Abstract
Nab-paclitaxel is a novel therapeutic agent, which was approved in combination with carboplatin in the first-line treatment of advanced non-small cell lung cancer (NSCLC) regardless of histologic subtype in the United States of America by the Food and Drug Administration in 2012 and by the European Commission in 2015. This approval was based on the results of a phase III clinical trial showing superior response rates compared with solvent-based paclitaxel in combination with carboplatin. This review will focus on the early development and clinical data to date supporting the use of nab-paclitaxel in advanced NSCLC. The clinical question central to this review is whether nab-paclitaxel has a place in the current therapeutic landscape of advanced NSCLC.
Keywords: Nab-paclitaxel, Non-small cell lung cancer (NSCLC), Carboplatin, Neuropathy, Squamous cell histology, Elderly
Lung cancer is the leading cause of cancer mortality in the United States of America and worldwide [1]. An estimated 221,200 new lung cancer cases will be diagnosed in 2015 in the United States of America alone, and 158,040 lung cancer deaths are estimated to occur [1]. Historically, palliative chemotherapy in the metastatic non-small cell lung cancer (NSCLC) setting resulted in modest survival prolongation and preservation of quality of life (QoL) [2]. Currently, platinum agents combined most commonly with tax-anes, gemcitabine, vinorelbine, or pemetrexed are the standard of care in advanced NSCLC [3]. In a large randomized clinical trial comparing four platinum-based regimens, all were associated with a similar efficacy, with overall response rates (ORRs) of about 19% and a median overall survival (OS) of 7.9 months [4]. In patients with newly diagnosed advanced NSCLC, cisplatin/pemetrexed and cisplatin/gemcitabine were associated with similar efficacy, though a histology specific survival benefit was noted in patients with non-squamous histology with pemetrexed-based therapy, while a survival detriment was noted in patients with squamous histology [5]. In general, solvent-based paclitaxel plus carboplatin (sb-PC) is the most commonly used taxane–platinum combination in the United States of America and is associated with a 15–32% ORR and a median OS of 7.9 to 10.06 months [4,6–9].
In a recent prospective observational study that captured real-world data on patients with advanced NSCLC receiving first-line platinum-based therapies across Europe, the median OS was 10.3 months in all patients. Patients in Europe were most commonly treated with platinum/pemetrexed (37.3%), followed by platinum/gemcitabine (23.6%), platinum/vinorelbine (19.7%), and platinum/taxane (19.4%); only 7% of patients received concomitant bevacizumab [10]. While the addition of bevacizumab to sb-PC for patients with non-squamous NSCLC is associated with improved efficacy, the incorporation of bevacizumab into first-line therapy in Europe has been limited [11]. In addition, the use of bevacizumab in patients with squamous NSCLC has been associated with excess toxicity in the form of pulmonary hemorrhage [7,12] and is not routinely used in the squamous subset.
The 130-nm albumin-bound nanoparticle formulation of paclitaxel (nab-paclitaxel [Abraxane]; Celgene, Summit, NJ) is a novel therapeutic agent, which was approved in the United States of America by the Food and Drug Administration (FDA) in 2012 in combination with carboplatin in the first-line treatment of advanced NSCLC regardless of histologic subtype. This approval was based on the results of a phase III trial showing superior response rates compared with sb-PC [13]. Recently, this regimen was also approved by the European Commission in 2015. This review will focus on the early development and clinical data to date supporting the use of nab-paclitaxel in advanced NSCLC.
1. Early development
Paclitaxel, a naturally occurring complex diterpenoid extracted from the bark of the western yew, Taxus brevifolia, stabilizes tubulin polymer and promotes microtubule assembly effectively inhibiting mitoses, motility, and intracellular transport [14–16]. Due to limited aqueous solubility, cremophor-based paclitaxel (solvent-based paclitaxel) is formulated with a cremophor EL/ethanol vehicle [17]. Solvent-based paclitaxel is associated with severe allergic, hypersensitivity, and anaphylactic reactions in humans and animals, and premedication with steroids and H1 and H2 receptor blockers are necessary to reduce the severity of these reactions [16,18–24]. The cumulative side-effects of steroids used as a premedication may contribute to treatment-related morbidity, while the cremophor EL solvent may contribute to chronic paclitaxel-induced peripheral neuropathy [25]. Cremophor and ethanol solvent leaches plasticizers from polyvinyl chloride (PVC) bags and infusion sets in routine clinical use, and solvent-based paclitaxel must be prepared and administered in either glass bottles or non-PVC infusion systems with in-line filtration [26]. In order to deliver paclitaxel in a more safe and convenient manner, the development of taxanes with improved solubility in aqueous solutions has been of great interest [27].
The lyophilized formulation of nab-paclitaxel is comprised of albumin and paclitaxel reconstituted in 0.9% NaCl. It is devoid of any solvents or ethanol with an average particle size of 130 nm allowing for intravenous administration without risk of capillary blockage [28]. Nab-paclitaxel caused tumour regression and was associated with prolonged survival in nude mice bearing human tumour xenografts with the highest level of sensitivity in lung xenografts, followed by breast, ovarian, prostate and colon models [17]. In this pre-clinical model, nab-paclitaxel was significantly less toxic than solvent-based paclitaxel; the LD50 and maximum tolerated dose (MTD) for nab-paclitaxel and solvent-based paclitaxel were 47 and 30 mg/kg/d and 30 and 13.4 mg/kg/d, respectively [17]. At equitoxic doses, nab-paclitaxel was associated with more complete regressions, longer time to recurrence, longer doubling time, and prolonged survival compared with solvent-based paclitaxel [17]. Nab-paclitaxel was also associated with higher exposure of the tumour to paclitaxel; at equal doses, tumour paclitaxel area under the curve (AUC) was 33% higher for nab-paclitaxel compared with solvent-based paclitaxel [17].
Nab-paclitaxel is comprised of a colloidal suspension of albumin and paclitaxel which likely enhances drug delivery of the cytotoxic agent to the cancer cells via a receptor-mediated transport mechanism. Trans-endothelial cell transport of albumin is mediated by binding of albumin to the gp60 receptor, which activates caveolin-1, resulting in formation of caveoli, which transport albumin and other plasma constituents across the endothelial cell to the interstitial space [29,30]. In support of this, the endothelial binding and transcytosis of paclitaxel are markedly higher for nab-paclitaxel compared with solvent-based paclitaxel and are abrogated by a known inhibitor of endothelial gp60 receptor/caveolar transport [17]. In addition, cremophor has been found to inhibit binding of paclitaxel to endothelial cells and albumin [17]. Therefore, the enhanced efficacy and intratumour delivery of nab-paclitaxel compared with solvent-based paclitaxel are likely from enhanced endothelial cell binding and transcytosis and inhibition of binding of paclitaxel to endothelial cells and albumin by cremophor. Another possible mechanism may be mediated by osteonectin (also known as secreted protein acid rich in cysteine [SPARC]), which shares sequence homology with gp60 [31,32]. Osteonectin has been shown to bind albumin and is expressed in a number of tumours [33–42]. Also, albumin accumulates in some tumours, possibly by binding to SPARC, thereby facilitating intratumour accumulation of albumin-bound drugs [43].
A phase I clinical trial in patients with advanced solid tumours evaluated doses of nab-paclitaxel ranging from 135 to 375 mg/m2 without premedication and with a cycle length of 21 d [44]. In 19 treated patients, no acute hypersensitivity reactions were observed during the infusion period. The dose-limiting toxicities (DLTs) of nab-paclitaxel at 375 mg/m2 consisted of sensory neuropathy, stomatitis, and superficial keratopathy. The MTD was thus determined to be 300 mg/m2. Hematological toxicity was mild and not cumulative. Another phase I clinical trial in patients with advanced solid tumours evaluated doses of nab-paclitaxel 80–200 mg/m2 once a week for 3 weeks, followed by 1 week of rest (4-week treatment cycle) without premedication [45]. In 39 treated patients, the MTDs for heavily and lightly pre-treated patients were 100 and 150 mg/m2, respectively. The DLTs were grade IV neutropenia and grade III peripheral neuropathy, respectively. In both phase I clinical trials, pharmacokinetic analyses demonstrated linear kinetics of paclitaxel over the dose ranges examined.
2. Nab-paclitaxel as first-line therapy for advanced NSCLC
Nab-paclitaxel was first approved in the United States of America for metastatic breast cancer based on a randomized phase III clinical trial comparing nab-paclitaxel 260 mg/m2 administered every 3 weeks with solvent-based paclitaxel 175 mg/m2 administered every 3 weeks [46]. Nab-paclitaxel was associated with a superior response rate and improved time to tumour progression compared with solvent-based paclitaxel, prompting interest in evaluating the safety and efficacy of single agent nab-paclitaxel in patients with advanced NSCLC. In a single-arm phase II clinical study, the safety and efficacy of single agent nab-paclitaxel at a dose of 260 mg/m2 was evaluated in patients with advanced NSCLC as first-line therapy [47]. In 43 patients enrolled, the ORR was 16% and disease control rate 49%, with a median time to progression of 6 months and a median survival of 11 months. Nab-paclitaxel was well tolerated with no severe hypersensitivity reactions noted despite the lack of premedication. Another phase I/II clinical trial aimed to determine the MTD and single-agent activity of nab-paclitaxel administered on a weekly basis to chemotherapy-naive patients with advanced NSCLC [48]. Dose levels of 100–150 mg/m2 were evaluated; at 150 mg/m2, the MTD was exceeded with DLTs of grade III sensory neuropathy and febrile neutropenia. On the phase II portion of the study, 40 patients were treated at 125 mg/m2, in whom the ORR was 30%, time to progression 5 months and OS 11 months. Both clinical trials warranted additional studies of nab-paclitaxel in the treatment of NSCLC.
In a dose-finding phase II clinical trial, previously untreated patients with advanced NSCLC were sequentially enrolled in seven cohorts evaluating a range of doses and schedules of nab-paclitaxel (every 3 week dosing ranging from 225 to 340 mg/m2 and weekly dosing ranging from 100 mg/m2 days 1, 8 and 15, 125 mg/m2 days 1, 8, and 15 and 140 mg/m2 days 1 and 8), in combination with carboplatin AUC 6 on day 1 of each 3 week cycle [49]. The most common treatment-related grade III adverse events were neutropenia (60%), neuropathy (19%), fatigue (9%), and thrombocytopenia (29%). No grade IV neuropathy or fatigue was noted. A 100 mg/m2 weekly nab-paclitaxel schedule produced less serious adverse events than other doses and schedules. Response rate was greater in the weekly versus q3w nab-paclitaxel cohorts (47% versus 30%). Median progression-free survival (PFS) ranged from 4.8 to 6.9 months, and OS ranged from 8.3 to 15.0 months (all cohorts). Patients receiving 100 mg/m2 weekly nab-paclitaxel achieved a 48% RR with 6.2 and 11.3 months PFS and OS, respectively. The favorable efficacy and safety profile led to a phase III, randomized, multicenter clinical trial comparing nab-paclitaxel 100 mg/m2 weekly plus carboplatin AUC 6 every 3 weeks (nab-paclitaxel/carboplatin [nab-PC]) with solvent-based paclitaxel 200 mg/m2 every 3 week plus carboplatin AUC 6 every 3 weeks (sb-PC).
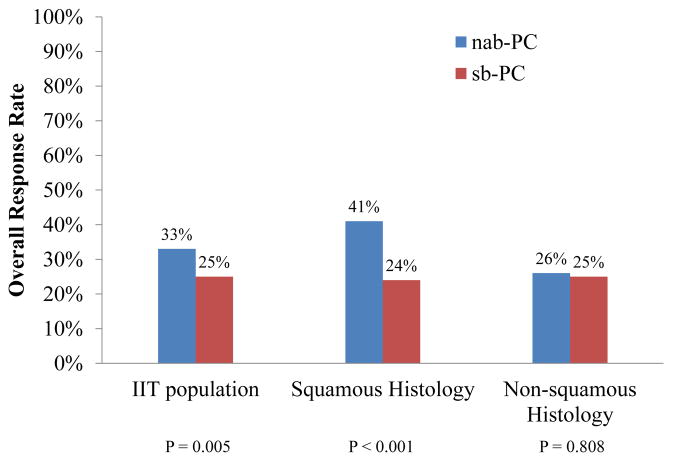
In this phase III clinical trial, untreated patients with stage III–IV NSCLC were accrued from institutions in Australia, Canada, Japan, Russia, Ukraine, and the United States of America and were randomized to nab-PC versus sb-PC with the primary end-point of ORR [13]. Randomization was stratified by disease stage (IIIB versus IV), patient age, gender, histology (squamous versus adenocarcinoma versus others), and geographic region. On the basis of independent assessment, nab-PC demonstrated a significantly higher ORR than sb-PC (33% versus 25%, P = 0.005, Fig. 1). Nab-PC was associated with a superior ORR in patients with squamous histology (41% versus 24%, P = 0.001) and was as effective in patients with non-squamous histology (ORR 26% versus 25%, P = 0.808) compared with sb-PC. PFS (6.3 versus 5.8 months, P = 0.214) and OS (12.1 versus 11.2 months, P = 0.271) were similar in the nab-PC versus the sb-PC arms, respectively. There was significantly less grade ≥III neutropenia, arthralgia, and myalgia in the nab-PC arm, but less thrombocytopenia and anemia in the sb-PC arm. The results of this phase III clinical trial indicated nab-PC was efficacious and associated with a superior ORR relative to sb-PC and led to the approval of nab-PC for the first-line treatment of advanced NSCLC in the United States of America by the FDA and more recent approval for this indication in the European Union.
Fig. 1.
Overall response rate of nab-paclitaxel/carboplatin (nab-PC) and solvent-based paclitaxel/carboplatin (sb-PC) on the basis of independent assessment in a randomized phase III clinical trial comparing nab-PC versus sb-PC in newly diagnosed advanced non-small cell lung cancer patients, in the intention-to-treat (ITT) population and by histology.
Regional differences in survival were noted in this phase III clinical trial [13]. Patients enrolled in Japan and Russia/Ukraine had no difference in OS between the treatment arms, while patients enrolled in North America has better OS with nab-PC compared with sb-PC (12.7 versus 9.8 months, respectively, P = 0.008). Regional differences in baseline characteristics, such as differing rates of oncogenic drivers, like epidermal growth factor receptor (EGFR) mutations amongst Asian and Caucasian populations, might have played a role in the differences in survival outcomes. Pharmacogenomic differences in the distribution of genes involved in paclitaxel metabolism and DNA repair, which are known to exist between Japanese and US populations, might also have influenced these regional differences in efficacy outcomes [50]. In an analysis of the 149 Japanese patients treated on this phase III clinical trial, the ORR was higher with nab-PC versus sb-PC (35% versus 27%), with non-statistically significant improvements in PFS and OS [51]. The toxicity profile of nab-PC in the Japanese mirrored that of the whole population with higher rates of grade ≥III treatment-related anemia and thrombocytopenia but less sensory neuropathy that in the nab-PC arm. Interestingly, patient survival seemed to favor the Japanese population over the whole population in both arms (16.7 versus 12.1 months for nab-PC arm, respectively, 15.9 versus 11.2 months for sb-PC arm, respectively). Adenocarcinoma histology was more prevalent in the Japanese population relative to the whole population (76% versus 49%, respectively); the percentage of patients with a performance status of 0 was higher (48% versus 23%, respectively), and a large proportion of Japanese patients received multiple post-study treatments (85% versus 54%, respectively) including EGFR tyrosine kinase inhibitor therapy. Pharmacogenomic data were not collected as part of this phase III clinical trial.
The Eastern Cooperative Group Clinical Trial E4599 demonstrated that the addition of bevacizumab to sb-PC is associated with superior efficacy compared with sb-PC alone [7]. Based on these results, a single-arm phase II clinical trial evaluated the addition to bevacizumab to carboplatin AUC 6 and nab-paclitaxel 300 mg/m2 every 21 d [52]. This regimen was associated with response rate of 31% with a stable disease rate of 54%; the PFS was 9.8 months and OS 16.8 months. The most frequent grades ≥III treatment-related toxicities were neutropenia and fatigue, indicating the combination of bevacizumab with nab-PC is clinically feasible and potentially warrants further study.
It is noteworthy in considering the efficacy and toxicity data of nab-PC versus sb-PC that direct comparisons of solvent-based paclitaxel given weekly versus every 3 weeks have demonstrated similar efficacy between treatment schedules with slightly different toxicity profiles. In a randomized phase II clinical trial in patients with advanced NSCLC treated with either solvent-based paclitaxel 225 mg/m2 in combination with carboplatin AUC 6 every 3 weeks or solvent-based paclitaxel 75 mg/m2 weekly in combination with carboplatin AUC 6 every 3 weeks, the ORR and survival outcomes were similar on both arms [53]. Weekly paclitaxel was associated with significantly more grade ≥III thrombocytopenia and grade ≥II anemia and less severe arthralgias and alopecia. While no difference was noted in the rates of peripheral neuropathy, patients receiving solvent-based paclitaxel every 3 weeks reported significantly more taxane therapy-related side-effects on the functional assessment of cancer therapy (FACT)-Taxane subscale. In a phase III clinical trial in patients with advanced NSCLC treated with either solvent-based paclitaxel 100 mg/m2 weekly (3 of 4 weeks) with carboplatin AUC 6 every 4 weeks versus solvent-based paclitaxel 225 mg/m2 with carboplatin AUC 6 every 3 weeks, there was no difference in efficacy between the different treatment schedules [54]. In the whole population and in the subset of elderly patients (age ≥70 years), grade ≥III anemia was more common with weekly administration and grades II and III neuropathy were less common [55].
2.1. Peripheral neuropathy
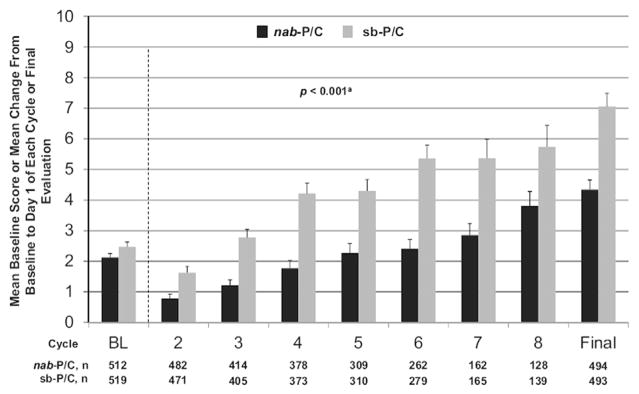
In the phase III clinical trial which evaluated nab-PC versus sb-PC in patients with previously untreated NSCLC, sensory neuropathy (all grades) occurred less frequently with nab-PC (46%) compared with sb-PC (62%, P = 0.001) [13]. In addition, there was significantly less severe (grade ≥III) sensory neuropathy with nab-PC compared with sb-PC. In patients experiencing grade ≥III neuropathy, median time to improvement to grade I was shorter in the nab-PC arm compared with the sb-PC arm (38 versus 104 d, respectively). More than 90% patients completed a baseline FACT-Taxane scale at baseline and at follow-up. There was a significant treatment effect favoring nab-PC over sb-PC over the entire course of treatment for the total score of all 16 items of the FACT-Taxane questionnaire (Fig. 2, p < 0.001) [56]. The mean change from baseline to final evaluation in the patient-reported FACT-Taxane scale, including a neuropathy subscale (P = 0.001), pain subscale (P = 0.001), and hearing loss subscale (P = 0.002), was significantly improved in the nab-PC arm versus sb-PC arm. In addition, deterioration in the patient-reported peripheral neuropathy subscore at or after the development of grade ≥III peripheral neuropathy was substantially less for nab-PC versus sb-PC, and median time to improvement to grade ≤I peripheral neuropathy tended to be shorter for nab-PC versus sb-PC. These results would indicate a favorable toxicity profile of this dose and schedule of nab-PC over sb-PC in patients with pre-existing or a predisposition to peripheral neuropathy. However, as previously noted, in general, the rates of neuropathy with paclitaxel given weekly versus every 3 weeks are lower [57].
Fig. 2.
Composite change for the Functional Assessment of Cancer Therapy-Taxane subscale at baseline and prior to dosing on day 1 of each cycle on a randomized phase III clinical trial comparing nab-paclitaxel/carboplatin (nab-PC) versus solvent-based paclitaxel/carboplatin (sb-PC) in patients with newly diagnosed advanced non-small cell lung cancer. Larger bars represent greater deteriorations from baseline as perceived by patients. Reprinted with permission from J Thorac Oncol. 2014;9: 83–90.
2.2. Squamous cell histology
A histology-specific benefit of nab-PC in patients with advanced NSCLC has been noted. In particular, there was a significant advantage in patients with squamous cell histology, in whom treatment options are limited. In patients with squamous cell histology, there was a 68% improvement in ORR with nab-PC compared with sb-PC (41% versus 24%, respectively, response rate ratio [RRR] 1.680, P < 0.001), in contrast to patients with adenocarcinoma (26% versus 27%, respectively, RRR 0.966, P = 0.814) [58]. In addition, there was also a numeric benefit for response for large cell carcinoma (33% versus 15%, respectively, RRR 2.167, P = 0.323) and histology not otherwise specified (24% versus 15%, RRR 1.593, P = 0.372); however, because of the relatively small numbers of patients, the differences were not statistically significant (although they may be clinically significant). A statistically significant interaction was noted between treatment effect and histology for ORR (P = 0.036). There was no improvement in PFS or OS for nab-PC versus sb-PC in patients with squamous cell histology or in patients with non-squamous histology, and no interaction was observed for histology and treatment effect for either PFS (P = 0.310) or OS (P = 0.928). The improvement in ORR with nab-PC is important because, in this setting, patients often have disease-related symptoms, such as cough, dyspnea, and chest pain, and tumour shrinkage may have a significant palliative effect which is particularly important in patients with a heavy burden of disease-related symptoms [59–61].
How nab-PC compares to other platinum doublet combinations in patients with squamous cell histology is as of yet unknown. In randomized phase II clinical trial conducted in China comparing nab-PC with carboplatin and gemcitabine, nab-PC was associated with a numerically higher ORR compared with carboplatin/gemcitabine (46.3% versus 30.4%, respectively, P = 0.085) and a non-statistically significant improvement in PFS (5.7 versus 4.8 months, respectively, P = 0.657); OS was not mature [62]. Necitumumab, a humanized EGFR monoclonal antibody, in combination with cisplatin/gemcitabine was recently associated with an OS advantage over cisplatin/gemcitabine alone in previously untreated patients with advanced squamous cell NSCLC [63]. A phase II clinical trial is currently underway to assess the safety and efficacy of necitumumab in combination with nab-PC in untreated advanced squamous cell NSCLC (NCT02392507).
2.3. The elderly
In the elderly population, platinum-based chemotherapy is associated with superior survival compared with single-agent chemotherapy in the first-line treatment of advanced NSCLC [64]. Despite these data, platinum-based therapy in the elderly has not been universally adopted due to the perceived burden of excess toxicity associated with this therapy [65–67]. Elderly patients with NSCLC may present with poor performance status and/or comorbidities. Due to the anticipated toxic effects, elderly patients are often under-represented in clinical trials. While the median age of all patients diagnosed with lung cancer is 70 years, the median age in large clinical trials is 59–63 years [68]. More effective and better-tolerated therapeutic options for the elderly are needed.
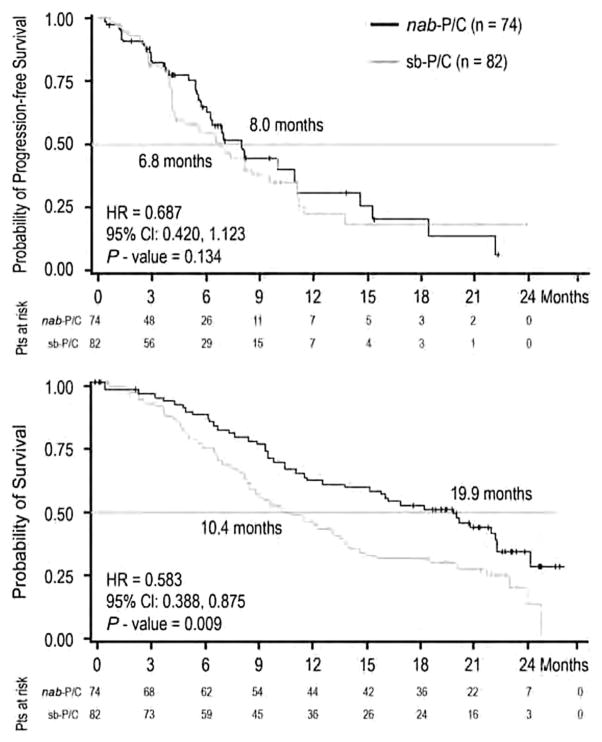
Fifteen percent of the 1052 patients enrolled on the phase III clinical trial comparing nab-PC with sb-PC were elderly (≥70 years of age) [58]. The ORR was higher with nab-PC compared with sb-PC regardless of age (age ≥70: 34% versus 24%, P =0.196, age <70: 32% versus 25%, P = 0.013). In elderly patients, nab-PC, while not associated with improvement in PFS (8.0 versus 6.8 months, P = 0.134, Fig. 3), was associated with a statistically significant improvement in OS (19.9 versus 10.4 months, P = 0.009, Fig. 3). In younger patients, PFS (6.0 versus 5.8 months, P =0.256) and OS (11.4 versus 11.3 months, P = 0.988) were similar in both arms. There was a significant interaction between age and treatment effect on OS (P = 0.018).
Fig. 3.
Kaplan–Meier curves of progression-free survival (top) and overall survival (OS, bottom) in the elderly (≥70 years of age) patients treated on a randomized phase III clinical trial comparing nab-paclitaxel/carboplatin (nab-PC) versus solvent-based paclitaxel/carboplatin (sb-PC) in patients with newly diagnosed advanced non-small cell lung cancer. Reprinted with permission from Annals of Oncology 24: 314–321, 2013.
In the elderly, nab-PC was associated with less neutropenia (P = 0.015) and arthralgia (P = 0.029) and increased anemia (P = 0.007) compared with sb-PC [58]. The rate of grade ≥III neutropenia was significantly less with nab-PC compared with sb-PC arms (55% versus 73%, respectively, P = 0.018). While the rates of grade ≥III neutropenia remained high from cycle 2 through cycle 6 in elderly patients who received sb-PC, the rates progressively declined in elderly patients receiving nab-PC. The overall rate of neuropathy was significantly less with nab-PC versus sb-PC (53% versus 67%, P =0.001), with a significantly lower rate of grade ≥III neuropathy (P = 0.007). Neuropathy tended to occur later in the course of treatment with nab-PC versus sb-PC with a median time to onset of 48.0 versus 24.5 d, respectively. Utilizing the patient-reported FACT-Taxane scale, significant treatment effects favoring nab-PC were noted for patient-reported neuropathy (P < 0.001), pain in hands and feet (P < 0.001), hearing loss (P = 0.022), and edema subscales (P =0.004). The survival benefit and tolerability of nab-PC compared with sb-PC would support nab-PC as the preferred platinum doublet in elderly advanced NSCLC patients.
In order to understand if this OS benefit with nab-PC in elderly patients extends to an improvement in QoL, a subsequent analysis examined quality-adjusted time without symptoms or toxicity (Q-TWiST) data collected from the phase III clinical trial of nab-PC versus sb-PC [69]. Using age-based subgroup data, nab-PC and sb-PC were compared with respect to ORR, OS, PFS, QoL, safety/toxicity, and Q-TWiST with age ≥60 and ≥70 years as cut points. Nab-PC compared with sb-PC was associated with a significantly improved ORR and prolonged OS, despite a non-significant improvement in PFS, among patients aged ≥60 years. Nab-PC was associated with improved QoL and less neuropathy, arthralgia, and myalgia but resulted in more anemia and thrombocytopenia. In terms of quality-adjusted time without symptoms or toxicity, nab-PC was associated with significant Q-TWiST benefits (11.1 versus 9.8 months, 95% confidence interval of gain: 0.2–2.6), with a relative Q-TWiST gain of 10.8% (ranging from 6.4% to 15.1% in threshold analysis). In patients ≥70 years age, nab-PC was associated with similar, though non-significant improvements in ORR, PFS, and Q-TWiST benefits and a significantly improved OS and QoL. These results would indicate the survival gains in the elderly NSCLC population with nab-PC are not at the expense of QoL and increased toxicity.
2.4. Diabetic advanced NSCLC patients
Though controversial, there has been suggestion in the literature that cancer patients with diabetes have worse outcomes compared with cancer patients without diabetes [70–72]. In a retrospective study of 442 patients with advanced NSCLC treated with platinum doublet therapy in the first-line setting, baseline diabetes was associated with inferior OS and PFS [72]. This may be due in part to difficulty in treatment delivery in this patient population due to the treatment-related toxicities which may be more pronounced in the diabetic lung cancer population. For instance, the DLT of taxanes is peripheral neuropathy which would be problematic in patients with pre-existing diabetic neuropathy. In addition, the requisite steroids with taxane therapy often result in worsening glycemic control in the diabetic patient or unmasking of a prediabetes or diabetes state.
Sixty-one of the 1052 randomized patients in the phase III clinical trial comparing nab-PC with sb-PC in the first-line treatment of advanced NSCLC had pre-existing diabetes [73]. While most patients with or without diabetes had grade 0 peripheral neuropathy at baseline, a higher percentage of patients with diabetes in the nab-PC versus the sb-PC arm had grade I peripheral neuropathy at baseline. In the diabetic subset, the ORR was 52% versus 27% (P = 0.046), PFS 10.9 versus 4.9 months (P = 0.016), and OS 17.5 versus 11.1 months (P = 0.057) for nab-PC versus sb-PC, respectively. The PFS advantage with nab-PC remained significant (P ≤ 0.036) after adjusting for histology, region, stage, race, and age, and the OS advantage with nab-PC remained significant for histology (P = 0.039). In patients with diabetes, grade ≥III peripheral neuropathy was higher with sb-PC compared with nab-PC (20% versus 7%, respectively). In patients without diabetes, sb-PC was also associated with a higher incidence of grade ≥III peripheral neuropathy compared with nab-PC. In both the diabetic and non-diabetic population, the time to onset of grade ≥III peripheral neuropathy was longer for patients treated with nab-PC versus sb-PC. Importantly, nab-PC was able to be delivered to patients with diabetes in an effective manner; the median number of nab-PC cycles was 6 in the diabetic population, which was the same for the intention-to-treat population and patients without diabetes [24]. While more diabetic patients receiving nab-PC had one or more dose reduction compared with sb-PC, dose intensity and cumulative dose were higher with nab-PC treatment. Though the number of diabetic patients treated on this phase III clinical trial is small, the results of this subset analysis would indicate superiority of nab-PC relative to sb-PC in the diabetic population.
2.5. Renal impairment
Renal impairment is a consideration in selecting first-line therapy for the advanced NSCLC patient, as the median age at diagnosis with lung cancer is 70 years, and patients with lung cancer have been noted to have an age-dependent decline in renal function and greater individual variability in creatinine clearance (CrCl) [74]. In the elderly, there has also been note of discrepancies in measured CrCl and estimated CrCl [74]. In addition, commonly used cytotoxic therapies in NSCLC, such as cisplatin and pemetrexed, are contraindicated in patients with significant renal impairment [75,76]. For both nab-paclitaxel and solvent-based paclitaxel, the main route of elimination is fecal excretion and renal excretion is, for the most part, complete by 48 h after dosing [77]. Renal impairment level is not a predictor of drug elimination of nab-paclitaxel or the development of neu-tropenia [78]. The pharmacokinetic parameters would indicate that dose reduction of nab-paclitaxel is not necessary in patients with a CrCl ≥30 ml/min [78].
In the phase III clinical trial comparing nab-PC with sb-PC in the first-line treatment of advanced NSCLC, 38% of patients had mild renal impairment (CrCl >50 to ≤80 mL/min) and 5% of patients had moderate renal impairment (CrCl ≤50 mL/min) [79]. The ORRs of nab-PC and sb-PC in patients with mild and moderate renal impairment were comparable to the ORRs seen in the overall population, but did not reach statistical significance (mild renal impairment: 35% versus 27%, respectively, P = 0.060; moderate renal impairment; 31% versus 19%, respectively, P = 0.300). There was a non-significant trend for longer OS and PFS for nab-PC versus sb-PC in these subsets. Similar to the overall population, patients with renal impairment experienced less grade ≥III neutropenia and sensory neuropathy, but more thrombocytopenia and anemia with nab-PC versus sb-PC. It would appear from this subset analysis that nab-PC is tolerable in patients with advanced NSCLC with mild and moderate renal impairment.
3. Future directions
While it is clear that nab-paclitaxel in combination with carboplatin is associated with better response rates compared with sb-PC in advanced NSCLC, understanding the biological rationale for this observation will be important to inform upon potential predictive bio-markers of response that may enhance patient selection. In a single-arm phase II clinical trial of carboplatin and nab-paclitaxel in a primarily squamous NSCLC population, correlative studies included immunohistochemistry for osteonectin and caveolin-1. In this patient population, the response rate was 38% with a PFS of 5 months and OS of 9.7 months. While no association was found for osteonectin expression in tumour or stroma with response rate or OS, higher caveolin-1 expression levels in tumour-associated stroma was associated with improved response rates (50% versus 20%, P = 0.036) and OS (P = 0.008). This observation that caveolin-1 may be predictive of nab-paclitaxel benefit is supported by preclinical data indicating a role for caveolin-1 in albumin-mediated endocytosis, through a gp60-dependent mechanism [30,80]. Further studies aimed at the development of caveolin-1 and additional bio-markers of nab-paclitaxel benefit are needed to improve patient selection.
In advanced NSCLC, there are ongoing clinical trials delineating the therapeutic benefit of nab-PC in patients with impaired performance status (NCT02289456), as well as addressing the role of maintenance nab-paclitaxel after initial nab-PC therapy in patients with squamous cell histology (NCT02027428). What is of particular interest is how nab-paclitaxel will be integrated with immune checkpoint inhibition, which is a highly active area of clinical development in both squamous and non-squamous NSCLC. Nab-paclitaxel is particularly attractive in combination with immune checkpoint therapy as there is no steroid premedication requirement. Ongoing clinical trials are also addressing the role of concurrent chemoradiotherapy with nab-paclitaxel/carboplatin in the treatment of inoperable stage IIIA/B NSCLC (NCT01757288).
4. Conclusions
Nab-paclitaxel is a novel therapeutic agent recently approved for the treatment of newly diagnosed advanced NSCLC patients in combination with carboplatin in the US and Europe. The clinical question central to this review is whether nab-paclitaxel has a place in the current therapeutic landscape of advanced NSCLC. It is clear from the literature to date that nab-PC compared with sb-PC has a firm place in the treatment of advanced NSCLC, particularly in selected patient populations (Table 1). It is, however, important to note that there is a paucity of data directly comparing nab-PC with alternative platinum doublets, such as platinum/pemetrexed, which are more commonly used in Europe. Also of critical importance is the cost-effectiveness of nab-PC compared with sb-PC. In the phase III clinical trial comparing nab-PC with sb-PC in NSCLC, a cost-of-care analysis from the US payer perspective demonstrated an incremental cost per life year gained in excess of $100,000 [81]. However, in patients who were 70 years of age or older and in those from North America, the cost per life year gained was reduced to $23,000 and $83,000, respectively, which is comparable to published incremental costs per life year gained of other recently approved NSCLC agents [81]. The efficacy data of nab-PC compared with sb-PC are clear; it is associated with a superior response rate in the advanced NSCLC population, which may be particularly important in a patient with a high burden of disease-related symptoms. Nab-PC appears to be more tolerable from the standpoint of peripheral neuropathy and is particularly effective in patients with squamous cell histology. It is notable that nab-PC is associated with a marked survival advantage compared with sb-PC in elderly NSCLC patients, a survival advantage that does not appear to be at the expense of excess toxicity, impaired QoL or excess financial burden. In patients with diabetes, nab-PC was associated with superior efficacy compared with sb-PC and might be particularly suited to this patient population due to the risk of peripheral neuropathy and the importance of glycemic control in the diabetic population.
Table 1.
Clinical characteristics of a patient with newly diagnosed advanced NSCLC best suited for nab-PC.
| 1. | Squamous cell histology |
| 2. | High burden of disease-related symptoms, which might be relieved by tumour response |
| 3. | Pre-existing neuropathy or at risk for the development of neuropathy |
| 4. | Elderly |
| 5. | Pre-existing diabetes or prediabetic state |
| 6. | Comorbid mild or moderate renal dysfunction |
nab-PC, nab-paclitaxel/carboplatin; NSCLC, non-small cell lung cancer.
Footnotes
Conflict of interest statement
Liza Villaruz has no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Burdett S, et al. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–25. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socinski MA, et al. Treatment of stage IV non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl 5):e341S–68S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller JH, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti GV, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 6.Kelly K, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19(13):3210–8. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti G, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(11):1835–42. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 9.Lilenbaum RC, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730) J Clin Oncol. 2005;23(1):190–6. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 10.Moro-Sibilot D, et al. Outcomes and resource use of non-small cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer. 2015;88(2):215–22. doi: 10.1016/j.lungcan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Carrato A, et al. Clinical management patterns and treatment outcomes in patients with non-small cell lung cancer (NSCLC) across Europe: EPICLIN-Lung study. Curr Med Res Opin. 2014;30(3):447–61. doi: 10.1185/03007995.2013.860372. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DH, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Socinski MA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 14.Wani MC, et al. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93(9):2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 15.Adams JD, et al. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr. 1993;(15):141–7. [PubMed] [Google Scholar]
- 16.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82(15):1247–59. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 17.Desai N, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 18.Arbuck SG, et al. Current dosage and schedule issues in the development of paclitaxel (Taxol) Semin Oncol. 1993;20(4 Suppl 3):31–9. [PubMed] [Google Scholar]
- 19.Dye D, Watkins J. Suspected anaphylactic reaction to Cremophor EL. Br Med J. 1980;280(6228):1353. doi: 10.1136/bmj.280.6228.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelderblom H, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–8. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz W, et al. Histamine release in dogs by Cremophor E1 and its derivatives: oxethylated oleic acid is the most effective constituent. Agents Actions. 1977;7(1):63–7. doi: 10.1007/BF01964882. [DOI] [PubMed] [Google Scholar]
- 22.Physicians’ Desk Reference. 53. New Jersey: Medical Economics Co., Inc; 1999. [Google Scholar]
- 23.Szebeni J, et al. Formation of complement-activating particles in aqueous solutions of Taxol: possible role in hypersensitivity reactions. Int Immunopharmacol. 2001;1(4):721–35. doi: 10.1016/s1567-5769(01)00006-6. [DOI] [PubMed] [Google Scholar]
- 24.Weiss RB, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–8. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 25.Windebank AJ, Blexrud MD, de Groen PC. Potential neurotoxicity of the solvent vehicle for cyclosporine. J Pharmacol Exp Ther. 1994;268(2):1051–6. [PubMed] [Google Scholar]
- 26.Waugh WN, Trissel LA, Stella VJ. Stability, compatibility, and plasticizer extraction of taxol (NSC-125973) injection diluted in infusion solutions and stored in various containers. Am J Hosp Pharm. 1991;48(7):1520–4. [PubMed] [Google Scholar]
- 27.Hidalgo M, et al. Phase I and pharmacokinetic study of BMS-184476, a taxane with greater potency and solubility than paclitaxel. J Clin Oncol. 2001;19(9):2493–503. doi: 10.1200/JCO.2001.19.9.2493. [DOI] [PubMed] [Google Scholar]
- 28.Abraxane Package Insert. Available from: http://www.abraxane.com/downloads/Abraxane_PrescribingInformation.pdf.
- 29.John TA, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L187–96. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 30.Schubert W, et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276(52):48619–22. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 31.Tiruppathi C, Finnegan A, Malik AB. Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells. Proc Natl Acad Sci U S A. 1996;93(1):250–4. doi: 10.1073/pnas.93.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnitzer JE, Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol. 1992;263(6 Pt 2):H1872–9. doi: 10.1152/ajpheart.1992.263.6.H1872. [DOI] [PubMed] [Google Scholar]
- 33.Kim YW, et al. Expression of osteopontin and osteonectin in breast cancer. J Korean Med Sci. 1998;13(6):652–7. doi: 10.3346/jkms.1998.13.6.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter PL, et al. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43(8):791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 35.Thomas R, et al. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6(3):1140–9. [PubMed] [Google Scholar]
- 36.Brown TJ, et al. Activation of SPARC expression in reactive stroma associated with human epithelial ovarian cancer. Gynecol Oncol. 1999;75(1):25–33. doi: 10.1006/gyno.1999.5552. [DOI] [PubMed] [Google Scholar]
- 37.Paley PJ, et al. Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol Oncol. 2000;78(3 Pt 1):336–41. doi: 10.1006/gyno.2000.5894. [DOI] [PubMed] [Google Scholar]
- 38.Ledda F, et al. The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol. 1997;108(2):210–4. doi: 10.1111/1523-1747.ep12334263. [DOI] [PubMed] [Google Scholar]
- 39.Massi D, et al. Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol. 1999;30(3):339–44. doi: 10.1016/s0046-8177(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka M, et al. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166(6):2495–9. [PubMed] [Google Scholar]
- 41.Yamashita K, et al. Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer. 2003;97(10):2412–9. doi: 10.1002/cncr.11368. [DOI] [PubMed] [Google Scholar]
- 42.Rempel SA, Ge S, Gutierrez JA. SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res. 1999;5(2):237–41. [PubMed] [Google Scholar]
- 43.Schilling U, et al. Design of compounds having enhanced tumour uptake, using serum albumin as a carrier–Part II. In vivo studies. Int J Rad Appl Instrum B. 1992;19(6):685–95. doi: 10.1016/0883-2897(92)90103-6. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim NK, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–44. [PubMed] [Google Scholar]
- 45.Nyman DW, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(31):7785–93. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 46.Gradishar WJ, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 47.Green MR, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17(8):1263–8. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 48.Rizvi NA, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol. 2008;26(4):639–43. doi: 10.1200/JCO.2007.10.8605. [DOI] [PubMed] [Google Scholar]
- 49.Socinski MA, et al. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(6):852–61. doi: 10.1097/jto.0b013e3181d5e39e. [DOI] [PubMed] [Google Scholar]
- 50.Gandara DR, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27(21):3540–6. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishino M, et al. Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer. 2013;79(3):283–8. doi: 10.1016/j.lungcan.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds C, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2009;4(12):1537–43. doi: 10.1097/JTO.0b013e3181c0a2f4. [DOI] [PubMed] [Google Scholar]
- 53.Socinski MA, et al. A randomized phase II trial comparing every 3-weeks carboplatin/paclitaxel with every 3-weeks carboplatin and weekly paclitaxel in advanced non-small cell lung cancer. Ann Oncol. 2006;17(1):104–9. doi: 10.1093/annonc/mdj016. [DOI] [PubMed] [Google Scholar]
- 54.Belani CP, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(3):468–73. doi: 10.1200/JCO.2007.13.1912. [DOI] [PubMed] [Google Scholar]
- 55.Ramalingam S, et al. Comparison of outcomes for elderly patients treated with weekly paclitaxel in combination with carboplatin versus the standard 3-weekly paclitaxel and carboplatin for advanced nonsmall cell lung cancer. Cancer. 2008;113(3):542–6. doi: 10.1002/cncr.23583. [DOI] [PubMed] [Google Scholar]
- 56.Hirsh V, et al. Patient-reported neuropathy and taxane-associated symptoms in a phase 3 trial of nab-paclitaxel plus carboplatin versus solvent-based paclitaxel plus carboplatin for advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(1):83–90. doi: 10.1097/JTO.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 57.Huang TC, Campbell TC. Comparison of weekly versus every 3 weeks paclitaxel in the treatment of advanced solid tumors: a meta-analysis. Cancer Treat Rev. 2012;38(6):613–7. doi: 10.1016/j.ctrv.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Socinski MA, et al. Safety and efficacy of weekly nab(R)-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(2):314–21. doi: 10.1093/annonc/mds461. [DOI] [PubMed] [Google Scholar]
- 59.de Marinis F, et al. Lung Cancer Symptom Scale outcomes in relation to standard efficacy measures: an analysis of the phase III study of pemetrexed versus docetaxel in advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(1):30–6. doi: 10.1097/JTO.0b013e31815e8b48. [DOI] [PubMed] [Google Scholar]
- 60.Bezjak A, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR. 21. J Clin Oncol. 2006;24(24):3831–7. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 61.Kris MG, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, et al. A phase II trial of first-line nab-paclitax-el/carboplatin versus gemcitabine/carboplatin in advanced squamous cell carcinoma of the lung (CTONG1002) J Clin Oncol. 2014;32:5s. suppl; abstr 8085. [Google Scholar]
- 63.Thatcher N, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763–74. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 64.Quoix E, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378(9796):1079–88. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 65.Davidoff AJ, et al. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2191–7. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 66.Lang K, et al. Trends and predictors of first-line chemotherapy use among elderly patients with advanced non-small cell lung cancer in the United States. Lung Cancer. 2009;63(2):264–70. doi: 10.1016/j.lungcan.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Hardy D, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199–211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 68.Langer CJ. Clinical evidence on the undertreatment of older and poor performance patients who have advanced non-small-cell lung cancer: is there a role for targeted therapy in these cohorts? Clin Lung Cancer. 2011;12(5):272–9. doi: 10.1016/j.cllc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Langer CJ, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer. 2015;113(1):20–9. doi: 10.1038/bjc.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasic L. Locally advanced non-small cell lung cancer - pretreatment of prognostic factors: disease stage, tumor histopathological characteristics, the patient related factors. Arch Oncol. 2007;15(1–2):19–23. [Google Scholar]
- 71.Hejleh T, et al. Survival of non-small cell lung cancer (NSCLC) patients with and without diabetes mellitus (DM): findings from the cancer care outcomes research and surveillance consortium (CanCORS) J Clin Oncol. 2013;31 doi: 10.1200/JCO.2012.43.7954. suppl abstr 6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inal A, et al. Is diabetes mellitus a negative prognostic factor for the treatment of advanced non-small-cell lung cancer? Rev Port Pneumol. 2014;20(2):62–8. doi: 10.1016/j.rppneu.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Hirsh V, et al. Weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small cell lung cancer: analysis of safety and efficacy in patients with diabetes. J Thoracic Oncology. doi: 10.1016/j.cllc.2016.04.002. Submitted. [DOI] [PubMed] [Google Scholar]
- 74.Ohara G, et al. Age-dependent decline in renal function in patients with lung cancer. Oncol Lett. 2012;4(1):38–42. doi: 10.3892/ol.2012.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pemetrexed Package Insert. Available from: http://uspl.lilly.com/alimta/alimta.html#ppi.
- 76.Cisplatin Package Insert.
- 77.Sparreboom A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11(11):4136–43. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 78.Chen N, et al. Pharmacokinetics and pharmacodynamics of nab-paclitaxel in patients with solid tumors: disposition kinetics and pharmacology distinct from solvent-based paclitaxel. J Clin Pharmacol. 2014;54(10):1097–107. doi: 10.1002/jcph.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langer CJ, et al. Weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: analysis of safety and efficacy in patients with renal impairment. Clin Lung Cancer. 2015;16(2):112–20. doi: 10.1016/j.cllc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Tiruppathi C, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272(41):25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 81.Spigel D, et al. Nab-paclitaxel in combination with carboplatin as first-line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC): an economic analysis. J Thorac Oncol. 2013;8 Supplement. [Google Scholar]