Abstract
Extensively drug-resistant (XDR) tuberculosis is becoming increasingly prevalent worldwide, but little is known about XDR tuberculosis in young children. In this Grand Round we describe a 2-year-old child from the USA who developed pneumonia after a 3 month visit to India. Symptoms resolved with empirical first-line tuberculosis treatment; however, a XDR strain of Mycobacterium tuberculosis grew in culture. In the absence of clinical or microbiological markers, low-radiation exposure pulmonary CT imaging was used to monitor treatment response, and guide an individualised drug regimen. Management was complicated by delays in diagnosis, uncertainties about drug selection, and a scarcity of child-friendly formulations. Treatment has been successful so far, and the child is in remission. This report of XDR tuberculosis in a young child in the USA highlights the risks of acquiring drug-resistant tuberculosis overseas, and the unique challenges in management of tuberculosis in this susceptible population.
Introduction
Extensively drug-resistant (XDR) tuberculosis is becoming increasingly prevalent worldwide. XDR tuberculosis is caused by bacterial strains that are resistant to isoniazid and rifampicin (multidrug resistant [MDR]), any fluoroquinolone, and at least one of three injectable second-line drugs (ie, amikacin, kanamycin, or capreomycin). XDR tuberculosis is very difficult to treat and associated with high mortality, especially in HIV-infected patients.1 XDR tuberculosis has been reported in 105 countries and is estimated to cause about 10% of cases of MDR tuberculosis.2 Although XDR tuberculosis is being increasingly reported, especially in urban areas in tuberculosis-endemic countries such as India, the absence of validated standards for drug-susceptibility testing (DST) remains a major challenge to diagnosis.3 Since international travel is becoming more common, the possibility of acquiring tuberculosis during travel and importation into low-prevalence settings is increasing. Moreover, active tuberculosis is a prominent disease in travellers, although definitive attribution of infection to the travel event is made difficult by the wide range of latency periods.4,5
Childhood tuberculosis constitutes 10–20% of all tuberculosis in high-burden countries,6 and accounts for 8–20% of tuberculosis-related deaths.7,8 However, since most children are sputum microscopy smear negative, and culture (when done) is not as sensitive as in adults, the burden of childhood tuberculosis and drug-resistant tuberculosis are probably underestimated. In particular, little is known about XDR tuberculosis in young children (aged ≤5 years), with only five reports with outcomes published worldwide.9–11 Moreover, young children are a susceptible population, with unique difficulties associated with the management of tuberculosis. Diagnosis is challenging because of the paucibacillary nature of infection and difficulties in obtaining appropriate clinical specimens, leading to diagnostic uncertainties and delays.12–16 Similarly, assessing response to treatment is particularly challenging in young children. Clinical response can sometimes be noted with suboptimal regimens, and microbiology cannot be used to monitor culture-negative disease. In this Grand Round, we report a case of XDR tuberculosis in a young child in the USA, after a 3 month visit to India. A recent study15 from this region in children younger than 5 years reported that four (57%) of the seven tuberculosis cases for which culture confirmation was possible, were due to drug-resistant strains. In view of the absence of clinical or microbiological markers, CT imaging was used to monitor response to an individualised drug regimen for XDR tuberculosis.
Case presentation
A previously healthy 2-year-old girl from the USA presented with a 2 week history of daily high fevers after a 3 month visit to India. On arrival in India, she was immunised with BCG. She stayed with her grandparents in an urban area and attended a local day-care facility. During the last week of her visit, she developed fevers that continued after her return to the USA. Clinical assessment showed a temperature of 39·9°C, tachycardia (172 beats per min), blood pressure of 95/49 mm Hg, tachypnoea (44 breaths per min), peripheral capillary oxygen saturation (SpO2) of 100% (in room air), weight of 12·7 kg, and no adventitious lungs sounds. Blood, urine, throat, and stool cultures were negative, as were malaria smears. The QuantiFERON-TB Gold In-Tube test (Cellestis, Chadstone, VIC, Australia) was positive and chest radiography showed a left lower lobe infiltrate. The concentration of C-reactive protein was high (12·1 mg/dL). A left lower lobe infiltrate and hilar adenopathy were seen on CT imaging (figure 1A; video 1). HIV testing was negative. Gastric aspirates were obtained for mycobacterial smear microscopy and culture on sequential days. Acid-fast bacilli stains were negative; however, in view of the high clinical suspicion, the child was started on first-line tuberculosis treatment with isoniazid, rifampicin, pyrazinamide, and ethambutol. The child improved clinically with resolution of fevers after 4 weeks, a decline in inflammatory markers, and weight gain (figure 2).
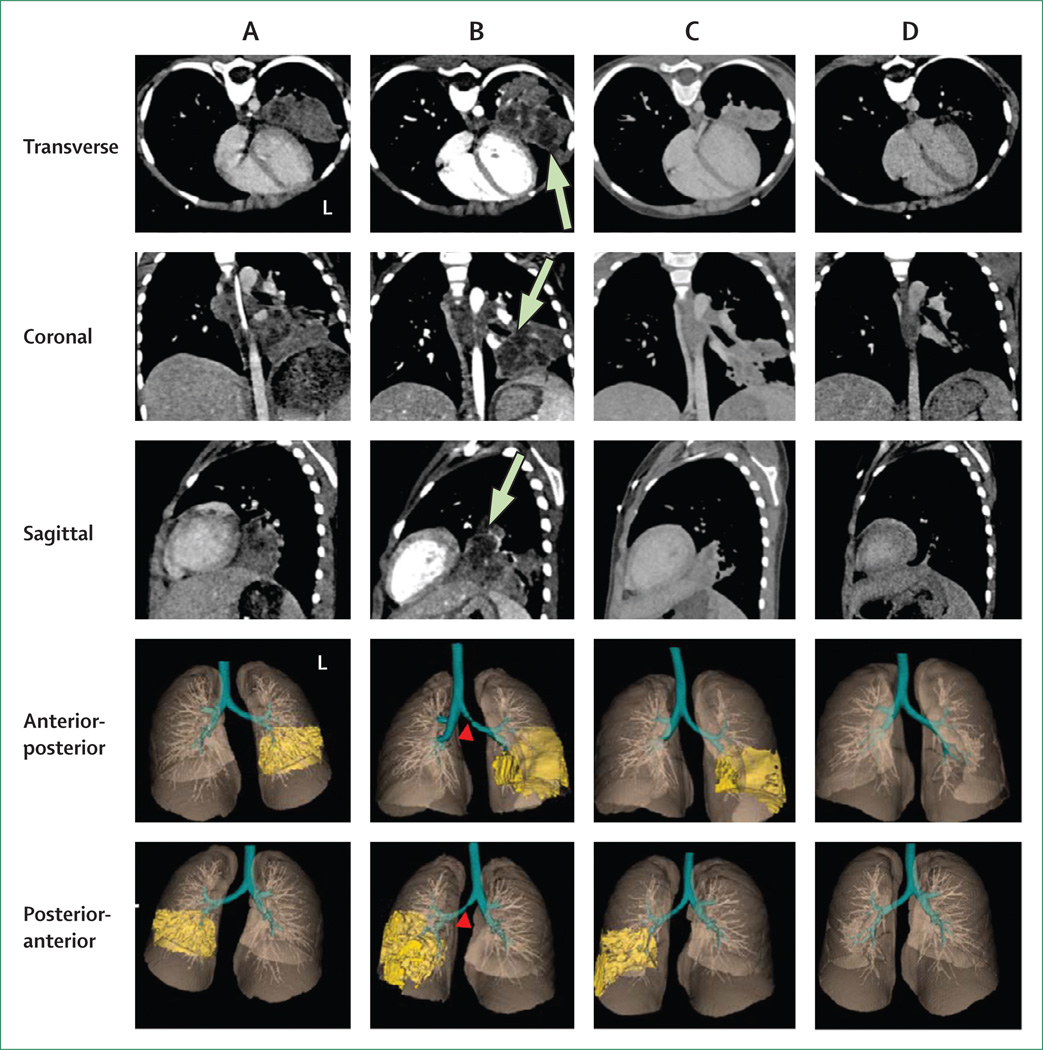
Figure 1. CT imaging.
CT imaging with intravenous contrast was done using the Definition FLASH (Siemens, Malvern, PA, USA) using a protocol customised for children. Lung segmentation and visualisation were done using VivoQuant 1·23 (inviCRO, Boston, MA, USA). The transverse, coronal, sagittal, and 3D views of the lung parenchyma and the pulmonary infiltrates in the left lung are shown. Each panel corresponds to CT done at: initiation of first-line tuberculosis treatment (A, day 0); initiation of individualised extensively drug-resistant (XDR) tuberculosis treatment (B, day 90); and 6 weeks (C, day 131) and 6 months (D, day 270) after initiation of XDR tuberculosis treatment. Several necrotic (hypodense) central areas can be seen in B (green arrows). Note the partial obstruction of the left main bronchus in B (red arrowheads). Marked improvement with resolution of necrotic areas is noted after 6 weeks (C) and near complete resolution of the infiltrate after 6 months (D) of XDR tuberculosis treatment. L = left side.
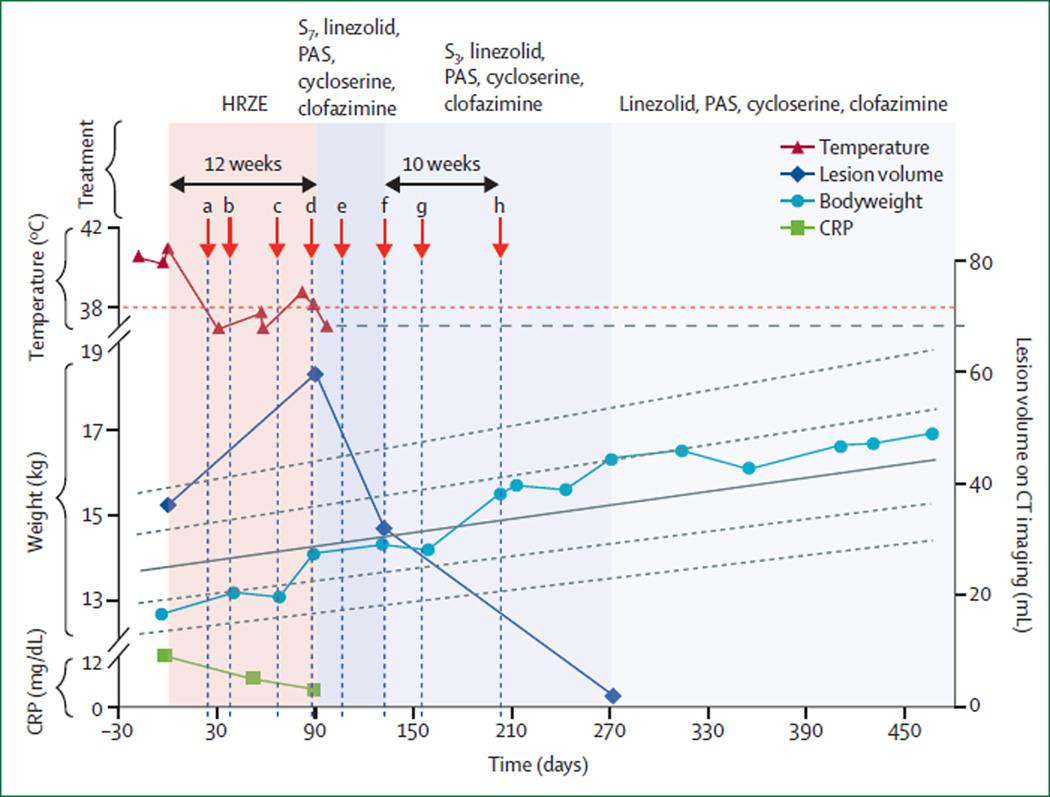
Figure 2. Clinical course.
The temperature, total bodyweight, lesion volume on CT imaging, and CRP concentration during the course of the illness and treatment are shown. The dashed red line shows the cutoff for fever; the child’s body temperature was not measured daily after it became normal, but she had a healthy body temperature thereafter (represented by the horizontal, dashed grey line). The solid grey line shows mean (0 Z score) female weight-for-age growth, and the dashed grey lines correspond to the 1, 0·5, −0·5, and −1 female weight-for-age Z scores. Red arrows denote: (a) growth of acid-fast bacilli in liquid broth; (b) identification of Mycobacterium tuberculosis complex by 16S rRNA sequencing; (c) persistent left lower lobe infiltrate on chest radiography; (d) Sanger sequencing and initial TREK panel confirming extensively drug-resistant strain; (e) agar proportion results (from the Maryland Department of Health and Mental Hygiene reference laboratory); (f) CT showing substantial reduction in lesion volume; (g) agar proportion results (from the National Jewish Health Mycobacteriology reference laboratory); (h) consistent weight gain. HRZE = isoniazid, rifampicin, pyrazinamide (discontinued at 8 weeks), and ethambutol. S7 = daily intravenous streptomycin. S3 = thrice-weekly intravenous streptomycin. PAS = para-aminosalicylic acid. CRP = C-reactive protein.
At 4 weeks, acid-fast bacilli were detected in one of four gastric aspirate cultures (Mycobacteria Growth Indicator Tube [MGIT] 960, Becton Dickinson, Sparks, MD, USA). Although the AccuProbe (GenProbe, San Diego, CA, USA) test was negative, subsequent 16S rRNA sequencing17 of the MGIT culture pellet identified it as a Mycobacterium tuberculosis complex. Chest radiography at 10 weeks showed a persistent left lower lobe infiltrate. Mycobacterial growth on Middlebrook 7H11 and Löwenstein-Jensen solid media was poor, and 12 weeks passed before final identification and preliminary DST results identified the isolate as XDR M tuberculosis (table 1). At this time, clinical assessment revealed a temperature of 38·2°C, heart rate of 126 beats per min, blood pressure of 100/57 mm Hg, respiratory rate of 20 breaths per min, SpO2 of 100% in room air, bodyweight of 14·1 kg, with decreased air entry and end-expiratory wheeze in the left lower lung field. CT imaging showed worsening infiltrate, several necrotic areas, and partial obstruction of the left main bronchus (figure 1B; video 2). Gastric aspirates were repeated, central venous access established, and the child started on an individualised drug regimen for XDR tuberculosis (25 mg/kg per day intravenous streptomycin, 20 mg/kg per day linezolid, 150 mg/kg per day para-aminosalicylic acid [PAS], 20 mg/kg per day cycloserine, 50 mg per day clofazimine, and vitamin B6). The child was discharged home 5 days after admission to the hospital. The drugs were given at home by a trained nurse or a parent, and directly observed by a nurse from the local health department.
Table 1.
Results of drug susceptibility testing
| Mutation(s) | Likelihood of phenotypic resistance |
MGIT (interpretation, critical concentration tested) |
TREK Sensititre plates (interpretation, MIC) |
Agar proportion* (interpretation, critical concentration tested) |
||
|---|---|---|---|---|---|---|
| Maryland State | National Jewish | |||||
| Rifampicin | rpoB (Ser531Leu) | 100% | Resistant, 1 µg/mL | Resistant, ≥16 µg/mL | Resistant, 1 µg/mL | Resistant, 1 µg/mL |
| Isoniazid | katG (Ser315Thr) | 100% | Resistant, 0·1 and 0·4 µg/mL |
Resistant, 2 µg/mL | Resistant, 1 µg/mL | Resistant, 0·2 and 1 µg/mL |
| Pyrazinamide | pncA (Asp12Glu) | Likely | Resistant, 100 µg/mL | ‥ | ‥ | ‥ |
| Ethambutol† | embB (Met306Ile) | 87% | Susceptible, 4 µg/mL | Susceptible, 4 µg/mL | Resistant, 5 µg/mL; susceptible, 10 µg/mL |
Susceptible, 7·5 µg/mL |
| Rifabutin | ‥ | ‥ | ‥ | Resistant, 16 µg/mL | ‥ | ‥ |
| Amikacin | rrs (Ala1401Gly) | 100% | ‥ | Resistant, ≥16 µg/mL | ‥ | Resistant, 6 µg/mL |
| Kanamycin | rrs (Ala1401Gly) | 100% | ‥ | Resistant, ≥40 µg/mL | Resistant, 6 µg/mL | Resistant, 6 µg/mL |
| Capreomycin | rrs (Ala1401Gly) | >45% | ‥ | ‥ | ‥ | Susceptible, 10 µg/mL |
| Streptomycin | ‥ | ‥ | Resistant, 1 µg/mL; susceptible, 4 µg/mL |
Susceptible, 2 µg/mL | Susceptible, 2 µg/mL | Susceptible, 2 and 4 µg/mL |
| Moxifloxacin | gyrA (Asp94Gly) | 100% | ‥ | Resistant, 4 µg/mL | ‥ | ‥ |
| Ofloxacin | gyrA (Asp94Gly) | 100% | ‥ | Resistant, 16 µg/mL | Resistant, 2 µg/mL | ‥ |
| Ethionamide |
InhA mutation not detected |
‥ | ‥ | Susceptible, 2·5 µg/mL | Susceptible, 10 µg/mL | Susceptible, 10 µg/mL |
| Cycloserine | ‥ | ‥ | ‥ | Susceptible, 4 µg/mL | ‥ | Susceptible, 60 µg/mL |
| PAS | ‥ | ‥ | ‥ | Susceptible, 0·5 µg/mL | ‥ | Susceptible, 8 µg/mL |
| Linezolid | ‥ | ‥ | ‥ | Susceptible, ≤1 µg/mL | ‥ | Likely susceptible, ≤4 µg/mL |
| Clofazimine‡ | ‥ | ‥ | ‥ | Susceptible, ≤0·125 µg/mL | ‥ | Susceptible, 0·25 µg/mL |
MIC determinations were done using MGIT and TREK Sensititre plates (ThermoScientific, Oakwood Village, OH, USA) at Johns Hopkins Hospitals (MD, USA). Sanger sequencing (complete panel) was used for molecular detection of drug resistance (Centers for Disease Control and Prevention, Atlanta, GA, USA). All TREK assays were done in duplicate or triplicate, and inoculum size was verified by determination of viable counts. Linezolid and clofazimine susceptibilities were not available (by any method) at the time of initiation of the individualised extensively drug-resistant tuberculosis treatment.
MGIT = mycobacteria growth indicator tube. MIC = minimum inhibitory concentration. PAS = para-aminosalicylic acid.
Agar proportion drug susceptibility testing was done at the Maryland Department of Health and Mental Hygiene (Maryland State) and the National Jewish Health Mycobacteriology (National Jewish) reference laboratories.
Breakpoint for ethambutol is 7·5 µg/mL.
Assay not clinically validated.
Since the child initially improved on standard first-line treatment, the gastric aspirate smears were negative at the time of initiation of XDR tuberculosis treatment, and cultures remained negative subsequently. Low-radiation exposure pulmonary CT imaging was used to assess the response to treatment 6 weeks after initiation of XDR tuberculosis treatment (appendix). Imaging revealed a marked reduction in the lesion volume (figure 2) with resolution of necrotic areas (figure 1C; video 3). The improvement in CT imaging was corroborated by consistent weight gain noted over the next few months, but which lagged behind CT results by 10 weeks (figure 2). After 6 months of treatment, CT imaging showed resolution of the infiltrate, with some residual fibrosis (figure 1D; video 4). On the basis of these results, which suggested that the chosen regimen was indeed effective, streptomycin was spaced to thrice weekly (from daily dosing) at 6 weeks, and then discontinued at 6 months. Linezolid, PAS, cycloserine, and clofazimine were continued. Close clinical and laboratory monitoring and age-appropriate hearing and vestibular testing were done. No side-effects other than hypothyroidism (attributed to PAS and that needed treatment with levothyroxine) and bronze skin discolouration (attributed to clofazimine) developed. The child received 18 months of directly observed treatment for XDR tuberculosis, and remains well in remission. However, follow-up will continue to ensure treatment has been successful.
Review and discussion
Travel-associated tuberculosis
Results from studies suggest that 0·9–2·7% of travellers presenting to health-care facilities with illness after travel have active tuberculosis.18,19 In one study, 5·5% (two of 36) of children (aged ≤16 years) with travel-associated illness requiring hospital admission had active tuberculosis.20 Transmission of infection has been reported on aeroplanes.21 Although the overall risk is low (0·05 per 100 000 passengers), travellers on flights from tuberculosis-endemic areas in Africa or India had a seven times higher risk of acquiring tuberculosis, because flights originating from these regions were more likely to have passengers with tuberculosis.22 The risk of transmission in travellers to tuberculosis-endemic areas is similar to that in the local population, with an incidence rate of 2·8 per 1000 person-months of travel for tuberculosis infection and 0·6 per 1000 person-months of travel for active tuberculosis disease.23 However, the risk is increased substantially when travelling for 90 days or longer.24–27 Moreover, children visiting friends and relatives overseas have a high risk of acquiring infection.28 In one study29 of children (aged ≤6 years), travel to tuberculosis-endemic countries in the preceding 12 months increased the risk of acquiring tuberculosis infection by almost four times. More than half (55%) of the 105 children who travelled to tuberculosis-endemic countries had stayed with their grandparents, presumably as travellers visiting friends and relatives. BCG vaccination and isoniazid prophylaxis have been suggested for the prevention of travel-associated tuberculosis, but no consensus has been reached on whether and how they should be used.30,31
Diagnosis
Young children are rarely able to produce sputum, and therefore three consecutive early morning gastric lavage specimens have long been regarded as the standard of care. However, some clinicians will forego obtaining invasive specimens because of their low yield. The sensitivities of different specimens and methods to diagnose pulmonary tuberculosis in children vary (table 2). Smear microscopy is the most widely available, but has a low sensitivity with a yield of less than 10% reported in most studies. Culture is the gold-standard method, with a wide range of sensitivities reported, but most studies report a sensitivity of 10–30%. GeneXpert (Xpert MTB/RIF, Cepheid, Sunnyvale, CA, USA) is an automated, cartridge-based test that detects M tuberculosis and rifampicin resistance. GeneXpert has high sensitivity for the detection of adult pulmonary tuberculosis,39 and WHO has widely recommended its use. Although GeneXpert is significantly more sensitive than smear microscopy alone, culture is more sensitive.14,32,37 In one study investigating pulmonary tuberculosis in children, GeneXpert failed to detect as many as 40% of culture-positive cases.37 Sputum induction has been shown to be safe, and as effective as gastric aspirates for the diagnosis of tuberculosis.33–35,38 However, one study reported that gastric lavage had better yields.16 The assessment of alternative patient samples, including nasopharyngeal aspirate and stool, is continuing.36,40 Multiple specimens are helpful at achieving the maximum yield.16,38,41 Urine tests for mycobacterial lipoarabinomannan by both ELISA and lateral flow were reported to be sensitive in adults with advanced HIV disease, but had low sensitivity and specificity in children.42,43
Table 2.
Diagnostic yields from clinical specimens in children with pulmonary tuberculosis
| Gastric lavage | Induced sputum | |||
|---|---|---|---|---|
| One specimen | Multiple specimens | One specimen | Multiple specimens | |
| Smear | 2·2% (1·4–6·9)12,16,32,33 |
7·0% (2.3–10·4)12,16,33 |
5·2% (3·5–8·0)16,33–36 |
6·7% (5·3–10)16,33,35,36 |
| GeneXpert | 4·2%32 | ‥ | 10·4% (3·9–12·6)14,35–37 |
11·4% (5·2–15·1)14,35–37 |
| Culture | 8·5% (6·1–42·0)12,16,32,33,38 |
15·0% (3·1–66·0)12,15,16,33,38 |
15·0% (3·0–38·0)14,16,33–36,38 |
18·3% (15·1–55·0)14,16,33,35,36,38 |
Data are median % (range).
In 2014, a review reported that the risk factors for acquiring MDR tuberculosis in children are similar to adults, but that many children with drug-resistant tuberculosis go undetected.44 The infrequency with which mycobacterial cultures and DST are done because of difficulties in obtaining appropriate specimens and low yields might be a reason for the low detection rate. GeneXpert can rapidly detect common rifampicin resistance mutations with the intent of being able to more rapidly initiate second-line treatment. Although GeneXpert is an important contribution to the rapid detection of drug resistance, it is less sensitive than liquid culture in children. Furthermore, a report has suggested that the current version of GeneXpert might not detect a substantial proportion of strains that could be resistant to rifampicin.45
Treatment and monitoring
The basic principles for treating XDR tuberculosis are similar to those for treating MDR tuberculosis.46,47 Tuberculosis drugs are chosen from five groups of drugs in a stepwise manner. The treatment regimen includes at least four drugs to which the isolate is regarded as susceptible, although five drugs are generally used for XDR tuberculosis. Surgery is considered in some situations, although good success with medical management alone has been reported in children.48 In view of the genotypic and phenotypic evidence of resistance to isoniazid, rifampicin, and pyrazinamide, the child’s improvement on standard first-line tuberculosis treatment in this report was attributed to monotherapy with ethambutol. Reports from the early 1960s suggested that adult patients with tuberculosis receiving monotherapy with ethambutol showed an initial bacteriological (but not radiological) improvement, followed by treatment failure because of the development of drug resistance.49 Streptomycin was included on the basis of initial phenotypic susceptibility results, and its established activity against M tuberculosis.50 Moreover, although the rrs mutation detected in the child’s isolate confers resistance to several aminoglycosides, it does not seem to confer cross-resistance to streptomycin.51 PAS and cycloserine were chosen because of their favourable susceptibility results. Furthermore, PAS has historically been used in combination with streptomycin with good outcomes.52,53 Linezolid was included because it has been shown to be highly effective in adults with refractory XDR tuberculosis54 and was well tolerated in a series describing treatment of 18 children with drug-resistant tuberculosis.48 Finally, clofazimine was included on the basis of evidence suggesting efficacy in treating drug-resistant tuberculosis.55 In view of the high minimum inhibitory concentration for ethionamide (2·5 µg/mL), and challenges in achieving sufficient serum concentrations in young children (maximum serum concentration [Cmax] ≥5 µg/mL),56 ethionamide was not included. As a result of discordant initial (genotypic and phenotypic) susceptibility results, and because no subsequent isolate was available for testing, ethambutol was not included. Delamanid and bedaquiline were considered; however, because of their unknown safety profile and the absence of pharmacokinetic data and child-friendly formulations, they were not used. Treatment of tuberculosis in young children is complicated by the scarcity of child-friendly drug formulations.57 Commercial PAS and cycloserine had to be custom formulated (opened, re-weighed, and unit dosed) and given with child-friendly foods (eg, apple sauce and chocolate pudding).57 Clofazimine, a hard-gel capsule, was swallowed whole by the child.
Historically, response to treatment for pulmonary tuberculosis is assessed clinically, by monitoring symptoms and weight gain, and by testing monthly sputum samples for smear or culture conversion. However, this assessment is particularly challenging in children with paucibacillary disease, in which clinical response can be seen with suboptimal regimens, and microbiology cannot be used to monitor culture-negative disease. Radiological imaging, especially chest radiography, is often used as complementary evidence of adequate treatment response. Compared with culture, which needs weeks or months, imaging is rapid and correlates well with disease progression and efficacy of tuberculosis treatments in animals58–60 and human beings.61–63 Serial CT imaging has been shown to be a good marker of response to tuberculosis treatments in adults.64,65 Although CT imaging has not yet been used to serially monitor tuberculosis treatments in young children, a retrospective study in infants diagnosed with pulmonary tuberculosis reported that CT imaging provided better visualisation of parenchymal lesions and lymphadenopathy than chest radiography.66 Chen and colleagues63 reported that CT imaging could be better than conventional (sputum) microbiology for monitoring response to MDR tuberculosis treatments in adults. In this study, quantitative changes in lesion volumes on CT imaging were predictive of treatment responses at both 2 and 6 months after initiation of treatment. Moreover, quantitative changes in 18F-fluorodeoxyglucose (18F-FDG) PET, which is a sensitive measure of metabolic activity, not only predicted treatment responses earlier, but also predicted long-term treatment outcomes. Pathogen-specific imaging techniques in development could have the potential to more precisely predict treatment response.67,68 CT is often perceived to deliver high levels of radiation. However, technological developments and the design of customised protocols for paediatric patients (dose modulation, lower tube voltage, and iterative reconstruction) have significantly lowered radiation exposure. For example, the effective dose for each chest CT in this child was 0·4–0·7 mSv, which is equivalent to 2 or 3 months of natural background radiation. Moreover, no sedation was needed because scan times were short (3 s). Low-radiation exposure protocols are routinely used for children at Johns Hopkins Hospitals, and could be applied more universally, including in developing countries.69
Outcomes
Treatment success is dependent on several factors such as host immunity, extent of drug resistance, and disease severity. However, favourable outcomes, which are defined as cure or treatment completion, are much lower (16–44%) in patients with XDR tuberculosis than in those with MDR tuberculosis. Furthermore, very high mortality (98%) was reported by Gandhi and colleagues1 in highly immunosuppressed adults with XDR tuberculosis and HIV co-infection. Data for treatment outcomes of XDR tuberculosis in young children are scarce, although case reports and expert opinion suggest they are likely to do better than adults.70 Five reports (three pulmonary and two meningitis) of XDR tuberculosis in children younger than 5 years with outcomes, have been published worldwide.9–11 One patient with tuberculosis meningitis died, but the other four were either cured or had culture conversion and were continuing treatment at the time the report was published. The children were reported to have tolerated the drugs well, with PAS-induced hypothyroidism being the most common side-effect. Liver toxic effects were reported in one child with both first-line and optimised treatment regimens. All diagnoses were delayed by 1 month to 1 year. Optimum duration of treatment for XDR tuberculosis in young children is not known; however, experts and guidelines recommend 18 months after culture conversion.46
In this report, no immediate contacts who were tested were reported to have active disease. Genotyping confirmed that the isolate was of east African Indian lineage, with no match to any previous isolate from the USA. Tuberculosis in young children is considered non-infectious,71 and adult patients with MDR tuberculosis are rendered non-infectious after 2 weeks of appropriate treatment.72 However, much debate occurred regarding the risk of this child to the general public, and the implications of public contact tracing, since current diagnostics cannot distinguish tuberculosis infection with resistant versus susceptible strains. A then 4-year-old sibling, who had extensive initial contact with the child described in this report, showed no signs of infection (negative QuantiFERON-TB testing initially and 6 months later) or disease. No other household member acquired infection, and all of them remain disease free.
In summary, this report of XDR tuberculosis in a young child in the USA highlights the risks of acquiring drug-resistant tuberculosis overseas. Empirical first-line treatment resulted in initial resolution of symptoms, but enabled disease progression. In view of the absence of clinical or microbiological markers, CT imaging was used to monitor and optimise an individualised XDR-tuberculosis drug regimen. Imaging showed marked improvement by 6 weeks, corroborated by consistent weight gain. However, the improvement in weight gain lagged behind CT imaging by 10 weeks, suggesting that CT imaging can serve as a rapid biomarker to monitor tuberculosis treatments. Treatment was complicated by the scarcity of child-friendly drug formulations and evidence-based dosing recommendations for some drugs, and controversy regarding the infectious risk of the child to the general public. Although treatment is complete, and the child is now in remission, this report highlights the unique difficulties associated with the management of drug-resistant tuberculosis in young children, a susceptible population for whom challenges in diagnosis, monitoring, and treatment could have fatal results.
Supplementary Material
Search strategy and selection criteria.
We identified data by searching PubMed for articles published in English with the terms “children” AND “tuberculosis” AND “diagnosis” AND “microbiology” between April 1, 2010, and Sept 5, 2015. Additional searches included the search terms “travel” AND “associated” AND “tuberculosis” (all articles until Sept 5, 2015), and “imaging” AND “tuberculosis” AND “therapy” OR “treatment” AND “correlates” OR “monitoring” (between April 1, 2010, and Sept 5, 2015). We reviewed identified articles and other relevant references from hand-searching of records.
Acknowledgments
JRS has been a member of a data safety monitoring board for Otsuka Pharmaceuticals, outside the submitted work. SKJ reports grants from the National Institutes of Health (NIH), during the conduct of the study, and grants from NIH and Gilead Biosciences, outside the submitted work. Additionally, SKJ has a patent pending (PCT/US13/059897).
This work was supported by the National Institutes of Health (NIH) Director’s Transformative Research Award R01-EB020539 (SKJ), NIH Director’s New Innovator Award DP2-OD006492 (SKJ), NHLBI R01-HL116316 (SKJ), and the Pediatric Infectious Diseases NIH Training grant (T32-AI052071). The funders had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript. We would like to thank the Centers for Disease Control and Prevention (Atlanta, GA, USA) for their support and for doing the Sanger sequencing (complete panel), and the Michigan Department of Community Health (Lansing, MI, USA) for genotyping. We would also like to thank Richard Chaisson and William Bishai (Johns Hopkins Hospitals) for comments, Pediatric House staff, Nursing and Pharmacy (Johns Hopkins Hospitals), Nancy Baruch, Lisa Paulos, Maureen Donovan, and Wendy Cronin (Maryland Department of Health and Mental Hygiene Center for TB Control and Prevention, MD, USA), and Bernard Farrell and Jayne McGunigale (Howard County Health Department, MD, USA).
Footnotes
See Online for video
See Online for appendix
Contributors
NS-A and SKJ were the primary physicians and managed the patient. NS-A and SKJ also did the scientific literature searches and wrote the initial draft. AJH was the clinical pharmacist and helped in paediatric dosing and formulations. JEB provided clinical reporting for CT and MM calculated the radiation dose. AAO did the CT image analyses. ELN and JRS were consultants for clinical management. NP processed microbiological specimens, isolated the organism, and did the MGIT and TREK assays. JHR and MS were responsible for the agar proportion assays. AMM helped with infection control and EM was in charge of directly observed treatment with the Health Department. NS-A and SKJ wrote the initial report and all coauthors participated in the editing of the report.
Declaration of interests
All other authors declare no competing interests.
References
- 1.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis report 2015. [accessed Oct 28, 2015]; http://www.who.int/tb/publications/global_report/en/
- 3.Michael JS, John TJ. Extensively drug-resistant tuberculosis in India: a review. Indian J Med Res. 2012;136:599–604. [PMC free article] [PubMed] [Google Scholar]
- 4.Schlagenhauf P, Weld L, Goorhuis A, et al. Travel-associated infection presenting in Europe (2008–12): an analysis of EuroTravNet longitudinal, surveillance data, and evaluation of the effect of the pre-travel consultation. Lancet Infect Dis. 2015;15:55–64. doi: 10.1016/S1473-3099(14)71000-X. [DOI] [PubMed] [Google Scholar]
- 5.Marienau KJ, Burgess GW, Cramer E, et al. Tuberculosis investigations associated with air travel: U. S. Centers for Disease Control and Prevention, January 2007-June 2008. Travel Med Infect Dis. 2010;8:104–112. doi: 10.1016/j.tmaid.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–263. [PubMed] [Google Scholar]
- 7.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(suppl 3):S184–S194. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367:348–361. doi: 10.1056/NEJMra1008049. [DOI] [PubMed] [Google Scholar]
- 9.Rose PC, Hallbauer UM, Seddon JA, Hesseling AC, Schaaf HS. Linezolid-containing regimens for the treatment of drug-resistant tuberculosis in South African children. Int J Tuberc Lung Dis. 2012;16:1588–1593. doi: 10.5588/ijtld.12.0322. [DOI] [PubMed] [Google Scholar]
- 10.Katragkou A, Antachopoulos C, Hatziagorou E, Sdougka M, Roilides E, Tsanakas J. Drug-resistant tuberculosis in two children in Greece: report of the first extensively drug-resistant case. Eur J Pediatr. 2013;172:563–567. doi: 10.1007/s00431-012-1811-8. [DOI] [PubMed] [Google Scholar]
- 11.Alsleben N, Garcia-Prats AJ, Hesseling AC, Willemse M, Donald PR, Schaaf HS. Successful treatment of a child with extensively drug-resistant tuberculous meningitis. J Ped Infect Dis. 2015;4:e41–e44. doi: 10.1093/jpids/piu120. [DOI] [PubMed] [Google Scholar]
- 12.Oberhelman RA, Soto-Castellares G, Gilman RH, et al. Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case-control study. Lancet Infect Dis. 2010;10:612–620. doi: 10.1016/S1473-3099(10)70141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockdale AJ, Duke T, Graham S, Kelly J. Evidence behind the WHO guidelines: hospital care for children: what is the diagnostic accuracy of gastric aspiration for the diagnosis of tuberculosis in children. J Trop Pediatr. 2010;56:291–298. doi: 10.1093/tropej/fmq081. [DOI] [PubMed] [Google Scholar]
- 14.Nicol MP, Workman L, Isaacs W, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11:819–824. doi: 10.1016/S1473-3099(11)70167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain SK, Ordonez A, Kinikar A, et al. Pediatric tuberculosis in young children in India: a prospective study. Biomed Res Int. 2013;2013:783698. doi: 10.1155/2013/783698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee A, Singh S, Lodha R, et al. Ambulatory gastric lavages provide better yields of Mycobacterium tuberculosis than induced sputum in children with intrathoracic tuberculosis. Pediatr Infect Dis J. 2013;32:1313–1317. doi: 10.1097/INF.0b013e31829f5c58. [DOI] [PubMed] [Google Scholar]
- 17.Cloud JL, Neal H, Rosenberry R, et al. Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries. J Clin Microbiol. 2002;40:400–406. doi: 10.1128/JCM.40.2.400-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansart S, Perez L, Vergely O, Danis M, Bricaire F, Caumes E. Illnesses in travelers returning from the tropics: a prospective study of 622 patients. J Travel Med. 2005;12:312–318. doi: 10.2310/7060.2005.12603. [DOI] [PubMed] [Google Scholar]
- 19.Monge-Maillo B, Norman FF, Perez-Molina JA, Navarro M, Diaz-Menendez M, Lopez-Velez R. Travelers visiting friends and relatives (VFR) and imported infectious disease: travelers, immigrants or both? A comparative analysis. Travel Med Infect Dis. 2014;12:88–94. doi: 10.1016/j.tmaid.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Hunziker T, Berger C, Staubli G, et al. Profile of travel-associated illness in children, Zurich, Switzerland. J Travel Med. 2012;19:158–162. doi: 10.1111/j.1708-8305.2012.00611.x. [DOI] [PubMed] [Google Scholar]
- 21.Driver CR, Valway SE, Morgan WM, Onorato IM, Castro KG. Transmission of Mycobacterium tuberculosis associated with air travel. JAMA. 1994;272:1031–1035. [PubMed] [Google Scholar]
- 22.Byrne N. Low prevalence of TB on long-haul aircraft. Travel Med Infect Dis. 2007;5:18–23. doi: 10.1016/j.tmaid.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Cobelens FG, van Deutekom H, Draayer-Jansen IW, et al. Risk of infection with Mycobacterium tuberculosis in travellers to areas of high tuberculosis endemicity. Lancet. 2000;356:461–465. doi: 10.1016/S0140-6736(00)02554-X. [DOI] [PubMed] [Google Scholar]
- 24.Cobelens FG, van Deutekom H, Draayer-Jansen IW, et al. Association of tuberculin sensitivity in Dutch adults with history of travel to areas of with a high incidence of tuberculosis. Clin Infect Dis. 2001;33:300–304. doi: 10.1086/321882. [DOI] [PubMed] [Google Scholar]
- 25.Kik SV, Mensen M, Beltman M, et al. Risk of travelling to the country of origin for tuberculosis among immigrants living in a low-incidence country. Int J Tuberc Lung Dis. 2011;15:38–43. [PubMed] [Google Scholar]
- 26.Mancuso JD, Tobler SK, Eick AA, Keep LW. Active tuberculosis and recent overseas deployment in the U. S. military. Am J Prev Med. 2010;39:157–163. doi: 10.1016/j.amepre.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Slopen ME, Laraque F, Piatek AS, Ahuja SD. Missed opportunities for tuberculosis prevention in New York City 2003. J Public Health Manag Pract. 2011;17:421–426. doi: 10.1097/PHH.0b013e31820759b8. [DOI] [PubMed] [Google Scholar]
- 28.Hendel-Paterson B, Swanson SJ. Pediatric travelers visiting friends and relatives (VFR) abroad: illnesses, barriers and pre-travel recommendations. Travel Med Infect Dis. 2011;9:192–203. doi: 10.1016/j.tmaid.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Lobato MN, Hopewell PC. Mycobacterium tuberculosis infection after travel to or contact with visitors from countries with a high prevalence of tuberculosis. Am J Respir Crit Care Med. 1998;158:1871–1875. doi: 10.1164/ajrccm.158.6.9804106. [DOI] [PubMed] [Google Scholar]
- 30.Rieder HL. Risk of travel-associated tuberculosis. Clin Infect Dis. 2001;33:1393–1396. doi: 10.1086/323127. [DOI] [PubMed] [Google Scholar]
- 31.Ritz N, Connell TG, Curtis N. To BCG or not to BCG? Preventing travel-associated tuberculosis in children. Vaccine. 2008;26:5905–5910. doi: 10.1016/j.vaccine.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 32.Bates M, O’Grady J, Maeurer M, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect Dis. 2013;13:36–42. doi: 10.1016/S1473-3099(12)70245-1. [DOI] [PubMed] [Google Scholar]
- 33.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–134. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 34.Moore HA, Apolles P, de Villiers PJ, Zar HJ. Sputum induction for microbiological diagnosis of childhood pulmonary tuberculosis in a community setting. Int J Tuberc Lung Dis. 2011;15:1185–1190. doi: 10.5588/ijtld.10.0681. i. [DOI] [PubMed] [Google Scholar]
- 35.Planting NS, Visser GL, Nicol MP, Workman L, Isaacs W, Zar HJ. Safety and efficacy of induced sputum in young children hospitalised with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2014;18:8–12. doi: 10.5588/ijtld.13.0132. [DOI] [PubMed] [Google Scholar]
- 36.Zar HJ, Workman L, Isaacs W, et al. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis. 2012;55:1088–1095. doi: 10.1093/cid/cis598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. Lancet Glob Health. 2013;1:e97–e104. doi: 10.1016/S2214-109X(13)70036-6. [DOI] [PubMed] [Google Scholar]
- 38.Hatherill M, Hawkridge T, Zar HJ, et al. Induced sputum or gastric lavage for community-based diagnosis of childhood pulmonary tuberculosis? Arch Dis Child. 2009;94:195–201. doi: 10.1136/adc.2007.136929. [DOI] [PubMed] [Google Scholar]
- 39.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicol MP, Spiers K, Workman L, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. 2013;57:e18–e21. doi: 10.1093/cid/cit230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Aghbari N, Al-Sonboli N, Yassin MA, et al. Multiple sampling in one day to optimize smear microscopy in children with tuberculosis in Yemen. PLoS One. 2009;4:e5140. doi: 10.1371/journal.pone.0005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicol MP, Allen V, Workman L, et al. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Glob Health. 2014;2:e278–e284. doi: 10.1016/S2214-109X(14)70195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroidl I, Clowes P, Reither K, et al. Performance of urine lipoarabinomannan assays for paediatric tuberculosis in Tanzania. Eur Respir J. 2015;46:761–770. doi: 10.1183/09031936.00003315. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383:1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Padilla E, Merker M, Beckert P, et al. Detection of drug-resistant tuberculosis by Xpert MTB/RIF in Swaziland. N Engl J Med. 2015;372:1181–1182. doi: 10.1056/NEJMc1413930. [DOI] [PubMed] [Google Scholar]
- 46.WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. [accessed Sept 5, 2015]; http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf?ua=1&ua=1. [PubMed]
- 47.Seddon J, Furin JJ, Gale M, et al. Caring for children with drug-resistant tuberculosis practice-based recommendations. Am J Respir Crit Care Med. 2012;186:953–964. doi: 10.1164/rccm.201206-1001CI. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Prats AJ, Rose PC, Hesseling AC, Schaaf HS. Linezolid for the treatment of drug-resistant tuberculosis in children: a review and recommendations. Tuberculosis. 2014;94:93–104. doi: 10.1016/j.tube.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Pyle MM. Ethambutol in the retreatment and primary treatment of tuberculosis: a four-year clinical investigation. Ann N Y Acad Sci. 1966;135:835–845. doi: 10.1111/j.1749-6632.1966.tb45526.x. [DOI] [PubMed] [Google Scholar]
- 50.Streptomycin in Tuberculosis Trials Committee. Streptomycin treatment of pulmonary tuberculosis. Br Med J. 1948;2:769–782. [PMC free article] [PubMed] [Google Scholar]
- 51.Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L. High level of cross-resistance between kanamycin, amikacin, and capreomycin among Mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother. 2009;53:5064–5068. doi: 10.1128/AAC.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox W, Sutherland I. A five-year assessment of patients in a controlled trial of streptomycin, para-aminosalicylic acid, and streptomycin plus para-aminosalicylic acid, in pulmonary tuberculosis. Q J Med. 1956;25:221–243. [PubMed] [Google Scholar]
- 53.Donald PR, Diacon AH. Para-aminosalicylic acid: the return of an old friend. Lancet Infect Dis. 2015;15:1091–1099. doi: 10.1016/S1473-3099(15)00263-7. [DOI] [PubMed] [Google Scholar]
- 54.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367:1508–1518. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dey T, Brigden G, Cox H, Shubber Z, Cooke G, Ford N. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2013;68:284–293. doi: 10.1093/jac/dks389. [DOI] [PubMed] [Google Scholar]
- 56.Thee S, Seifart HI, Rosenkranz B, et al. Pharmacokinetics of ethionamide in children. Antimicrob Agents Chemother. 2011;55:4594–4600. doi: 10.1128/AAC.00379-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peloquin CA, Durbin D, Childs J, Sterling TR, Weiner M. Stability of antituberculosis drugs mixed in food. Clin Infect Dis. 2007;45:521. doi: 10.1086/520011. [DOI] [PubMed] [Google Scholar]
- 58.Davis SL, Nuermberger EL, Um PK, et al. Noninvasive pulmonary [18F]-2-fluoro-deoxy-D-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob Agents Chemother. 2009;53:4879–4884. doi: 10.1128/AAC.00789-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ordonez AA, Pokkali S, DeMarco VP, et al. Radioiodinated DPA-713 imaging correlates with bactericidal activity of tuberculosis treatments in mice. Antimicrob Agents Chemother. 2015;59:642–649. doi: 10.1128/AAC.04180-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luna B, Kubler A, Larsson C, et al. In vivo prediction of tuberculosis-associated cavity formation in rabbits. J Infect Dis. 2015;211:481–485. doi: 10.1093/infdis/jiu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C. Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med. 2011;52:880–885. doi: 10.2967/jnumed.110.083709. [DOI] [PubMed] [Google Scholar]
- 62.Heysell SK, Thomas TA, Sifri CD, Rehm PK, Houpt ER. 18-Fluorodeoxyglucose positron emission tomography for tuberculosis diagnosis and management: a case series. BMC Pulm Med. 2013;13:14. doi: 10.1186/1471-2466-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen RY, Dodd LE, Lee M, et al. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci Transl Med. 2014;6:265ra166. doi: 10.1126/scitranslmed.3009501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tangg SJ, Zhang Q, Zheng LH, et al. Efficacy and safety of linezolid in the treatment of extensively drug-resistant tuberculosis. Jpn J Infect Dis. 2011;64:509–512. [PubMed] [Google Scholar]
- 65.Yanardag H, Tetikkurt C, Tetikkurt S, Demirci S, Karayel T. Computed tomography and bronchoscopy in endobronchial tuberculosis. Can Respir J. 2003;10:445–448. doi: 10.1155/2003/496296. [DOI] [PubMed] [Google Scholar]
- 66.Kim WS, Choi JI, Cheon JE, Kim IO, Yeon KM, Lee HJ. Pulmonary tuberculosis in infants: radiographic and CT findings. AJR Am J Roentgenol. 2006;187:1024–1033. doi: 10.2214/AJR.04.0751. [DOI] [PubMed] [Google Scholar]
- 67.Backus KM, Boshoff HI, Barry CS, et al. Uptake of unnatural trehalose analogs as a reporter for Mycobacterium tuberculosis. Nat Chem Biol. 2011;7:228–235. doi: 10.1038/nchembio.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinstein EA, Ordonez AA, DeMarco VP, et al. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med. 2014;6:259ra146. doi: 10.1126/scitranslmed.3009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahesh M. Advances in CT technology and application to pediatric imaging. Pediatr Radiol. 2011;41(suppl 2):493–497. doi: 10.1007/s00247-011-2169-1. [DOI] [PubMed] [Google Scholar]
- 70.The Emerging Threat of Drug-Resistant Tuberculosis in Southern Africa: Global and Local Challenges and Solutions: Summary of a Joint Workshop. [accessed Sept 5, 2015]; http://www.ncbi.nlm.nih.gov/books/NBK55582/ [PubMed]
- 71.Cruz AT, Medina D, Whaley EM, Ware KM, Koy TH, Starke JR. Tuberculosis among families of children with suspected tuberculosis and employees at a children’s hospital. Infect Control Hosp Epidemiol. 2011;32:188–190. doi: 10.1086/657940. [DOI] [PubMed] [Google Scholar]
- 72.Dharmadhikari AS, Mphahlele M, Venter K, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2014;18:1019–1025. doi: 10.5588/ijtld.13.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.