Abstract
Background
68Ga-triacetylfusarinine C (TAFC) and 68Ga-ferrioxamine E (FOXE) show great potential to be used as highly sensitive and selective tracers for Aspergillus infection imaging. Here we report on a comparison of the ex vivo biodistribution and small animal imaging of 68Ga-TAFC and 68Ga-FOXE versus 68Ga-colloid and 68Ga-citrate as unspecific control in mice.
Methods
The radiochemical purity of tested 68Ga labelled tracers was determined by RP-HPLC or ITLC-SG. Ex vivo biodistribution was studied in normal DBA/2 mice 30 min and 90 min p.i. Static and dynamic imaging were performed using µPET/CT.
Results
68Ga-TAFC and 68Ga-FOXE showed rapid renal excretion and low blood values even 90 min p.i. 68Ga-TAFC showed almost no retention in other organs while 68Ga-FOXE displayed some uptake in gastrointestinal tract. 68Ga-colloid and 68Ga-citrate revealed significantly different ex vivo biodistribution. 68Ga-colloid showed pronounced radioactivity retention in the liver, while 68Ga-citrate displayed high blood values and significant retention of radioactivity in highly perfused organs.
Conclusions
From the results, both 68Ga-TAFC and 68Ga-FOXE have excellent and significantly different in vivo behaviour compared to 68Ga-colloid and 68Ga-citrate. 68Ga-TAFC in particular confirmed its great potential use as a specific tracer for Aspergillus infection imaging.
Keywords: gallium-68, siderophores, colloid, citrate, µPET imaging, aspergillosis
INTRODUCTION
Triacetylfusarinine C (TAFC) and ferrioxamine E (FOXE) are common trihydroxamate-type siderophores. Siderophores are relatively low molecular weight compounds produced by bacteria, fungi and some plants for scavenging iron from the environment to make this vital mineral available to the microbial cell1,2. It has recently been recognised that iron plays a fundamental role in infection in general3 and in fungal infections in particular. Schrettl et al.4 reported that the siderophore system is essential for the virulence of Aspergillus fumigatus, which is the main pathogen responsible for invasive aspergillosis (IA) complications.
IA is a life-threatening infection, hazardous especially for haematopoietic stem cell and solid organ transplant recipients, as well as for patients with solid tumours and haematological malignancies5,6. Early and accurate diagnosis of IA is crucial for the survival of such affected patients7. Unfortunately, currently available methods for the diagnosis of IA lack sufficient specificity and/or sensitivity.
We have recently shown that various siderophores can be labelled with generator produced 68Ga (ref.8), based on the similarities between iron (III) and gallium (III). Gallium-68 is a positron emitter that has recently attracted great interest for molecular imaging applications using positron emission tomography (PET) (ref.9–11). It can be produced from a long shelf-life and cost-effective generator system. The physical half-life of 68Ga (67.7 min) permits production and application of most low molecular weight radiopharmaceuticals such as peptides, oligonucleotides, antibody fragments and potentially also siderophores.
68Ga labelled TAFC and FOXE show great promise as highly selective, highly sensitive tracers for Aspergillus infection imaging12,13. This could prove indispensable in the early, accurate diagnosis of IA. For translation into clinical practice more data on the pharmacokinetics and biodistribution of these promising new tracers are warranted. Here we report on a comparison of in vivo biodistribution and small animal imaging of selected 68Ga-siderophores (68Ga-TAFC and 68Ga-FOXE) versus radiochemical impurities, which can develop during 68Ga labelling (68Ga-colloid and 68Ga-acetate) and a representative of infection imaging agents (68Ga-citrate) as unspecific control in mice.
MATERIALS AND METHODS
Reagents
All chemicals obtained commercially were of the highest available purity and were used without further purification. TAFC and FOXE were gained from EMC microcollections GmbH (Tuebingen, Germany) and 68Ge/68Ga generator from Eckert & Ziegler Eurotope GmbH (Berlin, Germany).
Radiolabelling
68GaCl3 was obtained from the 68Ge/68Ga generator using 0.1N HCl as eluent. 300 µL of the fractionated generator eluate (20-80 MBq of 68GaCl3) was added to a mixture of 10 µL of TAFC (10 µg in water) or 20 µL of FOXE (20 µg in 10% ethanol) and 30 µL of 1.1M sodium acetate solution. After 15 min at room temperature (TAFC) or 20 min at 80°C (FOXE), 100 µL of 1.1M sodium acetate solution were added to increase the pH to 6-7.
68Ga-acetate/colloid was prepared using excess (150 µL) of 1.1M sodium acetate solution mixed with the fractionated 68Ge/68Ga generator eluate (300 µL) to reach a reaction pH of 6-7 for 15 min at room temperature.
68Ga-colloid was prepared by mixing a ‘Monday’ fractionated 68Ge/68Ga generator eluate (300 µL) with 0.5M sodium hydroxide (55 µL). The reaction mixture was incubated for 15 min at room temperature.
Fractionated 68Ge/68Ga generator eluate (300 µL) was mixed with 0.5M sodium citrate pH ~ 5 (80 µL) for the preparation of 68Ga-citrate. The reaction mixture was incubated for 15 min at room temperature.
Quality control
The radiochemical purity of the 68Ga labelled tracers was determined using reversed-phase high-performance liquid chromatography (RP-HPLC) and/or instant thin layer chromatography on silica gel impregnated glass fiber sheets (ITLC-SG). Dionex UltiMate 3000 (Thermo Scientific, Waltham, MA, USA) and GABI Star (Raytest, Straubenhardt, Germany) radiometric detector were used for RP-HPLC analysis of 68Ga-siderophores. A Nucleosil 120-5 C18 250 × 40 mm column (WATREX, Prague, Czech Republic) with a flow rate of 1 mL/min was used with the following gradient: acetonitrile (ACN)/0.1% trifluoracetic acid (TFA)/H2O: 0-2 min, 0% ACN; 2-15 min, 0-36% ACN; 15-18 min, 36-60% ACN; 18-19.5 min, 60% ACN; 19.5-20 min, 60-0% ACN; 20-24 min, 0% ACN.
ITLC-SG (Varian, Lake Forest, CA, USA) was used for 68Ga-citrate, 68Ga-acetate/colloid and 68Ga-colloid quality control. The radiochemical purity of 68Ga-citrate was determined by ITLC-SG using methanol/glacial acetic acid (9:1) as mobile phase (free 68Ga, Rf = 0 and 68Gacitrate, Rf = 1) (ref.14). Two methods were used for the assessment of 68Ga-acetate/colloid and 68Ga-colloid using ITLC-SG as previously described15. The distribution of radioactivity along the ITLC-SG strips was measured on a Cyclone Plus Storage Phosphor system (PerkinElemer, Waltham, MA, USA).
Animal experiments
All animal experiments were conducted in accordance with regulations and guidelines of the Czech Animal Protection Act (No. 246/1992) with the approval of the Czech Ministry of Education Youth and Sports (MSMT-18933/2013-1), and the institutional Animal Welfare Committee of the Faculty of Medicine and Dentistry of Palacky University in Olomouc. The studies were performed using DBA/2 mice (Anlab, Prague, Czech Republic).
Biodistribution in mice
Normal DBA/2 mice (female) were retro-orbitally (r.o.) injected with a dose of 1-2 MBq per mouse of the 68Ga labelled tracer. Animals were sacrificed by cervical dislocation 30 min and 90 min postinjection (p.i.). The organs (blood, spleen, pancreas, stomach, intestine, kidneys, liver, heart, lung, muscle and femur) were removed and radioactivity was measured in an automatic gamma counter (Wizard2, PerkinElmer, Waltham, MA, USA). The results are expressed as percentage of injected dose per gram organ (%ID/g).
Animal imaging
The positron emission tomography (PET) and computed tomography (CT) images were acquired with an Albira PET/SPECT/CT small animal imaging system (Bruker Biospin Corporation, Woodbridge, CT, USA) (ref.16). Radiolabelled tracers were administered retroorbitally into DBA/2 mice in a dose of 5-10 MBq per mouse. Mice were subsequently anaesthetized with isoflurane (FORANE, Abbott Laboratories, Abbott Park, IL, USA) (2% flow rate) and were kept under anaesthesia during the imaging. Static PET/CT imaging was carried out 5 min, 30 min and 90 min p.i. A 5-min PET scan (axial FOV 148 mm) was performed, followed by a CT scan (axial FOV 65 mm, 45 kVp, 400 µA, at 600 projections). Dynamic imaging was carried out immediately after the injection of 68Ga labelled tracer for 90 min (5-min PET scan per frame). Scans were reconstructed with the Albira software (Bruker Biospin Corporation, Woodbridge, CT, USA) using maximum likelihood expectation maximization (MLEM) and filtered backprojection (FBP) algorithms16,17. After reconstruction, the acquired data were viewed and analyzed with PMOD software (PMOD Technologies Ltd., Zurich, Switzerland). The 3D images were obtained using VolView software (Kitware, Clifton Park, NY, USA).
RESULTS
Radiolabelling and analytics
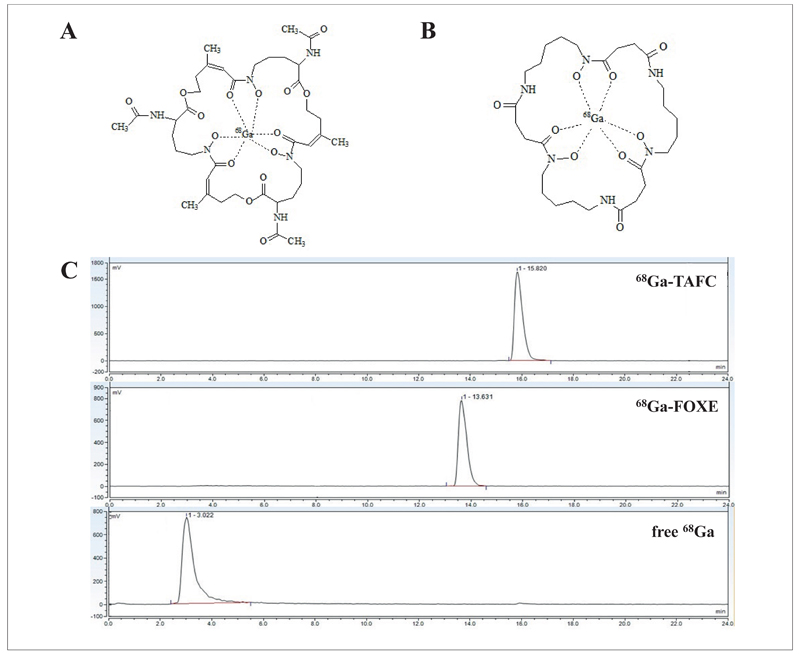
Both 68Ga labelled siderophores displayed high radiochemical purity (≥ 98%) and in vitro stability as previously described8. Fig. 1 shows the proposed chemical structures and radiochromatograms of 68Ga-TAFC and 68Ga-FOXE, with a single main peak for both 68Ga-siderophores (68Ga-TAFC retention time = 15.8 min and 68Ga-FOXE retention time = 13.6 min). Free 68Ga analyzed using RP-HPLC showed a single peak at the retention time (Rt) of 3 min as presented in Fig. 1. 68Ga-citrate, 68Ga-acetate/colloid and 68Ga-colloid were determined using ITLC-SG methods. 68Ga-citrate and 68Ga-colloid were prepared with a radiochemical purity of ≥ 95% and 68Ga-acetate/colloid mixture contained ≥ 75% of 68Ga-acetate.
Fig. 1.
Proposed chemical structures of 68Ga-TAFC (A) and 68Ga-FOXE (B) and HPLC-radiochromatograms of 68Ga-TAFC, 68Ga-FOXE and free 68Ga (C).
Ex vivo biodistribution
In normal DBA/2 mice (Table 1), both 68Ga-TAFC and 68Ga-FOXE showed rapid renal excretion with highest activity retained in kidneys (1.88±0.53 %ID/g for 68Ga-TAFC and 0.75±0.24 %ID/g for 68Ga-FOXE) and low blood values (0.24±0.10 %ID/g for 68Ga-TAFC and 0.04±0.01 %ID/g for 68Ga-FOXE) at 90 min after application. 68Ga-TAFC showed almost no retention in other organs, while 68Ga-FOXE displayed some uptake (0.92 ± 0.09 %ID/g (30 min p.i.) and 0.84±0.05 %ID/g (90 min p.i.)) in the gastrointestinal tract, which was confirmed by µPET imaging. 68Ga-acetate/colloid, 68Ga-colloid and 68Ga-citrate revealed significantly different ex vivo biodistribution compared to 68Ga-siderophores (see Table 1). High blood values (17.0±1.0 %ID/g at 90 min p.i.) and pronounced retention of radioactivity in highly perfused organs (4.1±1.1 %ID/g for spleen, 4.3±0.5 %ID/g for liver, 5.2±1.2 %ID/g for heart, 7.2±0.7 %ID/g for lung at 90 min p.i.) were found in mice injected with 68Ga-acetate/colloid. 68Ga-citrate showed comparable in vivo behaviour as 68Ga-acetate/colloid. The highest level of radioactivity was found in the blood (19.9±1.5 %ID/g at 90 min p.i.) and highly perfused organs revealed significant levels of retained activity (5.9±2.7 %ID/g for spleen, 3.8±0.3 %ID/g for liver, 9.0±0.7 %ID/g for heart, 10.9±1.4 %ID/g for lung at 90 min p.i.). In contrast, 68Ga-colloid displayed predominant liver uptake (36.2±2.7 %ID/g at 90 min p.i.).
Table 1.
Biodistribution of 68Ga-TAFC, 68Ga-FOXE, 68Ga-acetate/colloid, 68Ga-colloid and 68Ga-citrate in normal DBA/2 mice 30 min and 90 min p.i.
| Organ | 68Ga-TAFC | 68Ga-FOXE | 68Ga-acetate/colloid | 68Ga-colloid | 68Ga-citrate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 min | 90 min | 30 min | 90 min | 30 min | 90 min | 30 min | 90 min | 30 min | 90 min | |
| Blood | 2.92 ± 1.48 | 0.24 ± 0.09 | 1.04 ± 0.10 | 0.04 ± 0.01 | 21.7 ± 2.64 | 17.0 ± 1.00 | 10.8 ± 0.32 | 8.40 ± 1.04 | 20.8 ± 2.60 | 19.9 ± 1.53 |
| Spleen | 0.68 ± 0.12 | 0.09 ± 0.04 | 0.35 ± 0.13 | 0.05 ± 0.01 | 5.01 ± 0.06 | 4.05 ± 1.08 | 9.72 ± 2.68 | 5.91 ± 2.67 | 3.49 ± 0.70 | 4.16 ± 0.64 |
| Pancreas | 1.04 ± 0.39 | 0.13 ± 0.01 | 0.52 ± 0.23 | 0.06 ± 0.02 | 3.54 ± 0.45 | 2.57 ± 0.47 | 1.85 ± 0.42 | 1.51 ± 0.30 | 4.16 ± 0.16 | 3.70 ± 0.28 |
| Stomach | 1.64 ± 0.48 | 0.35 ± 0.41 | 0.63 ± 0.16 | 0.06 ± 0.01 | 2.35 ± 0.21 | 2.84 ± 0.45 | 1.38 ± 0.13 | 1.32 ± 0.01 | 3.21 ± 0.54 | 3.62 ± 0.29 |
| Intestine | 1.37 ± 0.35 | 0.49 ± 0.25 | 0.92 ± 0.09 | 0.84 ± 0.05 | 2.78 ± 0.42 | 3.85 ± 0.30 | 1.29 ± 0.15 | 1.55 ± 0.07 | 3.30 ± 0.38 | 4.29 ± 0.23 |
| Kidneys | 11.8 ± 3.60 | 1.88 ± 0.53 | 3.12 ± 0.29 | 0.75 ± 0.24 | 5.59 ± 0.27 | 4.44 ± 0.31 | 2.46 ± 0.63 | 2.73 ± 0.53 | 5.46 ± 1.40 | 5.79 ± 0.32 |
| Liver | 0.79 ± 0.30 | 0.11 ± 0.02 | 0.86 ± 0.02 | 0.13 ± 0.01 | 4.84 ± 0.85 | 4.34 ± 0.49 | 36.7 ± 5.92 | 36.2 ± 2.67 | 3.38 ± 0.34 | 3.86 ± 0.35 |
| Heart | 1.10 ± 0.36 | 0.08 ± 0.02 | 0.46 ± 0.12 | 0.05 ± 0.01 | 7.06 ± 0.71 | 5.25 ± 1.16 | 3.35 ± 0.31 | 2.65 ± 0.50 | 8.12 ± 1.28 | 9.05 ± 0.77 |
| Lung | 2.51 ± 0.97 | 0.27 ± 0.12 | 0.95 ± 0.15 | 0.10 ± 0.01 | 8.45 ± 0.33 | 7.24 ± 0.66 | 4.76 ± 0.89 | 5.57 ± 1.25 | 10.6 ± 3.76 | 10.9 ± 1.46 |
| Muscle | 0.65 ± 0.31 | 0.35 ± 0.15 | 0.22 ± 0.06 | 0.06 ± 0.03 | 1.22 ± 0.23 | 1.51 ± 0.51 | 0.68 ± 0.10 | 0.63 ± 0.06 | 1.79 ± 0.23 | 1.93 ± 0.19 |
| Femur | 0.55 ± 0.06 | 0.76 ± 0.49 | 0.15 ± 0.05 | 0.12 ± 0.09 | 1.64 ± 0.32 | 1.84 ± 0.24 | 1.20 ± 0.05 | 1.17 ± 0.07 | 2.51 ± 0.37 | 2.81 ± 0.50 |
Data are presented as % injected dose per gram organ (%ID/g ± Sd) (n = 3).
Animal imaging
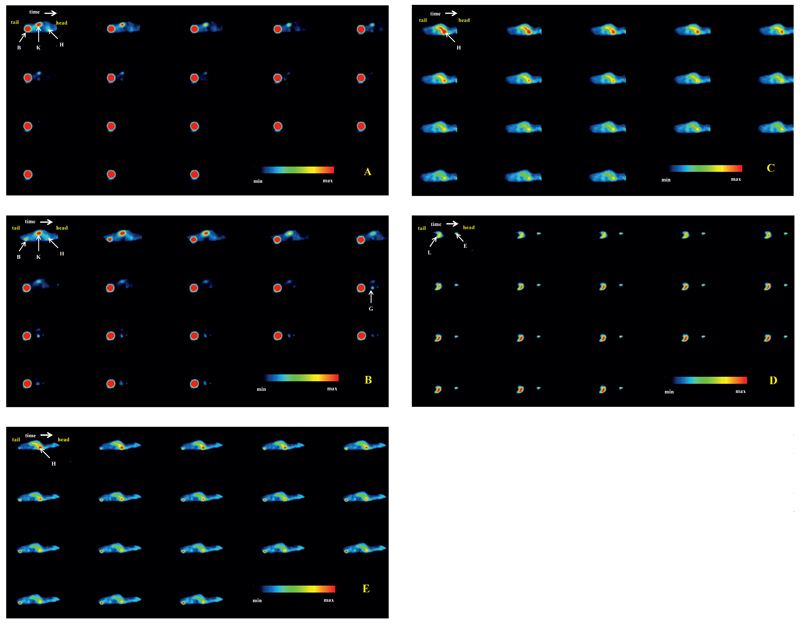
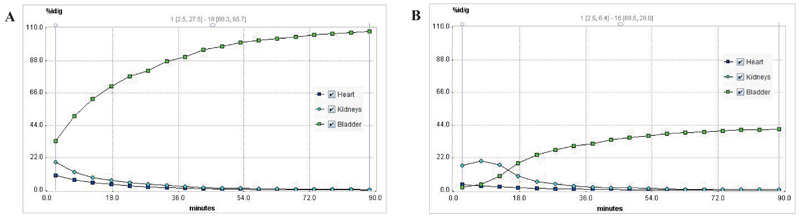
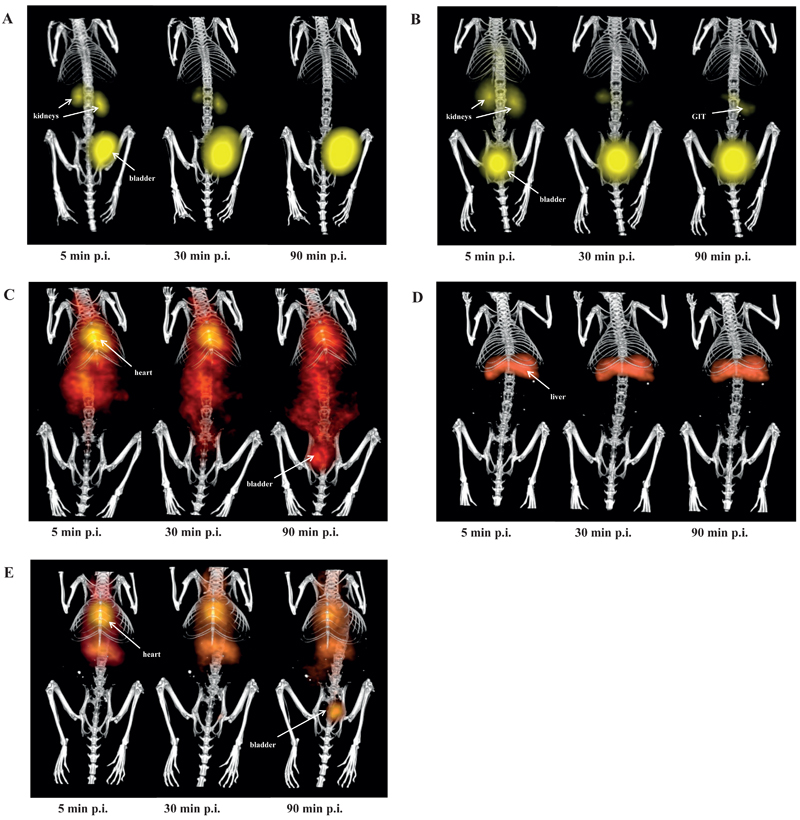
µPET imaging of normal DBA/2 mice confirmed rapid renal excretion of 68Ga-TAFC showing almost all injected activity in bladder at 45 min p.i. (Fig. 2 A and 3 A). 68Ga-FOXE revealed similar biodistribution compared to 68Ga-TAFC. The only relevant exception was an evident uptake of 68Ga-FOXE in gastrointestinal tract, conspicuous from 50 min frame of dynamic imaging (Fig. 2 B and 3 B). In contrast, both 68Ga-acetate/colloid and 68Gacitrate showed very slow in vivo kinetics with high retention of radioactivity in the blood pool (Fig. 2 C and E). 68Ga-colloid displayed rapid liver uptake slowly increasing over time (Fig. 2 D). Static imaging (Fig. 4) confirmed and supported the results gained from ex vivo biodistribution and dynamic imaging.
Fig. 2.
Dynamic µPET imaging of 68Ga-TAFC (A), 68Ga-FOXE (B), 68Ga-acetate/colloid (C), 68Ga-colloid (D) and 68Ga-citrate (E) up to 90 min p.i. (sagittal slices; injected dose: 5-10MBq; anaesthesia: 2% isoflurane; scan duration: 5-minute PET scan per frame (18 frames); B – bladder, E – eye, G – gastrointectinal tract, H – heart, K – kidney, L - liver).
Fig. 3.
Time activity curves of selected regions of interest (heart, kidneys, bladder) of 68Ga-TAFC (A) and 68Ga-FOXE (B).
Fig. 4.
Static µPET/CT imaging of 68Ga-TAFC (A), 68Ga-FOXE (B), 68Ga-acetate/colloid (C), 68Ga-colloid (D) and 68Ga-citrate (E) 5 min, 30 min and 90 min p.i. (supine position; injected dose 5-10MBq; anaesthesia: 2% isoflurane; scan duration: 5-minute PET scan followed by 15-minute CT scan).
DISCUSSION
The development of 68Ga-radiopharmaceuticals has increased enormously in the last five years. The renewed popularity of 68Ga was initiated mainly by the development of new, simple to use 68Ge/68Ga generator systems, by the fact that positron emission tomography (PET) has become a routine clinical and preclinical imaging modality and owing to the favourable chemical properties of 68Ga and developed 68Ga labelling strategies including automation11,18.
The commercially available 68Ge/68Ga generator from Eckert & Ziegler Eurotope GmbH (Berlin, Germany) used for our study provides 68Ga in its ionic form as 68Ga3+. Elution is performed using hydrochloric acid solution, since acidic conditions are required to inhibit hydrolysis of the 68Ga3+ ion (pH < 3), which is a requirement for chemical processing and successful radiolabelling10.
The in-house accessibility, favourable radiochemical properties of 68Ga3+ and chemical similarities with Fe3+, which is chelated by siderophores, led us to attempt 68Ga labelling of selected siderophores. These attempts were successful and we have shown that various siderophores can be labelled with 68Ga (ref.8). Moreover, we have demonstrated that 68Ga-TAFC and 68Ga-FOXE are highly selectively accumulated in the infected tissue in Aspergillus fumigatus rat infection model12,13 and 68Ga-TAFC revealed high in vitro specificity towards Aspergillus fumigatus19. Our studies showed, that 68Ga-TAFC in particular appears to be promising candidate for the noninvasive detection of Aspergillus infections by PET.
In this work, we studied in vivo kinetics and biodistribution of different 68Ga labelled tracers: 68Ga-siderophores (TAFC and FOXE), 68Ga-citrate, 68Ga-colloid and 68Ga-acetate/colloid. 68Ga-citrate was chosen as a representative of infection imaging tracer due to successful application of 67Ga-citrate for SPECT imaging in the past20 and recent reports on the clinical use of 68Ga-Citrate21. 68Ga-colloid is a radiochemical impurity, which can occur during 68Ga labelling at higher pH and could influence the biodistribution of improperly prepared 68Ga-siderophores. 68Ga-acetate/colloid mixture was studied to compare the in vivo behaviour of 68Ga-TAFC and 68Ga-FOXE versus reaction mixture used for 68Ga labelling of these siderophores.
68Ga-TAFC and 68Ga-FOXE showed high radiochemical purity and similar retention times using previously described RP-HPLC method12, while 68Ga-citrate, 68Ga-colloid and 68Ga-acetate/colloid displayed completely different analytical behaviour using ITLC-SG. Subsequent in vivo small animal imaging showed rapid elimination of both 68Ga-siderophores mainly via kidneys. This supports the findings of high in vivo stability of these complexes with only intact 68Ga-TAFC and 68Ga-FOXE found as urinary excretion products12. The only significant difference in the biodistribution of 68Ga-TAFC and 68Ga-FOXE was found in certain uptake of 68Ga-FOXE in gastrointestinal tract in later time points, which could be caused by the binding of 68Ga-FOXE to intestinal microflora. This hypothesis is supported by our previous study, testing of uptake specificity of 68Ga-TAFC and 68Ga-FOXE in various microorganisms19, which showed clear uptake of 68Ga-FOXE not only in Aspergillus fumigatus, but also in another tested fungal and bacterial species, indicating higher specificity of 68Ga-TAFC for imaging Aspergillus fumigatus infections. µPET/CT imaging of 68Ga-citrate in mice revealed slow excretion of the radioactivity with high blood pool values, which is in accordance with the investigations of Kumar et al.22, who have studied 68Ga-citrate for diagnostic imaging of infection in rats. 68Ga-acetate/colloid displayed comparable in vivo behaviour to 68Ga-citrate. Both citrate and acetate are weak chelators in vivo. 68Ga is rapidly released from the weak complex and bound to transferrin, ferritin and other iron-binding proteins23, which explain the high blood pool values for both tracers. The in vivo imaging of 68Ga-colloid showed clear rapid liver uptake slowly increasing in time. The ex vivo biodistribution data showed perfect correlation with in vivo dynamic and static imaging.
CONCLUSIONS
Both studied 68Ga labelled siderophores displayed excellent and significantly different in vivo behaviour compared to 68Ga-citrate, 68Ga-colloid and 68Ga-acetate/colloid, and especially 68Ga-TAFC confirmed its great potential to be used as specific tracer for Aspergillus infection imaging. These data on normal biodistribution and pharmacokinetics of 68Ga-TAFC and 68Ga-FOXE are essential for further translation of these promising compounds into the clinic.
ACKNOWLEDGMENTS
We would like to thank the staff of the Animal Facilities of Institute of Molecular and Translational Medicine of Faculty of Medicine and Dentistry of Palacky University in Olomouc. We gratefully acknowledge the financial support of Biomedreg project CZ.1.05/2.1.00/01.0030, National Programme of Sustainability LO1304 and the Austrian Science Foundation (FWF; grant L676-B18 to C.D. and I1346-B2 to H.H.).
Footnotes
Authorship contributions: MP, HH, CD: study design; MP, CD: manuscript writing; MP, AV: literature search; AV, MP, ZN: data collection; MP, LU: quality control; AV, MP, ZN: data analysis; MP, CD, HH, LU: data interpretation; AV, MP: figures, tables.
Conflict of interest statement: The authors state that there are no conflicts of interest regarding the publication of this article.
References
- 1.Neilands JB. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–6. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 2.Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–57. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 3.Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32:70–8. doi: 10.1046/j.1365-2362.2002.0320s1070.x. [DOI] [PubMed] [Google Scholar]
- 4.Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jöchl C, Moussa TA, Wang S, Gsaller F, Blatzer M, Werner ER, et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoSPathog. 2010;6(9):e1001124. doi: 10.1371/journal.ppat.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50:1559–67. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N. Risk assessment and prognostic factors for mouldrelated diseases in immunocompromised patients. J Antimicrob Chemother. 2011;66:i5–14. doi: 10.1093/jac/dkq437. [DOI] [PubMed] [Google Scholar]
- 7.Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743–55. doi: 10.1183/09031936.02.00256102. [DOI] [PubMed] [Google Scholar]
- 8.Petrik M, Haas H, Schrettl M, Helbok A, Blatzer M, Decristoforo C. In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl Med Biol. 2012;39:361–9. doi: 10.1016/j.nucmedbio.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fani M, André JP, Maecke HR. 68Ga-PET: a powerful generator based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3:53–63. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- 10.Decristoforo C. Gallium-68 – a new opportunity for PET available from a long shelf-life generator – automation and applications. Curr Radiopharm. 2012;5:212–20. doi: 10.2174/1874471011205030212. [DOI] [PubMed] [Google Scholar]
- 11.Velikyan I. Prospective of 68Ga-radiopharmaceutical development. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrik M, Haas H, Dobrozemsky G, Lass-Flörl C, Helbok A, Blatzer M, Dietrich H, Decristoforo C. 68Ga-Siderophores for PET imaging of invasive pulmonary aspergillosis: proof of principle. J Nucl Med. 2010;51:639–45. doi: 10.2967/jnumed.109.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrik M, Franssen GM, Haas H, Laverman P, Hörtnagl C, Schrettl M, Helbok A, Lass-Flörl C, Decristoforo C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur J Nucl Med Mol Imaging. 2012;39:1175–83. doi: 10.1007/s00259-012-2110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzello A, Di Pierro D, Lodi F, Trespidi S, Cicoria G, Pancaldi D, Nanni C, Marengo M, Marzola MC, Al-Nahhas A, Rubello D, et al. Synthesis and quality control of 68Ga citrate for routine clinical PET. Nucl Med Comm. 2009;30:542–5. doi: 10.1097/MNM.0b013e32832b9ac8. [DOI] [PubMed] [Google Scholar]
- 15.Ocak M, Antretter M, Knopp R, Kunkel F, Petrik M, Bergisadi N, Decristoforo C. Full automation of 68Ga labelling of DOTA-peptides including cation exchange prepurification. Appl Radiat Isot. 2010;68:297–302. doi: 10.1016/j.apradiso.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez F, Orero A, Soriano A, Correcher C, Conde P, González A, Hernández L, Moliner L, Rodríguez-Alvarez MJ, Vidal LF, Benlloch JM, et al. ALBIRA: a small animal PET/SPECT/CT imaging system. Med Phys. 2013;40:051906. doi: 10.1118/1.4800798. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez F, Moliner L, Correcher C, Gonzalez A, Orero A, Carles M, Soriano A, Rodriguez-Alvarez MJ, Medina LA, Mora F, Benlloch JM. Small animal PET scanner based on monolithic LYSO crystals: performance evaluation. Med Phys. 2012;39:643–53. doi: 10.1118/1.3673771. [DOI] [PubMed] [Google Scholar]
- 18.Rösch F. Past, present and future of 68Ge/68Ga generators. Appl Radiat Isot. 2013;76:24–30. doi: 10.1016/j.apradiso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Petrik M, Haas H, Laverman P, Schrettl M, Franssen GM, Blatzer M, Decristoforo C. 68Ga-Triacetylfusarinine C and 68Ga-Ferrioxamine E for Aspergillus infection imaging: uptake specificity in various microorganisms. Mol Imaging Biol. 2014;16:102–8. doi: 10.1007/s11307-013-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes DK. Nuclear medicine and infection detection: the relative effectiveness of imaging with 111In-oxine-, 99mTc-HMPAO-, and 99mTc-stannous fluoride colloid-labeled leukocytes and with 67Ga-citrate. J Nucl Med Technol. 2003;31:196–201. [PubMed] [Google Scholar]
- 21.Nanni C, Errani C, Boriani L, Fantini L, Ambrosini V, Boschi S, Rubello D, Pettinato C, Mercuri M, Gasbarrini A, Fanti S. 68Ga-citrate PET/CT for evaluating patients with infections of the bone: preliminary results. J Nucl Med. 2010;51:1932–6. doi: 10.2967/jnumed.110.080184. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V, Boddeti DK, Evans SG, Angelides S. 68Ga-Citrate-PET for diagnostic imaging of infection in rats and for intra-abdominal infection in a patient. Curr Radiopharm. 2012;5:71–5. doi: 10.2174/1874471011205010071. [DOI] [PubMed] [Google Scholar]
- 23.Silvola JM, Laitinen I, Sipilä HJ, Laine VJ, Leppänen P, Ylä-Herttuala S, Knuuti J, Roivainen A. Uptake of 68gallium in atherosclerotic plaques in LDLR-/-ApoB100/100 mice. EJNMMI Res. 2011;1(1):14. doi: 10.1186/2191-219X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]