Abstract
We determined the in vivo role of stromal-interacting-molecule-1 (STIM1) in the regulation of vascular function using endothelial-cell (EC)- and smooth-muscle (SM)-specific knockout mice. Systolic blood pressure and glucose levels were similar in all mice (Stim1SMC−/−, Stim1SMC−/+, Stim1EC−/−, Stim1EC−/+), but body weight was reduced in Stim1EC−/− and Stim1SMC−/− mice. The contraction of arteries in response to phenylephrine was significantly reduced in Stim1SMC−/− mice only. However, contraction to thromboxane and KCl was similar in all groups. The endothelium-dependent relaxation (EDR) was impaired in Stim1EC−/+ and drastically reduced in Stim1EC−/− mice while the endothelium-independent vasorelaxation was similar among all groups. Acute down regulation of STIM1 in arteries reduced EDR and the contractile response to phenylephrine, while the contractile response to thromboxane was not affected. NADPH oxidase activity was increased only in Stim1EC−/+ and Stim1EC−/− mice. Calcium (Ca2+) entry in endothelial cells stimulated with thrombin and histamine had the pharmacological features of store-operated Ca2+ entry (SOCE) and was dependent on STIM1 expression. We conclude that STIM1 plays opposing roles in vascular smooth muscle vs. endothelial cells in the regulation of vascular reactivity.
Keywords: STIM1, Ca2+ signaling, NADPH oxidase, vascular reactivity
Introduction
Vascular function is an important mechanism that regulates local blood flow and maintains normal functions of organs and tissue [16]. It is well established that cardiovascular diseases are associated with vascular endothelial and smooth muscle cell dysfunctions [2, 11]. The Ca2+ is a universal second messenger that regulates vascular function [11, 18] Stromal interacting molecule-1 (STIM1) has recently been identified as a central molecular component of store-operated Ca2+ entry (SOCE) in both endothelial cells and smooth muscle [1, 17, 23]. SOCE is mediated by the highly Ca2+ selective, Ca2+ release-activated Ca2+ (CRAC) current which allows Ca2+ influx across the plasma membrane upon the emptying of endoplasmic reticulum (ER) Ca2+ stores. STIM1 is the long-sought ER Ca2+ sensor that senses ER Ca2+ store depletion to activate Orai1, the store-operated Ca2+ channel located at the plasma membrane [11]. STIM1 expression is increased in vessels in hypertension and during neointima formation in animals [23, 9] and preventing STIM1 up-regulation inhibits neointima formation [23, 10, 3]. STIM1 was shown to be required for endothelial permeability in response to thrombin and bacterial lipopolysaccharides [8, 19]. Estrada et al reported decreased STIM1 protein expression and disrupted Ca2+ homeostasis in coronary endothelial cells from a mouse model of streptozotocin-induced hyperglycemia associated with severe weight loss and sickness [7]. However, the role of STIM1 in endothelial cells vs. smooth muscle cells in the basic mechanisms of microvascular homeostasis and reactivity has not been documented. Our results provide the first evidence that depending on the cell type considered, either vascular smooth muscle or endothelial cells, STIM1 plays a distinct role in the regulation of microvascular function highlighting the need to distinguish between endothelial and smooth muscle STIM1 in studies using animal models of vascular diseases.
Results and Discussion
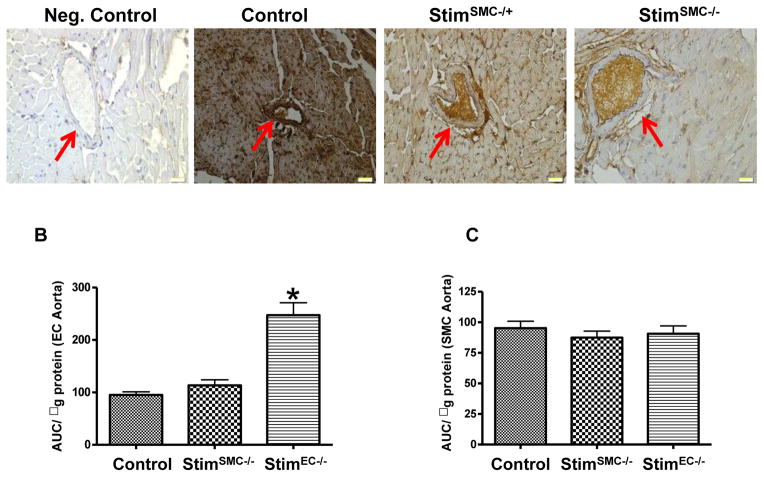
The specific knockout of STIM1 in smooth muscle cells (SMC) was accomplished using the SM22α-CreKI+ mouse that provides strong Cre expression in smooth muscle [22], which leads to a specific and complete deletion of Stim1 in smooth muscle when crossed to Stimflx/flx mice. The endothelial cell (EC) specific knockout mice (StimEC−/−) were generated by breeding Stim1flx/flx mice with TIE2-Cre mouse line. Figure 1A and Fig. 5A show the absence of STIM1 in Stim1SMC−/− and Stim1EC−/− mice in MRA and heart. Additionally, Western blot on lysates from mesenteric resistance arteries indicates a reduction in STIM1 expression in Stim1SMC−/− (Fig. 1B).
Figure 1. STIM1 deletion in SMC/EC and mesenteric resistance arteries (MRA) reactivity.
(N=8). Immunohistochemistry for STIM1 expression (A), Western blot analysis showing STIM1 expression (n=6) (B), percentage of contraction to potassium chloride (KCl) (C), thromboxane analogue (U46619) (D) and phenylephrine (PE) (E), endothelium-dependent relaxation to acetylcholine (ACh) (F) and endothelium-independent relaxation to sodium nitroprusside (SNP) (G). Nitrite/nitrates and cGMP levels (H, I) and NADPH oxidase activity (J) in control, Stim1SMC−/−, Stim1SMC−/+, Stim1EC−/− and Stim1EC−/+ mice. Red and green arrows indicate SMC and EC, respectively. *p< 0.05 for Stim1EC−/− vs. control, Stim1SMC−/−, Stim1SMC−/+ and Stim1EC−/+. #p< 0.05 for Stim1EC−/+ vs. control, Stim1SMC−/−, Stim1SMC−/+ and Stim1EC−/−.
Acute down regulation of STIM1 and MRA reactivity (N=8). Western blot for STIM1 expression (n=6) (K), Percentage of contraction to thromboxane analogue (U46619) (L) and phenylephrine (PE) (M), endothelium-dependent relaxation to acetylcholine (ACh) (N), NADPH oxidase activity (O) in control with or without acute down regulation of STIM1. *p< 0.05 for Control vs. siRNA STIM1
Figure 5. Immunostaining and NADPH oxidase activity (N=5).
Immunohistochemistry for STIM1 expression in heart section from control, Stim1SMC−/−, Stim1SMC−/+ (A); NADPH oxidase activity in EC and SMC isolated from aorta using laser capture microdissection in control, Stim1SMC−/− and Stim1EC−/− (B,C). *p< 0.05 for Stim1EC−/− vs. control and Stim1SMC−/−.
Blood glucose levels are similar in all groups of mice tested (Table 1). The heart weight/tibia length ratio was significantly low in Stim1SMC−/− and Stim1EC−/− compared to control and heterozygous mice (Table 1). These results indicate that Stim1SMC−/− and Stim1EC−/− have a small heart and could affect the physiopathology in a stress challenge conditions. The mechanism is still unclear. However, it has been reported that in vitro deletion of STIM1 in rat’s cardiomyocytes reduced growth factors levels [20]. Thus, it is likely that STIM1 regulates growth factors release that could dictate the heart weight. The systolic blood pressure is in the range of normotensive and is similar in all groups (Table 1) indicating that the absence of STIM1 in vascular endothelial cells or smooth muscle cells does not, on its own, cause hypertension, hypotension, hyperglycemia or hypoglycemia. STIM1 expression was enhanced in platelets from type 2 diabetic patients [21]. However, it has been shown that in mice infused with streptozotocin (STZ), a diabetic model known for causing severe sickness and weight loss, STIM1 expression was down regulated and associated with vascular dysfunction [7]. We previously reported microvascular endothelial and smooth muscle cell dysfunction in type 2 diabetic mice [4, 12, 5]. It is highly likely that the enhanced STIM1 expression in type 2 diabetes and hypertension could be causally involved in the impairment of arterial function. However, this has yet to be firmly demonstrated using a genetic approach.
Table 1.
Effect of specific STIM1 deletion in SMC vs. EC on body weight (BW), systolic blood pressure (SBP) and blood glucose levels (N=8).
| Control | Stim1SMC−/+ | Stim1SMC−/− | Stim1EC−/+ | Stim1EC−/− | |
|---|---|---|---|---|---|
| SBP | 103.12 ± 2.13 | 106.11 ± 1.21 | 102.33 ± 2.14 | 114.51 ± 1.01 | 120.70 ± 1.79 |
| Glucose | 133.13 ± 2.72 | 131.31 ± 3.27 | 135.83 ± 3.83 | 121.11 ± 2.12 | 125.17 ± 4.33 |
| BW | 24.22 ± 0.16 | 24.61 ± 0.24 | 20.64 ± 0.609* | 25.63 ± 0.28 | 19.29 ± 2.34* |
p< 0.05 for Stim1EC−/− and Stim1SMC−/− vs. control, Stim1SMC−/+ and Stim1EC−/+.
The body weight was significantly reduced in Stim1SMC−/− and Stim1EC−/− compared to controls and heterozygous (Table 1). These results indicate the potential contribution of STIM1 in the control of the body weight. These data are supported by previous studies indicating a reduction in body weight in Stim1SMC−/− mice [14].
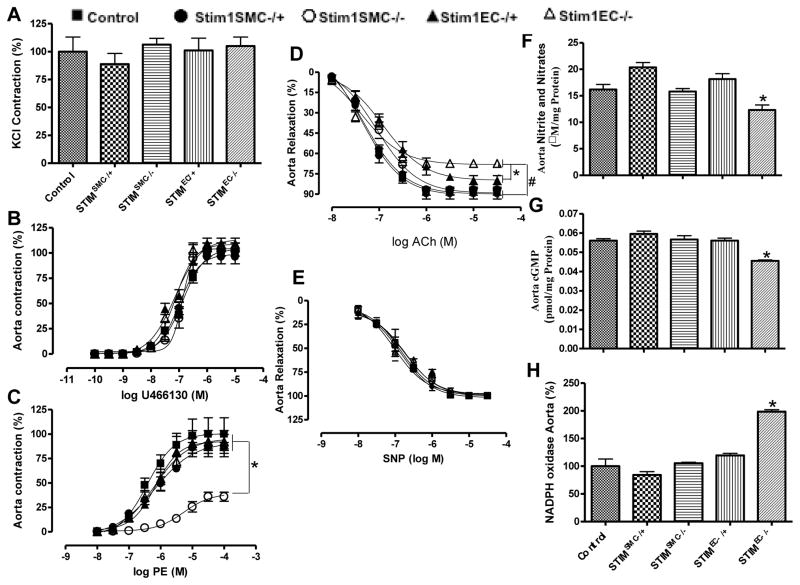
Microvascular smooth muscle cell, in vivo, responds to a variety of vasoconstrictors to maintain vascular tone. Thus, the vasoconstriction response to potassium chloride and thromboxane analogue (U46619) was similar in all groups of mice tested (Fig. 1C, D and Fig. 3A, B). However, the vasoconstriction in response to phenylephrine, a sympathetic stimulator, was drastically reduced in resistance and conductance arteries in Stim1SMC−/− mice only (Fig. 1E and Fig. 3C). This is likely caused by alteration of intracellular signaling in response to phenylephrine. STIM1 is likely an essential component of α-adrenoreceptor signaling in smooth muscle but it is not involved in thromboxane-mediated contractility. Our results are supported by previous studies showing that vasoconstriction in response to urotensin II, which binds to a Gq-protein coupled receptor, was significantly reduced when STIM1 was down regulated [6]. Similarly, the Gill group also found a reduction in vasoconstriction in response to phenylephrine in Stim1SMC−/− mice, albeit of smaller amplitude compared to our results [15]. The reduction in vasoconstriction in response to phenylephrine is independent of blood pressure since arterial blood pressure is similar in all groups indicating the specific role of STIM1 in sympathetic activity. The contraction in response to phenylephrine was not altered in mice lacking STIM1 in EC indicating that STIM1 in SMC specifically regulates vasoconstriction induced by sympathetic stimulation. STIM1 knockdown or knockout in essentially all cell type studied so far has been associated with inhibited agonist-mediated Ca2+ influx to a large number of membrane receptors, including alpha1 receptor-mediated Ca2+ influx specifically in smooth muscle, as recently reported [6].
Figure 3. Effect of specific STIM1 deletion in SMC vs. EC on thoracic aorta reactivity (n=8).
Percentage of contraction to potassium chloride (KCl) (A), thromboxane analogue (U46619) (B) and phenylephrine (PE) (C), endothelial-dependent to acetylcholine (ACh) (D) and endothelium-independent relaxation to sodium nitroprusside (SNP) (E) Nitrite/nitrates and cGMP levels respectively (F, G) and NADPH oxidase activity (H) in control littermates, Stim1SMC−/−, Stim1SMC−/+, Stim1EC−/− and Stim1EC−/+ mice. *p< 0.05 for Stim1EC−/− vs. control, Stim1SMC−/−, Stim1SMC−/+ and Stim1EC−/+.
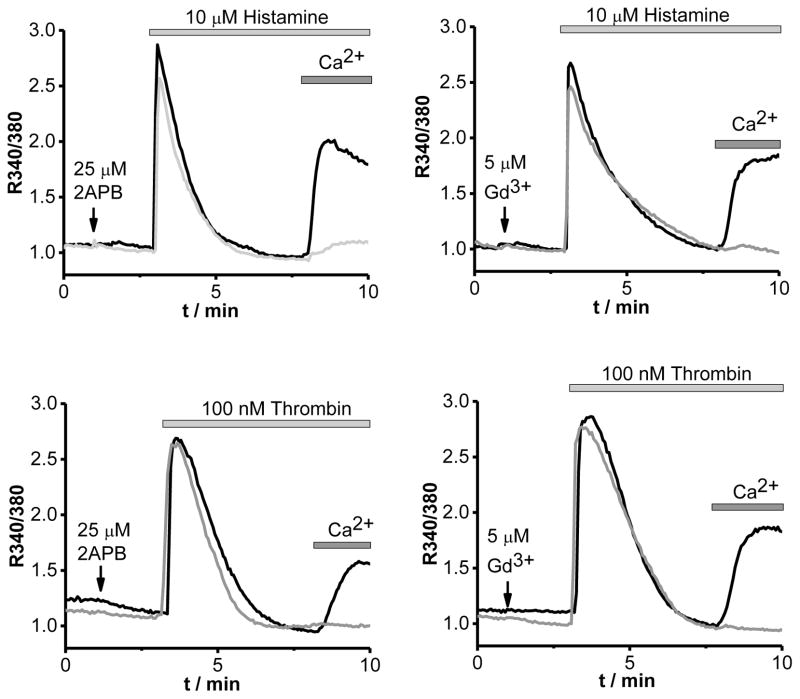
The arterial tone is mainly regulated by the contraction of SMC dictated by intrinsic mechanisms and factors released by endothelial cells. The endothelium-dependent relaxation was not affected in Stim1SMC−/− and Stim1SMC−/+ (Fig. 1F and Fig. 3D). However the endothelium-dependent relaxation was significantly reduced in Stim1EC−/+ and drastically inhibited in Stim1EC−/− (Fig. 1F and Fig. 3D). Our results indicate the differential effect of STIM1 in the regulation of microvascular reactivity. The deletion of STIM1 in either ECs or SMCs did not affect the sensitivity of SMCs to nitric oxide since the relaxation to a nitric oxide donor is similar in all groups of mice (Fig. 1G and Fig. 3D). Nitric oxide markers (Nitrite and cGMP) levels were significantly reduced while NADPH oxidase activity was augmented in Stim1EC−/− compared to the other groups of mice (Fig. 1H, I, J and Fig. 3F, G, H). These results indicate that STIM1 in ECs regulates eNOS- and oxidative stress-dependent pathways likely through control of NADPH oxidase activity, consistent with earlier reports [8]. Our results showed a slight increase in the systolic blood pressure that is similar between heterozygous Stim1EC−/+ and homozygous Stim1EC−/− mice. While the endothelium function was slightly but significantly reduced in the heterozygous group, it was severely reduced in the homozygous group. Therefore, the same slight increase in systolic blood pressure is unlikely to explain the differential effect on endothelial function between the two groups of mice. To rule out potential adaptive responses related to chronic down regulation of STIM1, we acutely down regulated STIM1 in vitro in endothelial and smooth muscle cells in resistance arteries using molecular knockdown (Fig. 1 K). Our results indicate a reduced phenylephrine-induced contraction, while the response to thromboxane was not affected (Fig. 1L, M) emphasizing the selective role of STIM1 in sympathetic vasoconstriction. These results are consistent with data in Stim1SMC−/− mice, ruling out any adaptive responses in vessels from knockout mice. Additionally, the in vitro acute down regulation of STIM1, reduced microvascular endothelium-dependent relaxation (Fig. 1N). Furthermore, NADPH oxidase activity was increased after acute down regulation of STIM1 (Fig. 1O). To strengthen our data, we used laser capture microdissection to isolate EC and SMC from aorta from all group of mice. Our data indicates an increase in NADPH oxidase activity in EC and no difference was reported in SMC (Fig. 5B, C). We also show that two vasoactive compounds known to produce NO, histamine and thrombin, activate Ca2+ entry in vascular endothelial cells (ECs) through a pathway pharmacologically-reminiscent of store-operated Ca2+ entry (SOCE), namely inhibition by the pyrazole BTP2 (10 μM; Fig. 2), 2-Aminoethoxydiphenyl borate (2-APB 25 μM, Fig. 4) and low concentrations of Gadolinium (Gd3+, 5μM, Fig. 4); thapsigargin is used throughout as a control (Fig. 2). Furthermore, molecular knockdown of STIM1 abrogated Ca2+ entry in ECs in response to both histamine and thrombin (Fig. 2). We have reported similar results on the requirement of STIM1 for receptor-mediated Ca2+ entry in human umbilical vein endothelial cells and human dermal microvascular endothelial cells upon stimulation with thrombin and vascular endothelial growth factor [1, 19]. Together, our results indicate that STIM1 in endothelial cells regulates nitric oxide and oxidative stress pathways and therefore the endothelium-dependent relaxation in resistance and conductance arteries.
Figure 2. Histamine- and thrombin-activated Ca2+ entry in human microvascular endothelial cells is mediated by STIM1.
A, Representative Ca2+ imaging traces depicting Ca2+ release (in the absence of extracellular Ca2+) and subsequent Ca2+ entry (upon addition of 2mM Ca2+ to the bath solution) in ECs stimulated with 10 μM histamine. ECs were transfected with either non-targeting siRNA (siNT) or STIM1 siRNA (A). The SOCE inhibitor, BTP2 at 10μM was added when the Ca2+ entry phase in ECs was maximal (grey arrows) followed by ionomycin (10 μM; black arrows); the latter is a Fura2 loading control. Traces shown represent an average from several ECs (see color-coded N for each trace). B, Statistical summary on extent of Ca2+ entry in ECs stimulated with histamine (N=71 and 98 for siNT and siSTIM1 respectively) taken from five independent experiments, including experiments described in A. C, D, representative Ca2+ imaging traces similar to those shown in A, but representing average Fura2 ratios from several ECs stimulated with either 100 nM thrombin (C) or 2 μM thapsigargin (D). *** Signifies p<0.001.
Fig. 4. Histamine and thrombin activate Ca2+ entry through SOCE in human microvascular endothelial cells (ECs).
Representative Fura2 Ca2+ imaging traces in ECs stimulated with either histamine (10 μM, top panels) or thrombin (100 nM, lower panels) using the standard Ca2+ off/Ca2+ on protocol. ECs were pre-incubated (grey traces) in the presence of either 2-APB (25μM, right traces) or Gd3+ (5μM, left traces) right before the addition of agonists (indicated by an arrow) and compared to control cells stimulated with agonists in the absence of inhibitors (black traces).
We conclude that STIM1 plays an important role in the control of vascular tone with distinct roles in vascular smooth muscle and endothelial cells. In smooth muscle, STIM1 is required for vasoconstriction in response to phenylephrine likely through a specific effect on sympathetic activity. However, STIM1 in endothelial cells is required for Ca2+-dependent vasorelaxation through regulation of nitric oxide and oxidative stress pathways. While previously published studies have reported altered STIM1 expression in hypertension and type II diabetes, the distinction between endothelial and smooth muscle cells has not been made under these conditions. Therefore, a careful assessment of these disease models with the mice described herein lacking STIM1 specifically in one of these two cell types is likely to help in the future specific targeting of this protein for the purpose of therapy of vascular diseases.
Material and Methods
All experiments were performed according to the American Guidelines for the Ethical Care of Animals and were approved by both SUNY Albany and EVMS Animal Care and Use Committee. Stim1 knockout in smooth muscle cells male mice (Stim1SMC−/−, 8 to 10 week-old) and their homologous heterozygous (Stim1SMC−/+) as well as male mice knockout for STIM1 in endothelial cells (Stim1EC−/−, 8 to 10 week-old) and their homologous/heterozygous (Stim1EC−/+) were generated in Dr. Mohamed Trebak laboratory using Stim1flx/flx mice provided by Dr. Stefan Feske, NYU [15]. All mice were housed in groups of five mice, maintained at a temperature of 23 ºC with 12 h light/dark cycles and fed a solid standard diet (Na+ content 0.4%) and water. The systolic blood pressure (SBP), body weight (BW), Heart weight (HW)/tibia length ratio and blood glucose levels were measured as previously described [14].
Mice were anaesthetized with isoflurane and then tissues (MRA and thoracic aorta) were immediately harvested, placed in PSS solution (composition in mM: NaCl 118; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4×7H2O 1.2; NaHCO3 25 and glucose 11, pH=7.4) and processed appropriately for further studies.
Vascular Reactivity
Intact Mesenteric resistance arteries (MRA) and intact thoracic aorta from control, Stim1SMC−/−, Stim1SMC−/+, Stim1EC−/− and Stim1EC−/+ mice were carefully cleaned from fat and connective tissue and then cut into rings (2 mm in length). Intact MRA and thoracic aorta were mounted in a small vessel dual chamber myograph for measurement of isometric tension at 37°C and oxygenation as previously described [20]. Cumulative concentration responses to phenylephrine (PE, 10−8-10−4 M) and thromboxane analogue (U46619, 10−10-10−5 M) were obtained. In other series of experiments, rings were pre-constricted with U46619 (3.10−7 mol/L) and at the steady maximal contraction, cumulative concentration response curves for acetylcholine (ACh, 10−8-3.10−5) and sodium nitroprusside (SNP, 10−8-3.10−5) were obtained. In other series of experiments, MRA from control mice were mounted in the wire myograph and incubated with siRNA for STIM1 for 4 hours to down regulate STIM1 expression, and then we performed vascular reactivity in response to phenylephrine (PE, 10−8-10−4 M), thromboxane analogue (U46619, 10−10-10−5 M) and acetylcholine (ACh, 10−8-3.10−5).
Ca2+ imaging
ECs were isolated and Fura2 imaging and data analysis were performed as described previously [1, 19].
Western blot analysis
Western blot analyses were performed for STIM1 expression using STIM1 monoclonal antibody (BD Biosciences). Blots were stripped and then re-probed with β-actin to verify the equal loading among the samples.
NADPH oxidase activity
NADPH oxidase activity in MRA and aorta from all groups was measured as previously described [13]. Additionally, we used laser-capture micro-dissection to study the NADPH oxidase activity in aortic EC and SMC independently.
Nitrates/nitrate and cGMP
cGMP and nitrates nitrites levels were measured in aorta and MRA tissues in all groups of mice as described previously [6].
Immunohistochemistry
Immunostaining was performed for STIM1, in paraffin sections of MRA and heart using specific antibodies, anti-STIM1 (1:200, BD Biosciences).
Statistical analysis
Results are expressed as mean ± SEM. Concentration-response curves were analyzed using either GraphPad Prism 4.0 or Origin 8.0 software. One-way or 2-way ANOVA was used to compare parameter when appropriate. Comparisons between groups were performed with t-tests when the ANOVA test was statistically significant. Values of P < 0.05 were considered significant. Differences between specified groups were analyzed using the Student’s t test (two-tailed) for comparing two groups with P < 0.05 considered statistically significant.
Acknowledgments
N/A
Sources of Funding
This work was supported by the NIH (HL095566 to KM and HL097111 to MT), AHA grants (14GRNT18880008 to MT and 16850060 to MK), and Applied Biophysics Inc. Troy, NY to JS.
Footnotes
Disclosures
None
References
- 1.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 3.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompré AM, Hulot JS. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2009;17:455–462. doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SK, Galán M, Kassan M, Partyka M, Trebak M, Matrougui K. Poly(ADP-ribose) polymerase 1 inhibition improves coronary arteriole function in type 2 diabetes mellitus. Hypertension. 2012;59:1060–1068. doi: 10.1161/HYPERTENSIONAHA.111.190140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domínguez-Rodríguez A, Díaz I, Rodríguez-Moyano M, Calderón-Sánchez E, Rosado JA, Ordóñez A, Smani T. Urotensin-II signaling mechanism in rat coronary artery: role of STIM1 and Orai1-dependent store operated calcium influx in vasoconstriction. Arterioscler Thromb Vasc Biol. 2012;32:1325–1332. doi: 10.1161/ATVBAHA.111.243014. [DOI] [PubMed] [Google Scholar]
- 7.Estrada IA, Donthamsetty R, Debski P, Zhou MH, Zhang SL, Yuan JX, Han W, Makino A. STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res. 2012;111:1166–1175. doi: 10.1161/CIRCRESAHA.112.275743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giachini FR, Chiao CW, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension. 2009;53:409–416. doi: 10.1161/HYPERTENSIONAHA.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo RW, Wang H, Gao P, Li MQ, Zeng CY, Yu Y, Chen JF, Song MB, Shi YK, Huang L. An essential role for stromal interaction molecule 1 in neointima formation following arterial injury. Cardiovasc Res. 2009;8:660–668. doi: 10.1093/cvr/cvn338. [DOI] [PubMed] [Google Scholar]
- 11.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassan M, Choi SK, Galán M, Bishop A, Umezawa K, Trebak M, Belmadani S, Matrougui K. Enhanced NF-κB activity impairs vascular function through PARP-1-, SP-1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes. 2013;62:2078–2087. doi: 10.2337/db12-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassan M, Galán M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancarella S, Potireddy S, Wang Y, Gao H, Gandhirajan RK, Autieri M, Scalia R, Cheng Z, Wang H, Madesh M, Houser SR, Gill DL. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 2013;27:893–906. doi: 10.1096/fj.12-215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittman RN. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 17.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruhle B, Trebak M. Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr. 2013;71:209–235. doi: 10.1016/B978-0-12-407870-3.00009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, González-Cobos JC, Zhang W, Matrougui K, Vincent PA, Trebak M. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci Signal. 2013:ra18. doi: 10.1126/scisignal.2003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–1334. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zbidi H, López JJ, Amor NB, Bartegi A, Salido GM, Rosado JA. Enhanced expression of STIM1/Orai1 and TRPC3 in platelets from patients with type 2 diabetes mellitus. Blood Cells Mol Dis. 2009;43:211–213. doi: 10.1016/j.bcmd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26:e23–4. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]