Abstract
In the recent years, noble nanoparticles have attracted and emerged in the field of biology, medicine and electronics due to their incredible applications. There were several methods have been used for synthesis of nanoparticles such as toxic chemicals and high energy physical procedures. To overcome these, biological method has been used for the synthesis of various metal nanoparticles. Among the nanoparticles, silver nanoparticles (AgNPs) have received much attention in various fields, such as antimicrobial activity, therapeutics, bio-molecular detection, silver nanocoated medical devices and optical receptor. Moreover, the biological approach, in particular the usage of natural organisms has offered a reliable, simple, nontoxic and environmental friendly method. Hence, the current article is focused on the biological synthesis of silver nanoparticles and their application in the biomedical field.
Keywords: Biological synthesis, Metal nanoparticles, Antibacterial activity, Protein degradation, DNA damage
INTRODUCTION
The study of nanomaterial has been emerging dramatically throughout the world in the 21st century due to their incredible applications in all spheres of human life (1). It has opened several arms in the development of new nanomaterials and examining their properties by tuning the particle size, shape and distribution (2,3). Metal nanoparticles have been extensively studied due to their specific characteristics such as catalytic activity, optical properties, electronic properties, antimicrobial properties and magnetic properties (4). Traditionally UV irradiation, aerosol technologies, lithography, laser ablation, ultrasonic fields, and photochemical reduction techniques have been used successfully to produce various metal nanoparticles such as gold, silver, platinum and palladium. However, considering the fast growth in the usage of nanomaterials in diverse fields, there is an urgent need to develop clean, nontoxic, simple and eco-friendly procedures for their synthesis.
Synthesis of noble metal nanoparticles, in particular silver nanoparticles (AgNPs) synthesis using natural organism has become a major research area in the field of nanotechnology. This may due to their simplicity of procedures, stability of nanoparticles, and their potential applications in chemical sensing, biological imaging, antimicrobial, gene silencing, drug delivery (5). Recently, several studies have reported natural polymers such as chitosan, starch and tannic acid as reducing agents for the synthesis of silver and gold nanoparticles (6,7). A vast array of biological resources including plants, algae, fungi, yeast, bacteria, and viruses has been studied so far for the intra and extracellular synthesis of silver, gold, platinum and titanium nanoparticles in different sizes and shapes were tabulated in Table 1. The major drawback of metal nanoparticles synthesis using plant extracts as reducing and stabilizing agent. This differs due to significant variation of biochemical compositions present in the plant extract of the same species differ from other part of the world. Therefore, identifying the biomolecules responsible for mediating the nanoparticles synthesis is a problem to overcome (8).
Table 1.
Biological synthesis of metal nanoparticles using various organisms
| Sources | Type of nanoparticles | Location | Size (nm) | References |
|---|---|---|---|---|
| A. Bacteria | ||||
|
| ||||
| Pseudomonas aeruginosa | Au | Extracellular | 15~30 | 9 |
| Pseudomonas stutzeri | Ag | Intracellular | 200 | 11 |
| Bacillus subtilis | Ag & Au | Intra & Extracellular | 5~10 | 12 |
| Shewanella oneidensis | U | Extracellular | 150 | 17 |
| Lactobacillus sp. | Ag & Au | Intracellular | 60 | 46 |
| Escherichia coli | CdS | Intracellular | 2~5 | 24 |
| Clostridium thermoaceticum | CdS | Intra & Extracellular | 2~5 | 47,48 |
| Rhodopseudomonas capsulate | Au | Extracellular | 10~20 | 49 |
| Escherichia coli DH5α | Au | Intracellular | 25~33 | 50 |
| Thermomonospora spp. | Au | Extracellular | 8 | 51 |
| Streptomyces albidoflavus | Ag | Intracellular | 10~14 | 52 |
| Klebsiella pneumonia | Ag | Extracellular | 5~32 | 53 |
|
| ||||
| B. Virus | ||||
|
| ||||
| Tobacco mosaic virus (TMV) | SiO2, CdS, PbS, Fe2O3 | Intra & Extracellular | 45~80 | 22 |
| M13 bacteriophage | ZnS and CdS | Intra & Extracellular | 50~100 | 23 |
|
| ||||
| C. Fungi | ||||
|
| ||||
| Phoma sp. 3.2883 | Ag | Extracellular | 71~74 | 54 |
| Fusarium oxysporum | Ag | Extracellular | 5~15 | 55 |
| Verticillium | Ag | Intracellular | 25 | 56 |
| Aspergillus fumigates | Ag | Extracellular | 5~25 | 57 |
| Trichoderma asperellum | Ag | Extracellular | 13~18 | 58 |
| Phaenerochaete chrysosporium | Ag | Extracellular | 50~200 | 56 |
| Fusarium oxysporum | Magnetite | Extracellular | 20~50 | 59 |
|
| ||||
| D. Yeast | ||||
|
| ||||
| Pichia jadinii | Au | Intracellular | 100 | 21 |
| Torulopsis | CdS | Intracellular | 2~5 | 24 |
| Candida glabrata | CdS | Intracellular | 200 | 60 |
| Schizosaccharomyces pombe | CdS | Intracellular | 1~1.5 | 60 |
|
| ||||
| E. Algae | ||||
|
| ||||
| Scencedesmus sp. | Ag | Extracellular | 15~20 | 61 |
| Chlorella vulgaris | Au | Extracellular | 9~20 | 62 |
|
| ||||
| F. Plant | ||||
|
| ||||
| Alfalfa sprouts | Ag | Intracellular | 2~20 | 26 |
| Cinnamomum camphora | Ag and Au | Extracellular | 55~80 | 45 |
| Azadirachta indica (Neem) | Ag/Au | Extracellular | 50~100 | 63,64 |
| Geranium leaves plant extract | Ag | Extracellular | 16~40 | 65 |
| Avena sativa (Oat) | Au | Extracellular | 5~85 | 66 |
| Aloe vera | Au | Extracellular | 50~350 | 67 |
|
| ||||
| G. Human cell lines | ||||
|
| ||||
| SiHa | Au | Intracellular | 20~100 | 33 |
| HeLa | Au | Intracellular | 20~100 | 33,34 |
| SKNSH | Au | Intracellular | 20~100 | 33 |
| HEK-293 | Au | Intracellular | 20~100 | 33,34 |
BACTERIA MEDIATED SYNTHESIS OF NANOPARTICLES
Soil is an extensively explored ecological niche for sources of microorganisms that are involved in various interactions. Among these, Metal-microbe interactions have important roles with fascinating applications such as bioremediation, biomineralization, bioleaching and microbial corrosion. However, recently that microorganisms have been explored as potential biofactory for synthesis of metallic nanoparticles such as cadmium, gold and silver (9,10). Among the microbes, the use of bacteria, like in this study, is rapidly gaining importance due to its growing success, ease of handling and genetic modification. Klaus et al. demonstrated that the Pseudomonas stutzeri AG259, isolated from a silver mine, produced silver nanoparticles of well-defined size and distinct morphology within the periplasmic space of the bacteria (11). In recent study various bacterial strains such as Bacillus amyloliquefaciens, Acinetobacter calcoaceticus, Escherichia coli and Bacillus megaterium could effectively induce the synthesis of silver nanoparticles (12,13). Biosynthetic methods can be categorized into intracellular and extracellular synthesis according to the place where nanoparticles are formed. Of which, the extracellular synthesis of nanoparticles is still continually emerging in order to understand the mechanisms of synthesis, easy downstream processing and rapid scale-up processing. For these reasons, a bacterial system could prove to be a potential source for the extracellular synthesis of metal nanoparticles instead of physical and chemical procedures.
VIRUS MEDIATED SYNTHESIS OF NANOPARTICLES
Viruses are unicellular organisms that hijack the replication machinery of the host cell and suspend most endogenous cellular activity. Their structure consists of nucleic acid, either DNA or RNA, which is surrounded by a protein shell that may or may not contain a lipid envelope. Viral genomes can be non-segmented, consisting of a single nucleic acid molecule, or segmented, consisting of more than one nucleic acid molecule. The nucleic acid molecules of a virus can be contained within a single virus or separated into multiple viruses. Viruses do not express their own ribosomal RNA. Viruses hold great promise in assembling and interconnecting novel nanosized components, allowing developing organized nanoparticle assemblies. Due to their size, monodispersity, and variety of chemical groups available for modification, they make a good scaffold for molecular assembly into nanoscale devices. Virus based nanocomposites are useful as an engineering material for the construction of smart nano-objects because of their ability to associate into desired structures including a number of morphologies. Viruses exhibit the characteristics of an ideal template for the formation of nano-conjugates with noble metal nanoparticles.
These bioinspired systems form monodispersed units that are highly amenable through genetic and chemical modifications. As nanoscale assemblies, viruses have sophisticated yet highly ordered structural features, which, in many cases, have been carefully characterized by modern structural biological methods. For many years animal viruses have been developed for material science, gene delivery and gene therapy purposes. More recently, other pathogens such as plant viruses, bacteriophages and viruses are increasingly being used for nanobiotechnology purposes because of their relative structural and chemical stability, ease of production, and lack of toxicity and pathogenicity in animals or humans (14). Biological scaffolds (viruses) hold great promise in assembling and interconnecting novel nanosized components, allowing such organized assemblies to interface with well-developed technologies such as lithography as nanotechnology develops (15). The cowpea mosaic virus (CPMV), for example, due to its size, monodispersity, and variety of chemical groups available for modification, makes a good scaffold for molecular assembly into nanoscale devices. The tobacco mosaic virus (TMV) was also used as bio-template, which has the shape of a linear tube, for assembly of various kinds of nanoparticles inside and outside the tubes. One can assemble gold nanoparticles onto the surfaces of polypeptide nanotubes while controlling their assembly position on the biomolecules using the specific affinities of the polypeptide sequences (16).
FUNGI MEDIATED SYNTHESIS OF NANOPARTICLES
Cell mass or extracellular components from fungi, such as Fusarium oxysporum, Aspergillus flavus, Aspergillus clavatus, and Penicillium brevicompactum (17,18) have been utilized for the reduction of silver ions to AgNPs. Filamentous fungi possess some distinctive advantages over bacteria due to high metals tolerance, wall binding capacity, and intracellular metal uptake capabilities (19). Previously, Vigneshwaran et al. (20) also showed that the use of Aspergillus flavus resulted in the accumulation of silver nanoparticles on the surface of its cell wall when incubated with silver nitrate solution for 72 hr. The average particle size was found to be 8.92 nm. The intracellular synthesis of gold nanoparticles produced by V. luteoalbum (21) showed morphologies of spherical, hexagonal and rods in the size range of 8.92~25 nm. Fungi are more advantageous compared to other microorganisms in many ways. Fungal mycelial mesh can withstand flow, pressure, agitation and other conditions in bioreactors or other chambers compared to plant materials and bacteria. These are fastidious to grow, easy to handle and easy for fabrication. The extracellular secretions of reductive proteins are more and can be easily handled in downstream processing. Since the nanoparticles precipitated outside the cell is devoid of unnecessary cellular components, it can be directly used in various applications.
YEAST MEDIATED SYNTHESIS OF NANOPARTICLES
Among the eukaryotic microorganism, yeast has been exploited mainly for the synthesis of semiconductors. Candida glubrata produced intracellularly monodispersed spherically shaped peptide bound CdS quantum crystallites of 20 Å by neutralizing the toxicity of metal ions by forming metal-thiolate complex with phytochelatins (22) and Schizosaccharomyces pombe also produced wurtzite-typed hexagonal lattice structured CdS nanoparticles in mid-log phase in the range of 1~1.5 nm (23). Kowshik et al. (24) first reported the synthesis of fcc structured CdS nanocrystallites exhibiting quantum semiconductor properties using yeast, Torulopsis sp. which intracellularly produced in the vacuoles with a dimension of 2~5 nm in spherical morphology when incubated with Pb2+ exhibiting λmax of 330 nm in UV-Vis spectrophotometer. These nanoparticles were used to fabricate diode heterojunction with poly (p-phenylenevinylene). In addition, baker’s yeast, S. cerevisiae was also reported to biosorb and reduces Au+ to elemental gold in the peptidoglycan layer of the cell wall in situ by the aldehyde group present in reducing sugars (25). Similarly, Pichia jadinii intracellularly formed gold nanoparticles of spherical, triangular and hexagonal morphologies throughout the cell mainly in the cytoplasm, of size 100 nm in 24 hr (26).
ALGAE MEDIATED SYNTHESIS OF NANOPARTICLES
Algae are eukaryotic aquatic oxygenic photoautotrophs, which produce its food through photosynthesis using sunlight producing oxygen as their by-products. Their photosynthesis machinery has been evolved from cyanobacteria via endosymbiosis. They are predominant primary producers in many aquatic environments. Among various algae, Chlorella sp. was found to accumulate various heavy metals such as cadmium, uranium, copper, and nickel. Chlorella vulgaris is a single-celled green algae belonging to phylum Chlorophyta, and the extracts of C. vulgaris showed anti-tumor properties (27). The dried algal cells were found to have a strong binding ability towards tetrachloroaurate (III) ions to form algal-bound gold, which was subsequently reduced to form Au(0). Nearly 88% of algal-bound gold attained metallic state and the crystals of gold were accumulated in the interior and exterior of cell surfaces with tetrahedral, decahedral and icosahedra structures. Though chemical synthesis produces nanoparticles more rapidly with well-controlled shape, size and dispersity, the use of toxic and expensive chemicals as reducing and capping agents restricts its use in biomedical applications.
In that case, optimizing the conditions like pH, temperature and metal ions (solute) concentration for expediting the biological synthesis of nanoparticles with narrow size and shape is mandatory. To date, only very few reports have been documented on the optimization in biological processes. A 28-kDa “gold shape-directing protein (GSP)” present in the extract of green algae, C. vulgaris, was used in the bioreduction and in the synthesis of size/shape controlled distinctive triangular and hexagonal gold nanoparticles. With an increase in the concentration of GSP, gold plates with lateral sizes up to micrometers were produced (28). The study of heavy metal biosorption by various algae showed that brown algae are superior compared to other autotrophs and algae (29).
PLANTS MEDIATED SYNTHESIS OF NANOPARTICLES
Indeed, a number of bacteria, fungi and yeast have been well-known for the synthesis of non-toxic noble nanoparticles. However the microbial mediated synthesis of nanoparticles is not industrially feasible as it requires expensive medium and maintenance of highly aseptic conditions. Hence, exploration of the plant systems as the potential bio-factories has gained heightened interest in the biological synthesis of nanoparticles. Hence, exploration into plant systems has been considered to be a potential bioreactor for synthesis of metal nanoparticles without using toxic chemicals.
Recently, we have reported that the various plant materials such as Rosmarinus officinalis (30), Sesbania grandiflora (31), Tribulus terrestris (32), used for biological synthesis of silver and gold nanoparticles. In continuation of the efforts for synthesizing gold nanoparticles by green route, here we present a report on the facile, rapid, and single pot aqueous biosynthesis of these nanoparticles using leaf extract of S. grandiflora L. (belonging to the family Fabaceae), which is commonly known as agati. The literature survey revealed that they are rich in various active ingredients such as water soluble antioxidant polyphenols, flavonoids etc, and research is still underway.
HUMAN CELL LINE MEDIATED SYNTHESIS OF NANOPARTICLES
Human cells are heterotrophic in nutrition. They need to be provided with energy for their survival. Human cancer cells and non-cancerous cells intracellularly produced some metal nanoparticles in vitro conditions that mimic their natural cellular environment. With an incubation of 1 mM of tetrachloroaurate solution, human cancer cells like SiHa (malignant cervical epithelial cells), SKNSH (human neuroblastoma) and HeLa (malignant cervical epithelial cells), and non-cancer cells like HEK-293 (non-malignant human embryonic kidney cells) synthesized gold nanoparticles in the size range of 20~100 nm. These nanoparticles were located in the cytoplasm and in the nucleus of the cells. The dimensions of these particles were smaller in nucleus compared to the cytoplasmic particles (33,34).
MECHANISM OF NANOPARTICLE FORMATION
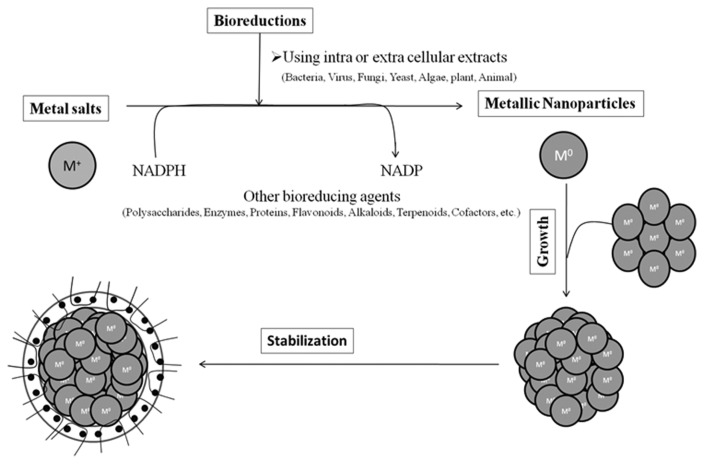
In producing nanoparticles using the intracellular and an extracellular extract of organisms, the extract is simply mixed with a solution of the metal salt at room temperature. The reaction is complete within minutes. Nanoparticles of silver, gold and other metals have been produced previously (35). Fig. 1 shows a picture of various organisms used for the biosynthesis of nanoparticles. The nature of the living extract, its concentration, the concentration of the metal salt, the pH, temperature and contact time are known to affect the rate of production of the nanoparticles, their quantity and other characteristics.
Fig. 1.
Mechanisms of nanoparticle synthesis.
APPLICATIONS OF METAL NANOPARTICLES IN ANTIBACTERIAL ACTIVITY
Mahendra et al. (36) reported that the silver nanoparticles show efficient antimicrobial property due to their extremely large surface area, which provides better contact with microorganisms. The nanoparticles penetrate the cell membrane or attached to the surface of the bacteria based on the size. Shekar et al. (37) reported that all AgNPs were found to be highly toxic to the bacterial strains and their antibacterial efficacy increased by lowering particle size. This effect was significantly enhanced as the size of nanoparticles approached the sub-10 nm range and, 5 nm AgNPs demonstrated the fastest bactericidal activity as compared to 7 nm and 10 nm AgNPs at their respective, Minimimum bactericidal concentration. The bacterial membrane contains sulfur-containing proteins and the silver nanoparticles interact with these proteins in the cell as well as with the phosphorus containing compounds like DNA. Silver has a greater affinity to bind with sulphur and phosphorus containing bio-molecules of the cell. Therefore, sulphur containing proteins in the cell membrane, inside the cells and phosphorus containing elements like DNA is likely to be the preferred sites for binding of silver nanoparticles (38). If the silver nanoparticles enter the bacterial cell it forms a low molecular weight region in the center of the bacteria to which the bacteria conglomerates thus, protecting the DNA from the silver ions. The nanoparticles preferably attack the respiratory chain, cell division finally leading to cell death. The nanoparticles release silver ions in the bacterial cells, which enhance their bactericidal activity (38–42).
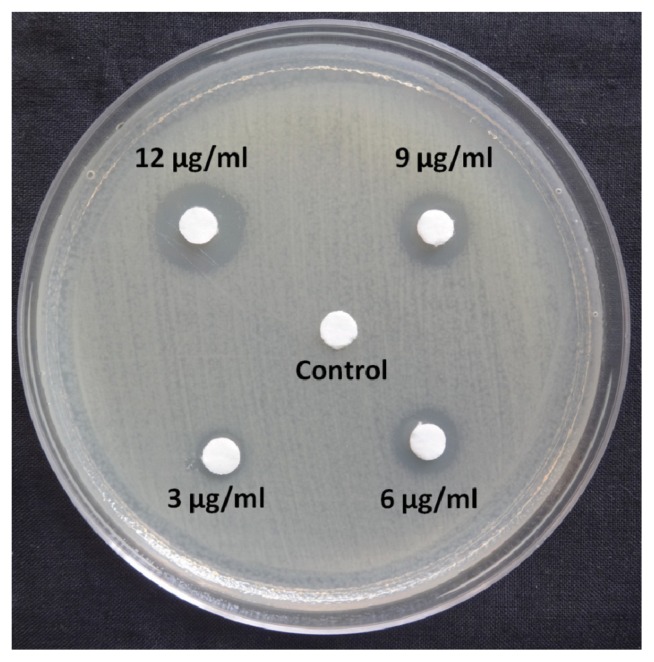
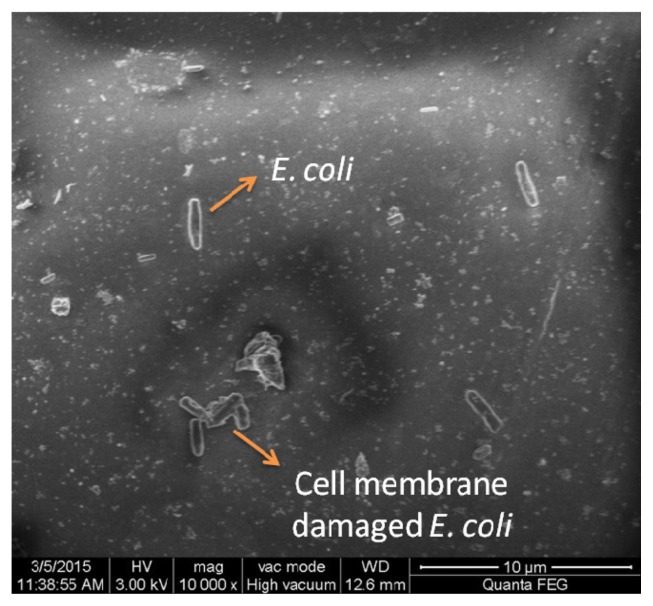
In the present study, we investigated whether these nanoparticles could induce nucleic acid DNA damage. For the assay of DNA damage, agarose gel electrophoresis was performed as per the manufacturer’s instructions using a commercially available DNA extraction kit (Bangalore Genei™, Bengaluru, India). The disc agar diffusion method was carried out to find the minimum inhibitory concentration of the AgNPs. Fig. 2 illustrates that 3 μg/mL shows the zone of inhibition around 7 mm including the disc (6 mm). 6 μg/mL which shows a little bigger zone of inhibition around 9 mm including the disc and this was taken for further analysis with field emission scanning electron microscopy (FE-SEM). Fig. 3 shows the FE-SEM images of the treated E. coli with the AgNPs. The morphological changes of E. coli was observed after the treatment with the 6 μg/mL AgNPs.. The rupture of cell membrane leading to bacterial cell death is clearly shown.
Fig. 2.
Disc diffusion test on Muller Hinton agar medium using AgNPs against E. coli.
Fig. 3.
FE-SEM images of E. coli treated with AgNPs (6 μg/mL).
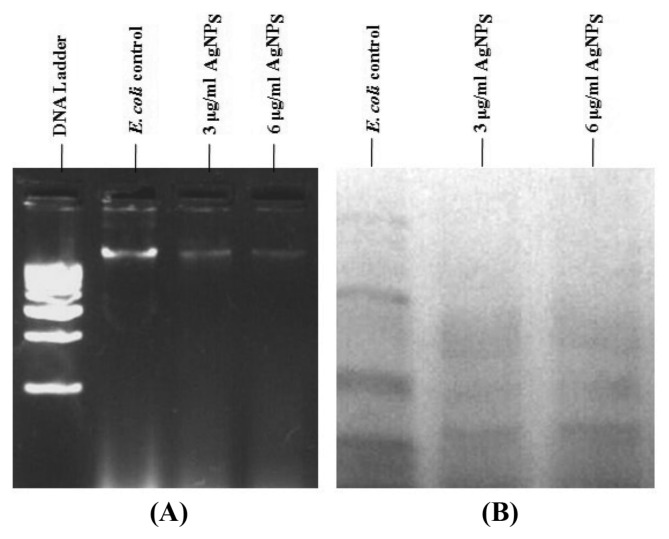
The bacterial cells of Gram negative, E. coli was harvested (before and after treatment with 6 μg/mL of AgNPs) by centrifugation at 8000 rpm for 10 min. The extracted DNA was analyzed for the occurrence of DNA damage. Surprisingly, 4 hr treated cells of E. coli showed remarkable DNA damage. However, the DNA of untreated cells remained intact shown in Fig. 4. It was observed that the amount of DNA damage within the cells increased with an increasing concentration of the AgNPs, and the damage to DNA from E. coli was higher than that Gram positive from B. subtilis, implying that E. coli may suffer greater membrane damage than B. subtilis. The various mechanisms by which silver ions were thought to act includes structural changes in the cell wall of bacteria, interactions with thiol groups in proteins and enzymes, and interruption of DNA replication due to damage of the DNA (43). Previously, Ramamurthy et al. (44) studied the effect of silver nanoparticles on E. coli, silver nanoparticles penetrate the nucleic acid DNA of E. coli and exhibit antibacterial effect by causing DNA damage at low concentrations. Specific DNA smearing is a characteristic feature of cell death.
Fig. 4.
(A) Agarose gel electrophoresis of E. coli control and treated cells for DNA Analysis, (B) SDS-PAGE Protein analysis of E. coli cells after treatment with AgNPs.
In agreement with the DNA damage, the amount of protein present in the cell suspension of the E. coli strain was analyzed by 12% SDS-PAGE. It was observed that for E. coli treated with AgNPs showed protein bands were missing when compared with the control. With the increase in the concentration of AgNPs, there was a further decrease in the protein band intensity shown in Fig. 4. The effect was more pronounced in case of E. coli bacteria than B. subtilis bacteria. Several reports propose that the affinity of AgNPs to the thiol groups of the proteins cause unfolding of the protein chain. This might result in the degradation of the proteins. Moreover the AgNPs are also implicated in the inhibition of the translational process by inhibiting various translation factors and hence preventing protein synthesis.
CONCLUSIONS
In recent years, there is an increased interest in studying the novel metal nanoparticles. In particular, biological nanoparticles have been reported to produce various sources possessing as an antibacterial activity. Increasing awareness towards green chemistry and biological processes has led to a desire to develop an environment-friendly approach for the synthesis of non-toxic nanoparticles. Unlike other processes such as physical and chemical methods, which involve hazardous chemicals. Biosynthesis of nanoparticles is cost-effective and eco-friendly approach. Therefore, biosynthesis of nanoparticles has been emerged as an important branch of nanobiotechnology. Due to their rich diversity, microorganisms have the innate potential for the synthesis of nanoparticles and they could be regarded as potential biofactories for nanoparticles synthesis. However, to improve the rate of synthesis and monodispersity of nanoparticles, factors such as microbial cultivation methods and downstream processing techniques have to be improved. Further, the combinatorial approach such as photobiological methods may be used. For instance, a great deal of effort has been put into the biosynthesis of nanoparticles, especially metal nanoparticles using plants. The use of plants and plant products as sustainable and renewable resources in the synthesis of nanoparticles is more advantageous over prokaryotic microbes, which need expensive methodologies for maintaining microbial cultures and downstream processing. Furthermore, the biosynthesized nanoparticle was explained the role of silver nanoparticles in antibacterial application against Gram negative bacterial strain E. coli.
ACKNOWLEDGMENTS
The author is acknowledging to the SRM University for providing the necessary chemicals and facilities to carry out this work.
REFERENCES
- 1.Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60:1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf, A. 2009;339:134–139. doi: 10.1016/j.colsurfa.2009.02.008. [DOI] [Google Scholar]
- 3.Das J, Velusamy P. Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora L. J Taiwan Inst Chem Eng. 2014;45:2280–2285. doi: 10.1016/j.jtice.2014.04.005. [DOI] [Google Scholar]
- 4.Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Wei D, Qian W. Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf B Biointerfaces. 2008;62:136–142. doi: 10.1016/j.colsurfb.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Xu H, Chen Z, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011:270974. doi: 10.1155/2011/270974. [DOI] [Google Scholar]
- 7.Dadosh T. Synthesis of uniform silver nanoparticles with a controllable size. Mater Lett. 2009;63:2236–2238. doi: 10.1016/j.matlet.2009.07.042. [DOI] [Google Scholar]
- 8.Shakeel A, Mudasir A, Babu LS, Saiqa I. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J Adv Res. 2015 doi: 10.1016/J.Jare.2015.02.007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husseiney MI, El-Aziz MA, Badr Y, Mahmoud MA. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A. 2007;67:1003–1006. doi: 10.1016/j.saa.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Priyadarshini S, Gopinath V, Meera Priyadharsshini N, Mubarakali D, Velusamy P. Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf B Biointerfaces. 2013;102:232–237. doi: 10.1016/j.colsurfb.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Klaus T, Joerger R, Olsson E, Granqvist CG. Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci USA. 1999;96:13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy AS, Chen CY, Chen CC, Jean JS, Chen HR, Tseng MJ, Fan CW, Wang JC. Biological synthesis of gold and silver nanoparticles mediated by the bacteria Bacillus subtilis. J Nanosci Nanotechnol. 2010;10:6567–6574. doi: 10.1166/jnn.2010.2519. [DOI] [PubMed] [Google Scholar]
- 13.Wei X, Luo M, Li W, Yang L, Liang X, Xu L, Kong P, Liu H. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresour Technol. 2012;103:273–278. doi: 10.1016/j.biortech.2011.09.118. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Cañizares MC, Monger W, Perrin Y, Tsakiris E, Porta C, Shariat N, Nicholson L, Lomonossoff GP. Cowpea mosaic virus-based systems for the production of antigens and antibodies in plants. Vaccine. 2005;23:1788–1792. doi: 10.1016/j.vaccine.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Blum AS, Soto CM, Wilson CD, Brower TL, Pollack SK, Schull TL, Chatterji A, Lin T, Johnson JE, Amsinck C, Franzon P, Shashidhar R, Ratna BR. An engineered virus as a scaffold for three-dimensional self-assembly on the nanoscale. Small. 2005;1:702–706. doi: 10.1002/smll.200500021. [DOI] [PubMed] [Google Scholar]
- 16.Yu L, Banerjee IA, Matsui H. Direct growth of shape-controlled nanocrystals on nanotubes via biological recognition. J Am Chem Soc. 2003;125:14837–14840. doi: 10.1021/ja037117i. [DOI] [PubMed] [Google Scholar]
- 17.Marshall M, Beliaev A, Dohnalkova A, David W, Shi L, Wang Z. C-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. Plos Biol. 2007;4:1324–1333. doi: 10.1371/journal.pbio.0040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SW, Mao C, Flynn CE, Belcher AM. Ordering of quantum dots, using genetically engineered viruses. Science. 2002;296:892–895. doi: 10.1126/science.1068054. [DOI] [PubMed] [Google Scholar]
- 19.Dias MA, Lacerda IC, Pimentel PF, de Castro HF, Rosa CA. Removal of heavy metals by an Aspergillus terreus strain immobilized in a polyurethane matrix. Lett Appl Microbiol. 2002;34:46–50. doi: 10.1046/j.1472-765x.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 20.Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–1418. doi: 10.1016/j.matlet.2006.07.042. [DOI] [Google Scholar]
- 21.Mariekie G, Anthony P. Microbial production of gold nanoparticles. Gold Bull. 2006;39:22–28. doi: 10.1007/BF03215529. [DOI] [Google Scholar]
- 22.Shenton W, Douglas T, Young M, Stubbs G, Mann S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater. 1999;11:253–256. doi: 10.1002/(SICI)1521-4095(199903)11:3<253::AID-ADMA253>3.0.CO;2-7. [DOI] [Google Scholar]
- 23.Mao C, Flynn CE, Hayhurst A, Sweeney R, Qi J, Georgiou G, Iverson B, Belcher AM. Viral assembly of oriented quantum dot nanowires. Proc Natl Acad Sci USA. 2003;100:6946–6951. doi: 10.1073/pnas.0832310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Microbial synthesis of semiconductor Cds nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng. 2002;78:583–588. doi: 10.1002/bit.10233. [DOI] [PubMed] [Google Scholar]
- 25.Awadalla FT, Pesic B. Biosorption of cobalt with the AMTTM metal removing agent. Hydrometallurgy. 1992;28:65–80. doi: 10.1016/0304-386X(92)90065-8. [DOI] [Google Scholar]
- 26.Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361. doi: 10.1021/la020835i. [DOI] [Google Scholar]
- 27.Hosea M, Greene B, Mcpherson R, Henzl M, Alexander MD, Darnall DW. Accumulation of elemental gold on the alga Chlorella vulgaris. Inorg Chim Acta. 1986;123:161–165. doi: 10.1016/S0020-1693(00)86339-2. [DOI] [Google Scholar]
- 28.Xie J, Lee JY, Wang DI, Ting YP. Identification of active biomolecules in the high-yield synthesis of single-crystalline gold nanoplates in algal solutions. Small. 2007;3:672–682. doi: 10.1002/smll.200600612. [DOI] [PubMed] [Google Scholar]
- 29.Mata YN, Blázquez ML, Ballester A, González F, Muñoz JA. Characterization of the biosorption of cadmium, lead and copper with the brown algae Fucus vesiculosus. J Hazard Mater. 2008;158:316–323. doi: 10.1016/j.jhazmat.2008.01.084. [DOI] [PubMed] [Google Scholar]
- 30.Das J, Velusamy P. Antibacterial effects of biosynthesized silver nanoparticles using aqueous leaf extract of Rosmarinus officinalis L. Mater Res Bull. 2013;48:4531–4537. doi: 10.1016/j.materresbull.2013.07.049. [DOI] [Google Scholar]
- 31.Das J, Das MP, Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta, Part A. 2013;104:265–270. doi: 10.1016/j.saa.2012.11.075. [DOI] [PubMed] [Google Scholar]
- 32.Gopinath V, Mubarakali D, Priyadarshini S, Meera PN, Noor T, Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf B Biointerfaces. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Anshup A, Venkataraman JS, Subramaniam C, Kumar RR, Priya S, Kumar TR, Omkumar RV, John A, Pradeep T. Growth of gold nanoparticles in human cells. Langmuir. 2005;21:11562–11567. doi: 10.1021/la0519249. [DOI] [PubMed] [Google Scholar]
- 34.Larios-Rodriguez E, Rangel-Ayon C, Castillo SJ, Zavala G, Herrera-Urbina R. Bio-synthesis of gold nanoparticles by human epithelial cells, in vivo. Nanotechnology. 2011;22:355601. doi: 10.1088/0957-4484/22/35/355601. [DOI] [PubMed] [Google Scholar]
- 35.Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using chenopodium album leaf extract. Colloids Surf, A. 2010;369:27–33. doi: 10.1016/j.colsurfa.2010.07.020. [DOI] [Google Scholar]
- 36.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Agnihotri S, Mukherji S, Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5–100 Nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014;4:3974–3983. doi: 10.1039/C3RA44507K. [DOI] [Google Scholar]
- 38.Park Y. A New Paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol Res. 2014;30:169–178. doi: 10.5487/TR.2014.30.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci. 2007;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 42.Song HY, Ko KK, Oh LH, Lee BT. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cell Mater. 2006;11:58. [Google Scholar]
- 43.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: Technological concepts and future applications. J Nanopart Res. 2008;10:507–517. doi: 10.1007/s11051-007-9275-x. [DOI] [Google Scholar]
- 44.Ramamurthy CH, Padma M, Samadanam ID, Mareeswaran R, Suyavaran A, Kumar MS, Premkumar K, Thirunavukkarasu C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf B Biointerfaces. 2013;102:808–815. doi: 10.1016/j.colsurfb.2012.09.025. [DOI] [PubMed] [Google Scholar]