.org/ 10.1016/j.brainresbull.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 60.Engstrom L, Ruud J, Eskilsson A, Larsson A, Mackerlova L, Kugelberg U, Qian H, Vasilache AM, Larsson P, Engblom D, et al.. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology 2012; 153:4849-61; PMID:22872578; http://dx.doi.org/ 10.1210/en.2012-1375 [DOI] [PubMed] [Google Scholar]
- 61.Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood 2006; 107:4000-2; PMID:16403908; http://dx.doi.org/ 10.1182/blood-2005-11-4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, et al.. Cellular and molecular bases of the initiation of fever. PLoS Biol 2006; 4:e284; PMID:16933973; http://dx.doi.org/ 10.1371/journal.pbio.0040284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z, Perlik V, Feleder C, Tang Y, Blatteis CM. Kupffer cell-generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol 2006; 290:R1262-70; PMID:16410400; http://dx.doi.org/ 10.1152/ajpregu.00724.2005 [DOI] [PubMed] [Google Scholar]
- 64.Eskilsson A, Mirrasekhian E, Dufour S, Schwaninger M, Engblom D, Blomqvist A. Immune-induced fever is mediated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J Neurosci 2014; 34:15957-61; PMID:25429137; http://dx.doi.org/ 10.1523/JNEUROSCI.3520-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. Relationship of EP(1-4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol 2000; 428:20-32; PMID:11058222; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 66.Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 2007; 10:1131-3; PMID:17676060; http://dx.doi.org/ 10.1038/nn1949 [DOI] [PubMed] [Google Scholar]
- 67.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, et al.. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 1998; 395:281-4; PMID:9751056; http://dx.doi.org/ 10.1038/26233 [DOI] [PubMed] [Google Scholar]
- 68.Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 2003; 551:945-54; PMID:12837930; http://dx.doi.org/ 10.1113/jphysiol.2003.048140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saper CB, Romanovsky AA, Scammell TE,. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci 2012; 15:1088-95; PMID:22837039; http://dx.doi.org/ 10.1038/nn.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 2005; 10:2193-216; PMID:15970487; http://dx.doi.org/ 10.2741/1690 [DOI] [PubMed] [Google Scholar]
- 71.Fabricio AS, Veiga FH, Cristofoletti R, Navarra P, Souza GE. The effects of selective and nonselective cyclooxygenase inhibitors on endothelin-1-induced fever in rats. Am J Physiol Regul Integr Comp Physiol 2005; 288:R671-7; PMID:15539607; http://dx.doi.org/ 10.1152/ajpregu.00532.2004 [DOI] [PubMed] [Google Scholar]
- 72.Bastos-Pereira AL, Leite MC, Fraga D, Zampronio AR. Central mediators involved in the febrile response induced by polyinosinic-polycytidylic acid: lack of involvement of endothelins and substance P. J Neuroimmunol 2015; 278:100-7; PMID:25595258; http://dx.doi.org/ 10.1016/j.jneuroim.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 73.Martins JM, Longhi-Balbinot DT, Soares DM, Figueiredo MJ, Malvar Ddo C, de Melo MC, Rae GA, Souza GE. Involvement of PGE2 and RANTES in Staphylococcus aureus-induced fever in rats. J Appl Physiol (1985) 2012; 113:1456-65; PMID:22936726; http://dx.doi.org/ 10.1152/japplphysiol.00936.2011 [DOI] [PubMed] [Google Scholar]
- 74.Chan CC, Panneton M, Taylor AM, Therien M, Rodger IW. A selective inhibitor of cyclooxygenase-2 reverses endotoxin-induced pyretic responses in non-human primates. Eur J Pharmacol 1997; 327:221-5; PMID:9200563; http://dx.doi.org/ 10.1016/S0014-2999(97)89664-1 [DOI] [PubMed] [Google Scholar]
- 75.Figueiredo MJ, Soares DM, Martins JM, Machado R Rde, Sorgi CA, Faccioli LH, Melo MC, Malvar Ddo C, Souza GE. Febrile response induced by cecal ligation and puncture (CLP) in rats: involvement of prostaglandin E2 and cytokines. Med Microbiol Immunol 2012; 201:219-29; PMID:22203392; http://dx.doi.org/ 10.1007/s00430-011-0225-y [DOI] [PubMed] [Google Scholar]
- 76.Damm J, Wiegand F, Harden LM, Gerstberger R, Rummel C, Roth J. Fever, sickness behavior, and expression of inflammatory genes in the hypothalamus after systemic and localized subcutaneous stimulation of rats with the Toll-like receptor 7 agonist imiquimod. Neuroscience 2012; 201:166-83; PMID:22116053; http://dx.doi.org/ 10.1016/j.neuroscience.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 77.Damm J, Wiegand F, Harden LM, Wenisch S, Gerstberger R, Rummel C, Roth J. Intraperitoneal and subcutaneous injections of the TLR9 agonist ODN 1668 in rats: brain inflammatory responses are related to peripheral IL-6 rather than interferons. J Neuroimmunol 2014; 277:105-17; PMID:25465287; http://dx.doi.org/ 10.1016/j.jneuroim.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 78.Shapiro L, Zhang XX, Rupp RG, Wolff SM, Dinarello CA. Ciliary neurotrophic factor is an endogenous pyrogen. Proc Natl Acad Sci U S A 1993; 90:8614-8; PMID:8378338; http://dx.doi.org/ 10.1073/pnas.90.18.8614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez-Valpuesta FJ, Myers RD. Fever produced by interleukin-11 (IL-11) injected into the anterior hypothalamic pre-optic area of the rat is antagonized by indomethacin. Neuropharmacology 1994; 33:989-94; PMID:7845555; http://dx.doi.org/ 10.1016/0028-3908(94)90157-0 [DOI] [PubMed] [Google Scholar]
- 80.Machado RR, Soares DM, Proudfoot AE, Souza GE. CCR1 and CCR5 chemokine receptors are involved in fever induced by LPS (E. coli) and RANTES in rats. Brain Res 2007; 1161:21-31; PMID:17604006; http://dx.doi.org/ 10.1016/j.brainres.2007.05.054 [DOI] [PubMed] [Google Scholar]
- 81.Osborn O, Sanchez-Alavez M, Dubins JS, Gonzalez AS, Morrison B, Hadcock JR, Bartfai T. Ccl22/MDC, is a prostaglandin dependent pyrogen, acting in the anterior hypothalamus to induce hyperthermia via activation of brown adipose tissue. Cytokine 2011; 53:311-9; PMID:21177120; http://dx.doi.org/ 10.1016/j.cyto.2010.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davatelis G, Wolpe SD, Sherry B, Dayer JM, Chicheportiche R, Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science 1989; 243:1066-8; PMID:2646711; http://dx.doi.org/ 10.1126/science.2646711 [DOI] [PubMed] [Google Scholar]
- 83.Minano FJ, Sancibrian M, Vizcaino M, Paez X, Davatelis G, Fahey T, Sherry B, Cerami A, Myers RD. Macrophage inflammatory protein-1: unique action on the hypothalamus to evoke fever. Brain Res Bull 1990; 24:849-52; PMID:2196977; http://dx.doi.org/ 10.1016/0361-9230(90)90150-X [DOI] [PubMed] [Google Scholar]
- 84.Fabricio AS, Rae GA, D'Orleans-Juste P, Souza GE. Endothelin-1 as a central mediator of LPS-induced fever in rats. Brain Res 2005; 1066:92-100; PMID:16360659; http://dx.doi.org/ 10.1016/j.brainres.2005.10.037 [DOI] [PubMed] [Google Scholar]
- 85.Fabricio AS, Rae GA, Zampronio AR, D'Orleans-Juste P, Souza GE. Central endothelin ET(B) receptors mediate IL-1-dependent fever induced by preformed pyrogenic factor and corticotropin-releasing factor in the rat. Am J Physiol Regul Integr Comp Physiol 2006; 290:R164-71; PMID:16123229; http://dx.doi.org/ 10.1152/ajpregu.00337.2005 [DOI] [PubMed] [Google Scholar]
- 86.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 2004; 44:525-57; PMID:14744257; http://dx.doi.org/ 10.1146/annurev.pharmtox.44.101802.121410 [DOI] [PubMed] [Google Scholar]
- 87.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, et al.. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A 2001; 98:2843-8; PMID:11226328; http://dx.doi.org/ 10.1073/pnas.051626398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 2000; 428:191-212; PMID:11064361; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 89.Bernardini GL, Richards DB, Lipton JM. Antipyretic effect of centrally administered CRF. Peptides 1984; 5:57-9; PMID:6608720; http://dx.doi.org/ 10.1016/0196-9781(84)90051-2 [DOI] [PubMed] [Google Scholar]
- 90.Opp M, Obal F Jr, Krueger JM. Corticotropin-releasing factor attenuates interleukin 1-induced sleep and fever in rabbits. Am J Physiol 1989; 257:R528-35; PMID:2789476 [DOI] [PubMed] [Google Scholar]
- 91.Nakamori T, Morimoto A, Murakami N. Effect of a central CRF antagonist on cardiovascular and thermoregulatory responses induced by stress or IL-1 β. Am J Physiol 1993; 265:R834-9; PMID:8238454 [DOI] [PubMed] [Google Scholar]
- 92.Strijbos PJ, Hardwick AJ, Relton JK, Carey F, Rothwell NJ. Inhibition of central actions of cytokines on fever and thermogenesis by lipocortin-1 involves CRF. Am J Physiol 1992; 263:E632-6; PMID:1415682 [DOI] [PubMed] [Google Scholar]
- 93.Werner MF, Souza GE, Zampronio AR. Nimesulide-induced antipyresis in rats involves both cyclooxygenase-dependent and independent mechanisms. Eur J Pharmacol 2006; 543:181-9; PMID:16814279; http://dx.doi.org/ 10.1016/j.ejphar.2006.05.029 [DOI] [PubMed] [Google Scholar]
- 94.Figueiredo MJ, Fabricio AS, Machado RR, Melo MC, Soares DM, Souza GE. Increase of core temperature induced by corticotropin-releasing factor and urocortin: a comparative study. Regul Pept 2010; 165:191-9; PMID:20691217; http://dx.doi.org/ 10.1016/j.regpep.2010.07.167 [DOI] [PubMed] [Google Scholar]
- 95.Fraga D, Machado RR, Fernandes LC, Souza GE, Zampronio AR. Endogenous opioids: role in prostaglandin-dependent and -independent fever. Am J Physiol Regul Integr Comp Physiol 2008; 294:R411-20; PMID:18032464; http://dx.doi.org/ 10.1152/ajpregu.00465.2007 [DOI] [PubMed] [Google Scholar]
- 96.Rothwell NJ. CRF is involved in the pyrogenic and thermogenic effects of interleukin 1 β in the rat. Am J Physiol 1989; 256:E111-5; PMID:2783532 [DOI] [PubMed] [Google Scholar]
- 97.Lundkvist J, Chai Z, Teheranian R, Hasanvan H, Bartfai T, Jenck F, Widmer U, Moreau JL. A non peptidic corticotropin releasing factor receptor antagonist attenuates fever and exhibits anxiolytic-like activity. Eur J Pharmacol 1996; 309:195-200; PMID:8874139; http://dx.doi.org/ 10.1016/0014-2999(96)00337-8 [DOI] [PubMed] [Google Scholar]
- 98.Kanashiro A, Figueiredo MJ, Malvar Ddo C, Souza GE. Cytokines, but not corticotropin-releasing factor and endothelin-1, participate centrally in the febrile response in zymosan-induced arthritis in rats. Brain Res 2015; 1610:12-9; PMID:25819555; http://dx.doi.org/ 10.1016/j.brainres.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 99.Bastos-Pereira AL, Fraga D, Ott D, Simm B, Murgott J, Roth J, Zampronio AR. Involvement of brain cytokines in zymosan-induced febrile response. J Appl Physiol (1985) 2014; 116:1220-9; PMID:24651990; http://dx.doi.org/ 10.1152/japplphysiol.01278.2013 [DOI] [PubMed] [Google Scholar]
- 100.Milton NG, Hillhouse EW, Milton AS. A possible role for endogenous peripheral corticotrophin-releasing factor-41 in the febrile response of conscious rabbits. J Physiol 1993; 465:415-25; PMID:8229843; http://dx.doi.org/ 10.1113/jphysiol.1993.sp019684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soares DM, Cristofoletti R, Melo MC, Lindsey CJ, Veiga-Souza FH, Fabricio AS, Souza GE, Cyclooxygenase-independent mechanism of ibuprofen-induced antipyresis: the role of central vasopressin V(1) receptors. Fundam Clin Pharmacol 2011; 25:670-81; PMID:21077948; http://dx.doi.org/ 10.1111/j.1472-8206.2010.00894.x [DOI] [PubMed] [Google Scholar]
- 102.Telegdy G. and Adamik A., Involvement of CRH receptors in urocortin-induced hyperthermia. Peptides 2008; 29:1937-42; PMID:18775757; http://dx.doi.org/ 10.1016/j.peptides.2008.07.028 [DOI] [PubMed] [Google Scholar]
- 103.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A 1989; 86:2863-7; PMID:2649896; http://dx.doi.org/ 10.1073/pnas.86.8.2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332:411-5; PMID:2451132; http://dx.doi.org/ 10.1038/332411a0 [DOI] [PubMed] [Google Scholar]
- 105.Kotsovolis G, Kallaras K. The role of endothelium and endogenous vasoactive substances in sepsis. Hippokratia 2010; 14:88-93; PMID:20596262 [PMC free article] [PubMed] [Google Scholar]
- 106.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 1994; 46:325-415; PMID:7831383 [PubMed] [Google Scholar]
- 107.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol 2008; 153 Suppl 2:S1-209; PMID:18347570; http://dx.doi.org/ 10.1038/sj.bjp.0707746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Audet M, Bouvier M. Restructuring G-protein- coupled receptor activation. Cell 2012; 151:14-23; PMID:23021212; http://dx.doi.org/ 10.1016/j.cell.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 109.Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol 2008; 86:485-98; PMID:18758495; http://dx.doi.org/ 10.1139/Y08-059 [DOI] [PubMed] [Google Scholar]
- 110.Fabricio AS, Silva CA, Rae GA, D'Orleans-Juste P, Souza GE. Essential role for endothelin ET(B) receptors in fever induced by LPS (E. coli) in rats. Br J Pharmacol 1998; 125:542-8; PMID:9806338; http://dx.doi.org/ 10.1038/sj.bjp.0702075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wanecek M, Weitzberg E, Rudehill A, Oldner A. The endothelin system in septic and endotoxin shock. Eur J Pharmacol 2000; 407:1-15; PMID:11050285; http://dx.doi.org/ 10.1016/S0014-2999(00)00675-0 [DOI] [PubMed] [Google Scholar]
- 112.Malvar Ddo C, Soares DM, Fabricio AS, Kanashiro A, Machado RR, Figueiredo MJ, Rae GA, de Souza GE. The antipyretic effect of dipyrone is unrelated to inhibition of PGE(2) synthesis in the hypothalamus. Br J Pharmacol 2011; 162:1401-9; PMID:21133897; http://dx.doi.org/ 10.1111/j.1476-5381.2010.01150.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol 1994; 55:97-179; PMID:8304236; http://dx.doi.org/ 10.1016/S0065-2776(08)60509-X [DOI] [PubMed] [Google Scholar]
- 114.Pease JE, Williams TJ. Chemokines and their receptors in allergic disease. J Allergy Clin Immunol 2006; 118:305-18; quiz 19-20; PMID:16890751; http://dx.doi.org/ 10.1016/j.jaci.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 115.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med 1998; 338:436-45; PMID:9459648; http://dx.doi.org/ 10.1056/NEJM199802123380706 [DOI] [PubMed] [Google Scholar]
- 116.Pessini AC, Santos DR, Arantes EC, Souza GE. Mediators involved in the febrile response induced by Tityus serrulatus scorpion venom in rats. Toxicon 2006; 48:556-66; PMID:16911816; http://dx.doi.org/ 10.1016/j.toxicon.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 117.IUPHAR-DB , IUPHAR/BPS Guide to Pharmacology. 2015, International Union of Pharmacology [Google Scholar]
- 118.Stievano L, Piovan E, Amadori A. C and CX3C chemokines: cell sources and physiopathological implications. Crit Rev Immunol 2004; 24:205-28; PMID:15482255; http://dx.doi.org/ 10.1615/CritRevImmunol.v24.i3.40 [DOI] [PubMed] [Google Scholar]
- 119.Viola A. and Luster A.D., Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol 2008; 48:171-97; PMID:17883327; http://dx.doi.org/ 10.1146/annurev.pharmtox.48.121806.154841 [DOI] [PubMed] [Google Scholar]
- 120.Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe SD, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 β. J Exp Med 1988; 168:2251-9; PMID:3058856; http://dx.doi.org/ 10.1084/jem.168.6.2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Menten P, Wuyts A, Van Damme J, Macrophage inflammatory protein-1. Cytokine Growth Factor Rev 2002; 13:455-81; PMID:12401480 http://dx.doi.org/ 10.1016/S1359-6101(02)00045-X [DOI] [PubMed] [Google Scholar]
- 122.Tavares E, Minano FJ. Macrophage inflammatory protein-1beta induces dexamethasone-unresponsive fever in rats. Neuroreport 1998; 9:2519-22; PMID:9721925; http://dx.doi.org/ 10.1097/00001756-199808030-00017 [DOI] [PubMed] [Google Scholar]
- 123.Soares DM, Veiga-Souza F Hiratsuka, Fabricio AS, Javier Minano F, Petto Souza GE. CCL3/macrophage inflammatory protein-1alpha induces fever and increases prostaglandin E2 in cerebrospinal fluid of rats: effect of antipyretic drugs. Brain Res 2006; 1109:83-92; PMID:16836983; http://dx.doi.org/ 10.1016/j.brainres.2006.06.026 [DOI] [PubMed] [Google Scholar]
- 124.Minano FJ, Armengol JA, Sancibrian M, Pomares F, Benamar K, Myers RD. Macrophage inflammatory protein-1 β and inducible nitric oxide synthase immunoreactivity in rat brain during prostaglandin E2- or lipopolysaccharide-induced fever. Ann N Y Acad Sci 1997; 813:272-80; PMID:9100893; http://dx.doi.org/ 10.1111/j.1749-6632.1997.tb51705.x [DOI] [PubMed] [Google Scholar]
- 125.Li S, Goorha S, Ballou LR, Blatteis CM. Intracerebroventricular interleukin-6, macrophage inflammatory protein-1 β and IL-18: pyrogenic and PGE(2)-mediated? Brain Res 2003; 992:76-84; PMID:14604775; http://dx.doi.org/ 10.1016/j.brainres.2003.08.033 [DOI] [PubMed] [Google Scholar]
- 126.Kielian T, Syed MM, Liu S, Phulwani NK, Phillips N, Wagoner G, Drew PD, Esen N. The synthetic peroxisome proliferator-activated receptor-gamma agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. J Immunol 2008; 180:5004-16; PMID:18354226; http://dx.doi.org/ 10.4049/jimmunol.180.7.5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dapunt U, Maurer S, Giese T, Gaida MM, Hansch GM. The macrophage inflammatory proteins MIP1alpha (CCL3) and MIP2alpha (CXCL2) in implant-associated osteomyelitis: linking inflammation to bone degradation. Mediators Inflamm 2014; 2014:728619; PMID:24795505; http://dx.doi.org/ 10.1155/2014/728619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tavares E, Minano FJ. RANTES: a new prostaglandin dependent endogenous pyrogen in the rat. Neuropharmacology 2000; 39:2505-13; PMID:10974335; http://dx.doi.org/ 10.1016/S0028-3908(00)00073-3 [DOI] [PubMed] [Google Scholar]
- 129.Boddeke EW, Meigel I, Frentzel S, Gourmala NG, Harrison JK, Buttini M, Spleiss O, Gebicke-Harter P. Cultured rat microglia express functional β-chemokine receptors. J Neuroimmunol 1999; 98:176-84; PMID:10430051; http://dx.doi.org/ 10.1016/S0165-5728(99)00096-X [DOI] [PubMed] [Google Scholar]
- 130.Tavares E, Minano FJ. Differential sensitivities of pyrogenic chemokine fevers to CC chemokine receptor 5 antibodies. Fundam Clin Pharmacol 2004; 18:163-9; PMID:15066130; http://dx.doi.org/ 10.1111/j.1472-8206.2003.00227.x [DOI] [PubMed] [Google Scholar]
- 131.Rothwell NJ, Hardwick AJ, Lindley I. Central actions of interleukin-8 in the rat are independent of prostaglandins. Horm Metab Res 1990; 22:595-6; PMID:2272606; http://dx.doi.org/ 10.1055/s-2007-1004981 [DOI] [PubMed] [Google Scholar]
- 132.Zampronio AR, Silva CA, Cunha FQ, Ferreira SH, Pela IR, Souza GE. Indomethacin blocks the febrile response induced by interleukin-8 in rabbits. Am J Physiol 1995; 269:R1469-74; PMID:8594951 [DOI] [PubMed] [Google Scholar]
- 133.Calkins CM, Bensard DD, Shames BD, Pulido EJ, Abraham E, Fernandez N, Meng X, Dinarello CA, McIntyre RC Jr. IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J Endotoxin Res 2002; 8:59-67; PMID:11981446 [PubMed] [Google Scholar]
- 134.Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol 1991; 104:765-7; PMID:1797337; http://dx.doi.org/ 10.1111/j.1476-5381.1991.tb12502.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lorenzetti BB, Veiga FH, Canetti CA, Poole S, Cunha FQ, Ferreira SH. Cytokine-induced neutrophil chemoattractant 1 (CINC-1) mediates the sympathetic component of inflammatory mechanical hypersensitivitiy in rats. Eur Cytokine Netw 2002; 13:456-61; PMID:12517731 [PubMed] [Google Scholar]
- 136.Minano FJ, Tavares E, Maldonado R. Role of endogenous macrophage inflammatory protein-2 in regulating fever induced by bacterial endotoxin in normal and immunosuppressed rats. Clin Exp Pharmacol Physiol 2004; 31:723-31; PMID:15554915; http://dx.doi.org/ 10.1111/j.1440-1681.2004.04086.x [DOI] [PubMed] [Google Scholar]
- 137.Kumar M, Nerurkar VR, Integrated analysis of microRNAs and their disease related targets in the brain of mice infected with West Nile virus. Virology 2014; 452-453:143-51; PMID:24606691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ko TM, Kuo HC, Chang JS, Chen SP, Liu YM, Chen HW, Tsai FJ, Lee YC, Chen CH, Wu JY, et al, CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ Res 2015; 116:876-83; PMID:25605650; http://dx.doi.org/ 10.1161/CIRCRESAHA.116.305834 [DOI] [PubMed] [Google Scholar]
- 139.Conroy AL, Gelvez M, Hawkes M, Rajwans N, Tran V, Liles WC, Villar-Centeno LA, Kain KC. Host biomarkers are associated with progression to dengue haemorrhagic fever: a nested case-control study. Int J Infect Dis 2015; S1201-9712(15)00195-2 [DOI] [PubMed] [Google Scholar]
- 140.Benamar K, Addou S, Yondorf M, Geller EB, Eisenstein TK, Adler MW. Intrahypothalamic injection of the HIV-1 envelope glycoprotein induces fever via interaction with the chemokine system. J Pharmacol Exp Ther 2010; 332:549-53; PMID:19906780; http://dx.doi.org/ 10.1124/jpet.109.160309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, Spiropoulou CF, Ebola hemorrhagic Fever: novel biomarker correlates of clinical outcome. J Infect Dis 2014; 210:558-66; PMID:24526742; http://dx.doi.org/ 10.1093/infdis/jiu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol 1993; 44:8-12; PMID:8393525 [PubMed] [Google Scholar]
- 143.Evans CJ, Keith DE Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science 1992; 258:1952-5; PMID:1335167; http://dx.doi.org/ 10.1126/science.1335167 [DOI] [PubMed] [Google Scholar]
- 144.Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A 1993; 90:9954-8; PMID:8234341; http://dx.doi.org/ 10.1073/pnas.90.21.9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 1995; 18:22-9; PMID:7535487; http://dx.doi.org/ 10.1016/0166-2236(95)93946-U [DOI] [PubMed] [Google Scholar]
- 146.Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol 1996; 371:85-103; PMID:8835720; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 147.Scammell TE, Elmquist JK, Griffin JD, Saper CB, Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci 1996; 16:6246-54; PMID:8815905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Appelbaum BD, Holtzman SG. Stress-induced changes in the analgesic and thermic effects of opioid peptides in the rat. Brain Res 1986; 377:330-6; PMID:3015351; http://dx.doi.org/ 10.1016/0006-8993(86)90876-0 [DOI] [PubMed] [Google Scholar]
- 149.Handler CM, Geller EB, Adler MW. Effect of mu-, kappa-, and delta-selective opioid agonists on thermoregulation in the rat. Pharmacol Biochem Behav 1992; 43:1209-16; PMID:1361992; http://dx.doi.org/ 10.1016/0091-3057(92)90504-9 [DOI] [PubMed] [Google Scholar]
- 150.Handler CM, Price RW, Geller EB, Adler MW. Effect of mu-selective opioid antagonists on MIP-1 β and IL-1 β-induced fever. Ann N Y Acad Sci 1998; 856:270-3; PMID:9917888; http://dx.doi.org/ 10.1111/j.1749-6632.1998.tb08336.x [DOI] [PubMed] [Google Scholar]
- 151.Spencer RL, Hruby VJ, Burks TF. Body temperature response profiles for selective mu, delta and kappa opioid agonists in restrained and unrestrained rats. J Pharmacol Exp Ther 1988; 246:92-101; PMID:2839673 [PubMed] [Google Scholar]
- 152.Cavicchini E, Candeletti S, Ferri S. Effects of dynorphins on body temperature of rats. Pharmacol Res Commun 1988; 20:603-4; PMID:2902649; http://dx.doi.org/ 10.1016/S0031-6989(88)80086-9 [DOI] [PubMed] [Google Scholar]
- 153.Broccardo M, Improta G. Hypothermic effect of D-Ala-deltorphin II, a selective delta opioid receptor agonist. Neurosci Lett 1992; 139:209-12; PMID:1319015; http://dx.doi.org/ 10.1016/0304-3940(92)90554-K [DOI] [PubMed] [Google Scholar]
- 154.Handler CM, Piliero TC, Geller EB, Adler MW. Effect of ambient temperature on the ability of mu-, kappa- and delta-selective opioid agonists to modulate thermoregulatory mechanisms in the rat. J Pharmacol Exp Ther 1994; 268:847-55; PMID:8113997 [PubMed] [Google Scholar]
- 155.Rawls SM, Cowan A. Modulation of delta opioid-evoked hypothermia in rats by WAY 100635 and fluoxetine. Neurosci Lett 2006; 398:319-24; PMID:16483716; http://dx.doi.org/ 10.1016/j.neulet.2006.01.017 [DOI] [PubMed] [Google Scholar]
- 156.Rawls SM, Hewson JM, Inan S, Cowan A. Brain delta2 opioid receptors mediate SNC-80-evoked hypothermia in rats. Brain Res 2005; 1049:61-9; PMID:15936000; http://dx.doi.org/ 10.1016/j.brainres.2005.04.074 [DOI] [PubMed] [Google Scholar]
- 157.Ghosh S, Geller EB, Adler MW. Interaction of cholecystokinin and somatostatin with a selective mu-opioid agonist and mu- and kappa-antagonists in thermoregulation. Brain Res 1997; 745:152-7; PMID:9037404; http://dx.doi.org/ 10.1016/S0006-8993(96)01144-4 [DOI] [PubMed] [Google Scholar]
- 158.Baker AK, Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther 2002; 302:1253-64; PMID:12183687; http://dx.doi.org/ 10.1124/jpet.102.037655 [DOI] [PubMed] [Google Scholar]
- 159.Yakimova KS, Sann H, Pierau FK. Neuronal basis for the hyperthermic effect of mu-opioid agonists in rats: decrease in temperature sensitivity of warm-sensitive hypothalamic neurons. Neurosci Lett 1996; 218:115-8; PMID:8945741; http://dx.doi.org/ 10.1016/S0304-3940(96)13133-5 [DOI] [PubMed] [Google Scholar]
- 160.Lin MT, Uang WN, Chan HK. Hypothalamic neuronal responses to iontophoretic application of morphine in rats. Neuropharmacology 1984; 23:591-4; PMID:6738828; http://dx.doi.org/ 10.1016/0028-3908(84)90035-2 [DOI] [PubMed] [Google Scholar]
- 161.Blatteis CM, Xin L, Quan N. Neuromodulation of fever: apparent involvement of opioids. Brain Res Bull 1991; 26:219-23; PMID:2012982; http://dx.doi.org/ 10.1016/0361-9230(91)90230-H [DOI] [PubMed] [Google Scholar]
- 162.Benamar K, Xin L, Geller EB, Adler MW. Blockade of lipopolysaccharide-induced fever by a mu-opioid receptor-selective antagonist in rats. Eur J Pharmacol 2000; 401:161-5; PMID:10924921; http://dx.doi.org/ 10.1016/S0014-2999(00)00424-6 [DOI] [PubMed] [Google Scholar]
- 163.Benamar K, McMenamin M, Geller EB, Chung YG, Pintar JE, Adler MW. Unresponsiveness of mu-opioid receptor knockout mice to lipopolysaccharide-induced fever. Br J Pharmacol 2005; 144:1029-31; PMID:15700026; http://dx.doi.org/ 10.1038/sj.bjp.0706145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Romanovsky AA, Shido O, Ungar AL, Blatteis CM. Peripheral naloxone attenuates lipopolysaccharide fever in guinea pigs by an action outside the blood-brain barrier. Am J Physiol 1994; 266:R1824-31; PMID:8024035 [DOI] [PubMed] [Google Scholar]
- 165.Tsai SM, Lin MT, Wang JJ, Huang WT. Pyrogens enhance β-endorphin release in hypothalamus and trigger fever that can be attenuated by buprenorphine. J Pharmacol Sci 2003; 93:155-62; PMID:14578583; http://dx.doi.org/ 10.1254/jphs.93.155 [DOI] [PubMed] [Google Scholar]
- 166.Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 2001; 297:688-95; PMID:11303059 [PubMed] [Google Scholar]
- 167.Nakashima T, Murakami T, Murai Y, Hori T, Miyata S, Kiyohara T. Naloxone suppresses the rising phase of fever induced by interferon-α. Brain Res Bull 1995; 37:61-6; PMID:7606480; http://dx.doi.org/ 10.1016/0361-9230(94)00259-2 [DOI] [PubMed] [Google Scholar]
- 168.Palma J, Abood ME, Barbe MF, Benamar K. Functional interaction between HIV-gp120 and opioid system in the preoptic anterior hypothalamus. Drug Alcohol Depend 2014; 134:383-6; PMID:24120859; http://dx.doi.org/ 10.1016/j.drugalcdep.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain 2002; 95:277-85; PMID:11839427; http://dx.doi.org/ 10.1016/S0304-3959(01)00410-9 [DOI] [PubMed] [Google Scholar]
- 170.Hurd YL, Keller E, Sotonyi P, Sedvall G. Preprotachykinin-A mRNA expression in the human and monkey brain: An in situ hybridization study. J Comp Neurol 1999; 411:56-72; PMID:10404107; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 171.Bost KL. Tachykinin-mediated modulation of the immune response. Front Biosci 2004; 9:3331-2; PMID:15358592; http://dx.doi.org/ 10.2741/1484 [DOI] [PubMed] [Google Scholar]
- 172.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD, Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 1997; 159:5654-60; PMID:9548509 [PubMed] [Google Scholar]
- 173.Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets 2006; 7:963-74; PMID:16918325; http://dx.doi.org/ 10.2174/138945006778019273 [DOI] [PubMed] [Google Scholar]
- 174.Harrison S, Geppetti P. Substance p. Int. J Biochem Cell Biol 2001; 33:555-76; http://dx.doi.org/ 10.1016/S1357-2725(01)00031-0 [DOI] [PubMed] [Google Scholar]
- 175.Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol 2009; 30:271-6; PMID:19427266; http://dx.doi.org/ 10.1016/j.it.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 176.Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol 1998; 86:80-6; PMID:9655475; http://dx.doi.org/ 10.1016/S0165-5728(98)00025-3 [DOI] [PubMed] [Google Scholar]
- 177.Cooke HJ, Fox P, Alferes L, Fox CC, Wolfe SA Jr., Presence of NK1 receptors on a mucosal-like mast cell line, RBL-2H3 cells. Can J Physiol Pharmacol 1998; 76:188-93; PMID:9635159; http://dx.doi.org/ 10.1139/y98-014 [DOI] [PubMed] [Google Scholar]
- 178.Lambrecht BN, Germonpre PR, Everaert EG, Carro-Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol 1999; 29:3815-25; PMID:10601989; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 179.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-α, interleukin 1-β, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides 2003; 37:355-61; PMID:14698678; http://dx.doi.org/ 10.1016/j.npep.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 180.Yaraee R, Ebtekar M, Ahmadiani A, Sabahi F. Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. Int Immunopharmacol 2003; 3:1883-7; PMID:14636837; http://dx.doi.org/ 10.1016/S1567-5769(03)00201-7 [DOI] [PubMed] [Google Scholar]
- 181.Berman AS, Chancellor-Freeland C, Zhu G, Black PH, Substance P primes murine peritoneal macrophages for an augmented proinflammatory cytokine response to lipopolysaccharide. Neuroimmunomodulation 1996; 3:141-9; PMID:8945730; http://dx.doi.org/ 10.1159/000097239 [DOI] [PubMed] [Google Scholar]
- 182.Tsuchida K, Shigemoto R, Yokota Y, Nakanishi S. Tissue distribution and quantitation of the mRNAs for three rat tachykinin receptors. Eur J Biochem 1990; 193:751-7; PMID:1701145; http://dx.doi.org/ 10.1111/j.1432-1033.1990.tb19396.x [DOI] [PubMed] [Google Scholar]
- 183.Gautreau A, Kerdelhue B. Simultaneous quantitation of substance P-encoding preprotachykinin alternatively spliced mRNAs and substance P receptor NK-1 mRNA by an RNase protection assay. Brain Res Brain Res Protoc 1998; 2:133-40; PMID:9473630; http://dx.doi.org/ 10.1016/S1385-299X(97)00036-6 [DOI] [PubMed] [Google Scholar]
- 184.Rewerski W, Misterek K, Dorociak A, Gumulka SW. Effect of intracerebral administration of substance P on body temperature. Acta Physiol Pol 1982; 33:185-8; PMID:6184946 [PubMed] [Google Scholar]
- 185.Blatteis CM, Xin L, Quan N. Neuromodulation of fever. A possible role for substance P. Ann N Y Acad Sci 1994; 741:162-73; PMID:7529971; http://dx.doi.org/ 10.1111/j.1749-6632.1994.tb23097.x [DOI] [PubMed] [Google Scholar]
- 186.Szelenyi Z, Szekely M, Balasko M. Role of substance P (SP) in the mediation of endotoxin (LPS) fever in rats. Ann N Y Acad Sci 1997; 813:316-23; PMID:9100901; http://dx.doi.org/ 10.1111/j.1749-6632.1997.tb51713.x [DOI] [PubMed] [Google Scholar]
- 187.Reis RC, Brito HO, Fraga D, Cabrini DA, Zampronio AR. Central substance P NK receptors are involved in fever induced by LPS but not by IL-1beta and CCL3/MIP-1alpha in rats. Brain Res 2011; 1384:161-9; PMID:21303668; http://dx.doi.org/ 10.1016/j.brainres.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 188.Wang Y, Novotny M, Quaiserova-Mocko V, Swain GM, Wang DH. TRPV1-mediated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol 2008; 294:R1517-23; PMID:18337316; http://dx.doi.org/ 10.1152/ajpregu.00005.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ng SW, Zhang H, Hegde A, Bhatia M. Role of preprotachykinin-A gene products on multiple organ injury in LPS-induced endotoxemia. J Leukoc Biol 2008; 83:288-95; PMID:17998302; http://dx.doi.org/ 10.1189/jlb.0807575 [DOI] [PubMed] [Google Scholar]
- 190.Bret-Dibat JL, Kent S, Couraud JY, Creminon C, Dantzer R. A behaviorally active dose of lipopolysaccharide increases sensory neuropeptides levels in mouse spinal cord. Neurosci Lett 1994; 173:205-9; PMID:7523997; http://dx.doi.org/ 10.1016/0304-3940(94)90184-8 [DOI] [PubMed] [Google Scholar]
- 191.Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104:2S-8S; discussion 21S-2S [DOI] [PubMed] [Google Scholar]
- 192.Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem 2007; 42:3-27; PMID:17612044; http://dx.doi.org/ 10.1007/1-4020-5688-5_1 [DOI] [PubMed] [Google Scholar]
- 193.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science 1996; 274:1383-5; PMID:8910280; http://dx.doi.org/ 10.1126/science.274.5291.1383 [DOI] [PubMed] [Google Scholar]
- 194.Scheuren N, Bang H, Munster T, Brune K, Pahl A. Weak inhibitors of cyclooxygenases may exert their antinociceptive effect by modulation of transcription factors. Adv Exp Med Biol 1997; 433:51-4; PMID:9561102; http://dx.doi.org/ 10.1007/978-1-4899-1810-9_9 [DOI] [PubMed] [Google Scholar]
- 195.Stuhlmeier KM, Li H, Kao JJ, Ibuprofen: new explanation for an old phenomenon. Biochem Pharmacol 1999; 57:313-20; PMID:9890559; http://dx.doi.org/ 10.1016/S0006-2952(98)00301-3 [DOI] [PubMed] [Google Scholar]
- 196.Cazanave S, Vadrot N, Tinel M, Berson A, Letteron P, Larosche I, Descatoire V, Feldmann G, Robin MA, Pessayre D. Ibuprofen administration attenuates serum TNF-α levels, hepatic glutathione depletion, hepatic apoptosis and mouse mortality after Fas stimulation. Toxicol Appl Pharmacol 2008; 231:336-43; PMID:18572215; http://dx.doi.org/ 10.1016/j.taap.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 197.Wilkinson MF, Kasting NW. Central vasopressin V1-receptors mediate indomethacin-induced antipyresis in rats. Am J Physiol 1989; 256:R1164-8; PMID:2785772 [DOI] [PubMed] [Google Scholar]
- 198.Wilkinson MF, Kasting NW, Central vasopressin V1-blockade prevents salicylate but not acetaminophen antipyresis. J Appl Physiol (1985) 1990; 68:1793-8; PMID:2361881 [DOI] [PubMed] [Google Scholar]
- 199.De Souza GE, Cardoso RA, Melo MC, Fabricio AS, Silva VM, Lora M, De Brum-Fernandes AJ, Rae GA, Ferreira SH, Zampronio AR. A comparative study of the antipyretic effects of indomethacin and dipyrone in rats. Inflamm Res 2002; 51:24-32; PMID:11852909; http://dx.doi.org/ 10.1007/PL00000278 [DOI] [PubMed] [Google Scholar]
- 200.Wilkinson MF, Kasting NW, Vasopressin release within the ventral septal area of the rat brain during drug-induced antipyresis. Am J Physiol 1993; 264:R1133-8; PMID:8322966 [DOI] [PubMed] [Google Scholar]
- 201.Dong J, Xie XH, Lu DX, Fu YM. Effects of electrical stimulation of ventral septal area on firing rates of pyrogen-treated thermosensitive neurons in preoptic anterior hypothalamus from rabbits. Life Sci 2007; 80:408-13; PMID:17054999; http://dx.doi.org/ 10.1016/j.lfs.2006.09.021 [DOI] [PubMed] [Google Scholar]
- 202.Richmond CA, The role of arginine vasopressin in thermoregulation during fever. J Neurosci Nurs 2003; 35:281-6; PMID:14593940; http://dx.doi.org/ 10.1097/01376517-200310000-00007 [DOI] [PubMed] [Google Scholar]
- 203.Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diflunisal: a review of its pharmacological properties and therapeutic use in pain and musculoskeletal strains and sprains and pain in osteoarthritis. Drugs 1980; 19:84-106; PMID:6988202; http://dx.doi.org/ 10.2165/00003495-198019020-00002 [DOI] [PubMed] [Google Scholar]
- 204.Maher A, El-Sayed NS, Breitinger HG, Gad MZ. Overexpression of NMDAR2B in an inflammatory model of Alzheimer's disease: modulation by NOS inhibitors. Brain Res Bull 2014; 109:109-16; PMID:25454121; http://dx.doi.org/ 10.1016/j.brainresbull.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 205.Nuernberg B., Koehler G., and Brune K., Pharmacokinetics of diflunisal in patients. Clin Pharmacokinet 1991; 20:81-9; PMID:2029803; http://dx.doi.org/ 10.2165/00003088-199120010-00006 [DOI] [PubMed] [Google Scholar]
- 206.Engstrom Ruud L, Wilhelms DB, Eskilsson A, Vasilache AM, Elander L, Engblom D, Blomqvist A. Acetaminophen reduces lipopolysaccharide-induced fever by inhibiting cyclooxygenase-2. Neuropharmacology 2013; 71:124-9; PMID:23545161; http://dx.doi.org/ 10.1016/j.neuropharm.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 207.C Malvar Ddo, Aguiar FA, Vaz Ade L, Assis DC, de Melo MC, Jabor VA, Kalapothakis E, Ferreira SH, Clososki GC, de Souza GE. Dipyrone metabolite 4-MAA induces hypothermia and inhibits PGE2 -dependent and -independent fever while 4-AA only blocks PGE2 -dependent fever. Br J Pharmacol 2014; 171:3666-79; PMID:24712707; http://dx.doi.org/ 10.1111/bph.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Soares DM, Figueiredo MJ, Martins JM, Machado RR, Sorgi C, Faciolli LH, Alves-Filho JC, Cunha FQ, Souza GE. A crucial role for IL-6 in the CNS of rats during fever induced by the injection of live E. coli. Med Microbiol Immunol 2012; 201:47-60; PMID:21643979; http://dx.doi.org/ 10.1007/s00430-011-0204-3 [DOI] [PubMed] [Google Scholar]


