Abstract
The multifaceted oncogene c-Myc plays important roles in the development and progression of human cancer. Recent in vitro and in vivo studies have shown that the p19Arf–Mdm2–p53 and the ribosomal protein (RP)–Mdm2–p53 pathways are both essential in preventing oncogenic c-Myc-induced tumorigenesis. Disruption of each pathway individually by p19Arf deletion or by Mdm2C305F mutation, which disrupts RP-Mdm2 binding, accelerates Eμ-myc transgene-induced pre-B/B-cell lymphoma in mice at seemingly similar paces with median survival around 10 and 11 weeks, respectively, compared to 20 weeks for Eμ-myc transgenic mice. Because p19Arf can inhibit ribosomal biogenesis through its interaction with nucleophosmin (NPM/B23), RNA helicase DDX5 and RNA polymerase I transcription termination factor (TTF-I), it has been speculated that the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways might be a single p19Arf–RP–Mdm2–p53 pathway, in which p19Arf activates p53 by inhibiting RP biosynthesis; thus, p19Arf deletion or Mdm2C305F mutation would result in similar consequences. Here, we generated mice with concurrent p19Arf deletion and Mdm2C305F mutation and investigated the compound mice for tumorigenesis in the absence and the presence of oncogenic c-Myc overexpression. In the absence of Eμ-myc transgene, the Mdm2C305F mutation did not elicit spontaneous tumors in mice, nor did it accelerate spontaneous tumors in mice with p19Arf deletion. In the presence of Eμ-myc transgene, however, Mdm2C305F mutation significantly accelerated p19Arf deletion-induced lymphomagenesis and promoted rapid metastasis. We found that when p19Arf–Mdm2–p53 and RP–Mdm2–p53 pathways are independently disrupted, oncogenic c-Myc-induced p53 stabilization and activation is only partially attenuated. When both pathways are concurrently disrupted, however, c-Myc-induced p53 stabilization and activation are essentially obliterated. Thus, the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 are non-redundant pathways possessing similar capabilities to activate p53 upon c-Myc overexpression.
INTRODUCTION
The Myc protein (c-Myc) is a helix-loop-helix leucine zipper transcriptional regulator, which forms specific DNA-binding heterodimers with the Max protein to control a variety of normal cellular functions.1 In addition, c-Myc facilitates the recruitment of Pol I (DNA polymerase I) to ribosomal DNA promoters,2 promotes the transcription of ribosomal proteins (RPs) by activating Pol II3 and activates Pol III-mediated transcription of 5S ribosomal RNA and transfer RNA.4 In addition, c-Myc is known to regulate ribosomal biogenesis through the transactivation of genes involved in ribosomal biogenesis, including RPs themselves.5 Increased c-Myc expression is frequently observed in human cancers and it can confer a growth advantage to cells by providing constitutive proliferative signals.6 However, c-Myc overexpression may also initiate an endogenous apoptotic program,7 and through this function trigger a potent tumor surveillance response that effectively opposes hyperproliferation by killing those cells in which c-Myc levels exceed a safe threshold.8
The p53 transcription factor is a critical tumor suppressor, and when activated it triggers cell cycle arrest, differentiation, apoptosis and senescence. The TP53 gene is mutated in ~ 50% of all human tumors and is often referred to as the ‘guardian’ of the genome.9,10 p53 is primarily regulated by the E3 ubiquitin ligase murine double minute 2 (Mdm2), which binds to p53 to both block its transactivation domain and to promote its ubiquitination and degradation. However, p53 enhances Mdm2 transcription, thus forming an autoregulatory feedback loop.11
The tumor suppressor p19 alternative reading frame (p19Arf, p14Arf in human) is transcribed from an alternative reading frame of the INK4a/ARF locus. p19Arf physically binds to Mdm2, inhibiting its E3 ligase activity, thereby stabilizing and activating p53, which constitutes a p19Arf–Mdm2–p53 signaling pathway.12 Oncogenic c-Myc driven tumors receive a selective advantage from inactivation of the p19Arf–Mdm2–p53 pathway, diminishing its protective checkpoint function and accelerating normal cell progression to malignancy; this accelerated progression to malignancy is observed in Eμ-myc;p19Arf−/− transgenic mice, a model for Burkitt’s lymphoma, which die of lymphoma within a few weeks of birth.13
Previous evidence has demonstrated that several RPs such as RPL5, RPL11 and RPL23 interact with Mdm2 to inhibit its E3 ligase function, thereby stabilizing and activating p53, suggesting an RP–Mdm2–p53 pathway.14 In vivo studies have established the physiological significance of the RP–Mdm2 interaction in responding to ribosomal stress to activate p53, providing evidence for a p53 checkpoint mediated by the RP–Mdm2 interaction, which is capable of monitoring the integrity of ribosome biogenesis.15 Furthermore, use of the Eμ-myc transgenic mouse model, which constitutively expresses c-Myc in the B-cell lineage and develops B-cell lymphoma at an early age,16 we have demonstrated that the RP–Mdm2–p53 pathway, similar to the p19Arf–Mdm2–p53 pathway, plays a critical role in preventing oncogenic c-Myc-induced lymphomagenesis.15
Previous studies have shown that p19Arf can disturb ribosomal biogenesis by interacting with nucleophosmin (NPM/B23) and inhibiting pre-ribosomal RNA processing.17,18 More recently, it has been shown that p19Arf inhibits the function of RNA polymerase I transcription termination factor19 and DDX5 RNA helicase 20 by preventing their nucleolar localization, thereby inhibiting ribosomal biosynthesis. These studies raise an interesting question as to whether the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways might function in a linear manner as a p19Arf–RP–Mdm2–p53 pathway, where p19Arf additionally activates p53 by inhibiting RP biosynthesis. As such, p19Arf deletion or Mdm2C305F mutation, which disrupts RP–Mdm2 binding, would result in similar consequences in the context of c-Myc overexpression. To gain insight into the interrelationship and possible cooperative mechanisms between p19Arf–Mdm2–p53 and RP–Mdm2–p53 pathways, we studied tumorigenesis in mice with disruption of each pathway individually and in combination.
RESULTS
Mdm2C305F mutation does not accelerate p19Arf deletion caused spontaneous tumor development
Previous studies have shown that mice with homozygous deletion of p19Arf (p19Arf−/−) are predisposed to spontaneous tumor development,21 whereas mice with homozygous mutation of Mdm2C305F (henceforth referred to as Mdm2m/m), which prevents Mdm2 binding to RPL5 and RPL11,22 do not develop spontaneous tumors.15 Because p19Arf interacts with and inhibits NPM/B23,17,18 RNA polymerase I transcription termination factor19 and DDX5.20 As these factors are all involved in ribosomal biosynthesis we speculated that the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways might be functionally interconnected. We crossed p19Arf−/− and Mdm2m/m mice to generate double-heterozygous Mdm2+/m;p19Arf+/− mice and then intercrossed the Mdm2+/m; p19Arf+/− mice to generate Mdm2m/m;p19Arf−/− compound mice. Of the 186 pups born from intercross of the double-heterozygotes, a predicted Mendelian inheritance was observed (Figure 1a, shown are only three genotypes for simplicity), indicating that concurrent disruption of p19Arf–Mdm2–p53 and RP–Mdm2–p53 pathways does not affect embryogenesis and early development.
Figure 1.

Disruption of the p19Arf–Mdm2–p53 pathway, but not the RP–Mdm2–p53 pathway, results in spontaneous tumor development. (a) The image shown are expected and observed birth ratios from a total of 186 mice obtained from Mdm2+/m;p19Arf+/− mice intercrosses (shown only three genotypes for simplicity). (b) Kaplan–Meier survival curves for WT, Mdm2m/m, p19Arf−/− and Mdm2m/m; p19Arf−/− mice are shown. Two to five mice of the same gender were housed in each cage, and the mice were observed over a 24-month period. Median survival time of p19Arf−/− and Mdm2m/m;p19Arf−/− mice was 16 and 19 months, respectively. There was no significant difference between survival of p19Arf−/− and Mdm2m/m;p19Arf−/− mice (analyzed by log-rank test, P = 0.06).
A survival study was carried out to examine the effect of concurrent p19Arf deletion and Mdm2C305F mutation on spontaneous tumor formation and lifespan in the mice. Consistent with previous studies, mice heterozygous for Mdm2C305F mutation (Mdm2+/m) and p19Arf deletion (p19Arf+/−) did not show any pathological or physiological changes. In addition, we did not observe any phenotypic changes in double-heterozygous Mdm2+/m; p19Arf+/− mice (data not shown). Consistent with previous reports,21 mice with homozygous deletion of p19Arf were predisposed to tumors, mostly lymphomas, and died shortly after the development of disease, resulting in a notably shorter lifespan compared to wild-type (WT) mice (Figure 1b). However, mice with homozygous Mdm2C305F mutation did not show observable differences in spontaneous tumor formation and lifespan from the WT littermates. Interestingly, double-homozygous compound mice (Mdm2m/m;p19Arf−/−) did not show accelerated spontaneous tumor formation compared to mice with only p19Arf deletion, instead they displayed slightly decelerated tumor formation (Figure 1b; log-rank test P = 0.06); the reasons for the deceleration remain unclear. We have noticed that the mean latency for survival of the p19Arf−/− mice in our experiment is 16 months, significantly longer than the previous report of ~ 10 months.21 One likely reason for the difference is that our mice are in >99% pure C57BL6 background as compared to a mixed 129svj/C57BL6 background in the previous study. It has been reported that C57BL6 mice are more resistant to spontaneous tumor formation than 129svj mice,23,24 and C57BL6 mice are more efficient in total leukocyte recruitment than 129svj mice,25 a function important in preventing lymphomagenesis.26
Concurrent disruption of the p19Arf–Mdm2–p53 and the RP–Mdm2-p53 pathways further accelerates oncogenic c-Myc-induced lymphomagenesis
To further investigate the consequence of disruption of the p19Arf–Mdm2–p53 and the RP–Mdm2-p53 pathways on tumorigenesis, we generated Eμ-myc transgenic mice on Mdm2m/m and p19Arf−/− background individually and in combination, and compared their tumor-free survival time. Eμ-myc;WT mice died of pre-B/B-cell lymphoma with a mortality curve consistent with previous studies (Figure 2).13,16 The median survival for Eμ-myc; p19Arf+/− mice was 15.6 weeks, significantly shorter than Eμ-myc; WT mice (Figure 2a, P = 0.003), whereas the median survival for Eμ-myc;Mdm2+/m mice was not significantly different from that of Eμ-myc;WT mice (Figure 2b, P = 0.5). In contrast, homozygous Eμ-myc;p19Arf−/− and Eμ-myc;Mdm2m/m mice developed aggressive, rapid onset pre-B/B-cell lymphoma with a mean survival of 10.1 and 11.6 weeks, respectively (Figures 2a and b), indicating that p19Arf–Mdm2 and RP–Mdm2 binding function as barriers to oncogenic c-Myc-induced tumorigenesis.
Figure 2.
c-Myc-induced lymphomagenesis is accelerated by Mdm2m/m or p19Arf−/− mutations, and is further accelerated by concurrent Mdm2m/m;p19Arf−/− mutation. (a) Survival of Eμ-myc transgenic p19Arf deletion mice. The median survival time for each genotype was as follows: Eμ-myc;WT (20.7 weeks), Eμ-myc;p19Arf+/− (15.6 weeks) and Eμ-myc;p19Arf−/− (10.1 weeks). Log-rank test, P = 0.003 between Eμ-myc;WT and Eμ-myc;p19Arf+/− mice; P<0.0001 between Eμ-myc;p19Arf+/− and Eμ-myc;p19Arf−/− mice. (b) Survival of Eμ-myc transgenic Mdm2C305F mutation mice. The median survival times were as follows: Eμ-myc;WT (20.7 weeks), Eμ-myc;Mdm2+/m (17.9 weeks) and Eμ-myc;Mdm2m/m (11.6 weeks). Log-rank test, P = 0.5 between Eμ-myc;WT and Eμ-myc;Mdm2+/m mice; P<0.0001 between Eμ-myc;Mdm2+/m and Eμ-myc;Mdm2m/m mice. (c) Survival of Eμ-myc;WT (20.7 weeks), Eμ-myc;Mdm2m/m (11.6 weeks), Eμ-myc;p19Arf−/− (10.1 weeks) and Eμ-myc;Mdm2m/m;p19Arf−/− (7.6 weeks) mice. Log-rank test, P = 0.0001 between Eμ-myc; Mdm2m/m and Eμ-myc;p19Arf−/− mice; P<0.0001 between Eμ-myc; p19Arf−/− and Eμ-myc;Mdm2m/m;p19Arf−/− mice. (d) Body weight of mice expressing Eμ-myc.
To determine if the RP–Mdm2–p53 and p19Arf–Mdm2–p53 represent two parallel pathways or a single linear p19Arf–RP–Mdm2–p53 pathway against Eμ-myc transgene-induced lymphomas, we generated Eμ-myc;Mdm2m/m;p19Arf−/− compound mice and evaluated the impact of the double mutation on mouse survival. Remarkably, Eμ-myc;Mdm2m/m;p19Arf−/− mice demonstrated a median survival of 7.6 weeks, evidently shorter than the lifespan observed in either Eμ-myc;Mdm2m/m or Eμ-myc;p19Arf−/− mice (Figure 2c). These data indicate that the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 likely represent two independent signaling pathways possessing non-overlapping tumor suppression functions. We noticed that the mean survival of Eμ-myc;p19Arf−/−mice is significantly shorter than that of Eμ-myc;Mdm2m/m mice (10.1 vs 11.6 weeks; Figure 2c, P = 0.0001). This indicates that in addition to directly inhibiting Mdm2 and activating p53, p19Arf may also partially exert its function through the RP–Mdm2–p53 pathway by inhibiting ribosomal biogenesis.17,18
No significant difference in body weight gain was observed among the genotypes in the early stages of the mouse lifespan (Figure 2d). In later stages, however, the Eμ-myc;p19Arf−/− mice grow larger, and the Eμ-myc;Mdm2m/m mice grow smaller than the Eμ-myc;WT control mice. The reason for this discrepancy is unclear. In the final stages of their life, the mice began to lose weight from cancerous cachexia, indicating that these Eμ-myc transgenic mice die of cachexia due to malignant tumor development.
Mdm2m/m;p19Arf−/− mice demonstrate accelerated c-Myc-induced tumorigenesis and metastasis
Splenomegaly is a common result of infiltration of lymphomas. Eμ-myc transgenic mice typically present a massive enlargement of the spleen.27 We measured the length of the spleens from mice at 9 weeks of age. As shown in Figure 3a, Eμ-myc;WT mice had an average spleen length of 1.6 cm, as compared to 1.1 cm spleen length in WT, non-transgenic mice. Both homozygous Mdm2C305F mutation and p19Arf deletion accelerated splenomegaly, with average spleen size increased in Eμ-myc;Mdm2m/m and Eμ-myc; p19Arf−/− transgenic mice to 2.4 and 2.5 cm, respectively. Compound mice harboring both Mdm2C305F mutation and p19Arf deletion demonstrated further accelerated splenomegaly, and the average spleen length of Eμ-myc;Mdm2m/m;p19Arf−/− mice was 3.8 cm at 9 weeks of age.
Figure 3.
Mdm2m/m;p19Arf−/− compound mice demonstrate accelerated c-Myc-induced tumorigenesis and metastasis. (a) Length of 9-week-old (±3 days) mouse spleens of WT (n = 5), Eμ-myc;WT (n = 5), Eμ-myc;Mdm2m/m (n = 5), Eμ-myc;p19Arf−/− (n = 5) and Eμ-myc;Mdm2m/m;p19Arf−/−(n = 5) mice. Spleen length was measured and the median lengths were as follows: WT (1.14 cm), Eμ-myc;WT (1.56 cm), Eμ-myc;Mdm2m/m (2.36 cm), Eμ-myc;p19Arf−/− (2.54 cm) and Eμ-myc;Mdm2m/m;p19Arf−/− (3.82 cm). Data are represented as mean ± s.e.m. and were analyzed by Student’s t-test. ***P<0.001; **P<0.01. (b) Hematoxylin and eosin (H&E) staining of spleen, liver and kidney tissues. WT mouse spleen serves as a positive control for normal spleen structure. Structure of spleens from Eμ-myc transgenic mice was destroyed by lymphocyte infiltration. Lymphocyte infiltration was observed as dark blue staining. The malignant cells are dispersed and the nuclei are larger than normal lymphocytes. Aggregates of small lymphocytes are observed in the livers and kidneys of Eμ-myc transgenic mice. Scale bar, 400 μm. (c) Representative Ki-67 staining of spleens from different genotypes of 9-week-old mice, as indicated. Brown staining indicates Ki-67-positive proliferating cells. Scale bar, 100 μm. The Ki-67 index (calculated as the percentage of Ki-67-positive tumor cells vs total cells in the view field from at least five randomly chosen fields along the edge of spleens) for the genotypes assayed are indicated in parentheses: WT (0.4), Eμ-myc; WT (2.8), Eμ-myc;Mdm2m/m (12.2), Eμ-myc;p19Arf−/− (14.6 ) and Eμ-myc;Mdm2m/m;p19Arf−/− (53.9) mice. Data are represented as mean ± s.e.m. ***P<0.001. (d) Representative TUNEL staining of spleens from different genotypes of 9-week-old mice. The percentage of TUNEL-positive cells in the view field was calculated from at least five randomly chosen fields along the edge of spleens and is indicated in parentheses: WT (8.4%), Eμ-myc;WT (14.6%), Eμ-myc;Mdm2m/m (13.6%), Eμ-myc;p19Arf−/− (14.4%) and Eμ-myc;Mdm2m/m;p19Arf−/− (6.2%). Data are represented as mean ± s.e.m. ***P<0.001; **P<0.01. Scale bar, 200 μm. (e) H&E staining of livers from different genotypes of similar metastasis stage. Mouse livers were collected at the time of death. Time of metastasis is indicated in parentheses: Eμ-Myc;WT (22 weeks), Eμ-Myc;Mdm2m/m (12.5 weeks), Eμ-Myc;p19Arf−/− (11 weeks) and Eμ-Myc;Mdm2m/m;p19Arf−/− (9 weeks). Scale bar, 400 μm.
We next examined the invasive nature of Eμ-myc-induced lymphomas in the spleen, liver and kidney tissues isolated from 9-week-old mice. Spleen metastasis occurred earlier than other organs, and the structure of the spleens in mice harboring Eμ-myc transgene was already destroyed at this age. As shown in Figure 3b, expression of the Eμ-myc correlates with an increased number of small lymphocytes within the cords and sinuses, as well as some nodular aggregates surrounding a small vessel, indicative of spleen metastasis. The most significant metastasis was observed in the spleen of Eμ-myc;Mdm2m/m;p19Arf−/− compound mice. Similar to the spleen, lymphocyte infiltration in the liver can be seen in each of the Eμ-myc transgenic mice, specifically in the region surrounding the central veins. Not surprisingly, the most obvious liver metastasis was observed in Eμ-myc;Mdm2m/m; p19Arf−/− compound mice (Figure 3b). Kidney metastasis usually occurs in advanced or late-stage lymphomas, and metastatic tumor cells were detected as diffuse and disorganized cells infiltrating between the glomerulus and tubules.28 As shown in Figure 3b, in WT, Eμ-myc;WT, Eμ-myc;Mdm2m/m and Eμ-myc; p19Arf−/− mice, kidneys revealed no discernible pathological phenotype, and lymphocyte infiltration is only obvious in the compound Eμ-myc;Mdm2m/m;p19Arf−/− mice. Together, these data indicate that individually disrupting the p19Arf–Mdm2–p53 pathway by p19Arf deletion or the RP–Mdm2–p53 pathway by Mdm2C305F mutation augments the invasive nature of Eμ-myc-induced lymphoma. Simultaneous disruption of both pathways, as seen in Eμ-myc;Mdm2m/m;p19Arf−/− mice, leads to even more malignant and aggressive lymphomagenesis.
To determine if cells in Eμ-myc-expressing spleens retained their proliferative capacity, Ki-67 staining was used to assess cellular proliferation by immunohistochemistry. As shown in Figure 3c, in the absence of the Eμ-myc transgene Ki-67 expression was barely detectable in the spleen of 9-week-old WT mice (Ki-67 index 0.4). In Eμ-myc;WT mice, we detected increased numbers of Ki-67-positive cells (Ki-67 index 2.8); the number of Ki-67-positive cells was further increased in spleens from Eμ-myc;Mdm2m/m and Eμ-myc;p19Arf−/− mice (Ki-67 index 12.2 and 14.6, respectively). In spleens of Eμ-myc;Mdm2m/m;p19Arf−/− compound mice the number of Ki-67-positive cells was even further increased (Ki-67 index 53.9).
To examine apoptosis in the spleen tumors of the transgenic mice, TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) immunohistochemical analysis was performed. Representative pictures of TUNEL-stained sections are shown in Figure 3d. Consistent with previous studies that Eμ-myc induces apoptosis in transgenic spleens,29 in spleens of 9-week-old Eμ-myc;WT, Eμ-myc;Mdm2m/m and Eμ-myc;p19Arf−/−mice, we detected high levels of TUNEL-positive apoptotic cells (14.6, 13.6, and 14.4%, respectively). However, in spleens of Eμ-myc;Mdm2m/m;p19Arf−/− compound mice the number of TUNEL-positive apoptotic cells decrease to 6.2%.
Local invasion and distant metastasis of lymphomas are commonly observed in Eμ-myc transgenic mice; we therefore analyzed liver tissues from individual mice for signs of metastasis. Eμ-myc transgenic mice were monitored for signs of moribund conditions,30 and moribund mice were killed and the livers were collected and examined for sign of metastasis. Tumors were detected in the livers of Eμ-myc;WT transgenic mice at an average age of 22 weeks (Figure 3e). In contrast, tumor onset in the liver was detected much earlier in Eμ-myc;Mdm2m/m, Eμ-myc;p19Arf−/−and Eμ-myc;Mdm2m/m;p19Arf−/− mice, with progressively shorter time of onset at 13, 11 and 9 weeks, respectively (Figure 3e). These results demonstrate that individually the Mdm2C305F mutation and p19Arf deletion promote local invasion and distant metastasis of Eμ-myc-induced lymphomas, with p19Arf deletion having a stronger effect than Mdm2C305F mutation, which is consistent with the shorter lifespan of the Eμ-myc;p19Arf−/− mice compared to the Eμ-myc;Mdm2m/m mice (Figure 2c). Concurrent Mdm2C305F mutation and p19Arf deletion further accelerates the aggressiveness of the lymphoma, resulting in an even shorter lifespan, consistent with the notion that the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 represent two non-redundant, independent pathways for tumor suppression.
The p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways function independently in oncogenic c-Myc-induced p53 activation
Oncogene c-Myc induces p53 to promote apoptosis, functioning as part of a major tumor suppression network. Because Eμ-myc-induced lymphomagenesis in mice can be further accelerated by concurrent disruption of the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways compared to disruption of either pathway alone, we reasoned that the two pathways could each individually be required for oncogenic c-Myc-induced p53 activation. To test this hypothesis, spleen extracts from 4-week-old, non-tumor-bearing Eμ-myc;WT, Eμ-myc;Mdm2m/m, Eμ-myc;p19Arf−/− and Eμ-myc;Mdm2m/m;p19Arf−/− transgenic mice, as well as from their non-transgenic counterparts, were analyzed for p53 expression. At this age the spleens harboring the Eμ-myc transgene are moderately hyperplastic, but still comparable in size and morphology to the spleens of non-transgenic mice (data not shown). The Eμ-myc transgene markedly induced p53 protein expression in WT mouse spleens (Figure 4a, lane 2), which is accompanied by increased expression of p19Arf, RPL5 and RPL11, all of which are known transcriptional targets of c-Myc.31,32 The Eμ-myc transgene also induced p53 expression in Eμ-myc;Mdm2m/m and Eμ-myc;p19Arf−/− spleens to a similar level, although slightly lower than what is detected in Eμ-myc;WT spleens (Figure 4a, compare lanes 2, 4 and 6). Remarkably, p53 induction was not observed in Eμ-myc;Mdm2m/m;p19Arf−/− compound mouse spleens (Figure 4a, lane 8). These data demonstrate that individual disruption of the p19Arf–Mdm2–p53 pathway by p19Arf deletion or the RP–Mdm2–p53 pathway by Mdm2C305F mutation does not block c-Myc induction of p53, while simultaneous disruption of both pathways blocks c-Myc-induced p53 expression. To ascertain whether these observations are Eμ-myc transgene specific, we infected early passage WT, Mdm2m/m, p19Arf−/− and Mdm2m/m; p19Arf−/− mouse embryo fibroblast (MEF) cells with retrovirus-expressing pBabe–c-Myc to determine if transient expression of c-Myc gives rise to similar results. Consistent with observations made in mouse spleens, transient expression of pBabe–c-Myc resulted in p53 induction in WT, Mdm2m/m and p19Arf−/− MEFs, but not in Mdm2m/m;p19Arf−/− MEFs (Figure 4b). Furthermore, the increased protein level of p53 was directly correlated with its transcriptional activity, indicated by S-18 phosphorylation of p53 in the spleens (p21 was undetectable in spleens; Figure 4a), and increased p21 protein level in pBabe–c-Myc infected MEFs (Figure 4b).
Figure 4.
The RP–Mdm2–p53 and p19Arf–Mdm2–p53 signaling pathways function independently in oncogenic c-MYC induction of p53. (a) Extracts from spleens of 4-week-old, non-tumor-bearing Eμ-myc;WT, Eμ-myc;Mdm2m/m, Eμ-myc;p19Arf−/− and Eμ-myc;Mdm2m/m;p19Arf−/− transgenic mice and spleens from their non-transgenic counterparts were analyzed by western blot. (b) Early passage WT, Mdm2m/m, p19Arf−/− and Mdm2m/m;p19Arf−/− MEFs were infected with retrovirus expressing either pBabe vector (−) or pBabe–c-Myc (+), selected by puromycin for 3 days, then allowed to recover for 48 h before harvesting for western blot analysis. (c–f) mRNA levels of p21 (c), Bax (d), Apaf1 (e) and TIGAR (f) in spleens of 4-week-old, non-tumor-bearing Eμ-myc;WT, Eμ-myc;Mdm2m/m, Eμ-myc;p19Arf−/− and Eμ-myc;Mdm2m/m;p19Arf−/− transgenic mice, and in spleens of their non-transgenic counterparts, analyzed by quantitative RT–PCR. All samples were analyzed in triplicate. Data are means ± s.d. calculated from three independent experiments and normalized to actin. Data are represented as mean ± s.e.m., and were analyzed by Student’s t-test. *P<0.1; **P<0.01; ***P<0.001; and NS indicates no significant difference.
To corroborate the protein analysis, we carried out quantitative RT–PCR to further examine p53 activity in 4-week-old, non-tumor-bearing Eμ-myc;WT, Eμ-myc;Mdm2m/m, Eμ-myc;p19Arf−/− and Eμ-myc;Mdm2m/m;p19Arf−/− mouse spleens and in spleens from their non-transgenic counterparts. We chose p53 targets involved in cell cycle regulation (p21), apoptosis induction (Bax and Apaf1) and metabolism regulation (TIGAR) to determine whether p53 is activated universally for its target genes. Induction of each of these p53 target genes was evident in Eμ-myc;WT, Eμ-myc;Mdm2m/m and Eμ-myc;p19Arf−/− mouse spleens, but not in Eμ-myc;Mdm2m/m;p19Arf−/− mouse spleens (Figures 4c and f), consistent with protein analysis. Together, our data indicate that not only can the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways be independently responsible for oncogenic c-Myc induction of p53, but also that the two pathways together contribute to most if not all p53 tumor suppressor response to c-Myc activation. Disabling both pathways results in undetectable p53 activation by oncogenic c-Myc overexpression.
DISCUSSION
The importance of p19Arf–Mdm2–p53 and RP–Mdm2–p53 signaling pathways in defending against oncogenic c-Myc-induced tumorigenesis is well established, and studies have shown that disruption of each of the two pathways by deletion or mutation accelerates the progression of malignancy in c-Myc-expressing mice.13,15 In this study, we generated and analyzed mice with concurrent disruption of these two pathways and compared p53 activity and tumorigenesis to mice with disruption of each pathway alone. The significance and implications of these findings are discussed below.
The p19Arf–Mdm2–p53 and the RP–Mdm2–p53 signaling pathways act independently in oncogenic c-MYC-induced p53 activation
The generation of Mdm2m/m mice has allowed for a detailed in vivo characterization of the role of RP–Mdm2 binding in p53 activation and tumor suppression. Unlike the disruption of the p19Arf–Mdm2–p53 pathway by p19Arf deletion, which results in spontaneous tumor development,33 disruption of the RP–Mdm2–p53 pathway by Mdm2C305F mutation does not result in spontaneous tumor development.15 The different tumorigenic potential between the two mouse models could be a result of the nature of the two pathways in each of the mouse models. In the p19Arf−/− mice the entire p19Arf gene is deleted, disrupting p19Arf–Mdm2 binding as well as all other p19Arf-related functions; in the Mdm2m/m mice, however, the single-point mutation in the Mdm2 is less disruptive, and while Mdm2C305F mutant protein cannot properly bind to RPL5/RPL11, it retains binding to RPL23,22 thus only reducing but not eliminating the functions of this pathway. Alternatively, it is conceivable that the p19Arf–Mdm2–p53 pathway primarily responds to deregulated oncogenes, which inflict a great risk of tumor formation if left unchecked, whereas the RP–Mdm2–p53 pathway may primarily respond to deregulated ribosome biosynthesis, which may play a lesser role in promoting cancerous growth.
In addition, it has been shown that p19Arf binds to and inhibits the ability of c-Myc to induce hyperproliferation and transformation through34,35 indicating loss of p19Arf could contribute to c-Myc-induced tumorigenesis by at least two mechanisms. Similarly, it has been shown that RPL11 binds to c-Myc and inhibits its transcriptional activity36 and that RPL5, cooperatively with RPL11, guides the RNA-induced silencing complex to c-Myc mRNA and mediates the degradation of c-Myc mRNA.37 These studies in combination with our results point to the importance of both p19Arf and RP signaling in c-Myc-induced lymphomagenesis. Our results agree with the notion that Eμ-myc-induced lymphomagenesis can be accelerated by disruption of negative regulators of c-Myc in each pathway in addition to disruption of p53 activation.
Our study also addresses the degree of cooperation between the p19Arf–Mdm2–p53 and RP–Mdm2–p53 pathways in p53 response to oncogenic c-Myc overexpression. When Mdm2C305F mutation or p19Arf deletion occur individually, c-Myc over-expression still elicits sufficient p53 response; however, when Mdm2C305F mutation and p19Arf deletion occur concurrently, c-Myc overexpression does not elicit p53 activation. Given the role of oncogenic c-Myc in promoting aberrant ribosomal biogenesis, the p19Arf-independent, c-Myc-mediated induction of p53 in the p19Arf−/− cells is conceivably dependent on c-Myc-induced overexpression of RPs, which activate p53 through interaction with Mdm2.15 Conversely, activation of p53 by c-Myc in Mdm2m/m mice is p19Arf dependent, as the p19Arf–Mdm2–p53 pathway remains intact in mice and c-Myc can induce p19Arf expression (Figure 4). Our data suggest that p53 responds to c-Myc overexpression via two independent signaling pathways: p19Arf–Mdm2–p53 and RP–Mdm2–p53. Each pathway conveys a share of c-Myc signaling to p53, and disruption of either one reduces, but not eliminates, p53 response to c-Myc overexpression. Consequently, disrupting either pathway will accelerate c-Myc-induced tumor formation, as illustrated by shortened survival times of Eμ-Myc;Mdm2m/m and Eμ-Myc;p19Arf−/− mice compared to Eμ-myc;WT mice. Disrupting both pathways further accelerates c-Myc-induced tumorigenesis, as illustrated by the comparatively shorter tumor-free lifespan of Eμ-Myc;Mdm2m/m; p19Arf−/− compound mice. Our data support a model in which the RP–Mdm2–p53 and p19Arf–Mdm2–p53 pathways represent two non-redundant, parallel mechanisms for p53 induction (Figure 5). Intriguingly, the lack of detectable p53 activation in the Eμ-myc; Mdm2m/m;p19Arf−/− compound mice implies that other active mechanisms transmitting oncogenic c-Myc signal to p53 either may not exist or be insufficient to raise p53 to a detectable level.
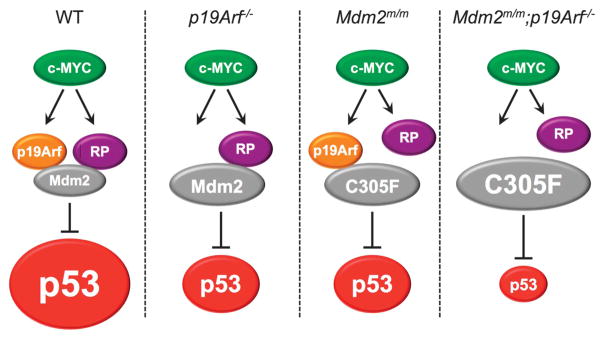
Figure 5.
A model depicting non-overlapping functions of the p19Arf–Mdm2–p53 and the RP–Mdm2–p53 pathways in oncogenic c-Myc-induced p53 activation. In WT cells, oncogenic c-Myc signal engages with both p19Arf–Mdm2–p53 and RP–Mdm2–p53 pathways, resulting in maximum Mdm2 inhibition and p53 stabilization and activation (first panel). In cells with p19Arf deletion (second panel) or Mdm2C305F mutation (third panel), c-Myc signal engages with one of the remaining pathways to induce p53, but to a lesser degree. In cells with concurrent p19Arf deletion and Mdm2C305F mutation, c-Myc overexpression cannot induce p53 to detectable levels (fourth panel).
The p19Arf–Mdm2 connection mediates a p53-dependent checkpoint in response to a broad range of oncogenic insults, including elevated expression of c-Myc, E2F1, Ras, E1A and BCR-ABL. However, the RP–Mdm2 connection provides evidence for a prevailing in vivo checkpoint for the integrity of ribosome biogenesis to invoke p53 response if the process goes awry. Given that overexpression of many oncogenes promotes fast growth and proliferation, which inevitably requires accelerated ribosomal biogenesis it is reasonable to speculate that the RP–Mdm2–p53 signaling pathway, in addition to responding to c-Myc overexpression, may also respond to other deregulated oncogenes that cause superfluous ribosomal biogenesis. The Mdm2m/m mouse model provides a convenient tool to investigate whether the RP–Mdm2–p53 pathway, as in the case of the p19Arf–Mdm2–p53 pathway, functions generally in oncogenic signaling surveillance, capable of responding to a broad range of deregulated oncogenes, or is only specific to oncogenic c-Myc.
MATERIALS AND METHODS
Ethics statement
Investigation has been conducted in agreement with the ethical standards according to the Declaration of Helsinki, national and international guidelines, and has been approved by the authors’ institutional review board.
Cell culture
Primary MEFs were isolated on embryonic (E) day 13.5 and grown in a 37 °C incubator with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplied with 10% fetal bovine serum and penicillin-streptomycin. For retroviral infections, WT, Mdm2m/m, p19Arf−/− and Mdm2m/m;p19Arf−/− MEFs were infected with retroviruses expressing c-MYC or pBabe control vector, and selected with puromycin (2.5 μg/ml) for 3 days. Infected MEFs were then allowed to recover for 48 h and harvested for analysis. MEFs at passage 4 were used for growth curves, western blotting and other analyses.
Mouse experiments
Mice were bred and maintained strictly under protocol (13-044) approved by the Institutional Animal Care and Use Committee in the University of North Carolina Animal Care Facility. Mdm2m/m females were bred with p19Arf−/− males to obtain Mdm2+/m;p19Arf+/− offspring, which were then crossed with Mdm2+/m;p19Arf+/− mice to obtain Mdm2m/m;p19Arf−/− compound mice. For c-MYC-mediated tumorigenesis study, Mdm2m/m; p19Arf−/− females were bred with Eμ-myc transgenic males to obtain Eμ-myc;Mdm2+/m;p19Arf+/− offspring, which were then crossed with Mdm2+/m;p19Arf+/− mice to obtain Eμ-myc;Mdm2m/m;p19Arf−/− compound mice. Eμ-myc mice were purchased from Jackson Laboratory (002728). For survival studies, mice were palpated regularly for early signs of inguinal lymph node enlargement and monitored for tumor progression and signs of morbidity. Moribund mice were humanely euthanized. Mouse tumors and organs were fixed in formalin for histopathology and snap frozen for protein and RNA extraction.
Protein analysis
For western blot, MEFs were lysed with 0.5% (Tergitol, Sigma, St Louis, MO, USA) NP-40 lysis buffer. For mouse tissue protein extraction, tissue from the spleen, thymus and lymphomas was ground by mortar and pestle with liquid N2, and protein was extracted with 0.5% NP-40 lysis buffer. Mouse monoclonal anti-Mdm2 (2A10, Calbiochem, Billerica, MA, USA), p53 (NCL-505, Novocastra, Buffalo Grove, IL, USA), actin (MAB1501, Chemicon International, Billerica, MA, USA), goat polyclonal anti-p53 (FL-393, Santa Cruz Biotechnology, Dallas, TX, USA) and rat monoclonal anti-p19Arf (5-C3-1, Santa Cruz Biotechnology) antibodies were purchased commercially. Rabbit polyclonal antibodies to p21 were gifts from Dr Yue Xiong (UNC-Chapel Hill). Rabbit polyclonal antibodies to L5 and L11 were made in house as previously described.22
Measurement of mouse tissue
Spleens, kidneys and livers from 9-week-old mice were excised, photographed and weighed. All procedures involving mice were carried out according to protocol 13-044, approved by the University of North Carolina Institutional Animal Care and Use Committee.
Histopathology
Animals were autopsied and all tissues were examined regardless of their pathological status. Spleen, kidney and liver tissue from Eμ-myc;WT, Myc; Mdm2m/m, Eμ-myc;p19Arf−/− and Eμ-myc;Mdm2m/m;p19Arf−/− transgenic mice, as well as tissue from non-transgenic counterparts were fixed overnight in 10% phosphate-buffered formalin and then transferred to 70% ethanol. Samples were sent to the UNC Histology Core Facility for paraffin embedding. Paraffin blocks were sectioned at 5-mm intervals for successive layers and stained with H&E for histopathology examination.
Proliferation analysis
Ki-67 immunohistochemical staining of mouse spleen samples was used to detect proliferating cells. Antigen retrieval for antibody on formalin-fixed paraffin sections was carried out by boiling paraffin samples in citrate buffer (pH 6.0) for 15 min. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in methanol for 10 min. Antibody detection was carried out with purified mouse anti Ki-67 primary antibody (BD Pharmingen, San Diego, CA, USA) and biotin-conjugated anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA, USA). Ki-67-expressed cells were stained brown color by biotin-peroxidase kit (VECTASTAIN Elite, Vector Laboratories). The ratio of positively stained cells to total cells was calculated. Student’s t-test (P<0.05 was considered significant) was used to compare the differences in proliferation levels between the different mouse genotypes.
Apoptosis analysis
Levels of apoptosis in mouse spleen sections were assessed by the TUNEL assay according to instructions (ApopTag Peroxidase in situ kit, S7100, Millipore, Temecula, CA, USA).
Quantitative real-time PCR
Total RNA was isolated using an RNeasy kit (74104, Qiagen, Hilden, Germany) and cDNA was synthesized using SuperScript III (18080400, Life Technologies, Waltham, MA, USA). qRT–PCR was performed with SYBR green master mix using the 7900HT fast real-time PCR system (Applied Biosystems, Waltham, MA, USA) according to manufacturers’ instructions. Data were collected and exported with SDS 2.2.2 software (Applied Biosystems). Relative expression was calculated using actin as an internal control as indicated. Primers used were as follows: p21, 5′-CCTGGTGATGTCCGACCTG-3′ and 5′-CCATGAGCGCATCGCAATC-3′; Bax, 5′-GGACAGCAATATGGAGCTGCAGAGG-3′ and 5′-GGAGGAAGTCCAGTGTCCAGCC-3′; Apaf1, 5′-CGGTGAAGGTGTGGAATGTCATTACCG-3′ and 5′-GGATTTCTCCATTGTCATCTCCAGTTGC-3′; TIGAR, 5′-CGATCTCACGAGGACTAAGCAGACC-3′ and 5′-GCCAAAGAGCTTTCCAAACCGCTGC-3′; and Actin, 5′-CCACAGCTGAGAGGGAAATCGTGC-3′ and 5′CCAGAGCAGTAATCTCCTTCTGCATCC-3′.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5 Software (Graph-Pad Software, San Diego, CA, USA). Kaplan–Meier survival curve was carried out to assess lifespan.
Acknowledgments
We thank Congying Wu, Yizhou He, Yong Liu, Aiwen Jin and Laura Tollini for their helpful advice and technical assistance. We are in debt to Yue Xiong, Guillermina Lozano, Gerard Evan, Charles Sherr and Norman Sharpless for their generosity in sharing mouse strains and reagents. This research was supported by grants from the National Institutes of Health (CA127770, CA100302 and CA167637) to YZ. This research was also supported by grants from the NSFC and Jiangsu Center for the Collaboration and Innovation of Cancer Biotherapy to YZ, the National S&T Major Project for Infectious Diseases of China (No. 2012ZX10002-017) to JD. NRC was supported in part by a grant from the National Institute of General Medical Sciences under award 5T32 GM007092.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 2.Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol. 2005;7:303–310. doi: 10.1038/ncb1225. [DOI] [PubMed] [Google Scholar]
- 3.Menssen A, Hermeking H. Characterization of the c-MYC-regulated transcriptome by SAGE: identification and analysis of c-MYC target genes. Proc Natl Acad Sci USA. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct activation of RNA polymerase III transcription by c-Myc. Nature. 2003;421:290–294. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- 5.Miliani de Marval PL, Zhang Y. The RP-Mdm2-p53 pathway and tumorigenesis. Oncotarget. 2011;2:234–238. doi: 10.18632/oncotarget.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mateyak MK, Obaya AJ, Adachi S, Sedivy JM. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 7.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 8.Packham G, Cleveland JL. c-Myc and apoptosis. Biochim Biophys Acta. 1995;1242:11–28. doi: 10.1016/0304-419x(94)00015-t. [DOI] [PubMed] [Google Scholar]
- 9.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 10.Meng X, Leslie P, Zhang Y, Dong J. Stem cells in a three-dimensional scaffold environment. Springerplus. 2014;3:80. doi: 10.1186/2193-1801-3-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 12.Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 13.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macias E, Jin A, Deisenroth C, Bhat K, Mao H, Lindstrom MS, et al. An ARF-independent c-MYC-activated tumor suppression pathway mediated by ribosomal protein-Mdm2 Interaction. Cancer Cell. 2010;18:231–243. doi: 10.1016/j.ccr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 17.Itahana K, Bhat KP, Jin A, Itahana Y, Hawke D, Kobayashi R, et al. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol Cell. 2003;12:1151–1164. doi: 10.1016/s1097-2765(03)00431-3. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto M, Kuo ML, Roussel MF, Sherr CJ. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol Cell. 2003;11:415–424. doi: 10.1016/s1097-2765(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 19.Lessard F, Morin F, Ivanchuk S, Langlois F, Stefanovsky V, Rutka J, et al. The ARF tumor suppressor controls ribosome biogenesis by regulating the RNA polymerase I transcription factor TTF-I. Mol Cell. 2010;38:539–550. doi: 10.1016/j.molcel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Saporita AJ, Chang HC, Winkeler CL, Apicelli AJ, Kladney RD, Wang J, et al. RNA helicase DDX5 is a p53-independent target of ARF that participates in ribosome biogenesis. Cancer Res. 2011;71:6708–6717. doi: 10.1158/0008-5472.CAN-11-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59:2217–2222. [PubMed] [Google Scholar]
- 22.Lindstrom MS, Jin A, Deisenroth C, White Wolf G, Zhang Y. Cancer-associated mutations in the MDM2 zinc finger domain disrupt ribosomal protein interaction and attenuate MDM2-induced p53 degradation. Mol Cell Biol. 2007;27:1056–1068. doi: 10.1128/MCB.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoag WG. Spontaneous cancer in mice. Ann NY Acad Sci. 1963;108:805–831. doi: 10.1111/j.1749-6632.1963.tb13421.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith GS, Walford RL, Mickey MR. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F 1 hybrids. J Natl Cancer Inst. 1973;50:1195–1213. doi: 10.1093/jnci/50.5.1195. [DOI] [PubMed] [Google Scholar]
- 25.White P, Liebhaber SA, Cooke NE. 129X1/SvJ mouse strain has a novel defect in inflammatory cell recruitment. J Immunol. 2002;168:869–874. doi: 10.4049/jimmunol.168.2.869. [DOI] [PubMed] [Google Scholar]
- 26.Shetty S, Bruns T, Weston CJ, Stamataki Z, Oo YH, Long HM, et al. Recruitment mechanisms of primary and malignant B cells to the human liver. Hepatology. 2012;56:1521–1531. doi: 10.1002/hep.25790. [DOI] [PubMed] [Google Scholar]
- 27.Sidman CL, Marshall JD, Harris AW. Genetic studies on Emu-myc transgenic mice. Curr Top Microbiol Immunol. 1988;141:94–99. doi: 10.1007/978-3-642-74006-0_13. [DOI] [PubMed] [Google Scholar]
- 28.Aoudjit F, Potworowski EF, St-Pierre Y. The metastatic characteristics of murine lymphoma cell lines in vivo are manifested after target organ invasion. Blood. 1998;91:623–629. [PubMed] [Google Scholar]
- 29.Eischen CM, Alt JR, Wang P. Loss of one allele of ARF rescues Mdm2 haploinsufficiency effects on apoptosis and lymphoma development. Oncogene. 2004;23:8931–8940. doi: 10.1038/sj.onc.1208052. [DOI] [PubMed] [Google Scholar]
- 30.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301–309. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 32.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 34.Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature. 2004;431:712–717. doi: 10.1038/nature02958. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Spears E, Boone DN, Li Z, Gregory MA, Hann SR. Domain-specific c-Myc ubiquitylation controls c-Myc transcriptional and apoptotic activity. Proc Natl Acad Sci USA. 2013;110:978–983. doi: 10.1073/pnas.1208334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai MS, Arnold H, Sun XX, Sears R, Lu H. Inhibition of c-Myc activity by ribosomal protein L11. EMBO J. 2007;26:3332–3345. doi: 10.1038/sj.emboj.7601776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao JM, Zhou X, Gatignol A, Lu H. Ribosomal proteins L5 and L11 co-operatively inactivate c-Myc via RNA-induced silencing complex. Oncogene. 2014;33:4916–4923. doi: 10.1038/onc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]