Abstract
Optical techniques are promising methods for measuring tissue temperature noninvasively due to the transparency of tissue to near infrared-light and the temperature dependent light-absorbing properties of endogenous absorbers, particularly water. Besides being noninvasive, the instruments are compact and portable, permitting bedside monitoring.
Keywords: absorption coefficient, brain temperature, piglet, reduced scattering coefficient, time-resolved near-infrared spectroscopy
Abbreviations
- CW
continuous wave
- TR-NIRS
time-resolved near-infrared spectroscopy
Clinical adaptation of mild hypothermia, in which the brain temperature is lower to 32–33°C, has been hampered by the lack of a reliable noninvasive method for measuring local brain temperature to adjust cooling and rewarming rate, since core measurements may not always reflect actual brain temperature, particularly during selective brain cooling techniques. Optical methods are promising techniques since they are safe and the instruments are compact and portable. In a recent paper,1 we showed the potential of time-resolved near-infrared spectroscopy (TR-NIRS) to measure brain temperature in newborn piglets. The developments of time-resolved methods have greatly improved the amount of information that can be acquired by taking advantage of discriminating photons based on their time-of-flight. This ability can be exploited to improve the sensitivity of TR-NIRS to deep brain temperature, especially for adults in whom the superficial layers of the head have substantial contribution to the measurements.
In the near-infrared region (650–1000 nm), the major endogenous tissue chromophores are water, oxy- and deoxy-hemoglobin. The aim of near-infrared spectroscopy is to quantify the concentrations of these chromophores in tissue, and this requires the ability to separate the effects of absorption from those of scattering. Fundamentally, the coefficients of absorption and reduced scattering can be determined by a series of reflectance measurements performed in one of the 3 general categories: continuous wave (CW) (with a constant intensity light source), frequency-domain (with a sinusoidal modulated source of light), and time-resolved (with a fast pulse of light).2 In fact, NIRS thermometry approaches based on broadband CW and diffuse optical spectroscopy have been investigated to predict tissue temperature in the adult forearm and breast, respectively, using the temperature response of water absorption peaks in the NIR spectral region.3-5 In principle, as pure water is heated the fraction of hydrogen bonds is reduced through thermal agitation of the water molecules, which overcomes the force of the hydrogen bonds. This in turn produces a blue-shift of the water absorption peaks. The water absorption peaks at ∼740, 840 and 970 nm decreases in amplitude by ∼0.8% °C−1, and shifts toward higher wavelengths with decreasing temperature.5 Water absorption spectra as a function of temperature are shown in Fig. 1A. Since 80–90% of the brain by mass is water, this fact suggests that brain temperature can be predicted by exploiting the temperature-dependency of water absorption of NIR light. Although temperature-dependent changes of hemoglobin NIR absorption spectra have also been reported,6 these changes are relatively small compared to the change in the water spectrum. Specifically, the amplitudes of the spectra of oxy- and deoxy-hemoglobin decrease by 0.1% °C−1 and 0.05% °C−1, respectively, when the temperature increases from 20–40°C.6
Figure 1.
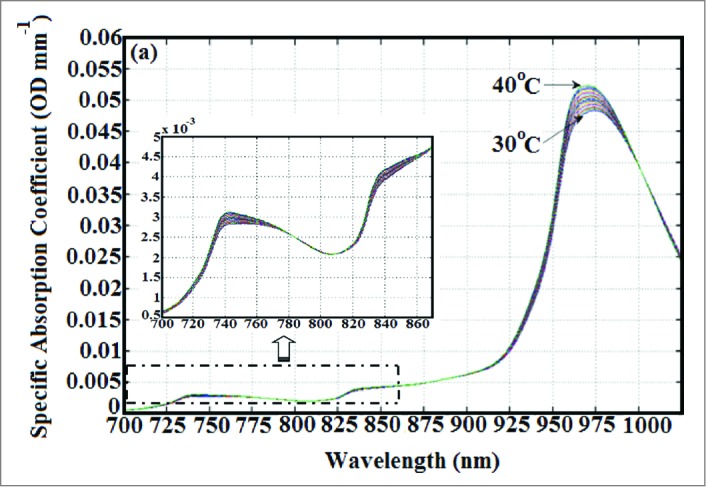
(A) NIR absorption spectra of pure water at various temperatures. (B) Close-up display of absorption spectra of pure water as a function of temperature in the wavelength range of 700–870 nm. These spectra were taken from the work of Hollis et al.5
Hollis et al. investigated the use of an in-vivo optical technique to predict tissue temperature. They used a broadband CW technique to measure reflectance over the spectral range of 650–1000 nm. The primary focus of their work was on the absorption peaks at 740 and 840 nm because the lower absorption of these peaks relative to the ∼970 nm peak allows for deeper penetration of light. Hollis et al. used the technique of principal component analysis to calibrate the pure water absorption spectrum against temperature to determine parameters associated with the temperature response that could then be used to predict temperature. Measurements performed on an adult forearm had a standard error of prediction of ∼1.2°C for the recovered temperature. Hollis et al. concluded that in order to improve results scattering should be accounted for and also using the water peak at ∼970 nm would provide more contrast although it would limit the light penetration depth in the tissue.5
Likewise, Chung et al.3 demonstrated a method that employs broadband diffuse optical spectroscopy to measure tissue temperature based on resolving water vibration frequency shifts in NIR water absorption spectra that occur with changes in temperature and macromolecular binding state. Diffuse optical spectroscopy unlike conventional reflectance methods acquires scattering-free absorption spectra, from 650 to 1000 nm, using a combination of frequency-domain and CW techniques to provide an absolute measure of absorption. The method focuses on the ∼970 nm water peak for greater contrast in temperature and uses the temperature isosbestic point at 996 nm to separate the macromolecular bound water contribution from the thermally induced spectral shift. This method has been tested in intralipid phantoms and human breast. The average difference between optical and thermistor measurements was 1.1°C ± 0.9°C in the temperature range of 28–48°C.3
We recently investigated the ability of TR-NIRS to measure temperature in tissue-mimicking phantoms (in-vitro) and brain tissue (in-vivo) during heating and cooling. TR-NIRS method has a number of advantages compare to CW and frequency-domain, specially the ability to distinguish early from late arriving photons which can provide better depth sensitivity.7 TR-NIRS temperature measurements were based on subtle changes in the NIR water absorption features at ∼740 and 840 nm. Although the temperature-dependent changes at these features are not as great as at ∼970 nm, this is compensated by the increased penetration depth at these wavelengths. TR-NIRS data were analyzed with the principal component analysis method, adapted from the work of Hollis et al.5, to predict tissue temperature. For in-vivo brain tissue temperature measurement, experiments were conducted on newborn piglets in which hypothermia was induced by gradual whole body cooling. The mean difference between the TR-NIRS and implanted thermocouple measurements was 0.5°C ± 1.6°C for the in-vivo measurements. In general, the accuracy of the temperature prediction by this technique can be improved by acquiring a continuous absorption spectrum which allows more accurate determination of chromophore concentration compared to discrete wavelengths as used in TR-NIRS. Improved spectral coverage can be obtained by combining TR-NIRS and broadband CW spectroscopy. In this configuration, spectral data would be acquired with CW spectroscopy while the TR data would provide absorption and scattering coefficients at a few selected wavelengths in the near-infrared region. These TR-NIRS derived coefficients are then used to calibrate the intensity of the CW measurements to estimate optical properties at all wavelengths in the spectral window of interest. Since the method is non-invasive and measurements can be obtained at the bedside in only a few minutes, it is believed that this technique could provide in-vivo brain temperature monitoring during hypothermia therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Bakhsheshi MF, et al. J Biomed Opt 2014; 19:057005; PMID:24817622; http://dx.doi.org/ 10.1117/1.JBO.19.5.057005 [DOI] [PubMed] [Google Scholar]
- 2. Delpy DT, et al. Philos Trans the Royal Soc B-Biol Sci 1997; 352:649-59; http://dx.doi.org/ 10.1098/rstb.1997.0046 [DOI] [Google Scholar]
- 3. Chung SH, et al. Phys Med Biol 2010; 55:3753-65; PMID:20551502; http://dx.doi.org/ 10.1088/0031-9155/55/13/012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung SH, et al. J Innov Opt Health Sci 2011; 4:361-72; PMID:22408653; http://dx.doi.org/ 10.1142/S1793545811001708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollis VS, et al. SPIE; 2001; 4250:470-81, San Jose, CA [Google Scholar]
- 6. Sfareni R, et al. Biochim Biophys Acta 1997; 1340:165-9; PMID:9252103; http://dx.doi.org/ 10.1016/S0167-4838(97)00042-3 [DOI] [PubMed] [Google Scholar]
- 7. Diop M, et al. Biomed Opt Express 2013; 4:447-59; PMID:23504445; http://dx.doi.org/ 10.1364/BOE.4.000447 [DOI] [PMC free article] [PubMed] [Google Scholar]


