Abstract
Purpose of review
Our objective is to describe prediction models for surgical patients who have suspected obstructive sleep apnea (OSA) at risk for postoperative respiratory complications and for surgical patients at risk for postoperative acute respiratory distress syndrome (ARDS).
Recent findings
Because of the increased rate of severe perioperative respiratory complications in patients with OSA, the American Society of Anesthesiologists issued practice guidelines for perioperative management. When OSA is diagnosed preoperatively, the rate of postoperative pulmonary complications is low and not associated with OSA severity. However, OSA continues to be an important risk because a substantial proportion of patients in the contemporary surgical population have undiagnosed OSA. Strategies based on preoperative and immediate postoperative clinical signs and symptoms can help identify patients with a high likelihood of OSA, postoperative desaturations, and pulmonary complications. ARDS is another serious postoperative complication associated with high mortality rate and limited treatment options, and its prevention is critical. Practice changes have led to a dramatic reduction in ARDS incidence. A recently developed prediction model can help identify high-risk patients.
Summary
Evidence is emerging that early identification of modifiable risk factors and implementation of ‘protective’ management strategies may lead to reduction of severe postoperative pulmonary complications.
Keywords: acute respiratory distress syndrome, hypercapnic respiratory failure, hypoxic respiratory failure, obstructive sleep apnea, prediction scores
INTRODUCTION
Specific etiologic factors for respiratory failure after surgery can be broadly categorized into those portending risk of hypercapnic respiratory failure [e.g., obstructive sleep apnea (OSA)] and those portending risk of hypoxic respiratory failure [e.g., acute respiratory distress syndrome (ARDS)]. Postoperative respiratory complications, including hypoventilation with hypercapnic respiratory arrest, are well documented in patients with OSA [1]. However, preoperative recognition of OSA or suspicion that the patient may have OSA allows for tailoring of perioperative management to minimize its impact on the postoperative course. Another serious complication is postoperative respiratory failure (PRF), especially when it arises from ARDS [2]. Of importance is the identification of patients at increased risk for this type of respiratory failure, because implementation of protective strategies, such as lung recruitment, ventilation with low tidal volumes, and restrictive fluid and transfusion practices, may reduce its occurrence [2,3■,4–6,7■■]. Our main objective is to describe approaches we use to identify patients at increased risk for postoperative OSA-associated complications and to describe factors that can facilitate the prediction of ARDS.
PREDICTING POSTOPERATIVE RESPIRATORY COMPLICATIONS IN PATIENTS WITH OBSTRUCTIVE SLEEP APNEA
Predictions of postoperative respiratory complications are based on patient characteristics, risks linked to comorbidities, especially OSA, as well as to the type of surgical procedure. Increased risk may be recognized with perioperative evaluation and may be mitigated with preventive measures.
Prevalence of obstructive sleep apnea in the surgical population
Because OSA is more prevalent in patients with advanced age and obesity – both characteristics frequently encountered in the contemporary surgical practice – perioperative complications related to OSA can be expected. Importantly, a large proportion of surgical patients have undiagnosed OSA, and an OSA-related complication may occur without anticipation. For example, OSA diagnosis was present in 15% of a cohort of bariatric surgical patients but, when assessed with polysomnography, was confirmed in 77% [8]. At the Mayo Clinic, where all bariatric patients undergo evaluation for sleep-disordered breathing, the incidence of OSA was 77.5% [9■].
Obstructive sleep apnea and postoperative complications
Anesthetic agents, sedatives, and opioids exaggerate airway obstruction and hypoventilation, which may lead to hypercapnic respiratory failure in patients with OSA. A large retrospective study found that surgical patients with OSA have a risk of postoperative tracheal intubation and mechanical ventilation that is several-fold higher than those without OSA [10]. In addition, investigators have shown that surgical patients with unrecognized OSA have higher rates of postoperative complications and ICU admissions and have longer hospital stays than those without OSA [11]. However, among bariatric surgical patients, when OSA was diagnosed preoperatively with polysomnography, management of OSA was optimized by treatment with noninvasive ventilation devices and when use of these devices continued during the postoperative period combined with vigilant monitoring, the overall complication rate was low and complications were not related to OSA severity [9■].
Perioperative diagnosis of obstructive sleep apnea
In 2006, the American Society of Anesthesiologists (ASA) published practice guidelines for the treatment of patients with OSA and recommended thorough preoperative evaluation and adjustment of anesthetic management to minimize postoperative respiratory depression [12]. The gold standard test for OSA diagnosis is overnight polysomnography [13]. Polysomnography yields the apneahypopnea index (AHI) score (an index of sleep apnea severity), and an AHI score greater than 5/h indicates the presence of OSA. The AHI score is the basis of the ASA rating of OSA severity as mild (AHI, 6–20/h), moderate (AHI, 21–40/h), and severe (AHI, ≥41/h) [12]. Of note, the AHI score of the American Academy of Sleep Medicine Task Force differs slightly from the one published by ASA [12]: no OSA (AHI, ≤4/h), mild OSA (AHI, 5–15/h), moderate OSA (AHI, 16–30/h), and severe OSA (AHI, ≥31/h) [14].
Polysomnography is expensive and of limited availability. Instead, overnight oximetry has been proposed as an OSA screening tool [15], with overnight oxygen desaturation index (ODI) scores greater than 10 desaturations per hour resulting in 93% sensitivity and 75% specificity to detect moderate-to-severe OSA [16]. However, some investigators show that overnight oximetry lacks diagnostic accuracy [17]. Its disadvantage is that, in patients for whom there is a high index of suspicion, a negative oximetry reading still needs confirmatory polysomnography.
Various screening tools of easily assessed clinical signs and symptoms have been used to identify patients at high risk for OSA. The Epworth Sleepiness Scale is a simple questionnaire that quantifies daytime sleepiness, a common symptom of OSA [18]. Correlation of the Epworth Sleepiness Scale scores with OSA continues to be controversial [18–20]. The Berlin Questionnaire queries the history of snoring, excessive daily sleepiness, and hypertension and takes into account demographic and anthropometric variables [21,22]. It has positive and negative predictive values of 77.9 and 44.9% for OSA and 31.5 and 92.8% for severe OSA, respectively [23].
The STOP (snoring, tiredness, observed apneas, high blood pressure) and the STOP-BANG (snoring, tiredness, observed apneas, high blood pressure, BMI >35 kg/m2, age >50 years, neck circumference >40 cm, and male sex, with a score of ≥2 positives for STOP or ≥3 positives for STOP-BANG indicating high risk of OSA) assessment tools have been widely used as OSA screening tools with positive and negative predictive values of 81.0 and 60.8% for OSA and 31.0 and 100% for severe OSA, respectively [24,25]. The 2006 ASA practice guidelines propose a 14-item screening tool that assesses OSA predisposing physical characteristics, signs of airway obstruction during sleep, and somnolence [12]. This tool has positive and negative predictive values of 72.1 and 38.2% for OSA and 27.9 and 90.9% for severe OSA, respectively [24].
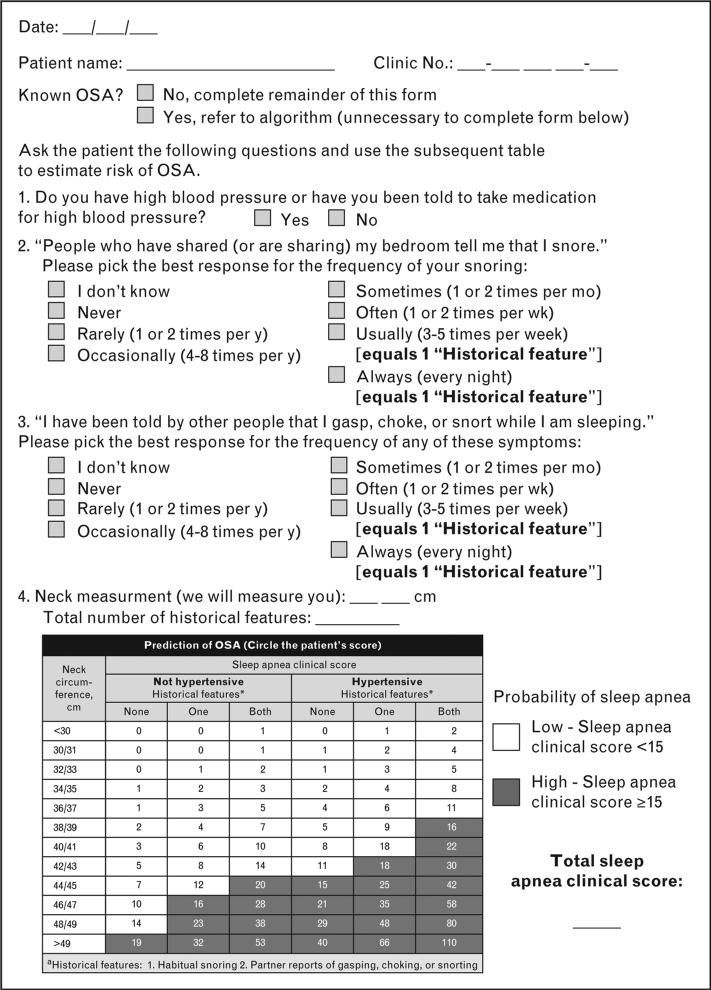
The Flemons criteria or sleep apnea clinical score (SACS) [26] shares similarities with STOP-BANG and the ASA screening tool in that it assesses hypertension, snoring, history of night-time airway obstruction, and neck circumference (Fig. 1) [26,27]. Patients with a high SACS have an 81% probability to have an AHI score greater than 10/h [26]. However, when Rowley et al. [28] evaluated four clinical screening questionnaires [26,29–31] and compared them to polysomnography, they found that for an AHI at least 10/h, the sensitivities ranged from 76 to 96%, the specificities from 13 to 54%, and the positive predictive value from 69 to 77%. Thus, a negative result from these tools does not exclude the possibility of OSA.
FIGURE 1.
Obstructive sleep apnea questionnaire. Adapted with permission from [27] and data from [26].
New technologies are emerging to diagnose OSA during sleep [32] and wakefulness [33] and are based on the analysis of tracheal sounds. The rationale behind these methods lies in the anatomical and physiological differences of the pharyngeal structures among individuals with and without OSA [34]. Such differences produce alterations in the inspiratory and expiratory airflow characteristics of the upper airways that are amenable to analysis with the power spectral density of the sound signal. However, although preliminary studies have used tracheal sounds with considerable accuracy, these methods are still experimental and need further validation.
Postanesthesia recovery in patients with known and suspicious obstructive sleep apnea
The 2006 ASA guidelines recommend that when OSA is highly suspected, the patient should undergo preoperative preparation and modified perioperative management [12]. The guidelines admit that the medical literature is insufficient to make specific recommendations regarding monitoring or postoperative disposition (monitored vs. nonmonitored wards) [12]. Given the low specificities of simple clinical assessment tools [28], monitoring of all patients in whom OSA is suspected would add considerable strain to healthcare. Yet, patients with unrecognized OSA have a substantial risk of respiratory complications [11].
So far, we have demonstrated that questionnaires and overnight pulse oximetry cannot exclude the presence of OSA precisely. Therefore, the only definitive test for OSA is polysomnography, but it is expensive and has limited availability. A possible solution for patients with OSA or suspected OSA was proposed by Gali et al. [27,35]. They described a distinctive, two-phase evaluation process that combines preoperative assessment with nursing respiratory assessments during phase I recovery from anesthesia, to identify patients at risk. In these studies, adult surgical patients with an expected hospital stay more than 48 h and without a known diagnosis of OSA were screened with SACS [26]. During phase I recovery, registered nurses continuously monitored patients during three 30-min periods for four specific assessments: hypoventilation, apnea, desaturations, and ‘pain/sedation mismatch’ (defined as the presence of moderate-to-severe pain occurring in a moderately sedated patient; Tables 1 and 2) [35–37]. Any patient who had a respiratory-specific event during any two 30-min periods was considered to have a ‘recurrent respiratory event’. Patients were then categorized into four groups on the basis of SACS preoperatively and recurrent respiratory events during phase I recovery.
Table 1.
Respiratory-specific assessment performed during phase I anesthesia recovery through postanesthesia care unit evaluation
| Assessment | Evaluation perioda | ||
|---|---|---|---|
| Bradypnea: <8 respirations per min (3 episodesb needed for ‘yes’) | Initial 30 min after extubation or PACU admission (whichever occurs later) | Second 30 min after initial evaluation (60 min after extubation or PACU admission) | Third 30 min after second evaluation (90 min after extubation or PACU admission) |
| Apnea: ≥10s (only 1 episode needed for ‘yes’) | |||
| Desaturations: pulse oxygen saturation <90% with nasal cannula (3 episodes needed for ‘yes’) | |||
| Pain/sedation mismatch: RASS score –3 to –5 and Pain Scale Scorec >5 (only 1 episode needed for ‘yes’) |
PACU, postanesthesia care unit; RASS, Richmond Agitation Sedation Scale.
The first respiratory assessment is obtained during the first 30 min after extubation. If no respiratory-specific assessments occur, the patient can be dismissed when discharge criteria are met (i.e., modified Aldrete criteria) [36]. However, if an event occurs during the first 30 min, the patient must have two consecutive evaluation periods (30 min each) free of further events before being discharged from phase I recovery. Patients who have recurrent events, but eventually meet phase I discharge criteria for care in the standard postoperative ward, have continuous pulse oximetry for at least 48 h.
Recurrent episodes are defined as any episode occurring at more than one evaluation period (not necessary to be same episode).
Pain Scale Score is a standard, 11-point scale that ranges from 0 (no pain) to 10 (worst pain imaginable).
Adapted with permission from [35].
Table 2.
Respiratory-specific assessment performed during phase I anesthesia recovery through Richmond Agitation Sedation Scale
| Score | Term | Description |
|---|---|---|
| +4 | Combative | Overtly combative or violent, immediate danger to staff |
| +3 | Very agitated | Pulls on or removes tubes or catheters, aggressive toward staff |
| +2 | Agitated | Frequent nonpurposeful movement or patient-ventilator dyssynchrony |
| +1 | Restless | Anxious or apprehensive, but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| –1 | Drowsy | Not fully alert, sustained (>10s) awakening, eye contact to voice |
| –2 | Light sedation | Briefly (<10s) awakens with eye contact to voice |
| –3 | Moderate sedation | Any movement (but no eye contact) to voice |
| –4 | Deep sedation | No response to voice, but any movement to physical stimulation |
| –5 | Unarousable | No response to voice or physical stimulation |
Adapted with permission from [37].
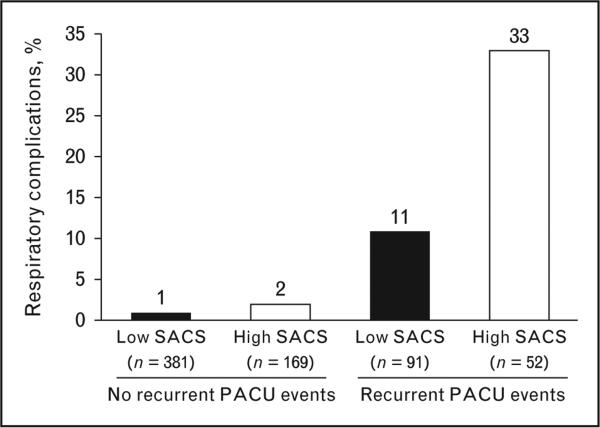
After discharge from phase I recovery, patients were monitored for episodes of ODI greater than 10 or other clinically meaningful respiratory complications (e.g., ICU admission for respiratory indication, noninvasive ventilatory support, pneumonia, respiratory arrest, respiratory therapy beyond clinical standards). In both studies, Gali et al. [27,35] found that patients with high SACS had more recurrent respiratory events in the recovery room. Episodes of ODI greater than 10 were more frequent among patients who had high SACS or had recurrent respiratory events. The likelihood of respiratory complications was 3.5-fold greater in patients with high SACS or patients who had a 21-fold increased chance of recurrent events (Fig. 2) [35]. The highest risk of complications was in patients with both high SACS preoperatively and recurrent events in the recovery room [35].
FIGURE 2.
Frequency of postoperative respiratory events. Events are shown in accordance with four patient groups defined by the combination of sleep apnea clinical score (SACS; low or high) and recurrent postanesthesia care unit (PACU) events (no or yes). A multiple logistic regression analysis included the SACS group and recurrent PACU events as explanatory variables and found that the likelihood of postoperative respiratory events was significantly associated with high SACS (odds ratio, 3.5; P = 0.001) and recurrent PACU events (odds ratio, 21.0; P < 0.001). Adapted with permission from [35].
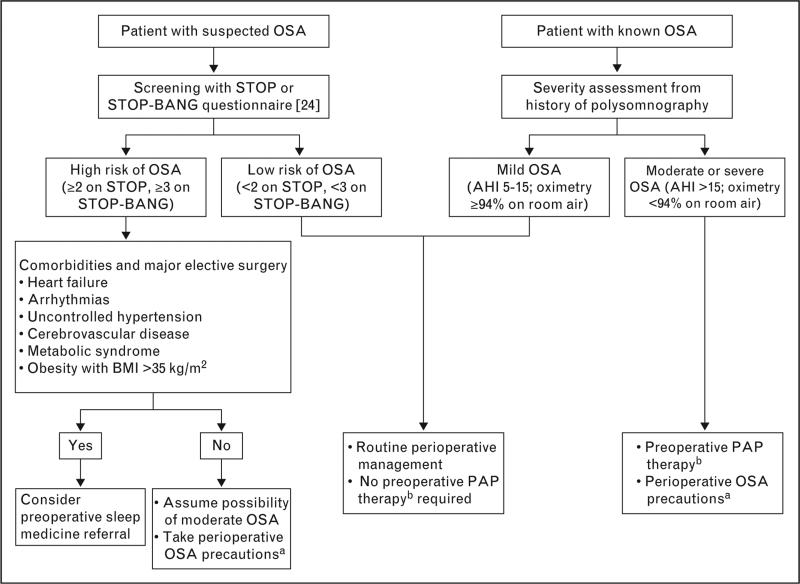
On the basis of these findings, Seet and Chung [38] proposed a postoperative management pathway for patients with known OSA or in whom OSA was suspected (Fig. 3). The pathway takes into consideration respiratory events in the recovery room. Although this approach has not been validated in prospective studies, it incorporates the two-phase assessment concept of Gali et al. [27,35] and then suggests an algorithm for triage to appropriate postoperative care.
FIGURE 3.
Perioperative management in known or suspected obstructive sleep apnea. AHI, apnea-hypopnea index; OSA, obstructive sleep apnea; PAP, positive airway pressure; STOP, snoring, tiredness, observed apneas, high blood pressure; STOP-BANG, snoring, tiredness, observed apneas, high blood pressure, BMI more than 35 kg/m2, age more than 50 years, neck circumference more than 40 cm, and male sex. a Perioperative OSA precautions include anticipation of possible difficult airway, use of short-acting anesthetic agents, opioid avoidance, verification of full neuromuscular block reversal, and extubation in a nonsupine position. b PAP therapy consists of continuous, bilevel, or autotitrating PAP. Adapted with permission from [38].
At Mayo Clinic, we have incorporated assessments of evaluation of respiratory-specific events in our discharge criteria from phase I anesthesia recovery (in addition to a modified Aldrete discharge criteria [36]). Any patient who has a respiratory-specific event must subsequently have two 30-min evaluation periods free of recurrent events before discharge from phase I recovery. In addition, any patient who has high SACS or recurrent respiratory events during phase I recovery but is identified as appropriate for discharge to a standard postsurgical ward is monitored remotely with continuous pulse oximetry for at least the first 48 postoperative hours.
PREDICTING POSTOPERATIVE ACUTE RESPIRATORY DISTRESS SYNDROME IN HIGH-RISK SURGICAL PATIENTS
A particularly concerning perioperative complication is PRF, which is defined as the failure to wean from mechanical ventilation within 48 h of a surgical procedure or an unplanned intubation/reintubation in the postoperative period [39■■]. Up to 5.4% of patients undergoing high-risk elective surgery may have PRF [40]. Numerous etiologic factors can lead to PRF, including hypercarbic respiratory failure from OSA, chronic obstructive lung disease exacerbation, and over-narcotization. A specific, common, and frequently lethal cause of PRF is ARDS, which accounts for approximately 35% of cases [40] and is characterized by injury to the alveolar-capillary barrier, with resultant alveolar flooding and hypoxemia [41]. Pathophysiologic mechanisms for ARDS include oxidative stress, lung deformation, inflammation, and intravascular coagulation [42]. Of note, the previously well-accepted American European Consensus Conference definitions for acute lung injury and ARDS [43] were revised recently by an ARDS Definition Task Force. In this revised Berlin Definition, acute lung injury is redefined as mild ARDS [44■■]. This modification was endorsed to help ensure that acute lung injury was not recognized inappropriately as a separate pathophysiologic entity.
The mortality rate of ARDS ranges from 25 to 45% [3■,40]. Recent estimates suggest that the incidence of postoperative ARDS is 3%, with the rate differing among different surgery types [45■]. Currently, supportive therapies continue to be the mainstay of ARDS management [46,47] and, therefore, ARDS prevention was identified as a key priority for the National Heart, Lung, and Blood Institute [48]. The importance of prevention was highlighted recently in a population-based study from Olmsted County, Minnesota [4]. This study demonstrated that over an 8-year period, the incidence of hospital-acquired ARDS decreased more than 50% despite the fact that critical illness was increasing in severity and that presenting critically ill patients had a higher prevalence of predisposing conditions for ARDS. This finding suggests that changes in hospital practices can reduce the incidence of this dreaded complication. However, a critical barrier to progress in the prevention of ARDS has been the lack of effective predictive models that can identify high-risk populations. Without early risk stratification, any ‘prevention strategy’ will be delivered either too late or to the wrong population.
Identifying risks of postoperative respiratory failure
Substantial work has been conducted to identify risks associated with PRF. A landmark study evaluated male patients undergoing noncardiac operations at 44 US Veterans Affairs medical centers [49]. The investigators developed and validated a respiratory failure risk index to facilitate the identification of high-risk surgical cohorts. Specific risks included the nature of the operation (i.e., abdominal aortic aneurysm repair, neurosurgery, and thoracic, upper abdominal, peripheral vascular, neck, and emergency surgical procedures), specific laboratory abnormalities (i.e., albumin, <3.0 g/dl; blood urea nitrogen, >30 mg/dl), and clinical characteristics (i.e., older age, low functional status, and chronic obstructive pulmonary disease). The model's predictive accuracy was good, with an area under the curve (AUC) for receiver operating characteristics of 0.84.
In an effort to address the study's limitation (restriction to male patients in the Veterans Affairs medical system) [49], the same group conducted a follow-up investigation that included a relatively limited number of non-Veterans Affairs institutions (128 Veterans Affairs medical centers and 14 non-Veterans Affairs academic medical centers) [50], but again, more than 80% of the study patients were men. The investigators again identified procedure type and urgency, increased age, chronic obstructive pulmonary disease, renal insufficiency, and hypoalbuminemia to be the risk factors for PRF. The ASA physical status, preoperative congestive heart failure, ascites, or sepsis, or a combination, were also strong predictors. In addition, factors such as preoperative dyspnea, cerebrovascular disease, hepatic dysfunction, smoking, alcohol abuse, and various laboratory abnormalities (e.g., elevated white blood cell count, hypernatremia, thrombocytopenia, anemia) were associated with PRF. However, the effect estimates for this latter group of predictor variables were somewhat modest, and such associations have been identified less consistently. Regardless, this 28-variable predictive model performed well with an AUC of 0.86 in the validation cohort.
More recently, Gupta et al. [39■■] evaluated the American College of Surgeon's National Surgical Quality Improvement Program database to identify robust preoperative risk factors for PRF while developing a parsimonious and more generalizable risk prediction model. Among the 468 795 patients in the combined derivation and validation datasets, PRF developed in 2.8%. Notably, the 30-day mortality rate was higher in patients who had PRF than in those who did not (25.6 vs. 0.98%). Variables identified as predictors included the procedure type and urgency, ASA physical status, dependent functional status, and preoperative sepsis. Despite a reduced number of variables, performance of the predictive algorithm remained good, with an AUC of 0.89 in the final model. Table 3 summarizes various risk factors for PRF.
Table 3.
Strength of evidence for associations among preoperative risk factors and development of postoperative respiratory failure
| Strong evidence | Moderate evidence | Fair evidence |
|---|---|---|
| Advanced age | Intermediate-risk proceduresa | Cerebrovascular disease |
| ASA physical status classification score >2 | Congestive heart failure | Impaired sensorium |
| High-risk proceduresb | Hypoalbuminemia | Hepatic dysfunction |
| Emergency procedures | Weight loss >10% | Preoperative dyspnea |
| Dependent functional status | Acute kidney injury/chronic kidney disease | Alcohol abuse |
| Chronic obstructive pulmonary disease | Ascites | Smoking |
| Sepsis |
ASA, American Society of Anesthesiologists.
Intermediate-risk surgical procedures include peripheral vascular surgery, lower abdominal surgery, ear-nose-throat surgery, and endocrine surgery.
High-risk surgical procedures include cardiac surgery, aortic aneurysm repair, thoracic surgery, upper abdominal surgery, and neurosurgery.
Although numerous studies have evaluated specific risks for postoperative ARDS [45■], development of models that can accurately identify persons at high risk for ARDS has not been emphasized historically. Recently, Gajic et al. [3■] stimulated interest in addressing this knowledge gap in the Lung Injury Prediction Score (LIPS) study. Their study was a large, multicenter prospective cohort investigation that evaluated patients admitted to the hospital from the emergency department with at least one major risk factor for ARDS. Major predisposing conditions for ARDS and potential ARDS modifying factors were collected and evaluated for potential inclusion in a prediction model. In the final model, validated ARDS risk factors (i.e., shock, aspiration, sepsis, pneumonia, high-risk surgical procedure, and high-risk trauma) and statistically significant and biologically plausible ARDS risk modifiers (i.e., alcohol abuse, obesity, hypoalbuminemia, chemotherapy, hypoxemia, tachypnea, acidosis, and diabetes mellitus) were combined to derive the LIPS.
The LIPS algorithm performed well, with a validation dataset AUC of 0.80 [95% confidence interval (CI) 0.77–0.84]. When evaluating model performance through an optimal LIPS cutoff score of 4, the LIPS facilitated identification of a cohort of patients with an ARDS rate of 17%. Unfortunately, the target population evaluated in the LIPS study (medical and surgical patients presenting to the emergency department with major risk factors for ARDS) precludes its use in the majority of surgical patients (e.g., patients undergoing elective surgery who do not have such major risk factors for ARDS as sepsis, pneumonia, pancreatitis).
To address this limitation, we recently developed and validated a risk prediction score that specifically targets patients undergoing elective high-risk surgery [45■]. To derive the Surgical Lung Injury Prediction (SLIP) model, we collected preoperative variables that had been associated previously with development of postoperative ARDS and evaluated them for inclusion in a final predictive algorithm. Consistent with prior reports [3■,40], the nature of the surgical procedure was the most influential factor in ARDS development (Table 4) [45■]. Additional predictive factors were diabetes mellitus, chronic obstructive pulmonary disease, gastroesophageal reflux, and alcohol abuse. The model accurately discriminated patients who had ARDS from those who did not, with an AUC of 0.82 (95% CI 0.78–0.86). The SLIP model (Table 5) [45■] generates a SLIP score that can be used to define groups at low, moderate, and high risk for postoperative ARDS. In the evaluated study population, the frequency of postoperative ARDS was 0.54% in the low-risk, 2.62% in the moderate-risk, and 12.2% in the high-risk groups.
Table 4.
Classification of cardiac, aortic vascular, and thoracic surgical procedures into low and high risk of acute respiratory distress syndrome
| Low-risk surgical procedures | ||
|---|---|---|
| Cardiac | Aortic vascular | Thoracic |
| Single valve repair | Primary abdominal aortic aneurysm repair | Video-assisted thoracoscopic surgery |
| ASD/VSD closure | Endovascular repair | Fundoplication surgery |
| Myectomy | Open lung biopsy | |
| Sternal wound revision | Wedge lung resection | |
| Pacemaker lead/device removal | Segmental lung resection | |
| High-risk surgical procedures | ||
|---|---|---|
| Cardiac | Aortic vascular | Thoracic |
| CABG | Descending thoracic aortic surgery | Multiple segmental lung resections |
| Valve replacement | Thoracoabdominal aortic surgery | Lobectomy |
| Multiple valve repair | Any revision aortic surgery | Multilobectomy |
| Pericardial resection | Pneumonectomy | |
| Ascending aortic/aortic arch repair | Esophagectomy | |
| Congenital heart repair | Lung decortication | |
| Cardiac transplantation | ||
| Cardiac reoperation | ||
ARDS, acute respiratory distress syndrome; ASD, atrial septal defect; CABG, coronary artery bypass grafting; VSD, ventricular septal defect. Adapted with permission from [45■].
Table 5.
Surgical Lung Injury Prediction model scoring criteria
| Criteria | SLIP pointsa |
|---|---|
| High-risk surgical procedure | |
| Cardiac | 19 |
| Vascular | 32 |
| Thoracic | 16 |
| Comorbidity | |
| Diabetes mellitus | 6 |
| COPD | 10 |
| GERD | 7 |
| Modifying condition | |
| Alcohol abuse | 11 |
COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; SLIP, Surgical Lung Injury Prediction.
The SLIP score is the summation of the SLIP points. Score less than 10 corresponds with low risk; 10-26, moderate risk; and ≥27, high risk. Adapted with permission from [45■].
A group of particular concern for ARDS development are patients who have severe trauma, in whom the prevalence of ARDS ranges from 12 to 30%, with a mortality rate as high as 80% [51–53]. Watkins et al. [53] studied trauma patients at risk for ARDS and identified advanced age, severity of illness, type of injury (e.g., blunt trauma, pulmonary contusion, flail chest), and need for massive transfusion as ARDS risks. Although the predictive accuracy of this model was modest [AUC 0.71 (95% CI 0.68–0.74)], the model did outperform previous ones that had attempted to predict ARDS in similar populations [54,55]. When evaluating the risk of trauma-related ARDS across studies, age, Injury Severity Score, massive transfusion, chest wall injuries, and pulmonary contusion appear to be relatively consistent predictors of ARDS [53–55].
CONCLUSION
Preoperative appreciation of factors associated with the development of severe postoperative respiratory complications allows us to tailor the perioperative care of high-risk patients that may lead to improved outcomes. We believe that patients with OSA and patients at high risk for development of postoperative ARDS represent specific groups that are amenable to preventive strategies.
KEY POINTS.
Patients with OSA are at increased risk for postoperative respiratory complications; however, when OSA is recognized, complications can be mitigated.
Formal diagnosis of OSA with polysomnography is cumbersome; thus, many surgical patients have unrecognized OSA.
Various simple clinical tools with variable sensitivities and specificities are used to attempt to identify patients at increased risk for OSA.
The ARDS is a common, life-threatening cause of postoperative hypoxemic respiratory failure, and because no effective treatment strategies exist, prevention is a major goal.
The accurate identification of high-risk patients is a key element in ARDS prevention, and recently published prediction models can facilitate this aim.
Acknowledgements
This work was supported by the Department of Anesthesiology, College of Medicine, Mayo Clinic, Rochester, MN 55905, USA.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 244–245).
- 1.Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–1563. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 2.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. Epub 2005 Apr 26. [DOI] [PubMed] [Google Scholar]
- 3.Gajic O, Dabbagh O, Park PK, et al. U. S. Critical Illness and Injury Trials & Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. Epub 2010 Aug 27. [This study details the development and validation of a LIPS that allows identification of patients at high risk for the ARDS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183:59–66. doi: 10.1164/rccm.201003-0436OC. Epub 2010 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Perez ER, Keegan MT, Brown DR, et al. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology. 2006;105:14–18. doi: 10.1097/00000542-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 7■■.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [This meta-analysis demonstrated that among patients without ARDS, protective ventilation with lower tidal volumes was associated with better clinical outcomes.] [DOI] [PubMed] [Google Scholar]
- 8.O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–26. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 9■.Weingarten TN, Flores AS, McKenzie JA, et al. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106:131–139. doi: 10.1093/bja/aeq290. Epub 18 October 2010. [This study found that the presence and severity of OSA were not associated with increased risk of postoperative respiratory complications in a cohort of bariatric surgical patients. The authors attribute these findings to their institution's practice of assessing all bariatric surgical patients for OSA with subsequent preoperative optimization, as well as appropriate postoperative management.] [DOI] [PubMed] [Google Scholar]
- 10.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–121. doi: 10.1213/ANE.0b013e3182009abf. Epub 2010 Nov 16. [DOI] [PubMed] [Google Scholar]
- 11.Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141:436–441. doi: 10.1378/chest.11-0283. Epub 2011 Aug 25. [DOI] [PubMed] [Google Scholar]
- 12.Gross JB, Bachenberg KL, Benumof JL, et al. American Society of Anesthesiologists Task Force on Perioperative Management. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093. doi: 10.1097/00000542-200605000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 15.Chiner E, Signes-Costa J, Arriero JM, et al. Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax. 1999;54:968–971. doi: 10.1136/thx.54.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung F, Liao P, Elsaid H, et al. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012. 114:993–1000. doi: 10.1213/ANE.0b013e318248f4f5. Epub 2012 Feb 24. [DOI] [PubMed] [Google Scholar]
- 17.Golpe R, Jimenez A, Carpizo R, Cifrian JM. Utility of home oximetry as a screening test for patients with moderate to severe symptoms of obstructive sleep apnea. Sleep. 1999;22:932–937. [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 20.Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology. 1999;52:125–131. doi: 10.1212/wnl.52.1.125. [DOI] [PubMed] [Google Scholar]
- 21.Netzer NC, Hoegel JJ, Loube D, et al. Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124:1406–1414. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 24.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 25.Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22:405–411. doi: 10.1097/ACO.0b013e32832a96e2. [DOI] [PubMed] [Google Scholar]
- 26.Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279–1285. doi: 10.1164/ajrccm.150.5.7952553. [DOI] [PubMed] [Google Scholar]
- 27.Gali B, Whalen FX, Jr, Gay PC, et al. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582–588. [PMC free article] [PubMed] [Google Scholar]
- 28.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–938. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 29.Crocker BD, Olson LG, Saunders NA, et al. Estimation of the probability of disturbed breathing during sleep before asleep study. Am Rev Respir Dis. 1990;142:14–18. doi: 10.1164/ajrccm/142.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 31.Viner S, Szalai JP, Hoffstein V. Are history and physical examination a good screening test for sleep apnea? Ann Intern Med. 1991;115:356–359. doi: 10.7326/0003-4819-115-5-356. [DOI] [PubMed] [Google Scholar]
- 32.Yadollahi A, Giannouli E, Moussavi Z. Sleep apnea monitoring and diagnosis based on pulse oximetry and tracheal sound signals. Med Biol Eng Comput. 2010;48:1087–1097. doi: 10.1007/s11517-010-0674-2. Epub 2010 Aug 24. [DOI] [PubMed] [Google Scholar]
- 33.Montazeri A, Giannouli E, Moussavi Z. Assessment of obstructive sleep apnea and its severity during wakefulness. Ann Biomed Eng. 2012;40:916–924. doi: 10.1007/s10439-011-0456-5. Epub 2011 Nov 9. [DOI] [PubMed] [Google Scholar]
- 34.Battagel JM, L'Estrange PR. The cephalometric morphology of patients with obstructive sleep apnoea (OSA). Eur J Orthod. 1996;18:557–569. doi: 10.1093/ejo/18.6.557. [DOI] [PubMed] [Google Scholar]
- 35.Gali B, Whalen FX, Schroeder DR, et al. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110:869–877. doi: 10.1097/ALN.0b013e31819b5d70. [DOI] [PubMed] [Google Scholar]
- 36.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–934. [PubMed] [Google Scholar]
- 37.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 38.Seet E, Chung F. Obstructive sleep apnea: preoperative assessment. Anesthesiol Clin. 2010;28:199–215. doi: 10.1016/j.anclin.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 39■■.Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140:1207–1215. doi: 10.1378/chest.11-0466. Epub 2011 Jul 14. [This study outlines risk factors for PRF and provides a contemporaneous risk prediction algorithm that can facilitate the identification of surgical patients who are at high risk for this complication.] [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Perez ER, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax. 2009;64:121–127. doi: 10.1136/thx.2008.102228. Epub 2008 Nov 6. [DOI] [PubMed] [Google Scholar]
- 41.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 42.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 43.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 44■■.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [This study uses recent data on ARDS epidemiology and pathophysiology to support development of the new Berlin Definition for ARDS as proposed by the ARDS Definition Task Force.] [DOI] [PubMed] [Google Scholar]
- 45■.Kor DJ, Warner DO, Alsara A, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011;115:117–128. doi: 10.1097/ALN.0b013e31821b5839. [This study summarizes previously identified risk factors for postoperative ARDS and presents a SLIP model that can be used to identify surgical patients who are at high risk for postoperative ARDS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 47.Wiedemann HP, Wheeler AP, Bernard GR, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. Epub 2006 May 21. [DOI] [PubMed] [Google Scholar]
- 48.Spragg RG, Bernard GR, Checkley W, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. Epub 2010 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery: the National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232:242–253. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson RG, Arozullah AM, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1188–1198. doi: 10.1016/j.jamcollsurg.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 51.Holland MC, Mackersie RC, Morabito D, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55:106–111. doi: 10.1097/01.TA.0000071620.27375.BE. [DOI] [PubMed] [Google Scholar]
- 52.Treggiari MM, Hudson LD, Martin DP, et al. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 2004;32:327–331. doi: 10.1097/01.CCM.0000108870.09693.42. [DOI] [PubMed] [Google Scholar]
- 53.Watkins TR, Nathens AB, Cooke CR, et al. Acute respiratory distress syndrome after trauma: development and validation of a predictive model. Crit Care Med. 2012;40:2295–2303. doi: 10.1097/CCM.0b013e3182544f6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller PR, Croce MA, Kilgo PD, et al. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68:845–850. [PubMed] [Google Scholar]
- 55.Navarrete-Navarro P, Rivera-Fernandez R, Rincon-Ferrari MD, et al. GITAN multicenter project. Early markers of acute respiratory distress syndrome development in severe trauma patients. J Crit Care. 2006;21:253–258. doi: 10.1016/j.jcrc.2005.12.012. [DOI] [PubMed] [Google Scholar]