Abstract
Background
The natural progression of balance decline in individuals with Parkinson disease (PD) is not well understood.
Objectives
We aimed to: 1) compare the utility of three standardized clinical measures for detecting balance decline over 1-year, 2) identify components of balance susceptible to decline, and 3) identify factors useful for predicting future balance decline.
Methods
Eighty people with PD (59% male; mean age 68.2 ± 9.3; Hoehn & Yahr range I-IV) completed Balance Evaluation Systems Test (BESTest), Mini-BESTest, and Berg Balance Scale (BBS) assessments. Baseline predictor variables included the MDS-UPDRS III sub-score, presence of freezing, 6-month fall history, age, gender, and physical activity. Balance and MDS-UPDRS III assessments were repeated at 6 (n=51) and 12 months (n=44).
Results
BESTest and Mini-BESTest score declined over 6 and 12 months (P<0.01). Postural responses, stability limits, and sensory orientation were most susceptible to decline. BBS score did not change (P>0.01). MDS-UPDRS III score was unchanged over 6 months (P>0.01), but declined over 12 months (P<0.01). Change in BESTest score over 6 months was related to baseline MDS-UPDRS III, H&Y, freezing, and fall history (P<0.05). Change in BESTest score over 12 months was related to baseline MDS-UPDRS III and freezing (P<0.05). Change in Mini-BESTest over 12 months was related to baseline MDS-UPDRS III and age (P<0.05).
Conclusions
The BESTest and Mini-BESTest were responsive to balance decline in individuals with PD and helped to identify decline in underlying balance components. Disease severity and freezing most consistently predicted balance decline in persons with PD.
Keywords: Parkinson disease, postural balance, outcome assessment, physical therapy, rehabilitation
Introduction
Much research has been directed at understanding the role of postural instability in falls and declining functional performance among people with Parkinson disease (PD). As the disease progresses, postural instability and falls become a major concern, given their association with reduced quality of life, physical inactivity, traumatic injuries, and mortality [1–3]. There is growing consensus that falls in PD are multifactorial, suggesting that many physical and cognitive impairments related to PD may contribute to the increased incidence of falls in this population [4–6].
Researchers previously used standardized clinical balance assessments, which are batteries composed of individual balance testing items, to examine various aspects postural instability and determine fall risk in PD. Leddy and associates reported that the Balance Evaluation Systems Test (BESTest) and the Mini-BESTest were accurate in identifying people with PD who have experienced falls in the 6 months prior to assessment [7]. Duncan and colleagues corroborated the utility of these measures as well as the Berg Balance Scale (BBS) prospectively over 6 months when determining fall risk in people with PD [8]. Kerr and colleagues noted the best sensitivity and specificity for predicting future falls in people with PD when combining assessments, which included measures of disease severity, balance, freezing of gait (FOG), orthostasis, and postural sway [9]. In addition, investigators have noted improvements in Mini-BESTest and BBS scores following exercise interventions in people with PD, suggesting these measures may be responsive to changes in balance [10, 11].
While progress has been made in understanding of measures to use when attempting to predict falls in PD, to our knowledge these measures have not been used to prospectively quantify rate and magnitude of balance decline in PD. Furthermore, it is unclear if certain aforementioned predictors of falls (i.e. motor symptom severity, freezing of gait) similarly predict balance decline in PD. Identification of the best measures to capture balance decline in PD is critical to determine appropriate interventions aimed at improving balance performance. Understanding specific, underlying components related to overall balance decline will help healthcare professionals refine their interventions to target specific balance impairments. It is also crucial to determine factors related to balance decline so that healthcare professionals can improve accuracy of prognoses with respect to fall risk. Improved accuracy could foster design of interventions tailored to reduce fall risk in people with PD.
The broad purpose of this study was to explore the natural progression of balance decline over a 1-year period in a cohort of individuals with PD. We had three objectives: 1) to compare the utility of three standardized clinical measures for detecting balance decline over 6 and 12 months, 2) to determine whether specific balance components were particularly susceptible to decline, and 3) to identify factors (e.g. disease severity, fall history) useful for predicting future balance decline.
Methods
Participants
Participants were recruited from the Washington University School of Medicine Movement Disorder Center and from the Volunteers for Health database for participation in a multi-center longitudinal study [12]. Sample size calculations were conducted to satisfy the requirements for the multi-center study [12]. Potential participants were included if they were over age 40 and diagnosed with idiopathic PD (Hoehn & Yahr (H&Y) Stages I-IV) [13–15]. Potential participants were excluded if they had 1) a history or presence of a neurological disorder other than PD, 2) a musculoskeletal injury limiting the ability to walk, or 3) any serious medical condition. Participants agreed to participate in laboratory assessments at: baseline, 6 months from baseline, and 12 months from baseline. All assessments were completed in the Locomotor Control Laboratory at Washington University School of Medicine.
Standards Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Human Research Protection Office at Washington University. All participants provided informed consent according to the policies and procedures of the Human Research Protection Office of Washington University.
Measures
The Balance Evaluation Systems Test (BESTest) is a comprehensive assessment battery containing 36 items grouped into 6 specific components of balance. Section I assesses biomechanical constraints (e.g. ankle strength and range of motion). Section II assesses stability limits/verticality (e.g. forward functional reach and sitting verticality and lean). Section III assesses anticipatory postural adjustments, (e.g. sit-to-stand and single limb stance). Section IV assesses postural responses (e.g. compensatory stepping reactions). Section V assesses sensory orientation (e.g. standing on firm and foam surfaces with the eyes open and eyes closed). Section VI assesses stability in gait (e.g. walking with head turns, and the dual-task Timed Up & Go). Each item is scored on a rank scale from 0 to 3, with 3 indicating no impairment of balance [16]. The BESTest total score is represented as a percentage, calculated by dividing the actual score by 108, the highest possible score. Section scores are also represented as percentages. Minimal detectable change (MDC) and Minimal Clinically Important Difference (MCID) values are not currently available.
Because the BESTest requires 45 minutes to administer in people with mild to moderate PD, it is often considered impractical for clinical use. The Mini-BESTest, however, is a condensed 14-item version of the BESTest with a maximum score of 28 [17]. Items from the Mini-BESTest represent four of the 6 sections presented in the BESTest, excluding biomechanical constraints and stability limits/verticality. Like the BESTest, the Mini-BESTest includes section scores, which are calculated as percentages. The BESTest and Mini-BESTest have high inter-rater and test-retest reliability when assessing balance in people with PD [7, 18]. The MDC for the Mini-BESTest is 3.5 points in people with balance disorders [19].
The Berg Balance Scale (BBS) [20] also has high inter-rater and test-retest reliability when assessing balance in people with PD [21]. The BBS, a widely accepted and frequently used clinical balance assessment, contains 14 items with a maximum score of 56. Each item is scored on a rank scale from 0 to 4, with 4 indicating no impairment [20]. BBS items include sit-to-stand, 360-degree turn to the right and left, and picking up an object from the floor. The MDC for the BBS in people with PD is 5 points [22].
Baseline factors potentially predictive of future balance decline were selected based on a previous study of fall risk in people with PD [23]. Demographic characteristics included age and gender. Motor sign severity and H&Y stage were determined using the Movement Disorders Society – Unified Parkinson Disease Rating Scale Section III Motor Examination (MDS-UPDRS III) [24]. Higher scores on the MDS-UPDRS III, the values of which range from 0–132, indicate more severe symptoms. Presence or absence of freezing more than one time in the past month was determined using participant response to item #3 of the Freezing of Gait Questionnaire (FOGQ) [25]. Physical activity during the previous week was quantified using the Physical Activity Scale for the Elderly (PASE) [26]. Higher scores on the PASE, the values of which range from 0 to more than 400, indicate greater physical activity. Fall history was collected using a forced response paradigm, in which participants were asked to report the frequency of falls in the previous 6 months as either never, once, 2–10 times, weekly, or daily. Participants reporting two or more falls in the previous 6 months were classified as fallers.
Procedures
All participants were assessed during a self-reported “on” phase of their anti-PD medication cycle, typically 1–1.5 hours after taking dopamine replacement. Levodopa equivalent daily dose (LEDD) [27] was calculated based on self-report of anti-PD medication use. Balance assessments were administered by a physical therapist at each time point. Due to overlap between items of each balance test, a custom scoring form was developed so that overlapping items were performed only once. The MDS-UPDRS III also was collected at each time point to provide a context for examining potential balance changes. Baseline predictor variables were collected in the following order: 1) MDS-UPDRS III, 2) FOGQ, 3) PASE, 4) fall history, and 5) demographics. Predictor variables were collected after the balance assessments in order to avoid influencing the rater’s assessment of balance performance. Testing sessions typically were completed in 2–2.5 hours.
Data Analysis
Only scores from those individuals completing the baseline and at least one follow up assessment were included the analysis. Descriptive statistics were used to characterize the sample at each time point. Raw change scores for the BESTest, Mini-BESTest, BBS, and MDS-UPDRS III were calculated at 6 months and at 12 months by first subtracting a participant’s score at the relevant time point from his or her baseline score. Percent change scores for these four measures also were calculated at an individual level (% change = raw change score / baseline score)*100). To account for attrition, paired, two-tailed t-tests (α = .01) were used to analyze group mean differences between different time points (i.e. baseline vs. 6 months or baseline vs. 12 months). When significant group-level balance changes existed, Spearman correlations were used to identify significant relationships (α = .05) between percent change in the relevant balance score (i.e., BESTest, Mini-BESTest, BBS) and baseline predictor variables (i.e., MDS-UPDRS III score, H&Y stage, presence or absence of freezing, PASE score, fall history, age, and gender). Variables significantly related to percent change in a given balance score during a particular time interval subsequently were entered into a multiple regression analysis to determine which factors were the best predictors of change.
Results
Eighty people with PD completed baseline assessments. Fifty-one and 44 participants returned to complete follow-up assessments at 6 and 12 months, respectively. Descriptive characteristics appear in Table 1. Demographic information for those who dropped out compared to those who completed the full 12 month study has been previously reported [28]. Overall, the group who dropped out was no different with respect to age or gender, but had greater disease severity (H&Y and MDS-UPDRS III) and a higher percentage of recurrent fallers than those who completed the study.
Table 1.
Baseline Values for Study Variables: a Factor of Sample Attrition.
| Baseline (n=80) | 6 Months (n=51) | 12 Months (n=44) | |
|---|---|---|---|
| Age | 68.20 ± 9.29 | 67.00 ± 8.77 | 66.55 ± 9.45 |
| % Male | 58.75 | 56.80 | 56.80 |
| H&Y (Stage(n)) | I(4), II(27), II.5(30), III(13), IV(6) | I(4), II(22), II.5(17), III(5), IV(3) | I(4), II(22), II.5(11), III(5), IV(2) |
| MDS-UPDRS III | 41.34 ± 14.68 | 38.55 ± 13.13 | 37.52 ± 12.81 |
| BESTest (%) | 70.31 ± 16.74 | 72.93 ± 16.05 | 75.76 ± 12.99 |
| Mini-BESTest | 17.81 ± 6.01 | 18.61 ± 5.74 | 19.73 ± 5.00 |
| BBS | 47.89 ± 8.13 | 49.08 ± 8.23 | 50.07 ± 6.30 |
| Freezer status (% sample) | 40.00 | 39.20 | 29.50 |
| PASE | 123.34 ± 95.47 | 129.50 ± 86.14 | 137.90 ± 112.76 |
| Fallers (% sample) | 31.25 | 27.50 | 20.50 |
| Levodopa Equivalent Daily Dose (LEDD) | 855.22 ± 573.59 | 888.33 ± 648.72 | 870.57 ± 683.43 |
All values are mean ± SD unless otherwise indicated. All values represent baseline values for the sample at each time point.
Change Over 6 Months
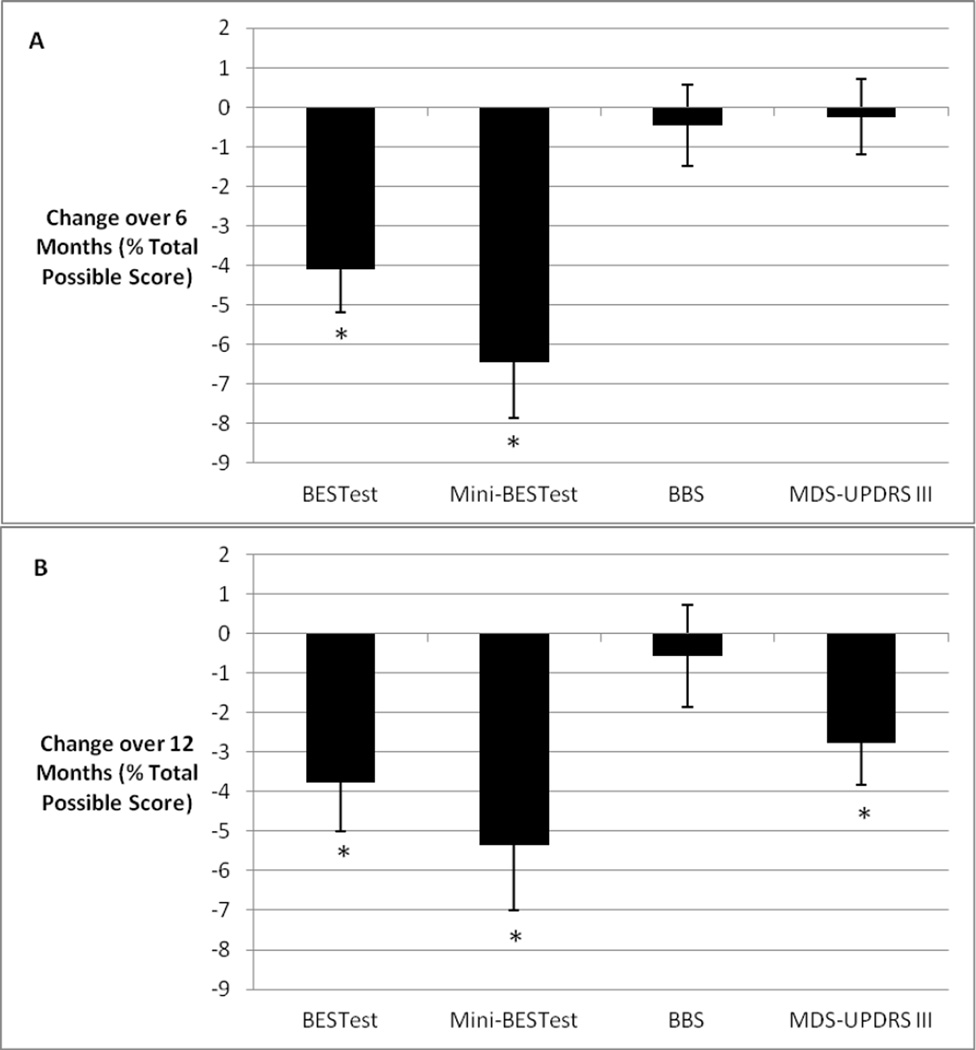
Balance declined significantly at 6 months compared to baseline as indicated by changes in BESTest (mean raw change: −4.08 ± 7.77; p<.001; 95% CI [−6.21, −1.95]) and Mini-BESTest (mean raw change: −1.81 ± 2.83; p<.001; 95% CI [−2.59, −1.03]) but not BBS scores (mean raw change: −0.25 ± 4.16; p=0.66; 95% CI [−1.39, 0.89]) (Figure 1A). There was no significant change in MDS-UPDRS III score during the same period (mean raw change: −0.31 ± 9.01; p=0.80; 95% CI [−2.78, 2.16]). With respect to change in specific balance systems over time, BESTest sections II, IV, and V demonstrated significant changes over 6 months (Table 2). Only section IV of the Mini-BESTest declined significantly over 6 months (Table 2).
Figure 1. Change in Scores Over Time.
Legend: Change in scores over 6 (A) and 12 (B) months. Change score values are means +/− SDs and are expressed as percent of total possible score for each measure. * - indicates significant change over 6 and 12 months.
Table 2.
Section scores (%) at baseline and 6 months for the BESTest and Mini-BESTest
| BESTest | Mini-BESTest | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | p value |
Baseline | 6 Months | p value | |
| I. Biomechanical Constraints | 62.48 ± 21.24 | 65.62 ± 24.38 | 0.92 | − | − | − |
| II. Stability Limits / Verticality | 82.26 ± 12.12 | 76.28 ± 16.48 | 0.0007 | − | − | − |
| III. Anticipatory Postural Adjustments | 72.11 ± 20.02 | 69.61 ± 24.12 | 0.08 | 63.07 ± 22.44 | 62.09 ± 27.90 | 0.35 |
| IV. Postural Responses | 71.24 ± 23.03 | 62.75 ± 27.25 | 0.0004 | 61.76 ± 27.14 | 45.75 ± 29.60 | 0.000002 |
| V. Sensory Orientation | 76.73 ± 21.27 | 70.46 ± 24.19 | 0.0008 | 76.14 ± 22.91 | 70.26 ± 21.68 | 0.01 |
| VI. Stability in Gait | 70.49 ± 21.40 | 67.13 ± 24.21 | 0.03 | 65.49 ± 24.92 | 61.18 ± 25.03 | 0.04 |
Six-month change in BESTest was significantly correlated with MDS-UPDRS III, H&Y, freezer status, and fall history at baseline (Table 3). Multiple regression analysis revealed that these four variables explained 31.93% of the variance in BESTest change.
Table 3.
Spearman Correlations Between Change in Balance Assessments and Baseline Characteristics at Six and 12 Months
| Six Months | 12 Months | |||
|---|---|---|---|---|
| BESTest | Mini-BESTest | BESTest | Mini-BESTest | |
| MDS-UPDRS III | −0.39** | −0.24 | −0.42** | −0.31* |
| H&Y | −0.28* | −0.17 | −0.18 | −0.08 |
| Freezer | −0.52** | −0.27 | −0.39** | −0.15 |
| PASE | 0.23 | 0.10 | 0.11 | 0.06 |
| Fall History | −0.30* | −0.04 | −0.14 | 0.08 |
| Age | −0.17 | −0.08 | −0.18 | −0.30* |
| Gender | 0.06 | 0.01 | −0.05 | 0.06 |
indicates p < .05;
indicates p < .01
Change Over 12 Months
Demographic information and baseline scores for the group that completed the 12-month assessment (n=44) are in Table 1. Balance declined significantly by 12 months compared to baseline, as indicated by changes in BESTest (mean raw change: −3.77 ± 8.12; p=0.004; 95% CI [−6.17, −1.37]) and Mini-BESTest (mean raw change: −1.50 ± 3.08; p=0.002; 95% CI [−2.41, −0.59]), but not BBS (mean raw change: −0.32 ± 4.77; p=0.62; 95% CI [−1.73, 1.09]) scores (Figure 1B). Motor impairment improved significantly (MDS-UPDRS III mean raw change: −3.65 ± 9.33; p=0.01; 95% CI [−6.41, −0.89]) during the same period. Over 12 months, scores in BESTest sections II, IV, and V and Mini-BESTest sections IV and V declined significantly (Table 4).
Table 4.
Section scores (%) at baseline and 12 months for the BESTest and Mini-BESTest
| BESTest | Mini-BESTest | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 Months | p value | Baseline | 12 Months | p value | |
| I. Biomechanical Constraints | 64.24 ± 20.08 | 72.72 ± 20.06 | 0.99 | − | − | − |
| II. Stability Limits / Verticality | 82.79 ± 11.26 | 77.71 ± 11.97 | 0.004 | − | − | − |
| III. Anticipatory Postural Adjustments | 74.37 ± 18.34 | 72.22 ± 21.83 | 0.10 | 65.53 ± 21.39 | 66.67 ± 23.29 | 0.68 |
| IV. Postural Responses | 75.25 ± 19.26 | 66.79 ± 26.24 | 0.001 | 65.53 ± 25.01 | 50.38 ± 30.18 | 0.00003 |
| V. Sensory Orientation | 81.52 ± 19.23 | 71.36 ± 22.80 | 0.00006 | 81.06 ± 20.83 | 73.86 ± 21.69 | 0.009 |
| VI. Stability in Gait | 74.46 ± 17.34 | 70.45 ± 23.32 | 0.04 | 70.00 ± 22.62 | 67.73 ± 25.78 | 0.22 |
Twelve-month change in BESTest was significantly correlated with baseline MDS-UPDRS III and freezer status (Table 3). Multiple regression analysis revealed that these two variables explained 20.48% of the variance in BESTest change over 12 months. Twelve-month change in Mini-BESTest was significantly correlated with the baseline MDS-UPDRS III score and age (Table 3). These two variables explained 8.36% of the variance in Mini-BESTest change over 12 months.
Discussion
In this study, we sought to compare the utility of three standardized balance assessments in detecting balance decline over a 1-year period, to determine specific components of balance susceptible to decline, and to determine baseline factors predictive of prospective balance decline. To our knowledge, this was the first study to compare multiple standardized clinical outcome measures in their ability to detect changes in balance in a cohort of people with PD.
Regarding our first objective, our findings indicated that the BESTest and Mini-BESTest, but not the BBS, were sufficiently responsive to reveal statistically significant group-level balance declines during both time periods. The results were consistent with previous reports that the BESTest and Mini-BESTest were more accurate than the BBS in both retrospective and prospective fall prediction for people with PD [7, 8]. For at least the Mini-BESTest, however, the group mean balance decline at 6 months (1.8 points) and at 12 months (1.5 points) did not exceed a previously published MDC value of 3.5 points generated from a sample of individuals with balance disorders [22]. Using this criterion, which was not specific to individuals with PD, it appeared that the magnitude of the group-level decline in Mini-BESTest score may have been within the margin of error for the measure. Nonetheless, a subset of the sample displayed balance declines on the Mini-BESTest in excess of the MDC at 6 months (n = 11) and at 12 months (n = 9), suggesting that at least for some individuals with PD, clinically relevant balance deterioration is likely to be ongoing and would benefit from frequent monitoring and rehabilitation.
Balance decline at 12 months occurred despite a significant reduction in motor impairment as reflected by MDS-UPDRS III score. The result was consistent with a previous balance study [29], and was in keeping with previous reports suggesting that postural instability appears to be resistant to levodopa therapy [30–32]. Alternatively, the result may also have been related to the relatively small number of items on the MDS-UPDRS III that address postural instability and gait difficulty. Furthermore, post-hoc analyses indicate LEDD [27] did not change over 6 months (−0.10% change; p=0.99), but significantly increased over 12 months (14.83% change; p=0.047), and this may underlie the small, significant improvement in MDS-UPDRS III over 12 months.
Regarding our second objective, several sub-components of balance appeared to be more susceptible to decline over 6 and 12 months than others. Section-specific analyses on the BESTest and Mini-BESTest revealed statistically significant declines in stability limits/verticality, postural responses, and sensory orientation. In clinical terms, these findings revealed that participants tended to have difficulties with maintaining balance while reaching or leaning, effectively taking a quick step in response to a sudden loss of balance, and remaining stable when standing in low light or soft surface conditions. These particular sub-components of balance may be important contributors to fall risk, and as such, provide important targets for rehabilitation.
Regarding our third objective, only baseline MDS-UPDRS III and freezer status were significantly associated with future decline in BESTest and Mini-BESTest scores at both 6 and 12 months. Researchers previously established that those with greater PD motor symptom severity or freezing of gait have more impaired balance performance [33, 34]. Our results build on this association and suggest that those with greater motor symptom severity or history of freezing may experience a more rapid decline in balance performance. Freezing of gait is also a consistent predictor of future falls [6, 35], while motor symptom severity is a weak predictor of future falls [36]. Interestingly, the most consistent predictor of future falls reported in the literature, a past history of falls, was only moderately correlated to change in BESTest score over 6 months.
It is important to note that the correlations at each time point between baseline factors and balance decline were weak to moderate. Furthermore, regression analysis revealed that MDS-UPDRS III, presence of freezing, and fall history combined to explain no more than 31.93% of the variance in both BESTest and Mini-BESTest decline over 6 and 12 months. The large amount of unexplained variance indicated that these factors may not necessarily predict balance decline. This is surprising with respect to presence of freezing [6] and fall history [36] as these two variables are commonly implicated as predictors of future falls. This apparent incongruence reinforces the idea that falls and postural instability, although strongly related, are not interchangeable constructs; other factors (i.e., cognitive, non-motor) may be more predictive of future balance decline.
Clinical Implications
Given the small but statistically significant decrements in balance noted over just 6 months, our results suggested that postural stability should be assessed using the BESTest or Mini-BESTest on a biannual basis in people with PD. This suggestion, which we have made previously [8] contrasts with recent recommendations to use the BBS or Tinetti Mobility Index to measure postural instability [37]. While we cannot speak to the utility of the Tinetti Mobility Index, results from our study and previous studies [7, 8, 18] suggested that the BESTest or Mini-BESTest may be preferred over the BBS in assessment of postural instability. Balance assessments at regular 6 month intervals, or when elevated fall risk is first identified [23], would allow for rapid detection of changes in balance and subsequent implementation of rehabilitation interventions designed to improve balance and prevent falls [38–42].
Our study results should be interpreted with caution given several limitations. First, the relatively large dropout at both 6 and 12 months yielded relatively small sample sizes at both time points. Comparison of demographic characteristics between participants who dropped out and those who completed the study indicates participants who withdrew had greater disease severity and fell more than those who completed the study. Accordingly, the decline in balance reported in this study may be underestimated. Secondly, all assessments were conducted with participants in the on phase of their anti-PD medication, which could potentially mask movement impairments specific to PD [43]. Future work should focus on exploring additional factors (i.e., cognitive, non-motor, and physical) that may predict prospective balance decline in people with PD and also determine if those with the postural instability/gait difficulty (PIGD) and tremor dominant subtypes of PD differ in their trajectory of balance performance as measured by the BESTest and Mini-BESTest.
Conclusion
Balance performance measured by the BESTest and Mini-BESTest declined over 6 and 12 months in people with PD. The decline was not detected by the BBS. The ability to maintain balance while reaching or leaning, while responding to a perturbation, and while standing without vision or on a soft surface were particularly susceptible to decline. Disease severity and freezing were the most consistent predictors of balance decline. Routine semiannual balance assessments using the BESTest or Mini-BESTest are recommended to closely monitor balance decline and to determine the need for rehabilitation.
Acknowledgments
This work was funded by the Davis Phinney Foundation, Parkinson’s Disease Foundation, NIH UL1 TR000448 and NIH R01 NS077959. General support was provided by the Greater St. Louis Chapter of the American Parkinson Disease Association (APDA) and APDA Center for Advanced PD Research at Washington University.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Bloem BR, Steijns JA, Smits-Engelsman BC. An update on falls. Curr Opin Neurol. 2003;16:15–26. doi: 10.1097/01.wco.0000053580.70044.70. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Bower JH, Maraganore DM, Oberg AL, Rocca WA. Fracture risk after the diagnosis of Parkinson's disease: Influence of concomitant dementia. Mov Disord. 2006;21:1361–1367. doi: 10.1002/mds.20946. [DOI] [PubMed] [Google Scholar]
- 3.Matinolli M, Korpelainen JT, Sotaniemi KA, Myllyla VV, Korpelainen R. Recurrent falls and mortality in Parkinson's disease: a prospective two-year follow-up study. Acta Neurol Scand. 2011;123:193–200. doi: 10.1111/j.1600-0404.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- 4.Grimbergen YA, Munneke M, Bloem BR. Falls in Parkinson's disease. Curr Opin Neurol. 2004;17:405–415. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- 5.Hiorth YH, Lode K, Larsen JP. Frequencies of falls and associated features at different stages of Parkinson's disease. Eur J Neurol. 2012 doi: 10.1111/j.1468-1331.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 6.Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord. 2009;24:1280–1289. doi: 10.1002/mds.22561. [DOI] [PubMed] [Google Scholar]
- 7.Leddy AL, Crowner BE, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther. 2011;35:90–97. doi: 10.1097/NPT.0b013e31821a620c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan RP, Leddy AL, Cavanaugh JT, Dibble LE, Ellis TD, Ford MP, Foreman KB, Earhart GM. Accuracy of fall prediction in Parkinson disease: six-month and 12-month prospective analyses. Parkinsons Dis. 2012;2013:237673. doi: 10.1155/2012/237673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- 10.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26:132–143. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 11.Smania N, Corato E, Tinazzi M, Stanzani C, Fiaschi A, Girardi P, Gandolfi M. Effect of balance training on postural instability in patients with idiopathic Parkinson's disease. Neurorehabil Neural Repair. 2010;24:826–834. doi: 10.1177/1545968310376057. [DOI] [PubMed] [Google Scholar]
- 12.Dibble LE, Cavanaugh JT, Earhart GM, Ellis TD, Ford MP, Foreman KB. Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC Neurol. 2010;10:110. doi: 10.1186/1471-2377-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol. 1992;32(Suppl):S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89:484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91:102–113. doi: 10.2522/ptj.20100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther. 2013;93:158–167. doi: 10.2522/ptj.20120171. [DOI] [PubMed] [Google Scholar]
- 20.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 21.Lim LI, van Wegen EE, de Goede CJ, Jones D, Rochester L, Hetherington V, Nieuwboer A, Willems AM, Kwakkel G. Measuring gait and gait-related activities in Parkinson's patients own home environment: a reliability, responsiveness and feasibility study. Parkinsonism Relat Disord. 2005;11:19–24. doi: 10.1016/j.parkreldis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88:733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 23.Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS. Three simple clinical tests to accurately predict falls in people with Parkinson's disease. Mov Disord. 2013;28:655–662. doi: 10.1002/mds.25404. [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 25.Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, Oertel W, Poewe WH, Stocchi F, Tolosa E. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. 2009;24:655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 26.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 28.Duncan RP, Leddy AL, Cavanaugh JT, Dibble LE, Ellis TD, Ford MP, Foreman KB, Earhart GM. Comparative utility of the BESTest, mini-BESTest, and brief-BESTest for predicting falls in individuals with Parkinson disease: a cohort study. Phys Ther. 2013;93:542–550. doi: 10.2522/ptj.20120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson's disease: a pilot longitudinal study. Gait Posture. 2012;36:471–476. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson's disease and their response to treatment. Br J Clin Pharmacol. 2012;74:267–283. doi: 10.1111/j.1365-2125.2012.04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord. 1996;11:509–521. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 32.Mancini M, Rocchi L, Horak FB, Chiari L. Effects of Parkinson's disease and levodopa on functional limits of stability. Clin Biomech (Bristol, Avon) 2008;23:450–458. doi: 10.1016/j.clinbiomech.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King LA, Priest KC, Salarian A, Pierce D, Horak FB. Comparing the Mini-BESTest with the Berg Balance Scale to Evaluate Balance Disorders in Parkinson's Disease. Parkinsons Dis. 2012;2012:375419. doi: 10.1155/2012/375419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, Tanner C. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–1721. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- 35.Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs. 2000;32:222–228. doi: 10.1097/01376517-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, Gray P, Kerr G, Bloem BR. A meta-analysis of six prospective studies of falling in Parkinson's disease. Mov Disord. 2007;22:1892–1900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 37.van der Marck MA, Klok MP, Okun MS, Giladi N, Munneke M, Bloem BR. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson's disease. Parkinsonism Relat Disord. 2014;20:360–369. doi: 10.1016/j.parkreldis.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Cheng EM, Tonn S, Swain-Eng R, Factor SA, Weiner WJ, Bever CT., Jr Quality improvement in neurology: AAN Parkinson disease quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology. 2010;75:2021–2027. doi: 10.1212/WNL.0b013e3181ff96dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.(NICE) NIfCE. Clinical practice guideline for the assessment and prevention of falls in older people. Royal College of Nursing: London: National Institute for Clinical Excellence. 2004 [PubMed] [Google Scholar]
- 40.Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JC, O'Rourke SD, Murray SM, Fung VS. The effects of an exercise program on fall risk factors in people with Parkinson's disease: a randomized controlled trial. Mov Disord. 2010;25:1217–1225. doi: 10.1002/mds.23082. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry. 2011;82:1232–1238. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G, Batya SS. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012;366:511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord. 2011;17:166–171. doi: 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]