Abstract
Circadian rhythm may be understood as a temporal organization that works to orchestrate physiological processes and behavior in a period of approximately 24 h. Because such temporal organization has evolved in the presence of predictable environmental clues, such as day length, tides, seasons, and temperature, the organism has confronted the natural selection in highly precise intervals of opportunities and risks, generating temporal programs and resetting mechanisms, which are well conserved among different taxa of animals. The present review brings some evidence of how these programs may have co-evolved in systems able to deal with 2 or more environmental clues, and how they similarly function in different group of animals, stressing how important temperature and light were to establish the temporal organizations. For example, melanopsin and rhodopsin, photopigments present respectively in circadian and visual photoreceptors, are required for temperature discrimination in Drosophila melanogaster. These pigments may signal light and temperature via activation of cationic membrane channel, named transient-receptor potential channel (TRP). In fact, TRPs have been suggested to function as thermal sensor for various groups of animals. Another example is the clock machinery at the molecular level. A set of very-well conserved proteins, known as clock proteins, function as transcription factors in positive and negative auto-regulatory loops generating circadian changes of their expression, and of clock-controlled genes. Similar molecular machinery is present in organisms as diverse as cyanobacteria (Synechococcus), fungi (Neurospora), insects (Drosophila), and vertebrates including humans.
Keywords: clock genes, opsins, photo-transduction, thermo-transduction, TRP channels
Abbreviations
- Two-APB
2-aminoethoxydiphenylborane
- Bmal1
Brain and Muscle Arnt-like
- CK 1 ε and δ
casein kinase 1 ε and δ
- Clock
Circadian Locomotor Output Cycles Kaput
- Cry
Cryptochrome1, 2
- D-box
destruction box
- DAG
diacylglycerol
- DD
constant darkness
- E-box
enhancer box
- IP3
inositol trisphosphate
- ipRGC
intrinsically photosensitive retinal ganglion cells
- LD
light-dark cycle
- NIH3T3 cells
established fibroblast cell line from Swiss mouse
- Npas2
Neuronal PAS (Per - Period circadian protein Arnt – Aryl hydrocarbon receptor nuclear translocator protein Sim – Single-minded protein) domain-containing protein 2
- PACAP
pituitary adenylyl cyclase activating peptide
- Per
Period1, 2, 3
- Per::Luc
Per gene containing luciferase gene in its promoter
- PIP2
phosphatidylinositol bisphosphate
- PLC
phospholipase C
- PRC
phase response curve
- Rev-erbα
NR1D1 (nuclear receptor subfamily 1
- group D
member 1)
- RORα
RAR-related orphan receptor α, also known as NR1F1 (nuclear receptor subfamily 1, group F, member 1
- SCN
suprachiasmatic nucleus
- TRP channels
transient-potential receptor cationic channels
- TRPA channels
ankyrin subfamily of Transient-Receptor Potential channels
- TRPC channels
canonical subfamily of Transient-Receptor Potential channels
- TRPM channels
melastatin subfamily of Transient-Receptor Potential channels
- TRPML channels
mucolipin subfamily of Transient-Receptor Potential channels
- TRPN channels
no mechanoreceptor potential C (NOMPC) subfamily of Transient-Receptor Potential channels
- TRPP channels,
polycystein subfamily of Transient-Receptor Potential channels
- TRPV channels
vanilloid subfamily of Transient-Receptor Potential channels
- TRPY channels
yeast subfamily of Transient-Receptor Potential channels
- U2OS
human bone osteosarcoma cell line
- UVA
ultraviolet A radiation
Introduction
Light and temperature are environmental physical entities that have empirically the same effects on endogenous biological clocks – together or separate, they function as a cue to adjust the organism physiology to exogenous time. Unicellular organisms may directly perceive these cues and translate them to changes in expression of a set of very-well conserved proteins, known as clock proteins. Multicellular organisms, on the other hand, have evolved specialized systems that indirectly detect these cues. As such, at the top of the phylogenetic tree, mammals, for example, are able to detect light only at retina, thus mammalian systemic tissues require internal signals to sustain circadian rhythm in concordance with light and dark cycles. These multiple organs constitute peripheral clocks that are hierarchically organized to advantageously display their function at the right time of day and, therefore, optimize resources. A caveat of biological clock functioning is the identification of this (or these) internal signal (s) that adjust multiple peripheral clocks to external time.
Buhr and co-workers1 proposed that temperature might work as universal internal signal to multiple peripheral clocks of mammals. A year later, Shen and colleagues2 identified that a paralogue mammalian photopigment – rhodopsin - participates in the behavioral thermo-discrimination of Drosophila, and it does so by opening a Transient Receptor Potential (TRP) channel. This may represent a random coincidence among players of photo/thermo perception or this may be a hint from evolution: what has been internalized to signal time-of- day to mammalian multiple clocks would be the same components described in invertebrates. However, in mammals this route (light → photoreceptors → central nervous system → multiple synapses → temperature information → TRP channels changes in clock genes of peripheral clocks) is now located in different systems. Here we will describe the function of endogenous biological clocks, how temperature and light affect them and the putative role of TRP channels in the entrainment mechanism of peripheral clocks of non-mammalian and mammalian vertebrates. TRP channels constitute a large family of channels that are sensitive to a wide range of stimuli and, importantly they are sensitive to light and temperature. Not surprising then, TRPC6 and 7, expressed in the retina of mammals participate in light perception whereas TRPV1 and TRPM8 are sensitive respectively to heat and cold.
Circadian Rhythms
Life exists as a continuous movement; animals and plants change according to the weather, the time of the day, the seasons, creating a scenario where the organisms constantly adjust to environmental alterations. The internal temporal organization properly synchronized with the ambient is essential for the health and survival of the organisms. Time as a variable is often neglected as one considers the interaction between the organism and its habitat, despite the fact that Earth is subject to geophysical cycles, such as light/dark cycles and seasons. Along evolution, the organisms faced natural selection in intervals of opportunity and adversity which were repeated with precise and predictable frequencies, favoring those bearing an innate timekeeping program responsible for circadian (circa=about; diem=day) oscillations.3 Therefore, the temporal organization, known as biological rhythm, exerts a determining character to the species viability.4
Endogenous rhythms are found in all animals studied to date, and can be seen as the manifestation of an endogenous biological clock, genetically coded, which may be synchronized with predictable periodic environmental cues, the so-called zeitgebers (time-giver from German5). Among these ambient cues, light/dark and temperature cycles are major agents to adjust the endogenous oscillations. In the absence of these external cues, for instance in constant dark, the endogenous clock assumes its own period, usually a little shorter or longer than the 24 hours set by the light/dark cycles or another zeitgeber. In this situation, the clock is said to be free-running, whereas when adjusted to a zeitgeber, it is entrained by that cue.3 Entrainment may also be observed by instantaneous shifts in response to transitions of the external time and, in this case, no changes in other parameters (e.g., amplitude, wave shape, period) of the underlying oscillator are observed, except by the phase.6
In mammals, the master biological clock is a pair of suprachiasmatic nuclei (SCN), located in the hypothalamus, which is daily reset by retinal inputs signaling the light/dark cycles. In the mammalian retina, a small subpopulation of ganglion cells expressing melanopsin is responsible for short-wavelength light detection and for conveying this information to the SCN via the retino-hypothalamic tract. This monosynaptic pathway signals the SCN through the release of glutamate and pituitary adenylyl cyclase activating peptide (PACAP7,8), entraining intrinsically rhythmic neurons to a 24 hour period.9 These nuclei are responsible to signal time-of-day information to other areas of the central nervous system and to peripheral tissues; both are considered peripheral clocks and will be discussed in the following sections.
Molecular mechanism of circadian rhythm
The intrinsic circadian rhythmicity is provided by a molecular clock based on the oscillation of transcription and translation of genes and proteins, the so-called clock genes: Clock (Circadian Locomotor Output Cycles Kaput), Bmal1 (Brain and Muscle Arnt-like), Per (Period1, 2, 3), and Cry (Cryptochrome1, 2).10 CLOCK and BMAL1 proteins form a heterodimer, which is a transcription factor of genes possessing E-box sequences, such as Per and Cry. PER and CRY proteins form oligomers, which are phosphorylated by casein kinase 1 (ε and δ) and traffic to the cell nucleus where they block CLOCK/BMAL1 action.11 Phosphorylated PER and CRY are tagged to be degraded in the proteasomes, and when PER and CRY are not sufficient to inhibit CLOCK/BMAL1, a new cycle begins. Two other genes, Rev-erbα and Rorα, also possess E-box sequence in their promoters and take part in another molecular loop of the clock core: The protein REV-ERBα inhibits whereas RORα activates Bmal1 transcription12 (Fig. 1). The induction of Per1 and Cry1 is triggered by light and lasts proportionally to light intensity.13 Accordingly, the electrical activity of SCN peaks during the subjective day14 in both nocturnal and diurnal species.15
Figure 1.
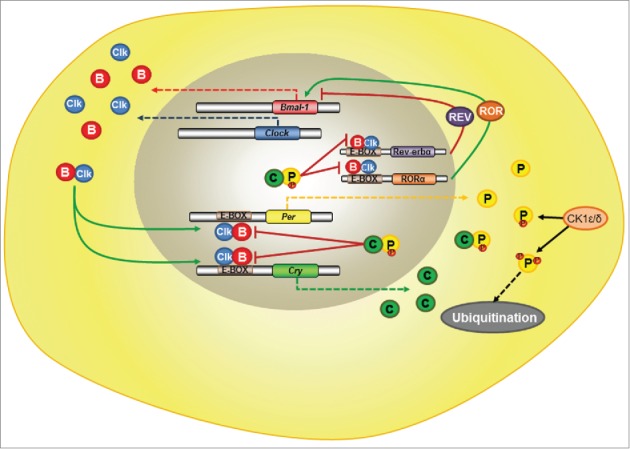
Schematic Model for the Molecular Clock Machinery in Mammals. The heterodimer CLOCK/BMAL1 is a transcription factor of E-box genes like Per, Cry, Rev-erbα and Rorα. PER and CRY proteins also form oligomers which may be phosphorylated by casein kinase 1 ε/δ, resulting in their traffic back to the nucleus, where they inhibit CLOCK/BMAL1 actions, or in their ubiquitination. Bmal1 transcription may be inhibited by the protein REV-ERBα , or activated by RORα. Clk = CLOCK protein; B = BMAL1 protein; C = CRY protein; P = PER protein; CK1 ε/δ = casein kinase1 ε/δ; small red circles attached to PER = phosphorylation sites; Solid black arrows = phosphorylation by CK1; Dashed black arrows=ubiquitination followed by protein degradation; Solid red blunt arrows = inhibition; Solid green arrows=stimulation; Dashed red, green, blue or yellow arrows=transcription and translation of the respective clock genes.
Interestingly, dissociated SCN neurons display a wide range of electrical activity periods, thus demonstrating the necessity of coupling of the autonomous rhythmic cells to guarantee their unisonous function.16,17 It has been recently proved that a subset of SCN neurons express the neuropeptide neuromedin, which is probably the intercellular mediator synchronizing SCN, indispensable to generate circadian rhythms.18
Since late 90s, a variety of cell types in culture have been reported to rhythmically express clock genes.19-27 Similarly to isolated SCN neurons, peripheral tissues also oscillate independently but are synchronized by the SCN. Nevertheless, in some cases, local cues are hierarchically superior to SCN to entrain peripheral organs.28
Although the molecular mechanism of the master clock is strikingly conserved in the peripheral clocks, some of the core genes may play more or less important role in the machinery.28 Because of the redundancy among Pers (1, 2 and 3) and between Clock and Npas2, for instance, the deletion of one of them may not cause any harm in one tissue, or in the SCN, but may be highly deleterious in others.29-32
Temperature compensation and temperature effect on central and peripheral clocks
Temperature compensation, persisting free-running rhythm and entrainment are considered the 3 hallmarks of circadian clocks functioning. The idea of temperature compensated pacemakers however was not promptly accepted as a characteristic of endogenous oscillator, and it was a major issue in the early days of circadian biological research. This feature assumes that to work accurately, a clock should display oscillation that remains resilient to daily changes of temperature. To support this assumption, period length (tau) of circadian rhythms needs to be resistant to changes in temperature. The mechanism responsible for temperature compensation repairs for the normal tendency of the rate of biochemical reactions to change with temperature, keeping therefore Q10 for temperature compensated oscillators close to 1.3,33
With the advance of a molecular technique that introduces a bioluminescent probe within the promoter of the gene of interest, much has been learned about the oscillation of central and peripheral clocks in mammals. Luciferase gene is inserted in Per1 (Per1::Luc) or Per2 (Per2::Luc) gene and every time that the gene is expressed, bioluminescence is produced in the presence of luciferin. Mammalian SCN neurons show robust circadian rhythm of neuronal firing rate and the period of this oscillation is not disturbed at different temperatures.34 Similarly, the period of transcriptional activity of SCN Period 2 gene is resilient to pulses of temperature, since bioluminescence recording from animals exposed to temperature cycles composed of 12 hours of 36°C and 12 hours of 38.5°C shows no phase-shift within 3 days.35 Network interactions among SCN neurons are required for temperature resistance in mammal SCN.1 In addition, robust rhythm of Per2::Luc is observed, in the SCN, at temperatures ranging from 31 to 37°C; period of this oscillation shows Q10 very close to 135. Furthermore, circadian rhythm period of locomotor activity that is determined by the central clock is temperature compensated in hibernated bats35 and in hypothermic rodents.36-39
Although temperature compensation has been claimed to explain this constancy of period found within SCN neurons, analyses of recordings from Per1::Luc activity in cultured rat SCN show controversially that circadian rhythm of expression may be entrained by temperature variation. Daily 1.5°C cycles of temperature and pulses of 34 to 37° C for 2 h during early and late subjective day induce phase-delays and advances of firing rate rhythm, respectively.40 In addition, cycles of warm and cool ambient temperature entrain free-running circadian rhythm of several species of mammals,41 and pulses of heat promote phase-shift in rhythmic locomotor activity in rats.42 These data indicate that SCN is not completely insensitive to temperature changes.
Temperature compensated rhythm is also reported in peripheral clocks at both single cell and tissue level. Period of Per1 promoter transcriptional activity circadian rhythm measured from rat-1cells is temperature compensated over the range of 28.5–36.5°C.43 Bioluminescence recording from pituitary gland, cornea, adrenal gland, and lung of Per2::Luc mice shows transcriptional activity rhythm that also remains unchangeable when measured at temperature ranging from 31 to 37°C.35
On the other hand, similar to what has been described to the central clock; peripheral clocks have retained some sensitivity to thermal stimuli. Brown and coworkers have shown that exposition of mice to environmental 37°C during nocturnal phase is not able to alter circadian rhythm of Per2 expression within SCN neurons but promotes phase-advance at liver and kidney.44 Cycles of heat with very low amplitudes, that simulate daily rhythms of body temperature, entrain circadian clock gene rhythm of NIH3T3 cells and primary tail tip fibroblasts.45-47
This non-observance of temperature compensated rule is also shown in invertebrates,48 in in vitro avian pineal,49,50 in in vitro mammalian retina,51 and in in vitro rat SCN neurons.34 In all these cases, comparison of phase response curves (PRCs) to light and heat pulses shows that both stimuli can induce phase shifts on circadian rhythms.
In face of these results, temperature compensated concept is, perhaps, the least consensual feature of circadian clock function. In order to correctly place this concept and to avoid generalization, one should consider differences between ectothermic and endothermic animals. It is also important to discriminate data obtained from whole organism regarding circadian rhythm of locomotor activity which, in mammals, translates SCN activity versus data from tissue explants or single cells, which reflect local clock functioning, which, may be controlled by the central clock in vivo.
In ectotherms, temperature may slightly affect the period of circadian rhythms.52,53 The period length of the reptile pineal circadian clock remains relatively constant over a range of temperatures in free-running conditions.52 Nevertheless, analysis of close phylogenetic avian clock genes (Per 2, Cry1, and Clock) of lizard eye and heart shows circadian rhythm under light-dark (LD) cycles and constant darkness (DD) either at 29°C or 6°C. In addition, exposition of these animals to low temperatures attenuates rhythmic expression of clock genes considerably, as well as raises their basal expression levels54 on those peripheral tissues. Furthermore, rhythmic expression of lPer2 in the SCN is strongly attenuated by exposure of the lizard to low temperatures.55 In zebrafish, cyclic temperature increases do not change the circadian rhythm period of Per4 gene expression but do alter the amplitude of gene expression, which has been proposed as a mechanism contributing to temperature compensation.56 Thus, temperature can be a potent zeitgeber in ectotherms.57
In endothermic animals, although the circadian period of some mammalian species remains unchangeable after pharmacological abolishment of homeothermy,3,37 temperature may alter circadian rhythms of peripheral and central clocks, as described above. One possible explanation for these differences may rely on the variation of internal temperature and how perception of the 2 most relevant zeitgebers – light and temperature - has evolved in these animals.
Homeothermic mammals regulate temperature homeostatically within a narrow limit in spite of large ambient temperature variations. Thermo-receptors present deep in the body, in the skin, and in the brain guarantee correction of thermo-deviation of internal temperature set point, through signals sent to thermoregulatory centers located in the preoptic/anterior hypothalamus, which generates, via feedback mechanisms, heat loss and/or heat production, resulting in slight variations of the temperature around an average.58 In addition, SCN directly acts on thermoregulatory hypothalamic areas, controlling variation of body temperature observed during the rest/active phase. Thus, body temperature exhibits a circadian rhythm as a result of homeostatic and rhythmic regulating mechanisms. In consequence, these animals experience limited range of internal temperature variation.59 Regarding light perception, mammalian has it restricted to the retina9; peripheral clocks therefore are not able to directly respond to light (Fig. 2). As a result, evolution of endothermic process may have lightened up selection pressure for temperature compensation of peripheral pacemakers, allowing them to be sensitive to very shallow thermal variations, as those observed in the circadian rhythm of body temperature.
Figure 2.
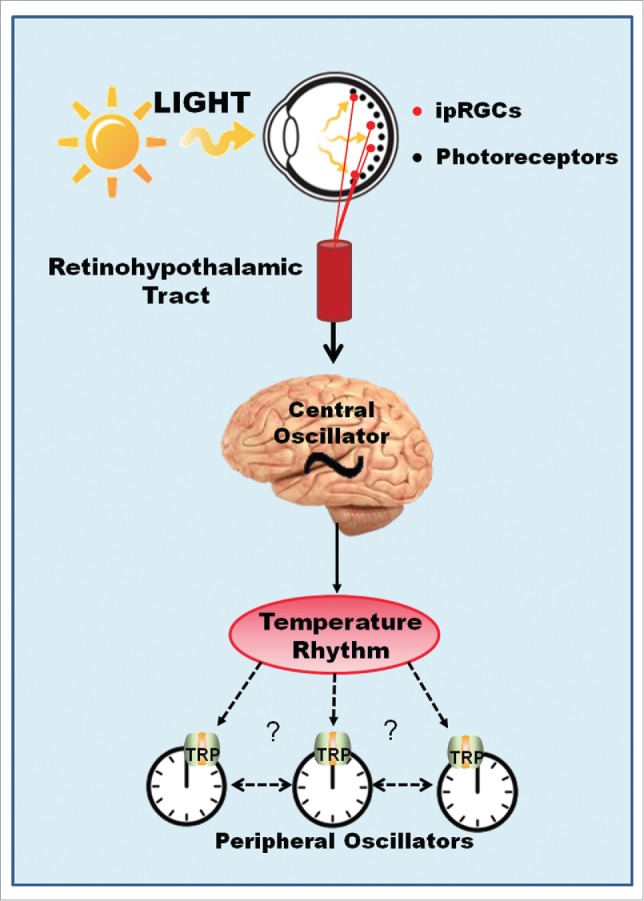
Hypothetical Model for Thermo-Entrainment of Mammalian Peripheral Clocks. Light perception in mammals is restricted to the retina. Circadian light is perceived by a set of intrinsically photosensitive ganglion cells of the retina (ipRGCs), which express melanopsin and depolarize in response to Ca2+ and Na+ influx through TRPC6 and TRPC7 channels. This information travels along the retinohypothalamic tract (RHT) and reaches the central oscillator resetting the clock molecular machinery. Circadian temperature variation is controlled by the central clock and may then be perceived by multiple peripheral clocks via thermo-TRP channels.
Temperature effects on circadian rhythm may be tissue specific, as it is the relevance of different component of molecular clock machinery.43 Homozygous Cry1 knockout mice display a short period length in locomotor activity, whereas dissociated SCN neurons (and also lung, liver, cornea and fibroblasts) from these mice are arrhythmic.60 Similar results were seen in lung and liver cells from Clock–/– animals.30,61 At the cellular level, however, knockdown of Bmal1, Clock, Cry1, Cry2, and Per1 of U2OS cells (an osteosarcoma cell line) all generated either arrhythmic cells or a clock with such low amplitude that is practically undetectable.62 Together, these results show that cellular oscillations may be not controlled as locomotor activity behavior. Temperature compensation may occur at the level of local clock as consequence of an intrinsic property63 that may not be preserved at the systemic level. This may also account for the differences found between circadian rhythms in vitro from cell lines and from cultured cells.
Temperature compensation remains an unsolved issue. Although the current models of clocks at the molecular level greatly advanced our understanding of how the 2 major zeitgebers – light and heat are interpreted by the endogenous clock, their relative relevance and their impact have still to be uncovered. As further discussed in following sections and represented in Figure 3, light and temperature may have certainly worked together to design our circadian systems.
Figure 3.
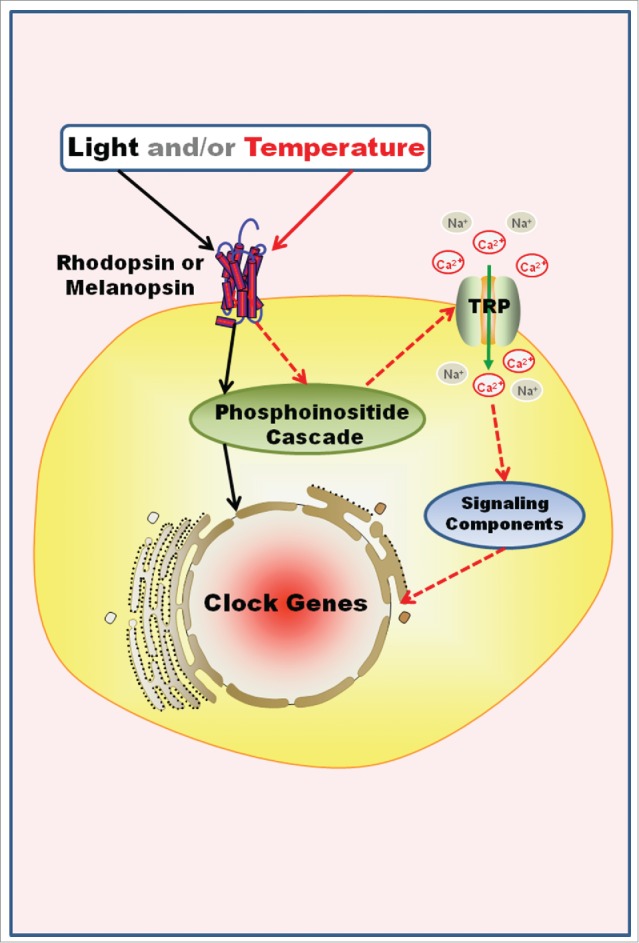
Hypothetical Model for the Co-Evolution of Entrainment Mechanisms. Invertebrates and non-mammalian vertebrates may be able to perceive light and/or temperature through opsins outside the classical photo- and thermo-receptors, integrate that information, and entrain the local clock in a single cell. For example, in Danio rerio embryonic cell line and Xenopus laevis melanophores, light increases Per expression and entrains the clock molecular machinery through a phosphoinositide cascade. In a speculative model, light and/or temperature opens TRPA1 channels after rhodopsin/melanopsin activation, probably through a phosphoinositide signaling as well, what could result in the reset of the molecular clock machinery. Solid black and red arrows=known steps of light and temperature signaling, respectively; dashed red arrows=temperature putative pathways of clock gene regulation.
The Role of TRP Channels in Thermal Responses
Understanding how temperature can modify biological rhythms at molecular level requires the knowledge of how organisms sense thermal variations. The thermal sensation, ability to perceive temperature, is one of the oldest sensory processes. All organisms, from bacteria to animals, possess mechanisms to perceive variations in ambient temperature and generate responses that are crucial for the survival of the species.64 At the basis of the thermal sensation we find a group of channels - highly conserved, present in all metazoan studied until now and involved in a series of organism “sensations” - called Transient Receptor Potential (TRP) channels.
The history of TRP channels began with the discovery of a dysfunction in Drosophila phototransduction cascade.65 The electroretinogram of these mutants exhibited atypical potentials in which, after photic stimulus, only a transient depolarization was observed, and 5 to 10 s after, basal values were recorded instead of the characteristic plateau. Minke demonstrated that this phenotype was not caused by a failure in the photopigment regeneration or by the activation of photosensitive channels.66,67 In fact, the defect was detected in a certain type of channel, and because the result was a transient receptor potential, animals showing this dysfunction were named TRP mutants.
In 1989, Montell and Rubin68 were able to unravel the culprit of this dysfunction, initiating the identification and characterization of more than 50 TRP channels found in a broad array of organisms. However, the confirmation that TRPs were indeed cationic channels, mainly for Ca2+, would still take a while to be established. The controversy was solved when a non-specific blocker of Ca2+ channels, lanthanum, was used in the retina fly, Calliphora, provoking a remarkable decline of the photoreceptor potential to the level observed in the dark after a light pulse.69
It's interesting that researchers searching for other sensorial mechanisms ended by bumping into members of the same family of cationic channels and the term TRP, initially used for Drosophila photoreceptors, remained until today. For instance, the vanilloid TRP channel 1-TRPV1- was found during the search of a receptor for capsaicin, an active molecule present in hot pepper which acts in nociceptors, triggering pain sensation and inflammation mediators.70,71,72 On the other hand, TRPV1 is associated with infrared sensation in vampire bats.73 Although some channels differ in their physiological features, all share quite the same architecture, formed by a tetramer composed of identical or similar subunits. TRPV174 and TRPA175 structures have been recently elucidated through elegant cryo-microscopy analyses. Both channels are homotetramers in which each monomer has 6 intramembrane segments, 2 of which form, with the loop between them, the cationic pore (Fig. 4). These channels are similar in structure to the voltage-gated channels, however it is still unknown whether the conformational changes are also similar.
Figure 4.
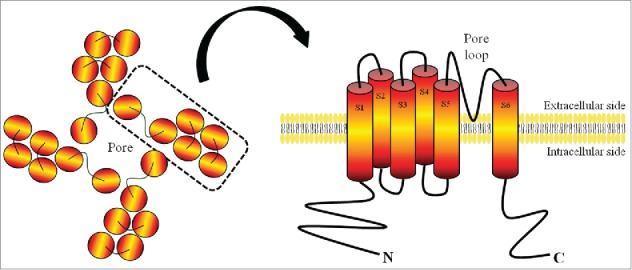
Schematic Structure of a TRP Channel Subunit. In general, TRP channels are composed of 4 identical or similar subunits, each consisting of 6 transmembrane segments with the cation pore between segments 5 and 6.
The first identified channels were named classical or canonical TRP channels, starting the formation of the TRPC subfamily. Currently, 28 different members of the large family of TRP channels have been identified in mammals. These channels were grouped into 7 subfamilies: TRPC (classical or canonical), TRPV (vanilloid), TRPM (melastatin) TRPA (ankyrin type), TRPP (polycystein) TRPML (mucolipin) and TRPN (no mechanoreceptor potential C (NOMPC)), while TRPY subfamily was identified only in yeast.76,77 Functional analyses of these channels have revealed their involvement in various types of sensory perception: thermal sensation, mechanosensation, chemosensation, nociception, as well as the perception of light.78
Among the members of the TRP family, many of them respond to thermal stimuli and consequently they were grouped in a subfamily named thermo-TRPs. These channels are non-selective, particularly permeable to calcium ions and are mainly present in cell membranes. In mammals, TRP channel activation is followed by an influx of calcium that can generate action potentials in sensory neurons, characterizing the beginning of the transduction process of thermal sensation.79 In addition to thermal response, thermo-TRPs can also be activated by chemical or physical stimuli, thus being characterized as multimodal receptors. Another characteristic of these proteins is that their expression is not restricted to sensory neurons; they may be presented in several types of tissues.80
The ten thermo-TRPs identified in mammals are members of different subfamilies: TRPV (TRPV1-TRPV4), TRPM (TRPM2, TRPM4, TRPM5 and TRPM8), TRPC5 and TRPA1, each one activated by different temperature levels.81 The mammalian thermo-TRPs activated by heat are TRPV1, TRPV2, TRPV3, TRPV4, TRPM2, TRPM4 and TRPM5, whereas TRPA1 and TRPM8 are activated by cold.64,79,82 Some of TRP properties confirm their participation in the mechanisms of temperature detection: (1) all thermo-TRP channels, once activated, generate a non-selective cationic current and a resulting membrane depolarization; (2) all thermo-TRPs except TRPM2, TRPM4, and TRPM5, are expressed in the peripheral nervous system; (3) each of these channels is activated in a narrow temperature range. Altogether, they cover a wide range of temperature to which mammals are exposed (Fig. 5): TRPV1 is activated by temperatures > 42oC, TRPV2 by temperatures > 52oC, TRPV3 by temperatures > 33oC, TRPV4 by temperatures between 27 and 42oC, TRPM2 by temperatures between 35 and 42oC, TRPM4 and TRPM5 by temperatures between 15 and 35oC, TRPM8 by temperatures < 25oC and TRPA1 by temperatures <17oC.83,84
Figure 5.
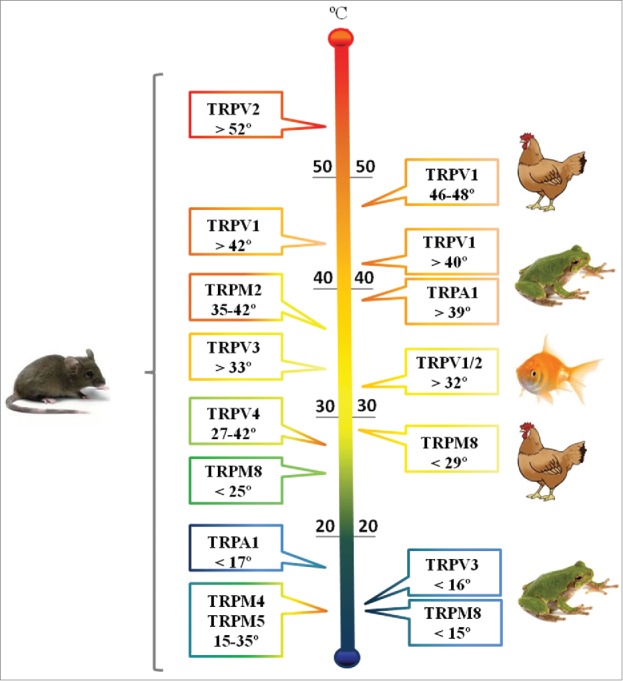
TRP Channel Families and the Range of Temperatures to which they Respond. Depending on the vertebrate class same channel family may span different temperature sets.
The advances of genome sequencing have revealed that each vertebrate class has its own repertoire of thermo-TRP homologues, and that the genes coding TRPV1 thru TRPV4, TRPM2, TRPM4, TRPM5 and TRPM8 are only found in vertebrates. The majority of the genes arose from a common ancestral of teleost fish and terrestrial vertebrates; the subsequent divergence resulted in a variety of thermo-TRP with different physiological properties.85 Although all vertebrates studied to date possess one copy of TRPV3, this gene has been lost in several teleost species.
In a thorough phylogenetic analysis, Saito and co-workers86 reported that teleost fish may present variable number of copies of TRPV1/2 genes. The data suggest that 2 copies were produced by genomic duplication in the teleost ancestral, one of them lost in some species such as Gasterosteus aculeatus and Danio rerio. Most mammals, Gallus gallus, and the lizard Anolis possess only one copy of each TRPV1 thru TRPV6.86 The same group found a few new TRP channels, namely TRPV8 and TRPV9 from Platypus sp and TRPV8 from the frog Xenopus tropicalis (for review see 87).
TRPV1 ortholog in D. rerio is necessary for the escape behavior from nociceptive heat above 32oC,88 but in some amphibian and reptile species, and in mammals TRPV1 is activated at temperatures above 40oC86,89 and in birds only at temperatures higher than 46–48oC.83,90 The channels related to cold detection also display different behaviors according to the species.91 For instance, in Xenopus tropicalis TRPM8 activation is triggered at 15oC whereas, in birds, it is sensitive to temperatures below 29oC, and in mammals to temperatures below 25°C92.
In addition to a role in thermoperception, TRP channels may represent a key component in rhabdomeric phototransduction pathways, not only in Drosophila photoreceptors,93 but also in Limulus,94 cephalopods95,96 and mammals.97
The Role of TRP Channels in Photoresponses
Colin Pittendrigh98 proposed the theory of “escape from light” in which high temperatures and UV radiation found during the light phase of the day would be harmful to the stability of enzymes and physiological processes. From this theory emerges the hypothesis that both light perception (photoreceptors) and circadian systems may have been evolved under the same selective pressures: daily cycles of light and temperature.3 Thus the theory of escape from light and its impact on the idea of co-evolution systems can be envisioned in several systems that will, in fact, go far beyond the light detection by the retina.
It is known that ultraviolet radiation, present in solar illumination, has profound damaging effects on human skin, causing photo-aging and skin cancer. In primary culture of human epidermal melanocytes, UVA radiation promotes calcium mobilization and early melanin synthesis only in the presence of 11-cis-retinal,99 what strongly suggests the involvement of an opsin in the UVA response. In fact, the presence of opsins including rhodopsin has been demonstrated in human melanocytes.99,100
To elucidate the meaning of opsin expression in peripheral tissues of mammals, a vertebrate class where light input is classically restricted to retina, like human melanocytes became crucial. It has been demonstrated that the signaling pathway triggered by UVA radiation involves the participation of a Gq protein, phospholipase C (PLC) and intracellular calcium; rhodopsin has been proposed as a candidate photopigment to translate UVA signal via activation of a retinal-dependent current mediated by TRPA1 channel.99,101,102
After the discovery of melanopsin in the mammalian retina, it became possible to understand how light signals to the endogenous biological clock. Studies of the signaling pathways triggered by the circadian photo-pigment melanopsin demonstrated the participation of TRP channels in response to light. This cascade recruits a Gq protein, followed by the activation of PLC and TRPC6 and C7 channels, resulting in membrane depolarization.97,103,104 As we said before, TRPC proteins form nonspecific cationic channels with substantial Ca2+ permeability, matching known features of the ipRGC light-activated channel.105 Pharmacological assays have demonstrated that 2-aminoethoxydiphenylborane (2-APB), an inositol trisphosphate (IP3) receptor and TRP channel antagonist, abolishes light responses in the ipRGCs in vitro and induces an acute knockdown of pupillary light reflex in vivo.103 Furthermore, evidence suggests that calcium permeability in rat ipRGCs can be suppressed by TRPC blockers102,106 (Fig. 6).
Figure 6.
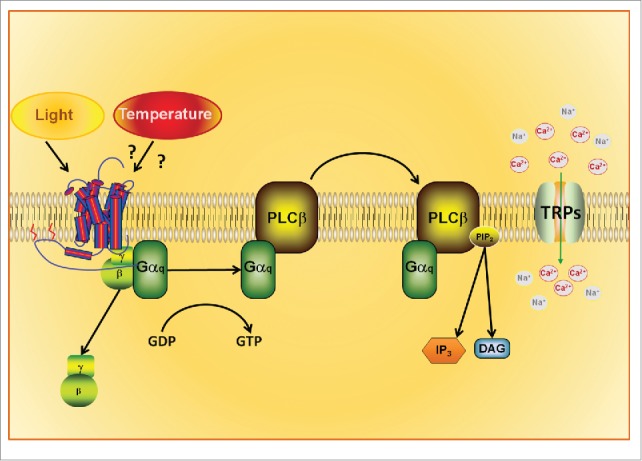
Hypothetical Signaling Pathway of the Thermo-Activation of Opsins. Recent data show the involvement of opsins (rhodopsin/melanopsin) and TRPA1 in temperature discrimination of Drosophila larvae. Temperature activates opsins by an unknown mechanism, triggering the phosphoinositide cascade. Depletion of PIP2 from the cell membrane leads to TRP channel opening and cation influx into the cytoplasm.
Heterologous cell systems have been used to express melanopsin and study the signaling pathway evoked by light. The participation of TRP channels in photo-transduction has also been demonstrated in these systems and their co-expression with melanopsin has been shown to result in a functional cascade,107,108 thus revealing the crucial physiological relevance of these channels.
TRP channel was discovered as a key component required for the light response in Drosophila photoreceptor cells. Analyses of this channel indicate that its loss of function changes the permeability to several cations, including a decreased Ca2+ influx in response to light.93,109 This phenotype, combined with the observation that fly vision requires PLC,110 similar to what is seen in the mammalian ipRGC,97,111 raised the possibility that TRP channels might be related with opsin photo-activation, involving the participation of PLC pathway that ultimately opens Ca2+ channel. However the mechanism by which these channels are activated has only been recently proposed.
In both Drosophila and mammalian models, there is a controversial issue concerning the mechanism through which stimulation of PLC leads to the activation of TRP. Two different hypotheses have been proposed: (1) TRP channels are activated by IP3 production; and (2) a rise in diacylglycerol (DAG) leads to the opening of TRP channels.97 In this regard, recent findings have shown that TRP channels can be activated by a combination of PLC signaling and phosphatidylinositol bisphosphate (PIP2) depletion. PIP2 depletion from the membrane lipid bilayer may promote a reduction of its area through cleavage of PIP2, resulting in a change of the mechanical properties of the membrane.112,113 Although in native models the participation of DAG has been apparently discarded, in heterologous systems, the opening of TRPC6 and TRPC7, which are involved in photo-responses, can be activated by DAG.114,115 Strengthening the controversy, the association of DAG and IP3 production with PIP2 depletion is reported to be involved with TRPC6 and TRPC7 opening in mammalian vascular smooth muscle.116,117 The mechanical ability to gating TRP channels is now proposed as a unifying activation mechanism among the distinct members of the TRP family. Besides mechano-activation of TRP channels in response to force applied to the cell, other non-mechanical stimuli such as temperature and light have been proposed to open these channels using the same strategy. Thus TRP channels have been considered as stretch-activated channels, even in cases that the initial stimulus is not mechanical and acts via an intracellular cascade.118
An important discovery that reinforces the interaction of light and temperature cycles is presented by Shen and colleagues.2 This group showed that temperature discrimination is dependent on rhodopsin and mediated by TRP channels in Drosophila, since rhodopsin-mutant animal loses this ability. In addition, thermo-sensitivity can be restored by melanopsin transfection; this role for rhodopsin/melanopsin is independent of light, as the assays for thermo discrimination were performed in constant darkness.2 This finding stands out as an important contribution to the understanding of the generation of circadian rhythms, since melanopsin, photopigment essential for the entrainment of circadian rhythms in mammals, is capable of interacting with Drosophila TRPA1 and reestablish thermo-sensitivity.
In fact, expression of TRPA1 in a subset of clock neurons in Drosophila brain determines the responses to temperature entrainment; lack of this channel impairs activity and alters the expression of Per gene.119 Why would be advantageous to have an indirect thermo perception? The convergence of components of 2 signaling pathways, opsins and TRP channels, may result in a considerable adaptation, since the former initiate the enzymatic cascade conferring the thermo-sensory system the capability to amplify even small temperature differences within the comfortable range. Whereas the direct activation of TRP channels by temperature appears to promote survival, since adaptation to harmful conditions might lead to physiological deregulation or death.120,121 Co-evolution of photoreception/thermoreception systems may be considered to explain the interaction of TRP channels and rhodopsin,122 thus expanding the current list of known temperature-sensitive proteins, to include light-sensitive proteins like rhodopsin (Fig. 6).
The Role of TRP Channels in Entrainment of Peripheral Clocks
Data about the role of thermo-TRP channels in biological rhythms has just begun to emerge and most available literature relies on experiments with Drosophila melanogaster. The first evidence was demonstrated by Shen and coworkers2 in 2011. Their experiments revealed that temperature discrimination in Drosophila larvae is dependent on TRPA1 channel. In the adult form TRPA1 is expressed in a subgroup of pacemaker neurons of the brain. The loss of TRPA1 impaired the temperature-induced syncronization and altered the expression of the clock gene Per in some pacemaker neurons.119 In peripheral tissues of Drosophila Pyx TRP channel (pyrexia transient receptor potential) located in sensory organs is responsible for the entrainment to lower cycles of temperature (16–20°C).123
Recently, in vertebrates and more specifically in the rainbow trout Oncorhynchus mykiss, the involvement of TRPV1 with rhythms of melatonin secretion has been demonstrated. The pharmacological blockade of pinealocyte TRPV1 inhibited melatonin secretion.124 Although the data linking thermo-TRP and biological rhythms are still scarce, and most of them were demonstrated in central clocks, valuable information has been provided to trace parallels between the role of thermo-TRP in central and peripheral synchronization, leading to necessary further investigation.
Conclusions and Perspectives
Light-dark cycles and temperature - major time-givers - have been dramatically altered in our 24-h society; now our biological clock is bombarded by artificial illumination, heating and cooling systems that maintain environment radically different from the one in which our entrainment mechanism has evolved. Among the consequences of such environmental changes is the increased incidence of pathologies such as cancer, cardiovascular disease, depression, obesity and diabetes. Several studies point out that the disruption of our endogenous biological clocks is the cause for this elevation: to mention one, clock mutant mice are obese and develop type II diabetes.125 Interestingly, aging TRPV1 knockout mice are also obese.126,127 These animals display a higher amplitude of circadian rhythm of core temperature,128 and they are more susceptible to deleterious effects of high fat diet, in terms of inducing obesity129and hypertension, reducing glucose tolerance. In addition, they show increased leptin, interleukin 10 and interleukin1β plasma levels.130
Considering the findings discussed in the present review about the involvement of TRP channels in thermo/photo responses in different groups of animals, we speculate that perception of time-giver signal may also follow a common mechanism. If so, TRP channels which have already been proven to mediate thermal and photic regulation of circadian rhythms in Drosophila,131 may be the missing bound in the entrainment mechanism of peripheral clocks in higher order metazoans.
Biographies
Maristela Oliveira Poletini, PhD.
She is assistant professor at Federal University of Minas Gerais, Brazil. Her lab focus on studying different cues for the synchronization of peripheral clocks, such as temperature, hormone and exercise in mammalian.
Maria Nathalia Moraes, PhD. She has a post-doc position in Dr Castrucci's lab, studying the interaction of opsins with TRP channels in photo- and thermo-responses in mammalian.
Bruno Cesar Ribeiro Ramos, PhD.
He is a post-doc in Prof. Castrucci's laboratory, investigating light signaling and clock-gene expression, and the correlations between light/temperature and opsins in peripheral clocks of teleost fish.
Rodrigo Jerônimo. He is graduated student at Dr Castrucci's laboratory, studying the temperature effect on clock genes of fish cell line.
Ana Maria de Lauro Castrucci, PhD.
She is a full professor of Physiology (retired) at the University of São Paulo. Her research contributed to understand the regulation and intracellular signaling of vertebrate pigment cells. Currently, her research focuses on the regulation of vertebrate peripheral clocks by hormones, light and temperature.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest are disclosed.
Funding
This work was partially supported by the Research Foundation of the State of São Paulo (FAPESP grant 2012/50214–4), the Research Foundation of the State of Minas Gerais (FAPEMIG grant APQ_01149–13), and the National Council of Research (CNPq grant 301293/2011–2).
References
- 1.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010; 330:379-85; PMID:20947768; http://dx.doi.org/ 10.1126/science.1195262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science 2011; 331:1333-6; PMID:21393546; http://dx.doi.org/ 10.1126/science.1198904 [DOI] [PubMed] [Google Scholar]
- 3.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol 1993; 55:16-54; PMID:8466172; http://dx.doi.org/ 10.1146/annurev.ph.55.030193.000313 [DOI] [PubMed] [Google Scholar]
- 4.Cerejido FB. Del Tiempo, Chronos, Freud, Einstein y los Genes. Folios Ediciones, Mexico 1988, 159 pp. [Google Scholar]
- 5.Aschoff J, Pohl H. Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschafften 1978; 65:80-4; http://dx.doi.org/ 10.1007/BF00440545 [DOI] [PubMed] [Google Scholar]
- 6.Daan S. The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms 2000; 15:195-207; PMID:10885874; http://dx.doi.org/ 10.1177/074873040001500301 [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Buchanan GF, Ding JM, Hannibal J, Gillette MU. Pituitary adenylyl cyclase-activating peptide: a pivotal modulator of glutamatergic regulation of the suprachiasmatic circadian clock. Proc Natl Acad Sci U S A 1999; 96:13468-73; http://dx.doi.org/ 10.1073/pnas.96.23.13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002; 418:935-41; PMID:12198538; http://dx.doi.org/ 10.1038/nature00965 [DOI] [PubMed] [Google Scholar]
- 9.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 2010; 90:1063-102; PMID:20664079; http://dx.doi.org/ 10.1152/physrev.00009.2009 [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Lahens NF, Balance HI, Hugles ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A 2014; 45:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko CH, Takahashi JS. Molecular components of mammalian circadian clock. Hum Mol Genet 2006; 15:R271-7; PMID:16987893; http://dx.doi.org/ 10.1093/hmg/ddl207 [DOI] [PubMed] [Google Scholar]
- 12.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 2014; 24:90-9; PMID:23916625; http://dx.doi.org/ 10.1016/j.tcb.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, et al.. Light- induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 1997; 91:1043-53; PMID:9428526; http://dx.doi.org/ 10.1016/S0092-8674(00)80494-8 [DOI] [PubMed] [Google Scholar]
- 14.Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res 1982; 247:154-8; PMID:7127113; http://dx.doi.org/ 10.1016/0006-8993(82)91041-1 [DOI] [PubMed] [Google Scholar]
- 15.Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007; 148:5648-55; PMID:17901231; http://dx.doi.org/ 10.1210/en.2007-0804 [DOI] [PubMed] [Google Scholar]
- 16.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995; 14:697-706; PMID:7718233; http://dx.doi.org/ 10.1016/0896-6273(95)90214-7 [DOI] [PubMed] [Google Scholar]
- 17.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 2010; 72:551-77; PMID:20148688; http://dx.doi.org/ 10.1146/annurev-physiol-021909-135919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld JE, et al.. Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron 2015; 85:1086-102; PMID:25741729; http://dx.doi.org/ 10.1016/j.neuron.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998; 93:929-37; PMID:9635423; http://dx.doi.org/ 10.1016/S0092-8674(00)81199-X [DOI] [PubMed] [Google Scholar]
- 20.Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature 2000; 404:87-91; PMID:10716448; http://dx.doi.org/ 10.1038/35003589 [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000; 288:682-5; PMID:10784453; http://dx.doi.org/ 10.1126/science.288.5466.682 [DOI] [PubMed] [Google Scholar]
- 22.Bartell PA, Miranda-Anaya M, Menaker M. Period and phase control in a multioscillatory circadian system (Iguana iguana). J Biol Rhythms 2004; 19:47-57; PMID:14964703; http://dx.doi.org/ 10.1177/0748730403261133 [DOI] [PubMed] [Google Scholar]
- 23.Costa A, Castanon-Cervantes O, Menaker M, Piccione G, Caola G. Daily rhythm of lactate dehydrogenase in the rat (Rattus norvegicus) carrying a Per1-luciferase transgene: assessment on serum and liver. Vet Res Commun 2005; 29:183-6; PMID:16244951; http://dx.doi.org/ 10.1007/s11259-005-0038-9 [DOI] [PubMed] [Google Scholar]
- 24.Davidson AJ, London BG, Block D, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens 2005; 27:307-11; PMID:15835394; http://dx.doi.org/ 10.1081/CEH-200048933 [DOI] [PubMed] [Google Scholar]
- 25.Farhat FP, Martins CB, Lima LH, Isoldi MC, Castrucci AML. Melanopsin and clock genes: regulation by light and endothelin in the zebrafish ZEM-2S cell line. Chronobiol Int 2009; 26:1090-119; PMID:19731108; http://dx.doi.org/ 10.3109/07420520903249005 [DOI] [PubMed] [Google Scholar]
- 26.Moraes MN, Poletini MO, Ramos BC, Lima LH, Castrucci AML. Effect of light on expression of clock genes in Xenopus laevis melanophores. Photochem Photobiol 2014; 90:696-701; PMID:24438110; http://dx.doi.org/ 10.1111/php.12230 [DOI] [PubMed] [Google Scholar]
- 27.Ramos BCR, Moraes MNCM, Poletini MO, Lima LHRG, Castrucci AML. From blue light to clock genes in zebrafish cells. PLoS One, 2014; 9(9):e106252; PMID:25184495; http://dx.doi.org/ 10.1371/journal.pone.0106252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Handb Exp Pharmacol 2013; 217:45-66; PMID:23604475; http://dx.doi.org/ 10.1007/978-3-642-25950-0_3 [DOI] [PubMed] [Google Scholar]
- 29.Shearman LP, Jun X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol 2000; 20:6269-75; PMID:10938103; http://dx.doi.org/ 10.1128/MCB.20.17.6269-6275.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 2006; 50:465-77; PMID:16675400; http://dx.doi.org/ 10.1016/j.neuron.2006.03.041 [DOI] [PubMed] [Google Scholar]
- 31.Debruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic clock. Nat Neurosci 2007a; 10:543-5; http://dx.doi.org/ 10.1038/nn1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pendergast JS, Yamazaki S. Masking responses to light in period mutant mice. Chronobiol Int 2011; 28:657-63; PMID:21793695; http://dx.doi.org/ 10.3109/07420528.2011.596296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney BM, Hastings JW. Effects of temperature upon diurnal rhythms. Cold Spring Harb Symp Quant Biol 1960; 25:87-104; http://dx.doi.org/ 10.1101/SQB.1960.025.01.009 [DOI] [PubMed] [Google Scholar]
- 34.Ruby NF, Burns DE, Heller HC. Circadian rhythms in the suprachiasmatic nucleus are temperature-compensated and phase-shifted by heat pulses in vitro. J Neurosci 1999; 19:8630-6; PMID:10493763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes BA, Pendergast JS, Yamazaki S. Mammalian peripheral circadian oscillators are temperature compensated. J Biol Rhythms 2008; 23:95-8; PMID:18258762; http://dx.doi.org/ 10.1177/0748730407311855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menaker M. The free running period of the bat clock; seasonal variations at low body temperature. J Cell Comp Physiol 1961; 57:81-6; PMID:13769330; http://dx.doi.org/ 10.1002/jcp.1030570204 [DOI] [PubMed] [Google Scholar]
- 37.Rawson KS. Effects of tissue temperature on mammalian activity rhythms. Cold Spring Harb Symp Quant Biol 1960; 25:105-13; http://dx.doi.org/ 10.1101/SQB.1960.025.01.010 [DOI] [PubMed] [Google Scholar]
- 38.Gibbs FP. Temperature dependence of rat circadian pacemaker. Am J Physiol 1981; 241:R17-20; PMID:7246797 [DOI] [PubMed] [Google Scholar]
- 39.Gibbs FP. Temperature dependence of the hamster circadian pacemaker. Am J Physiol 1983; 244:R607-610; PMID:6846568 [DOI] [PubMed] [Google Scholar]
- 40.Herzog ED, Huckfeldt RM. Circadian entrainment to temperature, but not light, in the isolated suprachiasmatic nucleus. J Neurophysiol 2003; 90:763-70; PMID:12660349; http://dx.doi.org/ 10.1152/jn.00129.2003 [DOI] [PubMed] [Google Scholar]
- 41.Francis AJ, Coleman GJ. The effect of ambient temperature cycles upon circadian running and drinking activity in male and female laboratory rats. Physiol Behav 1988; 43:471-7; http://dx.doi.org/ 10.1016/0031-9384(88)90121-7 [DOI] [PubMed] [Google Scholar]
- 42.Francis AJ, Coleman GJ. Phase response curves to ambient temperature pulses in rats. Physiol Behav 1997; 62:1211-7; http://dx.doi.org/ 10.1016/S0031-9384(97)00202-3 [DOI] [PubMed] [Google Scholar]
- 43.Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A 2003; 100:16089-94; http://dx.doi.org/ 10.1073/pnas.2536313100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 2002; 12:1574-83; PMID:12372249; http://dx.doi.org/ 10.1016/S0960-9822(02)01145-4 [DOI] [PubMed] [Google Scholar]
- 45.Saini C, Suter DM, Liani A, Gos P, Schibler U. The mammalian circadian timing system: synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol 2011; 76:39-47; http://dx.doi.org/ 10.1101/sqb.2011.76.010918 [DOI] [PubMed] [Google Scholar]
- 46.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev 2012; 26:567-80; PMID:22379191; http://dx.doi.org/ 10.1101/gad.183251.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saini C, Liani A, Curie T, Gos P, Kreppel F, Emmenegger Y, Bonacina L, Wolf JP, Poget YA, Franken P, et al.. Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes Dev 2013; 27:1526-36; PMID:23824542; http://dx.doi.org/ 10.1101/gad.221374.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edery I, Rutila JE, Rosbash M. Phase shifting of the circadian clock by induction of the Drosophila period protein. Science 1994; 263:237-40; PMID:8284676; http://dx.doi.org/ 10.1126/science.8284676 [DOI] [PubMed] [Google Scholar]
- 49.Zatz M, Lange GD, Rollag MD. What does changing the temperature do to the melatonin rhythm in cultured chick pineal cells? Am J Physiol 1994; 266:R50-58; PMID:8304555 [DOI] [PubMed] [Google Scholar]
- 50.Barrett RK, Takahashi JS. Temperature compensation and temperature entrainment of the chick pineal cell circadian clock. J Neurosci 1995; 15:5681-92; PMID:7643210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tosini G, Menaker M. The tau mutation affects temperature compensation of hamster retinal circadian oscillators. Neuroreport 1998; 9:1001-5; PMID:9601657; http://dx.doi.org/ 10.1097/00001756-199804200-00009 [DOI] [PubMed] [Google Scholar]
- 52.Menaker M, Wisner S. Temperature-compensated circadian clock in the pineal of Anolis. Proc Natl Acad Sci U S A 1983; 80:6119-21; http://dx.doi.org/ 10.1073/pnas.80.19.6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kondo T, Strayer CA, Kulkarni RD, Taylor W, Ishiura M, Golden SS, Johnson CH. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci U S A 1993; 90:5672-6; http://dx.doi.org/ 10.1073/pnas.90.12.5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vallone D, Frigato E, Vernesi C, Foà A, Foulkes NS, Bertolucci C. Hypothermia modulates circadian clock gene expression in lizard peripheral tissues. Am J Physiol Regul Integr Comp Physiol 2007; 292:R160-166; http://dx.doi.org/ 10.1152/ajpregu.00370.2006 [DOI] [PubMed] [Google Scholar]
- 55.Magnone MC, Jacobmeier B, Bertolucci C, Foa A, Albrecht U. Circadian expression of the clock gene Per2 is altered in the ruin lizard (Podarcis sicula) when temperature changes. Brain Res Mol Brain Res 2005; 133:281-5; PMID:15710245; http://dx.doi.org/ 10.1016/j.molbrainres.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 56.Lahiri K, Vallone D, Gondi SB, Santoriello C, Dickmeis T, Foulkes NS. Temperature regulates transcription in the zebrafish circadian clock. PLoS Biol 2005; 3:e351; PMID:16176122; http://dx.doi.org/ 10.1371/journal.pbio.0030351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vallone D, Lahiri K, Dickmeis T, Foulkes NS. Zebrafish cell clocks feel the heat and see the light! Zebrafish 2005; 2:171-87; PMID:18248192; http://dx.doi.org/ 10.1089/zeb.2005.2.171 [DOI] [PubMed] [Google Scholar]
- 58.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 2007; 292:R37-46; http://dx.doi.org/ 10.1152/ajpregu.00668.2006 [DOI] [PubMed] [Google Scholar]
- 59.Refinetti R. The circadian rhythm of body temperature. Front Biosci 2010; 15:564-94; http://dx.doi.org/ 10.2741/3634 [DOI] [PubMed] [Google Scholar]
- 60.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al.. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007; 129:605-16; PMID:17482552; http://dx.doi.org/ 10.1016/j.cell.2007.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol 2007b; 17:R538-9; http://dx.doi.org/ 10.1016/j.cub.2007.05.067 [DOI] [PubMed] [Google Scholar]
- 62.Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol 2009; 7:e52; PMID:19278294; http://dx.doi.org/ 10.1371/journal.pbio.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogenesch JB, Ueda HR. Understanding systems-level properties: timely stories from the study of clocks. Nat Rev Genet 2011; 12:407-16; PMID:21556016; http://dx.doi.org/ 10.1038/nrg2972 [DOI] [PubMed] [Google Scholar]
- 64.Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci 2014; 15:573-89; http://dx.doi.org/ 10.1038/nrn3784 [DOI] [PubMed] [Google Scholar]
- 65.Cosens DJ, Maning A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969; 224:285-7; PMID:5344615; http://dx.doi.org/ 10.1038/224285a0 [DOI] [PubMed] [Google Scholar]
- 66.Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975; 258:84-7; PMID:810728; http://dx.doi.org/ 10.1038/258084a0 [DOI] [PubMed] [Google Scholar]
- 67.Minke B. Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J Gen Physiol 1982; 79:361-85; PMID:7077289; http://dx.doi.org/ 10.1085/jgp.79.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 1989; 2:1313-23; PMID:2516726; http://dx.doi.org/ 10.1016/0896-6273(89)90069-X [DOI] [PubMed] [Google Scholar]
- 69.Hochstrate P. Lanthanum mimicks the trp photoreceptor mutant of Drosophila in the blowfly Calliphora. J Comp Physiol A 1989; 166:179-87. [DOI] [PubMed] [Google Scholar]
- 70.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 71.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998; 21:531-43; PMID:9768840; http://dx.doi.org/ 10.1016/S0896-6273(00)80564-4 [DOI] [PubMed] [Google Scholar]
- 72.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306-13; PMID:10764638; http://dx.doi.org/ 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- 73.Gracheva EO, Cordero-Morales JF, González-Carcacía JA, Ingolia NT, Manno C, Aranguren CI, Weissman JS, Julius D. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 2011; 476:88-91; PMID:21814281; http://dx.doi.org/ 10.1038/nature10245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013; 504:113-8; PMID:24305161; http://dx.doi.org/ 10.1038/nature12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015; 520:511-7; PMID:25855297; http://dx.doi.org/ 10.1038/nature14367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flockerzi V, Nilius B. TRPs: Truly remarkable proteins. Handb Exp Pharmacol 2014; 222:1-12; PMID:24756700; http://dx.doi.org/ 10.1007/978-3-642-54215-2_1 [DOI] [PubMed] [Google Scholar]
- 77.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 2007; 76:387-417; PMID:17579562; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol 2005; 1:85-92; PMID:16408004; http://dx.doi.org/ 10.1038/nchembio0705-85 [DOI] [PubMed] [Google Scholar]
- 79.Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci 2006; 29:135-61; PMID:16776582; http://dx.doi.org/ 10.1146/annurev.neuro.29.051605.112958 [DOI] [PubMed] [Google Scholar]
- 80.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci 2003; 4:529-39; PMID:12838328; http://dx.doi.org/ 10.1038/nrn1141 [DOI] [PubMed] [Google Scholar]
- 81.Saito S, Tominaga M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium 2015; 57:214-21; PMID:25533790; http://dx.doi.org/ 10.1016/j.ceca.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Elias A, Mrkonjic S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardó F, Plata C, Gaudet R, Vicente R, Valverde MA. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci U S A 2013; 110:9553-8; http://dx.doi.org/ 10.1073/pnas.1220231110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 84.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxius heat. Nature 1999; 398:436-41; PMID:10201375; http://dx.doi.org/ 10.1038/18906 [DOI] [PubMed] [Google Scholar]
- 85.Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics 2006; 27:219-30; PMID:16926268; http://dx.doi.org/ 10.1152/physiolgenomics.00322.2005 [DOI] [PubMed] [Google Scholar]
- 86.Saito S, Fukuta N, Shingai R, Tominaga M. Evolution of vertebrate transient receptor potential vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet 2011; 7:e1002041; PMID:21490957; http://dx.doi.org/ 10.1371/journal.pgen.1002041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shigeru S, Tominaga M. Functional diversity and evolutionary dynamics of thermoTRPchannels. Cell Calcium 2014; 57:214-21; PMID:25533790 [DOI] [PubMed] [Google Scholar]
- 88.Gau P, Poon J, Ufret-Vincenty C, Snelson CD, Gordon SE, Raible DW, Dhaka A. The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J Neurosci 2013; 33:5249-60; PMID:23516290; http://dx.doi.org/ 10.1523/JNEUROSCI.5403-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohkita M, Saito S, Imagawa T, Takahashi K, Tominaga M, Ohta T. Molecular cloning and functional characterization of Xenopus tropicalis frog transient receptor potential vanilloid 1 reveal its functional evolution for heat, acid, and capsaicin sensitivities in terrestrial vertebrates. J Biol Chem 2012; 287:2388-97; PMID:22130664; http://dx.doi.org/ 10.1074/jbc.M111.305698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘hot’ chili peppers. Cell 2002; 108, 421-430; PMID:11853675; http://dx.doi.org/ 10.1016/S0092-8674(02)00637-2 [DOI] [PubMed] [Google Scholar]
- 91.Gracheva EO, Bagriantsev SN. Evolutionary adaptation to thermosensation. Curr Opin Neurobiol 2015; 34C:67-73; http://dx.doi.org/ 10.1016/j.conb.2015.01.021 [DOI] [PubMed] [Google Scholar]
- 92.Myers BR, Sigal YM, Julius D. Evolution of thermal response properties in a cold-activated TRP channel. PLoS One 2009; 4:e5741; PMID:19492038; http://dx.doi.org/ 10.1371/journal.pone.0005741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 1992; 8:643-65; PMID:1314617; http://dx.doi.org/ 10.1016/0896-6273(92)90086-S [DOI] [PubMed] [Google Scholar]
- 94.Bandyopadhyay BC, Payne R. Variants of TRP ion channel mRNA present in horseshoe crab ventral eye and brain. J Neurochem 2004; 91:825-35; PMID:15525336; http://dx.doi.org/ 10.1111/j.1471-4159.2004.02773.x [DOI] [PubMed] [Google Scholar]
- 95.Monk PD, Carne A, Liu SH, Ford JW, Keen JN, Findlay JB. Isolation, cloning, and characterization of trp homologue from squid (Loligo forbesi) photoreceptor membranes. J Neurochem 1996; 67:2227-35; PMID:8931453; http://dx.doi.org/ 10.1046/j.1471-4159.1996.67062227.x [DOI] [PubMed] [Google Scholar]
- 96.Kington AC, Kuzirian AM, Hanlon RT, Cronin TW. Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. J Exp Biol 2015; 218:1596-602; PMID:25994635; http://dx.doi.org/ 10.1242/jeb.117945 [DOI] [PubMed] [Google Scholar]
- 97.Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci 2008; 31, 27-36; PMID:18054803; http://dx.doi.org/ 10.1016/j.tins.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 98.Pittendrigh CS. Biological clocks: the functions, ancient and modern, of circadian oscillations. Science in the Sixties. In Proc. 1965 Cloudcroft Symp. Air Force Office of Scientific Research, pp. 95-111. [Google Scholar]
- 99.Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol 2011; 21:1906-11; PMID:22055294; http://dx.doi.org/ 10.1016/j.cub.2011.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haltaufderhyde K. Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol 2015; 91:117-23; PMID:25267311; http://dx.doi.org/ 10.1111/php.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bellono NW, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci U S A 2013; 110:2383-8; http://dx.doi.org/ 10.1073/pnas.1215555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bellono NW, Najera JÁ, Oancea E. UV light activates a Gαq/11-coupled phototransduction pathway in human melanocytes. J Gen Physiol 2014; 143:203-14; PMID:24470488; http://dx.doi.org/ 10.1085/jgp.201311094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG, Hankins MW. Two-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci 2007; 27:3981-6; PMID:17428972; http://dx.doi.org/ 10.1523/JNEUROSCI.4716-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol 2008; 99:2522-32; PMID:18305089; http://dx.doi.org/ 10.1152/jn.01066.2007 [DOI] [PubMed] [Google Scholar]
- 105.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol 2003; 13:1290-8; PMID:12906788; http://dx.doi.org/ 10.1016/S0960-9822(03)00510-4 [DOI] [PubMed] [Google Scholar]
- 106.Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur J Neurosci 2006; 23:2477-87; PMID:16706854; http://dx.doi.org/ 10.1111/j.1460-9568.2006.04777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature 2005; 433:745-9; PMID:15674243; http://dx.doi.org/ 10.1038/nature03345 [DOI] [PubMed] [Google Scholar]
- 108.Kumbalasiri K, Rollag MD, Isoldi MC, Castrucci AM, Provencio I. Melanopsin triggers the release of internal calcium stores in response to light. Photochem Photobiol 2007; 83:273-9; PMID:16961436; http://dx.doi.org/ 10.1562/2006-07-11-RA-964 [DOI] [PubMed] [Google Scholar]
- 109.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron 1997; 19:1249-59; PMID:9427248; http://dx.doi.org/ 10.1016/S0896-6273(00)80416-X [DOI] [PubMed] [Google Scholar]
- 110.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol 1999; 15:231-68; PMID:10611962; http://dx.doi.org/ 10.1146/annurev.cellbio.15.1.231 [DOI] [PubMed] [Google Scholar]
- 111.Arshavsky VY, Lamb TD, Pugh EN. Jr G proteins and phototransduction. Annu Rev Physiol 2002; 64:153-87; PMID:11826267; http://dx.doi.org/ 10.1146/annurev.physiol.64.082701.102229 [DOI] [PubMed] [Google Scholar]
- 112.Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol 2010; 20:189-97; PMID:20116246; http://dx.doi.org/ 10.1016/j.cub.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 113.Hardie RC. Photosensitive TRPs. Handb Exp Pharmacol 2014; 223:795-826; PMID:24961970; http://dx.doi.org/ 10.1007/978-3-319-05161-1_4 [DOI] [PubMed] [Google Scholar]
- 114.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999; 397:259-63; PMID:9930701; http://dx.doi.org/ 10.1038/16711 [DOI] [PubMed] [Google Scholar]
- 115.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, et al.. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7 Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem 1999; 274:27359-70; PMID:10488066; http://dx.doi.org/ 10.1074/jbc.274.39.27359 [DOI] [PubMed] [Google Scholar]
- 116.Albert AP, Large WA. Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J Physiol 2006; 570:45-51; PMID:16195316; http://dx.doi.org/ 10.1113/jphysiol.2005.096875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ju M, Shi J, Saleh SN, Albert AP, Large WA. Ins(1,4,5)P3 interacts with PtdIns(4,5)P2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes. J Physiol 2010; 588:1419-33; PMID:20211974; http://dx.doi.org/ 10.1113/jphysiol.2009.185256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu C, Montell C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun 2015; 460:22-5; http://dx.doi.org/ 10.1016/j.bbrc.2015.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee Y, Montell C. Drosophila TRPA1 functions in temperature control of circadian rhythm in pacemaker neurons. J Neurosci 2013; 33:6716-25; PMID:23595730; http://dx.doi.org/ 10.1523/JNEUROSCI.4237-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Minke B. The history of the Drosophila TRP channel: the birth of a new channel superfamily. J Neurogenet 2010; 24:216-33; PMID:21067449; http://dx.doi.org/ 10.3109/01677063.2010.514369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci 2013; 92:394-403; http://dx.doi.org/ 10.1016/j.lfs.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Minke B, Peters M. Rhodopsin as thermosensor? Science 2011; 331:1272-3; PMID:21393531; http://dx.doi.org/ 10.1126/science.1203482 [DOI] [PubMed] [Google Scholar]
- 123.Wolfgang W, Simoni A, Gentile C, Stanewsky R. The pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster. Proc Royal Society B 2013; 230:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nisembaum LG, Besseau L, Paulin CH, Charpantier A, Martin P, Magnanou E, Fuentès M, Jesus Delgado M, Falcón J. In the heat of the night: Thermo TRPV Channels in the salmonid pineal photoreceptors and modulation of melatonin secretion. Endocrinology 2015; 156:4629-38; http://dx.doi.org/ 10.1210/en.2015-1684 [DOI] [PubMed] [Google Scholar]
- 125.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al.. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010; 466:627-31; PMID:20562852; http://dx.doi.org/ 10.1038/nature09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 2011; 31:1721-33; PMID:21289181; http://dx.doi.org/ 10.1523/JNEUROSCI.4671-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wanner SP, Garami A, Romanovsky AA. Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging 2011; 3:450-4; PMID:21483038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Szelényi Z, Hummel Z, Szolcsányi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci 2004; 19:1421-4; http://dx.doi.org/ 10.1111/j.1460-9568.2004.03221.x [DOI] [PubMed] [Google Scholar]
- 129.Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, Azuma Y, Wang HF, Tsitsilianos N, Shafiq U, et al.. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J 2015; 29:3182-92; PMID:25888600; http://dx.doi.org/ 10.1096/fj.14-268300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie SJ, Lalgi K, Fernandes ES, Gnudi L, et al.. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 2013; 61:246-52; PMID:23150506; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.112.201434 [DOI] [PubMed] [Google Scholar]
- 131.Bellemer A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature 2015; 22:227-43; http://dx.doi.org/ 10.1080/23328940.2015.1004972 [DOI] [PMC free article] [PubMed] [Google Scholar]


