Abstract
Background
Maternal vitamin D status has been associated with lower bone mass of the offspring in many, but not all, observational studies. However, proof that maternal vitamin D repletion during pregnancy improves offspring bone mass is lacking.
Methods
Between 06/10/2008 and 11/02/2014, we randomly assigned pregnant women with a serum 25-hydroxyvitamin D [25(OH)D] 25-100nmol/l at 12 weeks’ gestation to either 1000IU/day cholecalciferol or matched placebo from 14 weeks’ gestation until delivery. Serum 25(OH)D was measured at 14 and 34 weeks’ gestation. Neonatal whole body bone mineral, assessed within 2 weeks after birth (n=665) by dual-energy X-ray absorptiometry (DXA), was the primary outcome. Secondary pre-specified analyses explored interactions with study centre, maternal ethnicity, parity, compliance, protocol completion, baseline BMI, baseline 25(OH)D and change in 25(OH)D from 14 to 34 weeks; and offspring sex and season of birth.
Findings
We found no difference in neonatal whole body bone mineral content (BMC) of infants born to mothers randomised to 1000IU/day cholecalciferol compared with infants born to mothers randomised to placebo [61.6g (95%CI: 60.3, 62.8g) vs 60.5g (95%CI: 59.3, 61.7g) respectively, p=0.21].
Interpretation
Supplementation of mothers with 1000IU/day cholecalciferol during pregnancy did not lead to increased offspring whole body BMC compared with placebo.
Keywords: Vitamin D, cholecalciferol, supplementation, trial, osteoporosis, epidemiology, DXA, pregnancy, neonate
BACKGROUND AND OBJECTIVES
Osteoporosis is a devastating disease and its high prevalence makes it eminently suitable for population-wide public health interventions aimed at optimising bone health.1 There is increasing evidence that early growth, and factors acting in utero or during early infancy, may influence the trajectory of long-term skeletal accrual to peak bone mass.1 In particular, maternal serum 25(OH)-vitamin D [25(OH)D] concentrations in pregnancy have been associated with offspring bone morphology2-5 and bone mass6,7 up to young adulthood.8 The main determinant of 25(OH)D concentrations in most populations is UVB exposure to the skin, which varies markedly by season in temperate climes.9 Seasonal differences in neonatal BMC have been demonstrated,10,11 with effects potentially modified by vitamin D supplementation,11 and maternal UVB exposure during pregnancy has been positively associated with bone mass in childhood.6,12 However, not all studies have demonstrated a benefit of higher maternal 25(OH)D levels in pregnancy on childhood skeletal health.13-15 Whole body bone mineral content (BMC) is the recommended measure of bone mass in children; although of limited clinical utility in neonate due to the lack of normative data,16 infant DXA been widely used in research studies,17-20 where comparisons are internal. Childhood BMC is inversely related to childhood fracture risk,21; although data spanning from conception to peak bone mass in a single cohort are lacking, the current evidence-base supports tracking of bone mineral content over this time,22-25 and the magnitude of peak bone mass achieved is an important determinant of future fracture risk.26 The aim of the present trial was therefore to test the hypothesis that neonates born to mothers supplemented with vitamin D during pregnancy would have greater whole body bone mineral content (BMC) at birth than those of mothers who had not received supplementation.27 Given the previously documented importance of season for both 25(OH)D concentrations and childhood bone mass, we further hypothesised that there would be an interaction between season of birth and treatment effect.
METHODS
Trial design
A detailed description of The Maternal Vitamin D Osteoporosis Study (MAVIDOS) has been previously published.27 Briefly, MAVIDOS is a multicentre, double-blind, randomised, placebo-controlled trial of vitamin D supplementation in pregnancy in the United Kingdom. The study was conducted in accordance with guidelines laid down in the Declaration of Helsinki and was approved by the Southampton and South West Hampshire Research Ethics Committee. MAVIDOS was registered prospectively (ISRCTN:82927713; EUDRACT:2007-001716-23); full approval from UK MHRA was granted, and written, informed consent was obtained from all participants.
Participants
Pregnant women were recruited when attending for early pregnancy ultrasound screening at three study sites [University Hospital Southampton NHS Foundation Trust, Southampton, UK; Oxford University Hospitals NHS Trust, Oxford, UK; Sheffield Hospitals NHS Trust (University of Sheffield), Sheffield, UK] between 06/10/2008 and 11/02/2014. Inclusion criteria were: age over 18 years, singleton pregnancy, gestation less than 17 weeks based on last menstrual period (LMP) and ultrasound measurements, and aiming to give birth at the local maternity hospital. Women with known metabolic bone disease, renal stones, hyperparathyroidism or hypercalciuria, those with a diagnosis of cancer in the last 10 years, those unable to give informed consent or comply with the protocol, those taking medication known to interfere with fetal growth, those with fetal anomalies on ultrasonography and women already using >400IU/day vitamin D supplementation were excluded. A screening blood sample was obtained and analysed on the local NHS platforms and only 25 and 100nmol/l and serum calcium<2.75mmol/l were eligible to enrol in the study. All three laboratories (Southampton, Oxford, Sheffield) were accredited by the Vitamin D External Quality Assessment Scheme (DEQAS) (http://www.deqas.org/).
Trial assessments
Questionnaire and anthropometry
All participants received standard antenatal care, and were able to continue self-administration of antenatal multivitamins containing up to 400IU/day vitamin D. Women were assessed in detail at 14 and 34 weeks’ gestation including assessments of diet (including calcium and vitamin D intake), smoking, alcohol consumption, health, physical activity, medications, supplements (all interviewer-led questionnaires) and anthropometry.27 Anthropometric measurements of the newborns were obtained, and information on obstetric complications was extracted from maternity records.
Biochemical
Study blood samples were collected from the mother at 14 and 34 weeks’ gestation, and stored at −80°C after processing. Measurement of plasma 25(OH)D (Liaison RIA automated platform, Diasorin, Minnesota, USA), calcium, alkaline phosphatase, and albumin was undertaken centrally (MRC Human Nutrition Research, Cambridge, UK) in a single batch at the end of the study. Measurement of vitamin D binding protein is ongoing. Details of assay performance and quality control through participation in DEQAS, NIST and NEQAS are given elsewhere.28,29
DXA
The baby underwent DXA assessment at whole body and lumbar spine sites [Hologic Discovery (Hologic Inc., Bedford, MA, USA) or GE-Lunar iDXA (GE-Lunar, Madison, Wisconsin, US) with neonatal software] within 2 weeks after birth. In order to maximise scan quality, the infant was undressed, and clothed in a standard towel, fed and pacified before the assessment. Each instrument underwent daily QC, with cross-calibration between sites. The total radiation dose was estimated as 0.04mSv, equivalent to approximately 7 days’ exposure to background radiation in the UK. All DXA images were reviewed by two operators for movement artefacts and quality.
Interventions, randomisation and blinding
Women were randomised at 14 weeks’ gestation (or as soon as possible before 17 weeks’ gestation if recruited later) to either cholecalciferol 1000IU/day or matched placebo [Merck KGaA, (Darmstadt, Germany)/ Sharp Clinical Services (Crickhowell, UK; previously DHP-Bilcare)]. Packs were randomised in a 1:1 ratio in randomly permuted blocks of 10, starting randomly midway through the block, and sequentially numbered, by Sharp Clinical Services Ltd prior to delivery to the study sites, and then dispensed in order by each study pharmacist. Each pack contained sufficient capsules for the study duration and both the participant and research team were blind to treatment allocation throughout the study duration.
Outcomes
The primary outcome was whole body BMC of the neonate. Although we had originally planned to use whole body BMC adjusted for age, it was judged following further statistical review, in this randomised controlled trial setting, appropriate to include offspring age in a sensitivity analysis, rather than as the primary outcome. Secondary outcomes included maternal 25(OH)D concentration at 34 weeks’ gestation; change in 25(OH)D between 14 and 34 weeks’ gestation, neonatal whole body bone area and bone mineral density, and neonatal bone indices at the spine. In order to preserve statistical power, rather than perform separate analyses (as planned in original protocol) for those who completed the protocol, complied with treatment, demonstrated a rise in 25(OH)D, and stratification by baseline 25(OH)D, we explored these potential effect modifiers via their incorporation as interaction terms in regression models (described below). Safety analyses examined the frequency of adverse outcomes including: infection, nausea/vomiting, diarrhoea, abdominal pain, headache, hypertension, hypercalcaemia (greater than equal to 2.75mmol/l) at 34 weeks’ gestation, intrauterine growth restriction (IUGR), preterm birth (less than 37 weeks’ gestation), instrumental delivery, severe postpartum haemorrhage, stillbirth or neonatal death, congenital abnormalities.
Sample size
We estimated the sample size using the results from the Princess Anne Hospital Study,6 in which a difference of 0.42SD in whole body BMC was found between the infants of mothers who had been vitamin D deficient and those of mothers who had been vitamin D replete during pregnancy. Given this single observational study, we powered the trial conservatively, calculating that to detect 50% of this difference in whole body BMC at birth between the neonates of mothers who were deficient in vitamin D versus those replete in pregnancy (0.21SD or 3.5g), at the 5% significance level with 90% power, would require recruitment of 477 neonates in each arm.
Statistical analysis
We undertook analyses on an intention to treat (ITT) basis for all those with a neonatal DXA assessment; the analysis plan was published prior to unblinding of the study.27 At the request of the Data Monitoring Committee, an interim safety analysis of serum calcium concentration was requested after two years of recruitment, but no analysis of DXA outcomes was undertaken until follow-up of all participants had been completed. All data were checked for normality by visual inspection of histograms. Data were assumed to be missing at random. Comparison was made between treatment groups using Student t-test and Mann-Witney U test for normally and non-normally distributed outcomes respectively. Categorical outcomes were compared using χ2 test. DXA indices included neonatal whole body bone area, bone mineral content, bone mineral density, lean and fat. In order to assess bone mass independent of body size, we used bone mineral content adjusted for birth length in a regression model. Given the seasonal change in the 25(OH)-vitamin D observed in many previous studies, we hypothesised, a priori, that there might be an interaction between treatment and season of birth. We defined season of birth using the UK Meteorological Office classification, as winter (December-February), spring (March-May), summer (June-August) and autumn (September-November) [www.metoffice.gov.uk], and secondly explored differences in treatment effect by individual month of birth. We also investigated pre-specified interactions between treatment and offspring sex, and between treatment and ethnicity, as both these factors have been associated with variations in vitamin D metabolism. Since there is clear evidence of differences in body composition between first and subsequent offspring1, and an inverse relationship between body mass index and 25(OH)D concentration, we hypothesised interactions between each of these 2 variables and treatment. Finally we reasoned that treatment might be more effective in those who fully complied with the protocol and were compliant with medication, who had low concentrations of 25(OH)D at baseline, or in those with a greater change in 25(OH)D from 14 to 34 weeks, and that for a combination of reasons given above, there might be differences by study centre, providing the basis for the final 5 interaction analyses. In summary, the interactions tested were with: study centre, maternal ethnicity, parity, compliance, protocol completion, baseline BMI, baseline 25(OH)D and change in 25(OH)D from 14 to 34 weeks; and offspring sex and season of birth. All these interactions were explored in multivariable linear regression (with the independent variables, for example: treatment; season; treatment*season, and inclusion of no other covariates). In further analyses we adjusted bone outcomes for postnatal age at DXA. Given that the secondary analyses were pre-specified and hypothesis-based, and that the study was powered for the primary outcome, it was not judged appropriate to undertake correction for multiple testing, recognising that any statistically significant results from the secondary analysis would require further confirmation in future studies. With 10 analyses and an alpha of 0.05, we calculated that the probably of observing one or more false positive associations was 40% [equal to (1-0.95^10)*100]. All analysis was performed by SD, SRC and HMI using Stata v13.1 (Statacorp, College Station, Texas, USA). A p value of <0.05 was accepted as statistically significant.
Role of the funding source
The study was funded by Arthritis Research UK, UK Medical Research Council, UK National Institute for Health Research, and the Bupa Foundation. The original protocol incorporated suggestions from the Arthritis Research UK Clinical Trials Collaboration. The funders had no other role in the study and the corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Participants
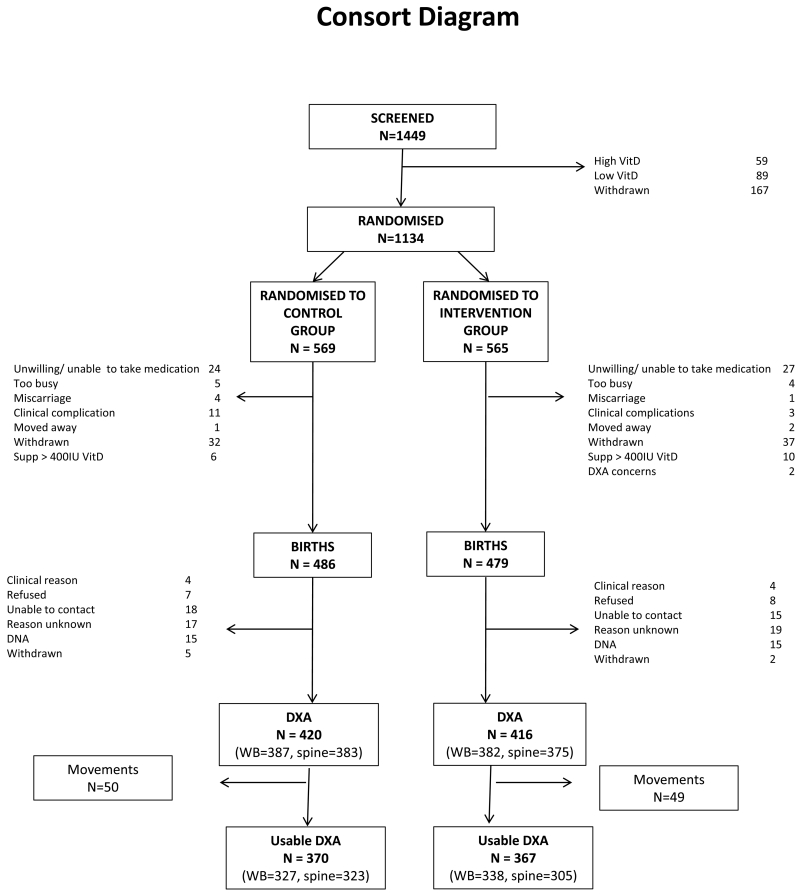
A total of 1449 women were initially eligible after screening and consented to a blood test to determine early pregnancy 25(OH)D status. Of these, 148 were ineligible to participate due to either 25(OH)D<25nmol/l (n=59) or 25(OH)D>100nmol/l (n=89). None had a plasma calcium >2.75 mmol/L. A further 167 women withdrew prior to randomisation. Thus, 1134 were randomised (Figure 1), of whom 965 (85.1%) remained in the study until delivery. A total of 836 (73.7%) neonates had a DXA scan. After excluding scans with significant movement artefact, DXA scan data were available for 737 neonates (65.0%), comprising 665 assessments at the whole body (intervention, n=338; placebo, n=327) and 628 at the lumbar spine (intervention, n=305; placebo, n=323), meaning that numbers were somewhat lower than specified in the original power calculation.
Figure 1.
Consort diagram
Maternal characteristics
Women in the treatment and placebo group at randomisation were of similar age, parity, educational attainment, smoking, exercise participation and ethnicity (Table 1). Height was also similar between the two groups, but weight, BMI and sum of skinfold thicknesses were greater in the placebo arm (Table 1). The women who remained in the trial until their baby was born were older (30.7 vs 28.9 years, p<0.001) and more likely to be white Caucasian (94.8% vs 89.2%, p=0.01), than those who withdrew. Women whose infants underwent DXA scanning tended to be older, and be less likely to smoke and have lower skinfold thicknesses (Supplementary Table 1), than women whose infants did not undergo DXA assessment.
Table 1.
Baseline maternal characteristics by randomisation group
| Placebo | Cholecalciferol (1000 IU/day) | |
|---|---|---|
| N | 569 | 565 |
| Age (years), mean±SD | 30.5 ± 5.2 | 30.5 ± 5.2 |
| Ethnicity, % Caucasian | 94.3 | 94 |
| Parity, % nulliparous | 43.9 | 43.6 |
| Current smoker, % | 8.2 | 8.3 |
| Educational attainment ≥ A level, % | 75.3 | 78.0 |
| Walking speed at least fairly brisk, % | 41.0 | 38.2 |
| Strenuous exercise ≥ once week, % | 14.0 | 15.7 |
| Height (cm), mean±SD | 165.8 ± 6.6 | 165.6 ± 6.4 |
| Weight (kg), median (IQR) | 71.4 (63.3-81.8) | 68.4 (60.9-79.5) |
| BMI (kg/m2), median (IQR) | 25.7 (23.0-30.0) | 24.7 (22.3-28.6) |
| Sum of all skinfold (mm), mean±SD | 84.0 ± 27.8 | 79.8 ± 27.9 |
| 25(OH)D (nmol/l), mean±SD | 45.9 ± 17.0 | 46.7 ± 17.7 |
| 25(OH)D>50nmol/l, % | 37.3 | 40.8 |
Neonatal bone indices, anthropometry and body composition
There was no difference in neonatal whole body bone mineral content (BMC) of infants born to mothers randomised to 1000IU/day cholecalciferol compared with infants born to mothers randomised to placebo [61.6g (95%CI: 60.3, 62.8g) vs 60.5g (95%CI: 59.3, 61.7g) respectively, p=0.21]. Similarly, there was no difference in bone area, bone mineral density, BMC adjusted for birth length, fat or lean mass of the neonate by treatment allocation (Table 2 and Supplementary Figure 1). There was no significant difference in neonatal bone indices at the spine, or birth weight, length, head or abdominal circumference between the two treatment groups (Table 2).
Table 2.
Anthropometry, whole body bone mineralisation and body composition in neonates born to mothers randomised to 1000 IU/day cholecalciferol or placebo from 14 weeks’ gestation until delivery. Data displayed as mean±SD, unless otherwise stated
| Placebo | Cholecalciferol (1000 IU/day) | p | |
|---|---|---|---|
| Obstetric data | |||
| N | 486 | 479 | |
| Male, N (%) | 251 (51.7) | 258 (53.9) | 0.49 |
| Birth weight (g) | 3518 ± 517 | 3481 ± 543 | 0.28 |
| Crown-heel length (cm) | 50.8 ± 2.3 | 50.6 ± 2.6 | 0.31 |
| Head circumference (cm) | 35.5 ± 1.5 | 35.4 ± 1.4 | 0.62 |
| Abdominal circumference (cm) | 32.7 ± 2.3 | 32.9 ± 2.2 | 0.16 |
| DXA | |||
| Whole body | |||
| N | 327 | 338 | |
| Age at DXA (days) | 7 ± 6 | 8 ± 7 | 0.12 |
| Bone Area (cm2) | 297.8 ± 37.3 | 301.6 ± 34.7 | 0.18 |
| BMC (g) | 60.5 ± 11.1 | 61.6 ± 11.7 | 0.21 |
| BMD (g/cm2) | 0.203 ± 0.019 | 0.203 ±0.022 | 0.96 |
| Lean (g) | 3014 ± 435 | 3055 ± 423 | 0.23 |
| Fat (g), median (IQR) | 374 (244-517) | 355 (235-564) | 0.97 |
Pre-specified secondary analyses
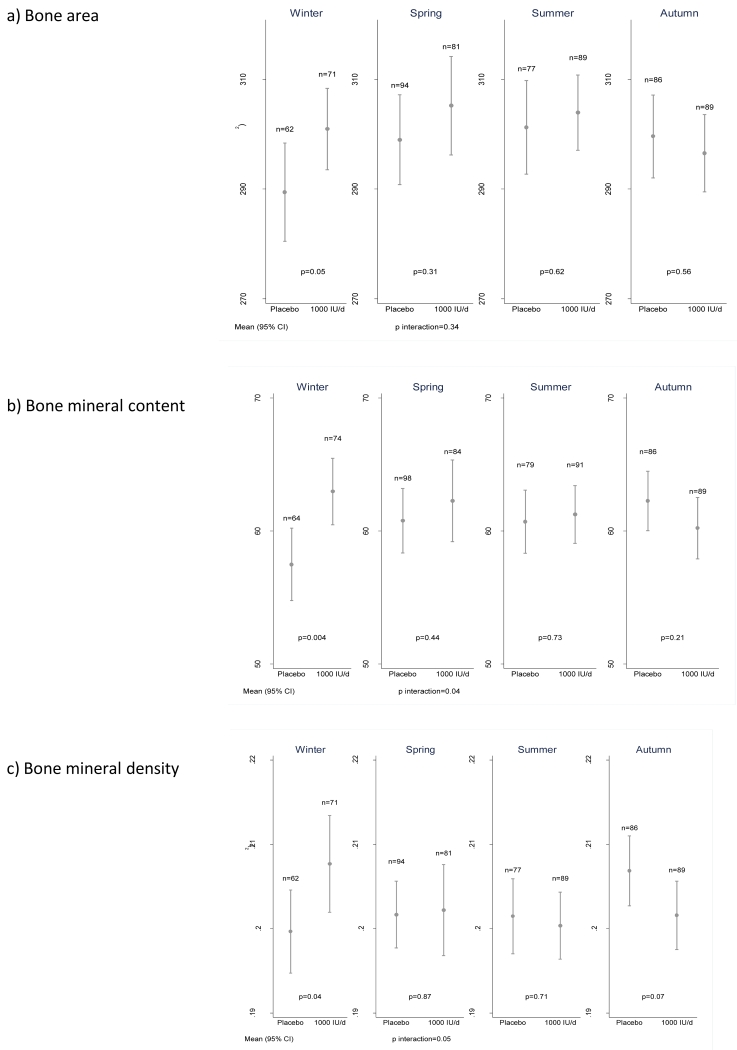
A priori, we hypothesized an interaction between treatment group and offspring season of birth.27 Supplementary Table 2 demonstrates the maternal baseline characteristics by treatment group and season of offspring delivery. The formal interaction term between treatment group and season of birth on offspring BMC was statistically significant (p=0.04) and the effect of treatment was of substantially greater magnitude [mean difference 5.5g (95%CI: 1.8, 9.1, p=0.004)] in winter months (December to February inclusive) than in the remaining seasons (Figure 2). A similar winter effect was observed for offspring whole body bone area (mean difference by group=11.5cm2, p=0.05), bone mineral density (mean difference by group=0.01g/cm2; p=0.04), bone mineral content adjusted for length (mean difference by group=3.7g, p=0.03) and indices of body composition (Supplementary Table 3 and Supplementary Figure 3). Results were similar for each of the 3 winter months (Supplementary Table 4), albeit with the statistical significance limited by the reduced sample size when stratified by individual month of delivery. Results were little changed after bone indices were adjusted for postnatal age at DXA. There was no evidence of a treatment season interaction for offspring birth length (p=0.95) or birth weight (p=0.19). Further pre-specified interactions for neonatal BMC between treatment and offspring sex (p=0.92); maternal BMI (p=0.91); maternal parity (p=0.95); recruitment centre (p=0.67); ethnicity (p=0.12); protocol completion (p=0.60); treatment compliance (p=0.70); baseline 25(OH)D status (p=0.67; Supplementary Table 5) and change in 25(OH)D (p=0.91) were not statistically significant. A further analysis of the effect of baseline 25(OH)D status on treatment efficacy within the winter group also demonstrated no statistically significant interaction (p=0.31).
Figure 2.
Neonatal whole body a) bone area, b) bone mineral content and c) bone mineral density by intervention group and season of birth. [Winter = December to February]. Data shown are mean and 95%CI.
Maternal 25(OH)D status
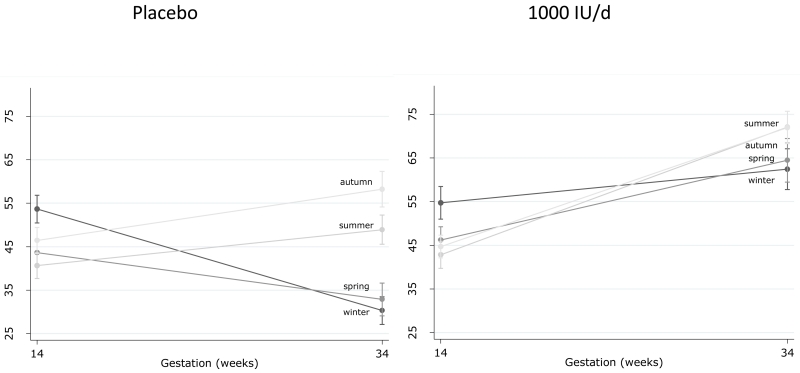
Baseline 25(OH)D status was similar in both groups (Table 1) and varied by season (Supplementary Table 6 and Supplementary Figure 4). Maternal 25(OH)D at 34 weeks’ gestation was significantly higher in the women who received 1000IU/day (68.2±21.9nmol/l) compared with placebo (43.4±22.4nmol/l), p<0.001. The percentage of participants with insufficient 25(OH)D (<50nmol/l) was similar at baseline (Table 1), but significantly lower at 34 weeks’ gestation in the intervention compared with placebo group (16.6% vs 63.5%, p<0.001). Furthermore, when the effect of vitamin D supplementation on maternal 25(OH)D status was explored by season of birth, the decline in 25(OH)D from 14 to 34 weeks’ gestation observed in placebo group women who delivered in winter and spring was not evident in the women, delivering in these same months, who received the vitamin D supplement (Figure 3). Frequency of non-protocol vitamin D containing supplement use did not vary by treatment group or season (Supplementary Table 7) and there was no effect of treatment on maternal adiposity (weight or skinfold thicknesses) at 34 weeks regardless of season (Supplementary Table 8).
Figure 3.
Maternal 25(OH)D status at baseline (14 weeks gestation) and 34 weeks’ gestation by randomisation to either 1000IU/day cholecalciferol or placebo and season of birth. [Winter = December to February]. Data shown are mean and 95%CI.
Safety analysis
Supplementary Table 9 documents the absolute numbers and percentages of adverse events by treatment group. Other than a greater proportion of women in the placebo group who had a report of severe postpartum haemorrhage, there were no differences in these safety measures.
DISCUSSION
Overall, we observed no effect of maternal supplementation with 1000IU/day cholecalciferol during pregnancy on the primary outcome (offspring neonatal bone mineral content). However the intervention clearly achieved maintenance of vitamin D repletion, and was safe. Furthermore, in a pre-specified secondary analysis we demonstrated an interaction between treatment and season of birth such that, for births in winter months, neonatal BMC, BA, BMD and body fat, but not birth weight or birth length, were greater amongst offspring of treatment versus placebo mothers. To our knowledge, this is the first published randomised controlled trial of vitamin D supplementation in pregnancy to include objective measures of offspring neonatal bone mass by DXA.
We undertook a large, population-based, double-blind, placebo-controlled, randomised trial, with standardised measures of vitamin D biochemistry and bone indices. However, there are some limitations that must be considered. First, we could not, as a result of stipulations made during the ethics approval process, include participants with 25(OH)D concentrations<25nmol/l. In addition, our study population did not include many members of ethnic minorities. If anything, both of these considerations are likely to bias towards the null hypothesis, but may reduce the generalisability of our findings. Second, DXA assessment in neonates presents some difficulties, as newborn babies are prone to move and have low absolute BMC. Appropriate software was used on each DXA instrument and DXA indices were cross-calibrated; the validity of the technique in small animals has been documented.30 Third, whilst we could not exclude the possibility that some participants were taking vitamin D additional to the study medication, supplement use was recorded and did not differ between the groups. Fourth, although the secondary analyses were pre-specified, and that the interaction with season is consistent with previous literature and is biologically plausible, the possibility of false positive results remains. This finding should therefore be interpreted with caution pending replication in other populations.
We identified only one previously published intervention study in which neonatal bone outcomes were measured,31 although its null result is difficult to interpret given its small size (n=64) and methodological limitations.7 Following the demonstration, by Javaid et al,6 of lower whole body BA, BMC and BMD, but not height or weight, at 9 years of age, in children born to mothers with 25(OH)D<25nmol/l in late pregnancy compared with those of replete mothers, other observational studies have documented positive associations between 25(OH)D status in pregnancy and newborn bone indices assessed by DXA,32,33 pQCT at birth4 and 18 months,5 and ultrasound measures of fetal femoral morphology.2,3 The persistence of such associations into adulthood has been recently demonstrated in the Western Australian Pregnancy Cohort (RAINE).8 In contrast, four other studies15,34-36 found no association between maternal 25(OH)D and infant bone mass, highlighting the need for this randomised controlled trial.
Variation of 25(OH)D with season has been well documented19,27-29 and was also observed in this cohort (Supplementary Figure 4). UVB exposure to the skin is a major determinant of circulating 25(OH)D concentrations in temperate climates such as the UK, and since 25(OH)D has a half life of around 3 weeks, the nadir appears in late winter/ early spring.37,38 In the present study, we observed a distinct fall in 25(OH)D concentration from 14 to 34 weeks of pregnancy in the placebo group, but a rise in the treatment group, when delivery occurred during this period. Indeed, there was a statistically significant effect of treatment on neonatal BA, BMC and BMD for births between December and February, consistent with relationships observed between maternal gestational UVB exposure and infant bone mass in our previous cohort study.6 Although fat mass was greater in neonates born in winter months to mothers in the treatment versus placebo groups, there was no evidence of a treatment-by-season interaction for birth length or birth weight; a treatment-by-season effect was apparent for BMC adjusted for birth length, suggesting that there is a specific influence on bone development rather than simply a generalised effect on birth size.
The majority of calcium mineral is accrued during the last trimester of pregnancy1, and our previous work has suggested that maternal factors (e.g. adiposity, physical activity, smoking, 25(OH)D status) within the last trimester are associated with offspring bone mineral content, in contrast to exposures in early pregnancy, where associations tend to be much weaker1. Furthermore data from the Southampton Women’s Survey demonstrate a seasonal difference in neonatal whole body BMC by season of birth, with winter births having lower BMC than summer births.39 It is therefore likely that low 25(OH)D is most important during the period of rapid bone mineral accrual in late pregnancy, consistent with effects of supplementation being observed where delivery is in the months of lowest 25(OH)D concentration. We therefore hypothesise that vitamin D supplementation, which reverses the drop in maternal 25(OH)D concentrations from 14 to 34 weeks in spring, and particularly winter births, therefore ameliorates the adverse effect of this decline on offspring bone mineral content, with the overall effect being one of removal of deficit rather than overall improvement. However, conversely, we did not find a statistically significant effect of treatment amongst spring births, which would have been expected from the timing of the 25(OH)D nadir. Given the relatively arbitrary nature of seasonal definition in relation to objectively measured UVB exposure, we feel that the best reconciliation of the findings relating to late pregnancy 25(OH)D in spring/winter with those relating to offspring bone in these seasons is that the supplement has the greatest effect in participants with an absolute decline in 25(OH)D above a notional threshold. Overall, these findings should be interpreted with caution, and replication of the treatment by season interaction in further studies will be required to delineate any messages for clinical care. The ongoing MAVIDOS childhood follow-up may also help to clarify this issue.
Notwithstanding, our findings inform public health policy, providing the first data from a large, blinded, randomised controlled trial with bone outcomes assessed by DXA, and demonstrating that overall, gestational supplementation with 1000 IU/day vitamin D does not benefit offspring neonatal bone mass. The intervention appeared safe, and although vitamin D supplementation appeared to be associated with a reduced incidence of severe postpartum haemorrhage, we suspect that this is a false positive finding as a result of misclassification, since these events were not adjudicated and it is very difficult to accurately assess postpartum blood loss in the typical clinical situation. This finding will therefore remain the subject of further investigation. Although BMD was lower in treatment than placebo births occurring during the autumn, this was not statistically significant (p=0.07) and such differences were not consistent across other DXA indices. Finally, although the dose used in our study is 2.5 times the standard UK recommendation of 400 IU daily in pregnancy, it is much lower than the highest doses used in several US studies (up to 4000 IU daily)7 and our results clearly demonstrate that such high doses are not required in order to achieve good levels of 25(OH)D repletion.
In conclusion, we found that supplementation of pregnant women with 1000 IU/day cholecalciferol from 14 weeks gestation until delivery of the baby does not lead to increased offspring neonatal BMC overall. Our demonstration of an interaction between treatment and season of delivery is consistent with previous data and biologically plausible, but should be replicated in further populations before its significance for public health can be fully appreciated. The overall safety of vitamin D supplementation during pregnancy is supported by our results.
Supplementary Material
Research in context.
Evidence before this study
We conducted a systematic review of studies relating maternal vitamin 25(OH)-vitamin D concentrations, UVB exposure, dietary vitamin D intake, or use of vitamin D supplements during pregnancy to maternal and offspring health outcomes.1 Major electronic databases (including, but not limited to, PubMed, Embase, Web of Science) were searched from their inception until June 2012. This was complemented by interrogation of grey literature and hand searching of reference lists. Two independent reviewers undertook all assessments, and the study was performed in accordance with PRISMA guidelines. We identified eight observational studies relating maternal gestational vitamin D status to offspring bone mass, all of which were assessed as being of medium to low risk of bias. Of these, five demonstrated a significant positive relationship between maternal vitamin D status and offspring bone outcomes2-6 [which included whole body, lumbar, femoral and tibial bone mineral content (BMC), and whole body and lumbar spine bone mineral density (BMD)]. Of the remaining studies, no significant association was observed between maternal vitamin D status and offspring radial and whole body BMC.7-9 Differences in study design did not permit meta-analysis. We identified one small intervention study,10 judged to be at high risk of bias, which found no difference in offspring forearm BMC (measured within five days of birth) between supplemented and un-supplemented mothers. We subsequently updated the search to August 2014, identifying two further observational studies, both judged to be low to medium risk of bias: one, using the ALSPAC cohort, demonstrated no association between maternal 25(OH)-vitamin D concentrations in pregnancy and offspring bone mass at 9 years.11 In contrast, the second study, from the Australian Raine cohort, documented positive relationships between maternal gestational 25(OH)-vitamin D concentrations offspring bone mass at 20 years.12
Added value of this study
There was no difference in the primary outcome (neonatal whole body BMC) between offspring born to mothers supplemented with vitamin D during pregnancy compared with mothers randomised to placebo. However, amongst the pre-specified secondary analyses, there was an interaction between treatment and season, with the suggestion of a benefit for offspring neonatal bone mineral content with treatment for deliveries during winter months. Although biologically plausible, this intriguing finding clearly requires replication in further studies before it can provide a basis for alterations to clinical care.
Implications of all the available evidence
Vitamin D supplementation during pregnancy is already recommended in many countries, including the UK. Observational studies have provided conflicting evidence regarding associations between maternal 25(OH)-vitamin D status and offspring intrauterine bone development. The MAVIDOS study, whilst negative for its primary outcome, has demonstrated that 1000 IU cholecalciferol daily is sufficient to ensure the majority of pregnant women are replete in 25(OH)-vitamin D, and that such a strategy is safe.
ACKNOWLEDGEMENTS
This work was supported by grants from the Arthritis Research UK (17702), Medical Research Council (4050502589), Bupa Foundation, National Institute for Health Research (NIHR) Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. IS and AP were funded by the Medical Research Council (MRC) (programme code U105960371). The work leading to these results was supported by the European Union’s Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreements numbers 289346 and 613977. CC and NCH are joint first author. We are extremely grateful to Merck GmbH for the kind provision of the Vigantoletten supplement. Merck GmbH had no role in the trial execution, data collection, analysis or manuscript preparation. The authors had full access to all study data.
Funding: Arthritis Research UK, MRC, Bupa Foundation and NIHR
AUTHORS’ CONTRIBUTIONS
C. Cooper contributed to study design, data collection and interpretation, study oversight and execution, and writing of the manuscript; N. Harvey contributed to study design, study oversight and execution, and preparation of the manuscript; N. Bishop contributed to study design, data interpretation, writing and critical appraisal of the manuscript; S. Kennedy contributed to study design, data interpretation, and drafting of the manuscript; A. Papageorghiou contributed to study design, data interpretation and drafting of the manuscript; I. Schoenmakers contributed to study design, biochemical analyses, data interpretation and manuscript drafting; R. Fraser is a member of the MAVIDOS study group, an investigator at the Sheffield site, and contributed in the preparation of the final manuscript; S. Gandhi is a principal investigator for the Sheffield site and contributed to data collection and analysis; A. Carr contributed to study design, data interpretation and manuscript drafting; S. D’Angelo conducted the statistical analyses; S. Crozier was involved in overseeing the analysis of the data and editing the manuscript; R. Moon contributed to management of the trial dataset and manuscript preparation; N. Arden was involved in the design, steering committee and preparation of the manuscript; E. Dennison contributed to study design, data collection, data interpretation and writing of the manuscript; K Godfrey contributed to study design, data collection, data interpretation and manuscript revision; H. Inskip contributed to protocol development and running of the study, overseeing the statistical analysis, commenting on drafts and approved the final version; A. Prentice contributed to study design, data interpretation and manuscript drafting; M.Z. Mughal contributed to review and revision of the manuscript; R. Eastell contributed to review and revision of the manuscript; D. Reid contributed to data interpretation, commenting n the paper and is Chair of the Trial Steering Committee; M.K. Javaid contributed to study design, data collection and interpretation and preparation of the manuscript. All members of the MAVIDOS Study Group contributed to manuscript preparation, study design and study execution.
DECLARATION OF INTERESTS
C. Cooper reports personal fees from ABBH, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier and Takeda, outside the submitted work. N. Harvey reports personal fees, consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma, outside the submitted work. N. Bishop reports remuneration from Internis Pharmaceuticals Ltd, outside the submitted work. S. Kennedy has nothing to disclose. A. Papageorghiou reports grants from Arthritis Research Council, during the conduct of the study. I. Schoenmakers has nothing to disclose. R. Fraser has nothing to disclose. S. Gandhi has nothing to disclose. A. Carr has nothing to disclose. S. D’Angelo has nothing to disclose. S. Crozier has nothing to disclose. R. Moon has nothing to disclose. N. Arden has received honoraria, held advisory board positions (which involved receipt of fees), and received consortium research grants, respectively, from: Merck, grants from Roche, personal fees from Smith & Nephew, Nicox, Flexion, grants from Bioiberica, Novartis, and personal fees from Bioventus and Freshfields, outside the submitted work. E. Dennison has nothing to disclose. K. Godfrey reports reimbursement for speaking at Nestle Nutrition Institute conferences, grants from Abbott Nutrition & Nestec, outside the submitted work; in addition, K. Godfrey has a patent Phenotype Prediction pending, a patent Predictive Use of CpG Methylation pending, and a patent Maternal Nutrition Composition pending, not directly related to this work. H. Inskip reports grants from Medical Research Council, Arthritis Research UK, European Union’s Seventh Framework Programme, during the conduct of the study; and while not directly receiving funding from other bodies, members of her team have received funding from the following companies from other work: Danone, Nestec, Abbott Nutrition. A Prentice has nothing to disclose. M.Z. Mughal has nothing to disclose. R. Eastell reports grants and personal fees from Amgen, grants from Department of Health, grants from AstraZeneca, grants, personal fees and non-financial support from Immunodiagnostic Systems, grants from ARUK/MRC Centre for Excellence in Musculoskeletal Ageing Research, grants from National Institute for Health Research, grants from MRC/AZ Mechanisms of Diseases Call, grants from MRC, grants and personal fees from Alexion, grants and other from National Osteoporosis Society, grants, personal fees and other from Roche, personal fees from Otsuka, Novartis, Merck, Bayer, Johnson & Johnson, Fonterra Brands, Janssen Research, personal fees and other from Eli Lilly, personal fees from Ono Pharma, Alere (Unipath), Chronos, personal fees and other from CL Biosystems, other from European Calcified Tissue Society, IOF CSA, personal fees from Teijin Pham Limited, other from ASBMR, personal fees from D-STAR, personal fees from GSK Nutrition, outside the submitted work. D. Reid has nothing to disclose. M.K. Javaid reports personal fees from Stirling Anglia, Consilient Health and Internis, outside the submitted work.
Footnotes
Trial registration: ISRCTN82927713, registered 11/04/2008
REFERENCES
- 1.Harvey N, Dennison E, Cooper C. Osteoporosis: a lifecourse approach. J Bone Miner Res. 2014;29:1917–25. doi: 10.1002/jbmr.2286. [DOI] [PubMed] [Google Scholar]
- 2.Mahon P, Harvey N, Crozier S, et al. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25:14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioannou C, Javaid MK, Mahon P, et al. The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab. 2012;97:E2070–7. doi: 10.1210/jc.2012-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 5.Viljakainen HT, Korhonen T, Hytinantti T, et al. Maternal vitamin D status affects bone growth in early childhood--a prospective cohort study. Osteoporos Int. 2011;22:883–91. doi: 10.1007/s00198-010-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 7.Harvey NC, Holroyd C, Ntani G, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technology Assessment. 2014;18:1–190. doi: 10.3310/hta18450. (Winchester, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu K, Whitehouse AJ, Hart PH, et al. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res. 2014;29:1088–95. doi: 10.1002/jbmr.2138. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Garabedian M. Vitamin D: Photobiology, Metabolism, Mechanisms of Action, and Clinical Applications. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Mineral Metabolism. ASBMR; Chicago: 2006. pp. 106–14. [Google Scholar]
- 10.Namgung R, Tsang RC, Lee C, Han DG, Ho ML, Sierra RI. Low total body bone mineral content and high bone resorption in Korean winter-born versus summer-born newborn infants. J Pediatr. 1998;132:421–25. doi: 10.1016/s0022-3476(98)70013-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsang RC. Seasonal vitamin D in African American and white infants. Am J Clin Nutr. 1999;69:159. doi: 10.1093/ajcn/69.1.159. [DOI] [PubMed] [Google Scholar]
- 12.Sayers A, Tobias JH. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab. 2009;94:765–71. doi: 10.1210/jc.2008-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey NC, Javaid MK, Inskip HM, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and bone-mineral content in offspring. Lancet. 2013;382:766. doi: 10.1016/S0140-6736(13)61827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey NC, Cooper C. Vitamin D: some perspective please. BMJ. 2012;345:e4695. doi: 10.1136/bmj.e4695. (Clin Res Ed) [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Wills AK, Fraser A, Sayers A, Fraser WD, Tobias JH. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet. 2013;381:2176–83. doi: 10.1016/S0140-6736(12)62203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Harvey NC, Javaid MK, Arden NK, et al. Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. Journal of Developmental Origins of Health and Disease. 2010;1:35–41. doi: 10.1017/S2040174409990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfrey K, Walker-Bone K, Robinson S, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res. 2001;16:1694–703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 19.Javaid MK, Godfrey KM, Taylor P, et al. Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J Bone Miner Res. 2004;19:56–63. doi: 10.1359/JBMR.0301211. [DOI] [PubMed] [Google Scholar]
- 20.Ay L, Jaddoe VW, Hofman A, et al. Foetal and postnatal growth and bone mass at 6 months: the Generation R Study. Clin Endocrinol (Oxf) 2011;74:181–90. doi: 10.1111/j.1365-2265.2010.03918.x. [DOI] [PubMed] [Google Scholar]
- 21.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–15. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 22.Harvey NC, Poole J, Taylor P, Godfrey KM, Cooper C. Skeletal growth tracks through childhood. Rheumatology. 2006;45(Suppl. 1) [Google Scholar]
- 23.Kalkwarf HJ, Gilsanz V, Lappe JM, et al. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95:1690–8. doi: 10.1210/jc.2009-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wren TA, Kalkwarf HJ, Zemel BS, et al. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164:1280–5. e2. doi: 10.1016/j.jpeds.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–7. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 27.Harvey NC, Javaid K, Bishop N, et al. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. Trials. 2012;13:13. doi: 10.1186/1745-6215-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redmond J, Palla L, Yan L, Jarjou LM, Prentice A, Schoenmakers I. Ethnic differences in urinary calcium and phosphate excretion between Gambian and British older adults. Osteoporos Int. 2015;26:1125–35. doi: 10.1007/s00198-014-2926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 30.Brunton JA, Weiler HA, Atkinson SA. Improvement in the accuracy of dual energy x-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res. 1997;41:590–6. doi: 10.1203/00006450-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Congdon P, Horsman A, Kirby PA, Dibble J, Bashir T. Mineral content of the forearms of babies born to Asian and white mothers. Br Med J (Clin Res Ed) 1983;286:1233–35. doi: 10.1136/bmj.286.6373.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey NC, Javaid MK, Poole JR, et al. Paternal skeletal size predicts intrauterine bone mineral accrual. J Clin Endocrinol Metab. 2008;93:1676–81. doi: 10.1210/jc.2007-0279. [DOI] [PubMed] [Google Scholar]
- 33.Weiler H, Fitzpatrick-Wong S, Veitch R, et al. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–61. doi: 10.1503/cmaj.1040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akcakus M, Koklu E, Budak N, Kula M, Kurtoglu S, Koklu S. The relationship between birthweight, 25-hydroxyvitamin D concentrations and bone mineral status in neonates. Ann Trop Paediat. 2006;26:267–75. doi: 10.1179/146532806X152782. [DOI] [PubMed] [Google Scholar]
- 35.Dror DK, King JC, Fung EB, Van Loan MD, Gertz ER, Allen LH. Evidence of associations between feto-maternal vitamin D status, cord parathyroid hormone and bone-specific alkaline phosphatase, and newborn whole body bone mineral content. Nutrients. 2012;4:68–77. doi: 10.3390/nu4020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr. 2009;98:1360–62. doi: 10.1111/j.1651-2227.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clinical Nutr. 2012;96:57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mavroeidi A, O’Neill F, Lee PA, et al. Seasonal 25-hydroxyvitamin D changes in British postmenopausal women at 57 degrees N and 51 degrees N: a longitudinal study. J Steroid Biochem Mol Biol. 2010;121:459–61. doi: 10.1016/j.jsbmb.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Moon RJ, Harvey NC, Davies JH, Cooper C. Vitamin D and bone development. Osteoporos Int. 2015;26:1449–51. doi: 10.1007/s00198-014-2976-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.