In plants, fungi, and invertebrates, antiviral RNAi is activated by processing viral double-stranded RNA (dsRNA) replication intermediates into virus-derived small interfering RNAs (vsiRNAs). Typically of a discrete length of ~22 nt, vsiRNAs form perfect duplexes, with 2 nt 3′ overhangs diagnostic of the Dicer-like RNase III activities that generate them across kingdoms. vsiRNAs are loaded into and guide Argonaute (AGO) nucleases in order to subsequently suppress virus accumulation (Ding and Voinnet, 2007). Attesting to the importance of antiviral RNAi, the virulence of plant and invertebrate viruses requires production of virus-encoded suppressors of RNAi (VSRs) that become dispensable for infection in RNAi-defective hosts (Ding and Voinnet, 2007). Comparatively, antiviral RNAi in mammals remains a hotly debated issue: virus-derived small RNAs (vsRNAs, unlike the vsiRNAs evoked above) isolated in numerous infections of mammalian differentiated cell lines do not conform to the definition of siRNAs; their random size-distribution and strong strand bias usually reflect the unspecific degradation of a single, abundant viral RNA strand (Parameswaran et al., 2010). Perhaps unsurprisingly, therefore, the replication of many viruses is unchanged in differentiated cells in which Dicer function is genetically disabled (Bogerd et al. 2014). This overall uncertainty has led to a hypothesis that the interferon response, which also detects viral dsRNA, may prevent dsRNA recognition by RNAi in mammals.
The above premises have led our groups to explore antiviral RNAi in pluripotent mouse embryonic stem cells (ESCs) that lack a potent interferon response (Maillard et al., 2013, Wang et al., 2014). Bona fide siRNAs were significantly enriched in the 5′ terminal genomic RNA regions of two distinct viruses, but, importantly, their accumulation was strongly attenuated upon ESC differentiation, leading Maillard et al. (2013) to suggest that multipotency might constitute another, and perhaps major, prerequisite to mammalian antiviral RNAi. Focusing on one of these viruses, Li et al. (2013) showed that highly abundant vsiRNAs, identical to those detected in ESCs, accumulated in systemically infected suckling mice, but only if a VSR inhibiting mammalian Dicer was disabled from the viral genome. vsiRNA accumulation in vivo correlated with the clearance of the VSR-deficient virus, suggesting that antiviral RNAi operates in vivo (Li et al., 2013). Importantly, replication of the VSR-deficient virus was partially restored in mouse ESCs lacking an RNAi response (Maillard et al., 2013). Rescue of VSR-deficient viruses in RNAi-deficient hosts has been the defining experiment in all model organisms to conclude the antiviral nature of RNAi (Ding and Voinnet 2007), and so it was concluded that RNAi is indeed antiviral, at least in mouse ESCs. Mammalian antiviral RNAi studies are also complicated by the fact that several viral genomes interact directly with host-encoded, Dicer-dependent microRNAs (miRNAs). Specific host miRNAs may either enhance or inhibit virus infection, as shown for hepatitis C virus in hepatocytes and vesicular stomatitis virus (VSV) in murine macrophages, respectively (Jopling et al., 2005; Otsuka et al., 2007).
In a recent manuscript focusing on VSV, Backes et al. (2014) concluded that the in vitro and in vivo mammalian responses to infection are independent of silencing by either vsiRNAs or host-encoded miRNAs and that, consequently, RNAi therapies, including their virus-based formulations, should now be considered safe to mammals and humans. However, our analysis shows that these conclusions are not supported by the experiments presented for the following reasons.
Virus Choice
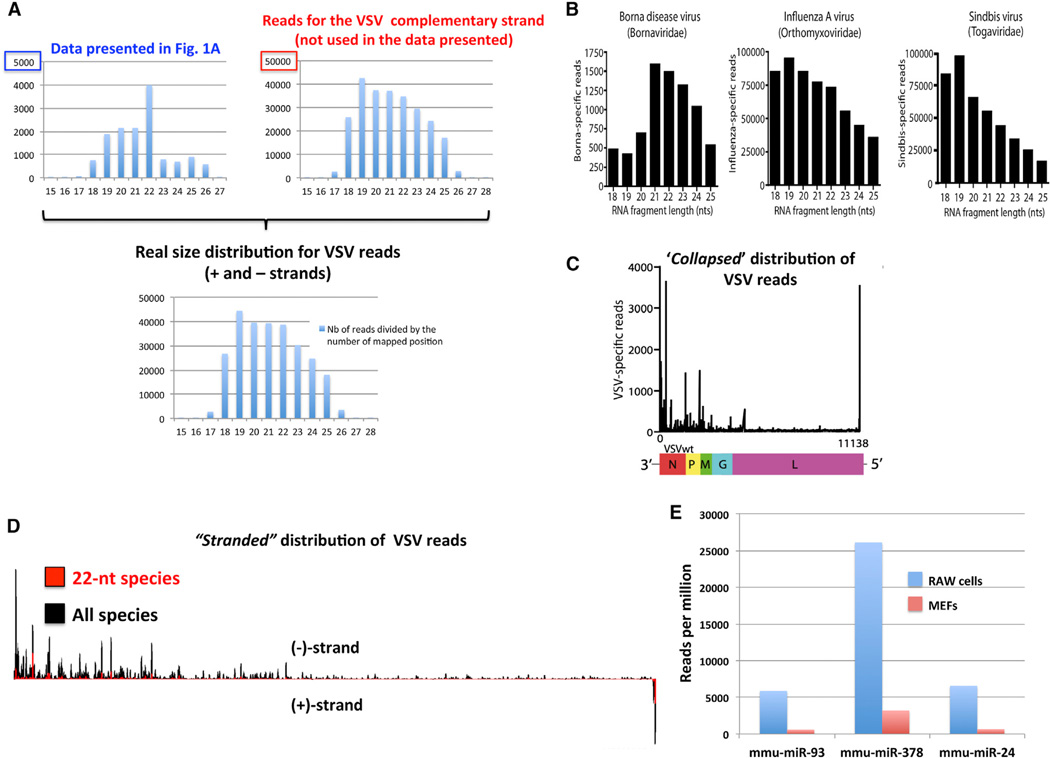
As shown previously (Parameswaran et al., 2010), the authors found that vsRNAs from three distinct RNA viruses do not display any size preference indicative of RNAi activity in differentiated mouse cells. By contrast, they claim that vsRNAs from VSV (a negative-strand RNA virus) in infected mouse embryonic fibroblasts (MEFs) are dominantly 22 nt in length and enriched at the ends of the genome, features they deem “reminiscent of the RNAi-like activity recently described in mammalian cells” (Maillard et al., 2013; Li et al., 2013). However, our analysis of the sequencing reads shows that the authors presented the size distribution only for the low-abundance VSV positive strand, whereas the genomic distribution for both positive- and negative-strand vsRNAs was collapsed onto a virtual VSV negative genome. When reads from both viral strands are analyzed and their polarity is considered—the norm in all studies conducted thus far—the sequenced vsRNAs display a random size-distribution and strong negative-strand bias (Figures 1A–1D), indicating that they are RNA degradation products not different from those of the other three viruses tested by the authors or indeed from other vsRNAs previously characterized in mammalian differentiated cells (Parameswaran et al., 2010). Therefore, the assertion that VSV-derived vsRNAs are reminiscent of mammalian vsiRNAs is misleading, and, more importantly, the authors’ choice of VSV to further investigate antiviral RNAi unjustified (additional concerns are discussed in brief in the Supplemental Information).
Figure 1. VSV-Derived Small RNAs Are Mere Degradation Fragments.
(A) Our analysis of VSV-derived reads from both viral strands reveals the absence of any particular length bias. Note also that reads from the nonanalyzed strand are more abundant by at least one order of magnitude.
(B) The size distribution of VSV-derived reads shown in (A) is now not distinguishable from that of the other viruses tested by the authors (panel extracted from Backes et al., 2014).
(C) The VSV reads presented in Backes et al. (2014) are somehow collapsed onto a virtual negative viral genomic RNA and thus intrinsically uninformative about the respective contribution of each viral strand to vsRNA production.
(D) A stranded genomic distribution of VSV reads upon our reanalysis of the author’s data reveals a strong read bias for the negative strand, which is much more abundant in infected cells owing to VSV replication. Note that the genomic distribution of VSV reads remains unchanged if all read sizes (18–25 nt) are compared to the 22 nt-only reads. This, along with the strong strand bias, shows that VSV-derived vsRNAs are simply degradation products.
(E) Comparison of the respective accumulation of miR-93, miR-24, and miR-378 in noninfected MEFs and RAW cells. Both data sets were generated via Illumina deep sequencing of comparable depth and normalized as reads per million. The RAW cell data are from Zheng et al. (2012). The MEFs data are from the Voinnet laboratory.
Test of Silencing Suppression by VSV
Backes et al. (2014) then argue they test whether VSV encodes an inhibitor of silencing to ensure that small RNAs can target VSV in the context of their mammalian systems. Their observations that recombinant VSV can (1) be targeted by an endogenous miRNA and (2) produce an artificial miRNA were used to conclude that “VSV does not encode an inhibitor of small RNA silencing.” However, the authors ignore the results of identical experiments conducted previously with plant viruses encoding VSRs targeting both the miRNA and siRNA pathways: these viruses can produce functional miRNAs engineered in their genome and are also efficiently targeted by endogenous miRNAs despite expression of potent VSRs (Simón-Mateo and García, 2006; Tang et al., 2013). Virus-induced gene silencing of host mRNAs is also routinely achieved with VSR-encoding viruses and hairpin-derived silencing immunizes transgenic crops against aggressive diseases from VSR-proficient viruses (Simón-Mateo and García, 2006). Therefore, the rationale behind those experiments is flawed, and the results presented cannot be used to conclude that VSV does not encode a VSR or that an engineered expression of a heterologous VSR must enhance VSV replication.
In Vitro Effects of VP55 on VSV Levels
The authors previously identified a vaccinia virus protein, VP55, which specifically degrades miRNAs and siRNAs but only if they are loaded into an AGO complex and not protected by 2′-O-methylation. Here, Backes et al. (2014) engineer VSV variants in order to express VP55 (VSV-VP55) or a control insert (VSV-Ctrl) and compare their accumulation in order to assess the potential contribution of antiviral RNAi and host miRNAs to restricting VSV in MEFs. They observe no change in accumulation of VSV-VP55 versus VSV-Ctrl, a result they argue is surprising in light of “previous studies [that] have implicated host miRNAs in the direct silencing of VSV and RNAi activity in fibroblasts (Li et al., 2013; Otsuka et al., 2007).” This leads them to make the statement “these results suggest that small RNAs do not significantly impact the mammalian cellular response to virus infection in vitro.” However, none of the data presented support these assertions.
First, Otsuka et al. (2007) conclusively showed that miR-93, miR-24, and, to a lesser extent, miR-378 have direct antiviral effects on VSV in RAW cells, in which these miRNAs were shown to effectively accumulate, unlike 21 other miRNAs with predicted complementarity to the VSV genome. Thus, the only reported effects of miRNAs on VSV in vitro implicate three discrete miRNAs found in one specific cell type. Therefore, to reach any conclusion, the authors must prove that at least one of the above-mentioned miRNAs indeed accumulates in infected MEFs to levels comparable to those of infected RAW cells; this is clearly not the case in noninfected cells (Figure 1E). Alternatively, they must first conclusively establish which miRNAs, if any, might productively restrict VSV in MEFs, as did Otsuka et al. (2007) with miR-93, miR-24, and miR-378 in RAW cells. Besides, given that RAW cells are used throughout their paper, we question why the authors did not provide the results of VSV-VP55 infections in these cells, a legitimately expected and decisive experiment. In the absence of these results, their data are inconclusive.
Second, the only vsRNAs detectable in VSV-infected MEFs are heterogeneous RNA degradation products (Figures 1A and 1B), of which neither the 3′-methylation- nor the AGO-loaded status is known. Given its substrate specificity, VP55 is highly unlikely to alter the accumulation of these degradation products that have not been assigned any role in antiviral defense thus far. Therefore, this experiment is bound to be inconclusive regarding the potential contribution of RNAi to the cellular restriction of VSV in MEFs.
In Vivo Effects of VP55 on VSV Levels
A final set of experiments showing no difference in accumulation between VSVVP55 and VSV-Ctrl in whole-mouse lungs leads the authors to suggest “a functional antiviral RNAi system is not a physiological contributor to mammalian antiviral defenses.” However, this statement is again unfounded, given that VSV-derived vsRNAs accumulating in vivo are “comparable to those observed in fibroblasts” and thus most likely RNA degradation products unaffected by VP55. Moreover, because the identity of the VSV-infected lung cells and their miRNA repertoire are undetermined, the experiment is also inconclusive regarding potential host miRNA-mediated effects on VSV in vivo.
The authors themselves admit that “it is difficult to prove the absence of a biological activity,” and indeed the inference made in their manuscript’s title is not supported by a definitive experiment. One emerging aspect apparently not considered by Backes et al. (2014) is the likely contextual nature of mammalian RNAi in vivo: only specific cell niches and tissues—possibly with features of adult stem cells—are likely to accumulate significant levels of bona fide vsiRNAs (Pare and Sullivan, 2014; Wang et al., 2014). Identifying these cell niches and the biochemical features of their associated vsiRNAs in vivo is therefore an absolute prerequisite if the types of experiments conducted by Backes et al. (2014) are to be meaningful. Likewise, the significance of host miRNA-virus interactions will only be apparent in cells with well-established virus-complementary miRNA repertoires. If mammalian antiviral RNAi is the attribute of only a fraction of infected cells, does this make those cells less important? On these premises, the final conclusion of the authors on the innocuous nature of mammalian RNAi-based therapies appears highly premature and dangerous.
Supplementary Material
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information contains Supplemental Discussion and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.10.029.
REFERENCES
- Backes S, Langlois RA, Schmid S, Varble A, Shim JV, Sachs D, tenOever BR. Cell Rep. 2014;8:114–125. doi: 10.1016/j.celrep.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Skalsky RL, Kennedy EM, Furuse Y, Whisnant AW, Flores O, Schultz KL, Putnam N, Barrows NJ, Sherry B, et al. J. Virol. 2014;88:8065–8076. doi: 10.1128/JVI.00985-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SW, Voinnet O. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu J, Han Y, Fan X, Ding SW. Science. 2013;342:231–234. doi: 10.1126/science.1241911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard PV, Ciaudo C, Marchais A, Li Y, Jay F, Ding SW, Voinnet O. Science. 2013;342:235–238. doi: 10.1126/science.1241930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Kang YJ, Jiang Z, Du X, Cook R, et al. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, Heissmeyer V, Einav S, Jackson W, et al. PLoS Pathog. 2010;6:e1000764. doi: 10.1371/journal.ppat.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare JM, Sullivan CS. PLoS Pathog. 2014;10:e1003865. doi: 10.1371/journal.ppat.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Mateo C, García JA. J. Virol. 2006;80:2429–2436. doi: 10.1128/JVI.80.5.2429-2436.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Lai Y, Liu Y. Methods Mol. Biol. 2013;975:99–107. doi: 10.1007/978-1-62703-278-0_8. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang J, Acharya D, Paul AM, Bai F, Huang F, Guo YL. J. Biol. Chem. 2014;289:25186–25198. doi: 10.1074/jbc.M113.537746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Chen DS, Wu YQ, Xu XJ, Zhang H, Chen CF, Chen HC, Liu ZF. Int. J. Biol. Sci. 2012;8:1013–1022. doi: 10.7150/ijbs.3836. http://dx.doi.org/10.7150/ijbs.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.