Abstract
Unravelling the functional operation of neuronal networks and linking cellular activity to specific behavioural outcomes are among the biggest challenges in neuroscience. In this broad field of research, substantial progress has been made in studies of the spinal networks that control locomotion. Through united efforts using electrophysiological and molecular genetic network approaches and behavioural studies in phylogenetically diverse experimental models, the organization of locomotor networks has begun to be decoded. The emergent themes from this research are that the locomotor networks have a modular organization with distinct transmitter and molecular codes and that their organization is reconfigured with changes to the speed of locomotion or changes in gait.
Locomotion is the motor function that allows humans and other animals to interact with their surroundings. It takes the form of swimming in fish, flying in insects and birds, and over-ground locomotion in limbed animals, and is the output of numerous integrated brain activities that allow the animal to find its way, escape predators or search for food.
Although locomotion might seem effortless, it is a complex motor behaviour that involves the concerted activation of a large number of limb and body muscles. The planning and initiation of locomotion take place in supraspinal areas, including the cortex1, the basal ganglia2–4, the midbrain5,6 and the hindbrain7–9, but the precise timings and patterns of locomotor movements in vertebrates are generated by activity in neuron assemblies that are located in the spinal cord itself10,11 (FIG. 1). These neurons receive activating inputs from the brain and are able to produce the rhythms and patterns of locomotion that are conveyed to motor neurons and then to the axial and limb muscles, as first shown by Thomas Graham Brown more than 100 years ago in the cat12 and later confirmed in all vertebrates13. Additional layers of regulation come from the cerebellum, modulatory signals9,14–16 and sensory feedback17,18.
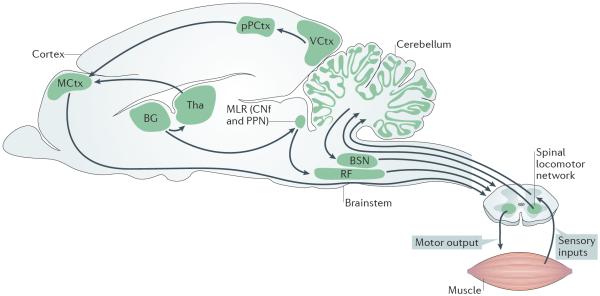
Figure 1. Organization of neuronal control of locomotion in vertebrates.
The selection and initiation of locomotor behaviour involves various regions of the brain and brainstem. Output neurons of the basal ganglia (BG) project both to the thalamus (Tha) — which sends projections to the motor cortex (MCtx) and other cortical areas — and to areas in the brainstem, including the mesencephalic locomotor region (MLR)2–4. Initiation of locomotion is thought to be mediated by the activity of neurons in the MLR7,9, including the cuneiform nucleus (CNf) and the pedunculopontine nucleus (PPN). MLR neurons project to neurons in the reticular formation (RF) in the hindbrain7,9. Neurons in the RF project to locomotor networks in the spinal cord that execute locomotion. Descending fibres from the vestibular and rubrospinal spinal pathways (brainstem nuclei (BSN))8 maintain posture and modulatory signals9,14–16 that regulate the ongoing locomotor activity. The cerebellum coordinates locomotor behaviour by mediating movement-generated feedback and internal feedback, as well as by modulating the activity in the descending pathways8. Proprioceptive sensory feedback modulates the activity of the spinal locomotor network17. Cortical activity (MCtx) provides visuomotor (VCtx) correction of locomotion via the posterior parietal cortex (pPCtx)1. Figure is adapted with permission from REF. 165, Springer.
Much of the early work on spinal locomotor networks was carried out in cats, in which it was shown that monoamine precursors could evoke locomotor-like neural activity in spinal cords that were isolated from the brain and sensory organs9,18,19. Vertebrate locomotion is now studied in several vertebrate models. Owing to their relatively limited number of neurons, less complex range of motor behaviours than those associated with limbs, and the possibility of performing comprehensive connectivity studies in vitro, the adult lamprey and the young tadpole have provided detailed insights into the organization of the spinal circuits that are involved in swimming11,20–26. However, important advances in our understanding of the organization of spinal locomotor networks in mammals are now emerging10,16,20,25,27–37. These advances have mostly been the result of combining electrophysiology with molecular mouse genetics to identify and/or to manipulate the activity of components of the spinal locomotor networks. In this way, it has been possible to probe these large-scale networks and reveal both molecular and physiological aspects of network organization in a behavioural context and to link neuronal populations to specific aspects of the behaviour. A similar approach has been instigated in the genetically tractable zebrafish20,25,27,29,30,38.
In this Review, I focus on these new advances in understanding locomotion, with an emphasis on comparing what is known about the spinal locomotor network organization in these model systems of non-limbed and limbed animals. I highlight general principles and differences in the intrinsic organization of vertebrate locomotor networks and underscore the functional network reorganization that may occur with changes in speed of locomotion or changes in gait. Finally, I point to unresolved issues regarding the functional operation of these fundamental motor networks.
Key features of locomotor networks
The spinal locomotor circuit is charged with the task of driving groups of motor neurons rhythmically in such a way that their concerted activity leads to appropriate motor output. In non-limbed animals, this involves the coordination of axial bends along the body; in limbed animals, it additionally involves the coordination of muscle activity within a limb and between pairs of limbs. Together with rhythm-generating circuits that may drive the activity in the network and set the speed of locomotion, left–right pattern-generating circuits are needed to secure the coordination between undulating movements on both sides of the body in non-limbed locomotion and between limbs in limbed locomotion. In limbed locomotion, flexor–extensor pattern-generating circuits are needed for intra-limb coordination. This functional modularity — with rhythm generation and two aspects of pattern generation — has served as a vantage point for deciphering the intrinsic organization of the locomotor networks.
Left–right coordinating circuits
Appropriate locomotion requires the coordination of muscle activities on the left and right sides of the body. Groups of commissural neurons (CNs) that have their axons crossing the midline provide the lines of communication that are needed to link bilateral activity. In limbed animals, changes in speed are often followed by changes in gait that involve changes in left–right coordination. A significant feature of the integrated control of CNs during locomotion is, therefore, to provide changes in left–right coordination at different speeds of locomotion. An understanding of these dynamic network configurations is starting to arise in which diverse groups of CNs are organized in a modular manner to secure alternating and synchronous activity in bilateral pairs of limbs during different locomotion speeds.
Dedicated CN populations encode gaits
The CNs that are involved in motor control in mammals are localized in the ventral spinal cord and constitute a heterogenous group of neurons with respect to both their projection patterns39–41 and their transmitter phenotype (that is, being glutamatergic, glycinergic or GABAergic42–44).
Crossed inhibition in mammals may be accomplished in two ways: directly by inhibitory CNs acting on motor neurons (or interneurons) or indirectly by excitatory CNs, which act on premotor inhibitory neurons28,45–47. By contrast, crossed excitation may be obtained by excitatory CNs acting directly on motor neurons (or interneurons)28,45–47. The dual inhibitory pathway is tuned to support alternating — out-of-phase — muscle activity across the cord, whereas the excitatory pathway is tuned to support synchronous — in-phase — muscle activity. Accordingly, this complex CN system has been proposed to be involved in segmental left–right alternation and in promoting in-phase firing of motor neurons on either side of the spinal cord during locomotion in tetrapods46,47. Experiments using the genetic ablation of identified CNs have provided broad support for this conjecture.
V0 neurons, which are characterized by their early expression of the transcription factor developing brain homeobox 1 (DBX1)48,49, constitute a large proportion of the CNs in the ventral spinal cord. Genetically driven ablation of V0 neurons abolishes the ability to produce left–right alternating gaits at all frequencies of locomotion50, which complements observations of disrupted left–right alternation when Dbx1 is deleted in spinal neurons49. The V0 population is subdivided into inhibitory V0D neurons that derive from paired box protein 7 PAX7-positive (PAX7+) progenitor cells and excitatory V0V neurons that derive from PAX7-negative (PAX7−) progenitor cells and that later express homeobox even-skipped homologue protein 1 (EVX1)48–50 (FIG. 2a). Through the use of selective ablation of these two populations it was shown50 that the inhibitory V0D population secures hindlimb alternation at low locomotor frequencies, whereas the excitatory V0V population maintains hindlimb alternation at high frequencies of locomotion (FIG. 2b). The functional importance of these findings becomes clear when the V0-related deficits are compared with spontaneous locomotor gaits that are observed in wild-type mice. Wild-type mice display four basic gaits: two alternating gaits, walk and trot; one synchronous gait, bound; and an intermediate gait, gallop51,52. The four gaits are expressed at different frequencies of locomotion, with walk expressed at the lowest frequency and bound at the highest (FIG. 2c). Walk and trot are phenotypically different, although both show alternation between bilateral pairs of limbs: the diagonal front and hind legs move forwards and backwards together in trot, whereas three limbs are simultaneously on the ground during walking. During bound, pairs of hindlimbs and pairs of forelimbs are moved in synchrony, whereas during gallop the pairs of hindlimbs and forelimbs are slightly out of phase. In the V0-deleted animals, bound is the only gait that can be executed at all speeds of locomotion51 (FIG. 2c). In V0V-deleted animals, trot is completely absent but walk is present at low frequencies of locomotion, and gallop and bound are present at higher speeds of locomotion51 (FIG. 2c). This analysis suggests that the activities of the V0D and V0V populations lead to two distinct alternating gaits, walk and trot, that are expressed in non-overlapping frequencies of locomotion. By contrast, synchronous activity is the result of non-V0 CNs. This functional dichotomy of the V0 population resembles the organization of the electrophysiologically described dual inhibitory pathway28,45–47.
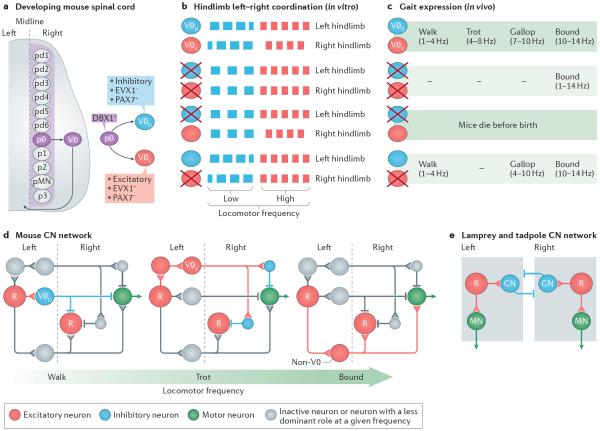
Figure 2. Multiple left–right coordination circuits.
Diverse commissural neurons (CNs) control left–right coordination at different frequencies of locomotion in mice. a | V0 CNs develop from the p0 progenitor domain in the ventral spinal cord. There are five specific progenitor domains (p0–p3 and pMN) in the ventral cord and six in the dorsal cord (pd1–pd6), each of which is characterized by differential expression of transcription factors (see REFS 29,84,166). When the progenitor cells mature, they migrate laterally and become neurons or motor neurons. p0 progenitors express developing brain homeobox 1 (DBX1) and develop into inhibitory dorsal V0 neurons (V0D neurons), which express paired box protein 7 PAX7 (PAX7+) but not homeobox even-skipped homologue protein 1 (EVX1) (EVX1−), and excitatory ventral V0 neurons (V0V neurons), which are PAX7− but EVX1+. b | Genetic ablation of V0 neurons leads to the loss of hindlimb alternation at all locomotor frequencies in vitro, as indicated by the switch from out-of-phase left–right locomotor-like activity in wild-type mice (two upper rows) to in-phase locomotor-like activity (third and fourth rows). V0D neurons secure alternation at low frequencies: deletion of V0D neurons leads to synchronous left–right locomotor-like activity at low frequencies but maintained alternation at high frequencies of locomotor-like activity (fifth and sixth rows). V0V neurons secure hindlimb alternation at high frequencies of locomotion: deletion of the V0V neurons leads to synchronous left–right activity at high frequencies but maintained alternation at low frequencies of locomotor-like activity (seventh and eight rows). c | In the presence of V0 neurons, mice express four gaits at different frequencies of locomotion: walk, trot, gallop and bound. When V0 neurons are ablated, mice only express bound at all frequencies of locomotion, whereas when V0V neurons are ablated, mice lack trot but can express walk and gallop, which is now expressed in the frequency range at which trot is normally expressed. V0D neuron-ablated animals do not survive postnatally and therefore their gaits were not tested. d | Proposed recruitment of V0D- and V0V-related pathways in response to increased locomotor frequency. At low frequencies of locomotion that correspond to walk (left-hand panel), inhibitory V0D CNs are activated by rhythm-generating neurons (R) on the same side of the cord. Their activation leads to the inhibition of locomotor networks on the other side of the cord, including motor neurons (MNs). At higher frequencies of locomotion that correspond to trot excitatory (middle panel), V0V commissural neurons are recruited. Their activation causes the inhibition of locomotor networks on the other side of the cord, including MNs via local inhibitory neurons (blue). At very high frequencies of locomotion that correspond to bound (right-hand panel) left–right synchrony is secured by excitatory non-V0 neurons (red), which are possibly V3 neurons that originate from single-minded homologue 1 (SIM1)-expressing progenitor cells54. Note that a single neuron in the diagrams represents a group of neurons. e | Proposed CN network in lamprey and tadpole. The core of the network is made up of inhibitory CNs that cross the midline and inhibit excitatory neurons rhythm-generating neurons and MNs on the other side of the cord (as indicated by the square box). Part a is based on data from REFS 48–50. Part b is based on data from REF. 50. Part c is based on data from REF. 51. Part d is based on data from REFS 47,50,51,83,87. Part e is based on data presented in REFS 11,20,24,26.
To attain alternation, the V0D–V0V populations need to impose actions on locomotor-related neurons on the other side of the cord. A recent modelling study has incorporated the V0D–V0V population pathways in a general model of the limb locomotor network and has proposed a scheme for the connectivity and recruitment of the different CNs during different speeds of locomotion53. Such connectivity and recruitment patterns await further studies.
The experiments and the modelling studies do suggest, however, that the V0D and V0V populations are recruited in an ascending order as speed increases (FIG. 2d). Thus, at low speeds of locomotion, the V0D population is active and results in walk (FIG. 2d), whereas at higher speeds of locomotion, the V0V populations become active and result in trot (FIG. 2d). At the highest speed of locomotion, the left–right synchronizing circuits that lead to bound (FIG. 2d) become active while the actions of the left–right alternating circuits are suppressed or overridden. The molecular identity of this excitatory left–right synchronizing circuit has not yet been determined. In addition to V0V CNs, the only known group of excitatory CNs in the ventral spinal cord is V3 neurons, which express the transcription factor single-minded homologue 1 (SIM1). The V3 CNs project directly to contralateral motor neurons and interneurons54 and are rhythmically active during locomotion55. When V3 neurons are removed from the cord, spinal locomotor activity shows increased variability in the locomotor burst amplitude and period with no clear disruption of left–right alternation. However, the involvement of these cells in left–right synchrony has not been directly tested, and it therefore remains to be seen whether this function can be ascribed to V3 neurons or whether it is secured by an as yet molecularly undefined group of excitatory CNs.
Of relevance to speed-dependent changes in gait, a genome-wide association study in horses identified a mutation in doublesex- and mab-3-related transcription factor 3 (Dmrt3), which leads to the expression of a truncated form of DMRT3 (REFS 56,57). When the mutation is homozygotic in Icelandic horses, these animals express a gait that is normally not expressed, namely, pace (which is characterized by the legs on the same body side moving forwards or backwards in synchrony). However, when this mutation is homozygotic in other breeds of horse, it leads to the expression of ambling gaits, which are various gaits that are characterized by three to four hooves being on the ground but with a faster speed than walk. The Dmrt3 mutation is also abundant in harness race horses, presumably allowing them to run faster in a trot before switching to a gallop. DMRT3 is expressed in interneurons in the spinal cord, including inhibitory CNs that originate from dorsal dI6 progenitor cells. Both flexor and extensor and left–right coordination are affected in mice with the Dmrt3 mutation and their maximal speed of locomotion is reduced. The variability of the effects on gait (involving the addition of new gaits in some horse breeds but the prevention of gait transitions in others) combined with fate change or transdifferentiation of neurons induced by the gene mutant makes it difficult to ascribe a specific function to the dI6 neuronal population during normal locomotion. To address this issue, it would be advantageous to selectively activate or inactivate dI6 neurons in mice rather than rely on gene mutation, which may not remove neurons from the network but which may lead to transdifferentiation.
Left–right coordination of axial muscles
In young tadpoles and adult lampreys, left–right alternation is thought to be organized by inhibitory glycinergic CNs that have projections to motor neurons and locomotor circuit neurons on the contralateral side11,23,58 (FIG. 2e). Active inhibitory CNs on one side of the body inhibit motor activity by inhibiting CNs, excitatory neurons and motor neurons on the other side of the body, providing an axial bend. Glutamatergic CNs have been described in the tadpole: dorsolateral CNs that carry information from skin sensory neurons across the midline of the cord59 and CNs that are active during struggling60, a form of rhythmic activity in which an undulating wave propagates from the tail to the head. In the lamprey61, glutamatergic CNs are also present and thought to be involved in promoting left–right synchrony11. Although the molecular code for CNs has not been delineated in lampreys and tadpoles, it has been at least partly deciphered in larval zebrafish62. As in mice, the V0 population is divided into inhibitory V0D neurons and glutamatergic V0V neurons. There are four morphological classes of V0V neurons, one of which is the multipolar commissural descending (MCoD) neurons that seem to be involved in rhythm generation rather than in left–right alternation (see below). Decisive information about the role of the other classes of inhibitory and excitatory V0 neurons for left–right coordination has not yet been obtained in zebrafish larvae30,63,64.
The difference between the leg-less locomotor network and the mammalian locomotor network is the lack of evidence for the existence of an indirect inhibitory pathway mediated by excitatory CNs for axial locomotor networks in leg-less animals. The extra layer in the left–right alternating circuits that is observed in limbed animals seems to be required in order to execute specific alternating gaits that are expressed at different speeds of locomotion. A recent report using monosynaptically restricted trans-synaptic labelling from axial and limb muscles in mice indeed showed that inhibitory commissural premotor circuits are more dominant in axial muscles than in limb muscles65, possibly a remnant of these functional differences.
Excitatory neurons set the tempo
Studies in both limbed and non-limbed animal models have provided strong evidence that the rhythm, as well as the rhythmic drive, in the locomotor circuit and onto motor neurons comes from activity in ipsilaterally projecting excitatory (and in most species solely glutamatergic) neurons9–11,20,24–27,29. Therefore, blocking fast glutamatergic transmission leads to the cessation of rhythmic activity, and a coordinated rhythm can for the most part persist in one-half of the spinal cord, suggesting that CNs are not needed for rhythm generation per se9–11,20,24,29.
The cardinal feature that characterizes rhythm-generating neurons is that their selective activation should be able to initiate the rhythm and/or change the frequency of the ongoing rhythm. Conversely, a selective reduction in the number of rhythm-generating neurons or their broad removal should reduce the frequency of the ongoing locomotor rhythm or completely block its expression. These criteria are not completely exclusive: activation of an initiating descending command system may also evoke locomotion and/or change its frequency. Similarly, the inactivation of permissive neuronal systems may reduce locomotor frequency. However, these general criteria have served as guidelines for identifying groups of excitatory neurons as part of the rhythm-generating circuits.
Rhythm generation in tadpoles and lampreys
In young tadpoles and adult lampreys, electrophysiological and anatomical studies have identified groups of locomotor-related ipsilaterally projecting excitatory neurons11,20,24,26,66–69. Their intrinsic connectivity includes electrical coupling and reciprocal excitation in tadpoles67,68 (FIG. 3a) and excitatory synaptic coupling in lampreys70 (FIG. 3b). On the basis of their target connections in the spinal cord (which include motor neurons and CNs), their cellular properties and modelling studies, these excitatory neurons have been proposed to constitute the rhythm-generating core in both tadpoles and lampreys11,20,24,26 (FIG. 3a,b). In lampreys, rhythm-generating networks are represented throughout the ~100 spinal segments, with one unit in each segment11, whereas in tadpoles, the rhythm-generating network seems to be restricted to the rostral spinal cord and lower brainstem, although excitatory neurons are also found throughout the spinal cord24,26. Owing to limited genetic access in lampreys and tadpoles, no attempts have been made to manipulate the excitatory circuits, and only anecdotal reports exist about perturbations of individual neurons that lead to changes in the ongoing rhythm. Thus, in the strictest sense, the causal role of the excitatory neurons in generating the rhythm has not been directly shown in these species. With optogenetic methods now being used in tadpoles71 and the possibility of carrying out genetic manipulation with high fidelity in almost any animal72, this issue might soon be addressed.
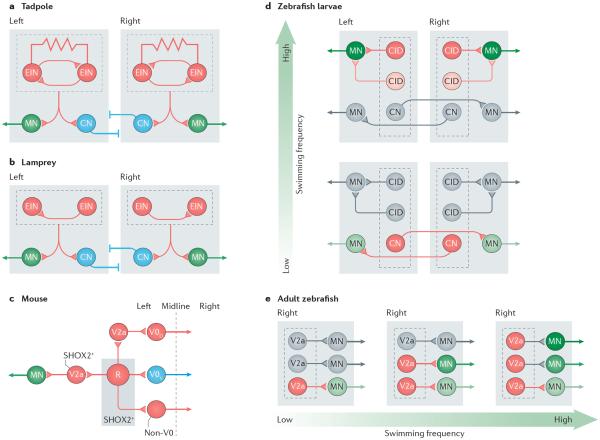
Figure 3. Organizational and molecular delineation of rhythm-generating circuits.
Rhythm-generating circuits are excitatory in all the vertebrates that have been investigated. a,b | In tadpoles, the circuit is composed of reciprocally and electrically connected glutamatergic and cholinergic neurons (excitatory interneurons (EINs)) that are located in the hindbrain and the spinal cord. In lampreys, EINs are glutamatergic, with synaptic connections to other EINs, and are located in each segment along the spinal cord. EINs drive motor neurons (MNs; which project to the muscles) and inhibitory commissural neurons (CNs; with axons projecting to the other half of the cord) on the same side of the cord. Inhibitory neurons with connections on the same side of the cord are omitted in the diagrams (for example, see REF. 26). c | Proposed circuits for the rhythm-generating circuit in the mouse spinal cord are shown. The rhythm-generating circuit (R) is composed of neurons that express the transcription factor short stature homeobox protein 2 (SHOX2). Rhythm-generating circuits drive left–right alternating circuits (V0D–V0V), including V2a neurons that express the transcription factor ceh 10 homeodomain containing homologue (CHX10), and neurons that are both CHX10- and SHOX2-positive (V2a SHOX2+) that presumably connect to motor neurons. Rhythm-generation circuits also drive left–right synchronizing circuits (non-V0, possibly of the V3 class). Only the left side of the circuit is shown. Blocking the synaptic output of SHOX2+ neurons or optogenetic silencing these neurons disrupts the rhythm without completely abolishing it, suggesting that as yet unidentified EINs contribute to the rhythm. d | The rhythm-generating circuits in zebrafish larvae are composed of CNs that belong to the excitatory multipolar commissural descending type (MCoD) neurons and circumferential ipsilateral descending (CID) neurons, which are analogues of V0V and V2a mouse neurons, respectively. The MCoDs (CNs) are active at low swimming frequencies but are silenced as the swimming frequencies increase (>40 Hz). The probability of CID (V2a) neuron firing increases with frequency, with the most dorsal neuron active at the highest frequencies (60–90 Hz). Laser ablation of MCoD neurons abrogates slow swimming, whereas ablation of dorsal CID (V2a) neurons abolishes high frequency swimming frequency. e | In the adult zebrafish, three groups of rhythm-generating V2a neurons innervate slow, intermediate and fast MNs. The three groups of V2a neurons are recruited incrementally (as indicated by the colour change) in a modular manner that reflects an ordered recruitment of slow, intermediate and fast MNs as the speed of swimming increases. Parts a and b are based on data from REFS 11,20,24,26,67. Part c is based on data from REFS 50,83,87,92. Part d is based on data from REFS 30,63,64,98,99. Part e is based on data from REFS 25,102.
Rhythm generation in mammals: multiple contributors
The role of excitatory neurons in rhythm generation has been tested in mice through the use of optogenetic activation or inactivation of the spinal excitatory neurons73,74. Optogenetic activation of the excitatory neurons initiates locomotor-like activity, whereas inhibition of these neurons blocks such activity, providing strong evidence that excitatory neurons are both sufficient and necessary for rhythm generation in the mammalian spinal cord.
When spinal excitatory neurons are optogenetically activated in a regionally confined manner, the rhythmic motor output is limited to restricted flexor-related or extensor-related motor neurons74. These findings are incompatible with a classic `half-centre model' that is composed of flexor and extensor networks that are mutually connected but in which the individual half-centres (flexor or extensor) are unable to burst without reciprocal activity19. These results are also at odds with the idea of a localized rhythmogenic centre75,76 or a dominating flexor-burst model, which relies on a single rhythmogenic flexor circuit that drives the motor neurons of the flexors and at the same time inhibits tonic activity in the motor neurons of the extensors35,77. Rather, the evidence implies that the rhythm-generating circuits in rodents — at least when these circuits are optogenetically activated — comprise multiple rhythm-generating circuits or modules that are functionally arranged in close association with the motor neuron pools that they control.
This concept shares similarities with the unit-burst generator concept proposed by Grillner13 to explain the organization of flexor and extensor activity around joints during locomotion in cats and to account for the segmental rhythm generation during swimming in lampreys11. The presence of multiple flexor and extensor rhythm-generating modules provides large flexibility to the network composition78, including the intrinsic capability of the spinal cord to control flexor and extensor motor neurons in a sequential recruitment pattern that is different from pure flexion and extension78–80: a capability that is laid down early in ontogeny and that is already present at birth, at least in rodents78,80 (however, also see REF. 81). The presence of flexor-and extensor-rhythm generation does not exclude the fact that flexor bursting might be predominant under certain circumstances. Thus, locomotor activity is readily modelled with dominant flexor bursting53 and when the composite flexor-and extensor-related lateral motor column is genetically converted into a flexor-related motor column, flexor bursting is still possible in the absence of extensor bursting80,82; this has led to the speculation that flexor bursting is the phylogenetic expression of burst activation of the axial muscles in leg-less animals80,82.
In an attempt to define which of the many groups of excitatory neurons in the spinal cord are involved in rhythm generation, different subpopulations of neurons have been experimentally targeted. The first excitatory group that has been targeted is the V2a neurons83. These neurons express the transcription factor ceh-10 homeodomain containing homologue (CHX10; also known as VSX2)29,37,84 and make up a large contingent of excitatory ipsilaterally projecting neurons in the locomotor region of the spinal cord85,86. However, their chronic elimination does not change the frequency of the ongoing rhythm, although left–right alternation is affected in the absence of V2a neurons, especially at higher frequencies of locomotion83,87. V2a neuron-ablated animals also show an alternating gait at low speeds of locomotion and synchronous gaits in the frequency range in which trot is normally expressed87, much like the V0V-deleted animals50,51. Furthermore, V2a neurons project to V0V neurons83 and to motor neurons88. They are also active over a wide range of locomotor frequencies89,90 but fire more strongly at higher frequencies91, which is compatible with a subpopulation of V2a neurons being involved in directly driving the V0V pathway at higher speeds of locomotion53 (FIG. 3c). So, rather than being rhythm generating, the V2a neurons seem to be downstream of the rhythm-generating neurons.
A recent study linked another ipsilaterally projecting excitatory interneuron population that is marked by the expression of the transcription factor short stature homeobox protein 2 (SHOX2) to rhythm generation in rodents92. SHOX2-expressing (SHOX2+) neurons partially overlap with V2a CHX10-expressing neurons so that the expression of SHOX2 and CHX10 divides neurons into three constituent populations of excitatory neurons in the ventral spinal cord: V2a SHOX2-negative (SHOX2−), V2a SHOX2+ and nonV2a SHOX2+ neurons92. Optogenetic silencing or the blockade of the synaptic output of the SHOX2+ neuronal population substantially perturbed the rhythm without completely blocking it or changing the coordination of the locomotor pattern. By contrast, genetic ablation of the V2a SHOX2+ neuronal population had no effect on the rhythm but affected the amplitude modulation. Thus, by deduction, it seems that nonV2a SHOX2+ neurons participate in rhythm generation in rodents, whereas the V2a SHOX2+ neurons directly drive motor neurons, which is different from the role of the V2a SHOX2− neurons that secure left–right alternation (FIG. 3c). This key role of SHOX2+ neurons in the locomotor network is supported by their rhythmic activity and connectivity, including synaptic connections between SHOX2+ neurons92.
The studies of V2a and SHOX2+ neurons show that excitatory neurons in the mouse locomotor network have diverse roles, and that rhythm generation is not mediated by a homogenous group of excitatory neurons. It is unclear which other groups of excitatory neurons, aside from the SHOX2+ neurons, constitute the rhythm-generating circuit, although neurons expressing the transcription factor HB9 (basic helix–loop–helix domain containing, class B, 9) may be candidates. HB9-expressing neurons constitute a small group of rhythmically active neurons in the ventral spinal cord with rhythmogenic cellular properties and connectivity that support a role in rhythm generation35,93–96. However, selective perturbation of the activity of this group has not so far been reported. Another group of excitatory neurons are the dI3 neurons, which represent one of the six classes of dorsally derived interneurons. These cells directly project to motor neurons, but elimination of synaptic output from the dI3 neurons does not affect locomotion97. Therefore, the molecular identity of other contributing neurons to rhythm generation in the rodent spinal cord remains elusive. Similarly, only rudimentary knowledge exists regarding the possible contribution of excitatory neurons in the cat spinal cord to rhythm generation10.
Rhythm generation: swimming speed
In zebrafish larvae, two groups of excitatory neurons have been shown to be involved in rhythm generation and the excitatory drive in locomotor circuits: the MCoD neurons (which belong to the V0V group of neurons and the circumferential ipsilaterally descending (CiD) neurons63,64. The CiD neurons express a transcription factor that is homologous to CHX10, the V2a marker, in mice98. The CiD neurons (or V2a) neurons provide dual synaptic and gap-junction-mediated excitation of motor neurons on the same side of the cord, and in zebrafish larvae they are distributed in a ventro-dorsal pattern that is related to their recruitment order. Zebrafish larvae produce short bouts of swimming when they are startled, and these bouts begin with high bending frequencies (70–90 Hz) of the axial muscles that then slow during the bout (to around 15–20Hz). The most dorsal CiD-V2a neurons are only recruited at the highest swimming frequencies (the beginning of the bout) and drop out as the swimming frequency slows down, when more ventral CiD-V2a neurons are engaged. These ventral CiD-V2a neurons in turn fire less reliably at the very slowest swimming frequencies when the MCoD neurons are rhythmically active63,64,98. The MCoDs provide dual synaptic and gap-junction-mediated excitation of motor neurons on the other side of the cord63,64. When MCoD neurons are laser ablated from the cord, the ability to produce slow swimming is severely reduced64. These findings suggest that MCoD neurons regulate slow swimming and that the CiD-V2a neurons have differential involvement in the regulation of medium to high swimming frequencies (FIG. 3d). Consistent with these data, selective laser ablation of the most dorsal CiD-V2a neurons has no effect on slow and medium frequency swimming64 but the absence of these neurons does reduce the maximal frequency of swimming that can be obtained99. Moreover, randomized laser ablation of CiD-V2a neurons in the larvae spinal cord increases the threshold for evoking swimming and reduces the maximal frequency of locomotion that can be obtained, leaving the presence of mostly slow swimming99. By contrast, the optogenetic activation of V2a neurons causes stable slow (around 20 Hz) swimming with little frequency modulation100. The reason for this observed discrepancy in the frequency control between the ablation and activation experiments remains to be resolved.
The experiments in zebrafish larvae suggest that rhythm generation has a modular organization that is distributed in to two morphologically and molecularly distinct groups of neurons that are active at different speeds of locomotion (FIG. 3d). Even in the V2a group of neurons, further functional subdivisions may appear. For example, in zebrafish larvae, subsets of rhythm-generating V2a neurons have targeted outputs with mutually exclusive inputs to motor neurons innervating either ventral or dorsal axial musculature101. In adult zebrafish, V2a neurons are divided into three circuits that are recruited at increasing swimming speed, securing the activation of motor neurons with increasing recruitment thresholds102,103 (FIG. 3e).
The V2a and V0V neurons seem to have attained a different role in the control of locomotion during evolution. In legged animals these neuronal types do not seem to be directly involved in rhythm generation but are instead involved in pattern generation. No obvious explanation exists for this lack of evolutionary conservation but it underscores the point that, although the known molecular markers may determine many aspects of cellular function (for example, transmitter phenotypes and axonal projections), they do not transfer the full complement of the functional phenotypes with the additional layer of control that is observed when locomotion involves legs. Moreover, although the rhythm-generating excitatory neurons that are found in tadpoles and lampreys may correspond to the rhythm-generating V2a neurons in zebrafish, the homologue of MCoD-V0V neurons has not yet been described in these species. Therefore, the combined evidence from experiments in zebrafish may suggest a finer-grained organization of the rhythm-generating circuits that drive axial muscle activity — with respect to the diversity of cellular types involved and speed-dependent recruitment pattern — than has previously been appreciated from the earlier studies in tadpoles and lampreys. Similarly, it remains to be resolved in mammals whether different groups of rhythm-generating excitatory neurons are responsible for driving the left–right coordinating circuits that are expressed at different speeds of locomotion or different gaits.
Cellular mechanisms contribute to rhythm generation
Identifying excitatory neurons as rhythm generating does not alone address in what way the rhythm is generated. Rhythm generation is often thought of as being generated by cellular properties in combination with circuit architecture. Three main mechanisms have been proposed to account for the rhythmogenesis itself: the pacemaker mechanism, in which some neurons have inherent rhythmic bursting capability; the network mechanism, in which the rhythm emerges as a result of interactions between neurons; and the combination of these two mechanism96,104,105. Pacemaker properties that are conditional on the presence of glutamate and that arise from the activation of the NMDA glutamate receptors (NMDARs) have been described in spinal cord neurons and motor neurons in lampreys, rodents and amphibians106–110. In lampreys, NMDA-induced pacemaker activity has a role in generating slow swimming11, and in tadpoles NMDAR activation in excitatory neurons sustains swimming24,110. It is uncertain whether NMDAR-mediated properties have a pivotal role for rhythm generation in mammals because blockade of their activation does not prevent rhythmic activity111, and they are not needed for inducing rhythmic activity112.
The persistent sodium current (INaP), which is native to many spinal cord neurons, including excitatory neurons, has been suggested to be involved in rhythm generation in mammals. It is activated in the sub-threshold range of the transient sodium current that underlies action potentials and it promotes pacemaker properties113–116. The expression of INaP may be regulated as a consequence of changes in extracellular calcium and potassium concentrations during locomotion, suggesting that INaP-mediated pacemaker properties are activated during network activity117. Blockade of the persistent sodium conductance severely affects the ability to produce a rhythmic motor output114,115, and expression of INaP in excitatory neurons in network models readily endows the network with rhythmogenic properties53. However, blocking INaP impairs the spiking mechanism in neurons, including motor neurons115. Consequently, neurons are converted from multi-spiking to single-spiking neurons. Altogether, although there is evidence that points to a role for INaP in promoting rhythm generation in cooperation with network properties, similar to what has been suggested (and debated) for rhythm generation in respiratory networks105,118, definitive proof is still unavailable. To address this issue, a conditional genetic approach that targets the INaP in specific interneuron populations may be needed. Moreover, a number of other cellular properties may contribute to pattern and phase transitions (BOX 1). The role of these cellular properties for network function also needs to be further evaluated.
Coordinating flexors and extensors
During limbed locomotion, the motor neurons that control flexor and extensor muscles around different joints within a limb need to be activated in a precise alternating and sequential pattern. This allows the limb to be flexed and cleared from the ground and to be brought forwards during the swing phase before it is extended to support the stance phase. In general terms, when a group of flexor motor neurons around a joint is inhibited, the corresponding extensor motor neurons around the same joint are excited, and vice versa. During locomotion, both flexor and extensor motor neurons receive alternating inhibitory and excitatory drive119,120 and in the absence of any inhibition, flexors and extensors are contracted in synchrony36. This push–pull organization implies that rhythmic alternation of inhibition and excitation in flexors and extensors is driven by rhythm-generating circuits the activity of which is out of phase: that is, alternating. The flexor–extensor alternation appears to be organized in layers with circuits directly upstream of flexor and extensor motor neurons and circuits between flexor and extensor rhythm-generating modules.
Reciprocal inhibitory neurons encode alternation
A group of inhibitory interneurons that are directly upstream of motor neurons are reciprocal-Ia-inhibitory neurons (rIa-INs). rIa-INs are activated by stretch-activated group Ia afferents from muscle spindles and they receive inputs from the same Ia afferents that mono-synaptically excite the motor neurons121. rIa-INs inhibit antagonist motor neurons in a reciprocal manner so that the rIa-INs that are activated from muscle spindles in flexor muscles around a joint inhibit the extensor motor neurons around the same joint and vice versa. The pair of rIa-INs around a joint also mutually inhibit each other and are inhibited by Renshaw cells121. The rIa-INs, therefore, have a reciprocal inhibitory connectivity with pairs of extensor and flexor motor neurons. The general organization of rIa-INs was first described in cats121 and was later outlined in mice122,123. rIa-INs are rhythmically active during locomotion and, because of their reciprocal connectivity pattern, they have long been proposed to be a major source of the rhythmic inhibition of motor neurons during locomotion124–126. Experiments in mice have provided support for this idea. Through the use of a mouse model in which all the synaptic output from glutamatergic rhythm-generating networks was genetically quenched, one study123 showed that a minimal inhibitory network including rIa-INs is sufficient to coordinate flexor–extensor motor neuron alternation123. The authors propose that this reciprocal circuit is downstream of the excitatory rhythm-generating network and that it contributes markedly although perhaps not exclusively47,127 to the rhythmic inhibition that reaches flexor and extensor motor neurons during locomotion (FIG. 4a).
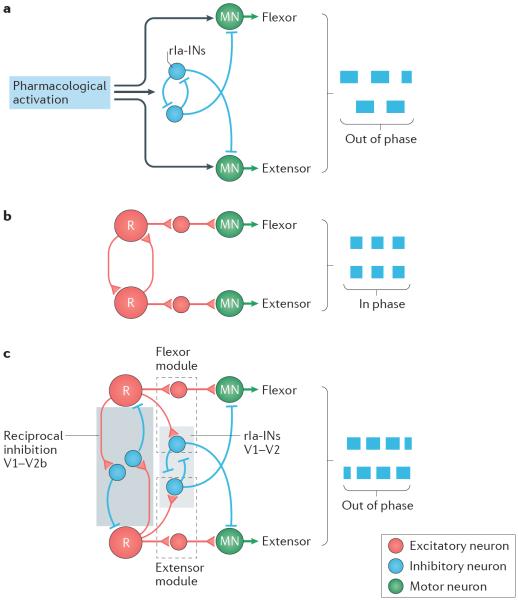
Figure 4. Multiple levels of flexor–extensor antagonism.
During limbed locomotion, motor neurons (MNs) that control flexor and extensor muscles around different joints within a limb must be activated in alternation. This flexor and extensor antagonism is generated by the activity of inhibitory neurons that are active at multiple levels in the locomotor network. a | One synapse away from flexor and extensor MNs are reciprocal Ia-inhibitory neurons (rIa-INs), which reciprocally inhibit antagonist MNs and each other. A minimal network of rIa-INs may coordinate flexor–extensor alternation (out of phase; denoted by blue boxes) in the isolated spinal cord in the absence of excitatory rhythm-generating circuits when appropriately activated by drugs. b | When two major groups of inhibitory neurons in the ventral spinal cord, V1 and V2b inhibitory neurons, are genetically ablated, all flexor–extensor alternation is lost leaving only flexor–extensor synchrony during locomotor-like activity (in phase; blue boxes). Excitatory neurons of different kinds provide premotor rhythmic excitation. `R' refers to rhythm-generating neurons. c | Schematic showing multiple levels of control of flexor–extensor alternation with all circuit elements intact. A module comprising excitatory neurons and rIa-INs (dashed boxes) receives input from excitatory rhythm-generating circuits and provides rhythmic excitation and inhibition to flexor and extensor antagonism, respectively. Inhibitory neurons belonging to the V1 and V2b classes of neurons (blue box) provide reciprocal inhibition between flexor and extensor rhythm generators. rIa-Ins also belong to the V1 and V2b classes134. Part a is based on data from REF. 123. Part b is based on data from REF. 134. Part c is based on data from REFS 119,123,134.
Premotor excitatory and inhibitory activity is modular
Inhibition alternates with excitation both in flexor and extensor motor neurons. In rodents, multiple sources of this excitation exist that possibly involve V2a SHOX2+ neurons88,92, V3 neurons54, dI3 neurons88,97, excitatory CNs47 and dorsally located neurons that originate in cells expressing the homeobox gene Lbx1 (REF. 128). All of these neurons project to motor neurons and the genetic ablation of some of these neuron populations54,92 compromises the robustness of locomotor modulation. In the cat spinal cord, excitatory group I neurons have been proposed to be involved in rhythmic excitation129,130. Analysis of the rhythmic excitatory and inhibitory synaptic inputs to flexor and extensor motor neurons suggest that the premotor inhibitory and excitatory locomotor networks have a modular reciprocal organization119. Recent findings using trans-synaptic labelling of flexor and extensor motor neurons lend support to this idea128. The authors observed spatial segregation between extensor and flexor premotor interneurons, providing anatomical evidence for a modular organization of flexor and extensor antagonism directly upstream of motor neurons.
Dual identity of flexor–extensor alternating circuits
The nature of the alternation between flexor and extensor rhythm-generating circuits has recently been addressed in genetic ablation experiments. Two major groups of ipsilaterally projecting inhibitory neurons are involved, the V1 neurons derived from progenitor cells that express the transcription factor engrailed homeobox 1 (EN1)131 and the V2b neurons that are derived from progenitor cells that express the transcription factor GATA binding protein 2 (GATA2)132. The genetic ablation or silencing of V1 neurons from the spinal cord has no apparent effect on flexor–extensor patterning but is followed by a marked decrease in locomotor frequency, which is ascribed to a permissive effect of the V1 neurons on rhythm generation133. Similarly, when synaptic output is blocked from V2b neurons, there is no effect on flexor and extensor alternation134. However, when the neuronal synaptic output of both V1 and V2b neuron populations is blocked, flexor–extensor alternation collapses into a synchronous flexor–extensor pattern with preserved left–right alternation134 (FIG. 4b). This study134 also showed that V1 and V2b neurons together account for all rIa-INs, although only approximately 20% of the V2b interneurons and 30% of the V1 neurons belong to the rIa-IN class. Because of this heterogeneity, the study did not address the question of whether flexor–extensor alternation is determined by the V1 and V2b populations as a whole or by the activity of specific V1 and V2b cell types. A follow-up study, in which the V1 and V2b neurons were genetically eliminated in the spinal cord only, demonstrated that V1 and V2b neurons may have a differential control of flexor–extensor motor output, with V1 interneurons dominantly inhibiting flexor activity and V2b neurons dominantly inhibiting extensor activity135. It seems likely that the abrogation of V1 and V2b neuron classes has at least two effects: the removal of the direct rhythmic inhibition that is conveyed to the flexor (V1) or extensor (V2b) motor neurons through rIa-INs circuits, and the synchronization of flexor and extensor bursting by silencing the reciprocal inhibitory circuits — mediated by non-rIa-INs of the V1 and/or the V2b class — that function to keep flexor and extensor rhythm-generating circuits firing out of phase (FIG. 4c). This study, therefore, clearly identifies the reciprocal inhibitory circuits in the mammalian locomotor network that are needed to generate flexor–extensor alternation. The reciprocal inhibitory circuits directly upstream of the motor neurons are organized together with excitatory neuronal counterparts in functional flexor and extensor modules (FIG. 4c) (see above). The nature of the excitatory coupling between flexor and extensor rhythm-generating circuits that is revealed in the absence of V1 and V2b neurons remains to be determined.
Flexor–extensor coordination is also influenced by the frequency or speed of locomotion. Thus, the duration of the stance phase decreases as the frequency of locomotion increases while the duration of the swing phase remains almost unchanged. Although modelling studies have suggested that at least part of this modulation may be explained by stronger bursting capability in rhythm-generating flexor circuits than in rhythm-generating extensor circuits53, the neuronal underpinning of this speed-dependent asymmetric modulation of stance and swing phase is still unknown.
V1 and V2b neurons have developmental homologues in the spinal cords of zebrafish and Xenopus laevis136–138. Experiments in these species suggest that the V1 (EN1) neurons provide recurrent inhibition to nearly all the types of neurons that control axial muscles in each side of the nervous system, possibly curtailing their rhythmic firing pattern. The presence of these neuronal types in non-limbed vertebrates suggests a preserved molecular code but that these classes of cells are recruited to additional new functions specifically controlling the flexor–extensor alternation in limbed animals.
Proprioception step-by-step
Even though the activity of the locomotor network can produce most of the precise timing and phasing of the muscle activity that is needed to locomote in the absence of sensory information, its activity is regulated by sensory signals — in particular, by sensory information that is generated through the active movements of the limb or the bending of the body17. Prominent examples of such movement-activated receptors include the proprioceptive input from stretch-sensitive muscle spindles (group Ia/II) and from force-sensitive muscle Golgi tendon organs (GTOs) in limbed animals or from stretch-activated edge cells on the lateral border of the spinal cord in fish139. The inputs from these receptors promote transitions between opposite locomotor phases.
Studies in cats have indicated that proprioceptive input from flexor hip muscles may enhance the flexor burst activity during locomotion140,141, whereas inputs from GTOs in ankle extensor muscles may inhibit flexor burst generation17,142,143. In the late stance phase when the limb is unloaded, the inhibitory signal from GTOs in ankle extensor muscles decreases, whereas the activity in afferents around the hip joints increases because the hip is stretched (FIG. 5a). Together, these signals facilitate the transition from stance to swing phase by promoting the activity in flexor motor neurons. This regulation of phase transition affects the rhythm-generating circuits, showing that these afferent proprioceptive signals become integrated components of the function of the locomotor networks in the intact moving animal (FIG. 5a).
Figure 5. Proprioceptive input directs stance and swing phase transitions.
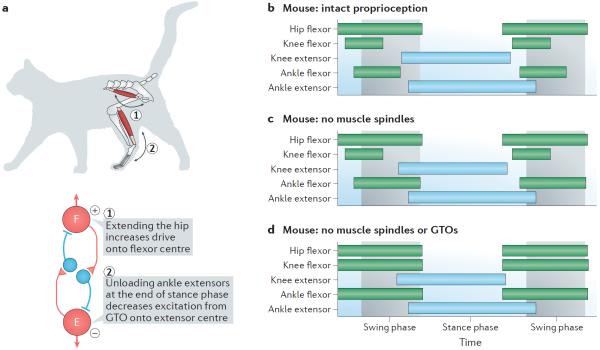
The activity of the locomotor network is regulated by sensory signals such as proprioceptive sensory information that is generated by active movement. a | Proprioceptors in hip muscles are activated when the hip is flexed and extended. When the hip is fully extended (step 1) there is maximal stretch-mediated proprioception from flexor hip muscles, which increases the excitation of the flexor (F) rhythm generator. Unloading of the ankle extensor Golgi tendon organs (GTOs) at the end of the stance phase (step 2) decreases the drive to the extensor (E) rhythm generator. The hip and ankle proprioceptors therefore act in synergy on the locomotor network to increase excitation and to decrease inhibition of the flexor rhythm generator at the end of the stance phase, promoting swing-to-stance phase transition. b–d | Genetic elimination of stretch-activated (from muscle spindles) and force-activated (from GTOs) proprioception leads to changes in locomotor patterns in mice. The green and blue bars indicate the times of activation for hip, knee and ankle flexors and extensors with respect to the swing phase (light grey) and stance phase (light blue). Elimination of muscle spindle afferents prolongs ankle flexor swing-to-stance phase transition, whereas elimination of both the muscle spindles and the GTOs leads to phase advance of knee and ankle flexors with respect to the hip flexor, corresponding to a removal of the indirect inhibition of the flexor rhythm generator from GTOs. Part a based on data from REFS 17,140–143. Parts b–d are based on data from REFS 144,145.
Owing to experimental limitations, it has not been possible to differentiate between the contribution of muscle spindles and the GTOs on phase transition in freely moving cats. However, recent experiments in mice have shed new light on this intricate regulation by selectively ablating proprioceptive feedback from muscle spindles or proprioception from both muscle spindles and GTOs144,145. The elimination of sensory feedback from muscle spindles leads to prolonged activity in ankle flexors, which affects the swing-to-stance phase transition (with little or no effect on the stance-to-swing phase transition) and decreases the flexion of the hip144,145 (FIG. 5b,c). The latter effect is compatible with a reduced hip flexor burst promotion, but the effect on swing-to-stance phase transition was not predicted based on results from previous studies. By contrast, in the absence of proprioception from both muscle spindles and GTOs144, the typical delayed and sequential activation of knee and ankle flexors with respect to hip flexors is lost, supporting a specific action of GTOs in regulating the stance-to-swing phase in the ankle and knee, similar to that which has been proposed in the cat (FIG. 5d). These experiments thus indicate that GTOs have a dominant effect on the stance-to-swing transition and that muscle spindles contribution less to this process. However, in the absence of muscle spindle proprioceptive inputs, the ability to regain locomotor capability after spinal cord injury is strongly impaired145. Thus, locomotion in mammals requires ongoing proprioceptive feedback to ensure that the intrinsically generated motor pattern is appropriately timed according to the biomechanical state of the limb.
Edge cells in lampreys provide an equivalent of proprioception in mammals. They have crossed inhibitory and ipsilateral excitatory effects in the locomotor network11. When one side is bent, the crossed inhibition helps to terminate the activity on the other side while excitation promotes activity on the same side. Activity in this dual stretch-activated system thus supports phase switching between the left and the right sides during swimming.
Conclusions and perspectives
Moving from an era that was dominated by electrophysiology, the vertebrate locomotor field is now being driven forwards by a combined electrophysiological and molecular genetic approach that has made it possible to decode the network organization in considerably different ways from before and to assign function to designated populations of neurons in a more direct way. This increased fidelity has provided substantial advances in the knowledge of the intrinsic organization of large-scale spinal networks in limbed animals and has allowed for comparisons with the network organization in leg-less animals that have more limited numbers of cells in the spinal cord.
The comparison of the network organization of the key circuit elements in limbed and non-limbed animals reveals both commonalities and differences. The commonalities extend to the basic components of inhibitory left–right alternating circuits and excitatory neurons that are involved in rhythm generation. The differences include: left–right alternating circuitries that have multiple components in legged animals but which are dominated by one component in fish; rhythm-generating neurons that originate from developmentally diverse progenitors in fish and mice; the elaborate reciprocal network circuits that are involved in flexor–extensor coordination that have been found in legged animals but that have not yet been found for axial muscles in non-legged animals; and the multi-layered nature of the legged locomotor network versus the mono-layer outline of the non-legged locomotor network.
A recurrent theme, however, is that locomotor networks, whether they control swimming or over-ground locomotion, are built around modules of rhythm- and pattern-generating networks that may be further functionally organized in to sub-modules. Such functional network organization may take place at the level of the pattern generator in which left–right coordinating circuits are recruited at different speeds of locomotion or at the level of rhythm-generating circuits in which different excitatory neurons are active or dominantly active at different locomotor gaits50,51,63,64,83,87,102,146. The presence of these speed- and/or task-dependent network reconfigurations of both non-limbed and limbed vertebrate locomotor networks underscores both the necessity of studying the locomotion in different contexts to gain a full understanding of how the locomotor networks are functionally organized and the high degree of flexibility or plasticity of the spinal locomotor networks. This insight reiterates what has been known for years from studies of rhythmic networks in invertebrates with few neurons, such as the stomatogastric ganglion that controls gut movements in crustaceans147. The cellular mechanisms for rhythmogensis itself are not generally understood across phyla but seem to depend on intertwined cellular and network properties that are dynamically regulated. The currently available pharmacology targets these cellular properties on a broad scale, and a more cell-specific approach is needed to disentangle the effect on the different neuronal components in the network. Using genetic tools to selectively target proprioceptors, it is now possible to unravel the effect of sensory modalities on locomotion. The resolution of these techniques will probably increase so that sensory sub-modalities can be targeted148. The prediction from these type of experiments is that the sensory modulation of locomotor networks is of the upmost importance for the rehabilitation of lost motor function, for example, following spinal cord injury.
The developmental code that specifies broad categories of spinal interneurons has provided important entry points to the molecular and genetic analysis of the network. However, spinal neurons come in many types, and new molecular markers149,150 for the different populations of inhibitory and excitatory neurons are needed to probe the functional groups of pattern- and rhythm-generating neurons. This need may be met by performing RNA sequencing with high sensitivity for individual cells or for a population of cells151 in the spinal cord together with the use of intersectional strategies that spatially narrow the cell populations that are targeted152.
A better description of the organization and cellular origin of the locomotor-initiating systems directly upstream of the spinal locomotor network is essential to address which neurons in the locomotor network receive the commands. This problem is circular and will require reiterating in both bottom-up and top-down studies. The development of cell-driven genetic tracing in the cord and brainstem and the use of optogenetic153 and chemogenetic techniques applied both in the isolated cord and in the behaving animal154–156 belong to the experimental armamentarium needed to approach this issue. The knowledge gained from applying such techniques will define a functional connectome, which will allow researchers to determine how behavioural motor selection is accomplished. Moreover, the increased possibility of imaging large populations of cells in behaving animals157–159 promises new insights into how cell populations are recruited and how they work together to produce behaviour.
Decoding the intrinsic function of spinal locomotor networks in vertebrates has moved forwards with an increasing pace since they were initially demarcated more than 100 years ago. There are, however, still many steps to take, which will ultimately lead to a better understating of how we as humans move.
Box 1 | Ionic currents involved in phase transition and burst termination.
Neurons are equipped with various ionic currents that may contribute to rhythmicity and patterning. These currents confer pacemaker properties and promote bursting (see main text), initiate phase transitions and promote burst termination104,160–164.
There are three currents that may affect phase transitions: transient low threshold calcium current (IT), hyperpolarization-activated inward current (Ih) and transient potassium current (IA). IT is activated around the resting potential of the cell and rapidly inactivates. This inactivation is removed by inhibition, and upon release of inactivation IT causes rebound excitation, promoting phase transition. Ih is activated by synaptic inhibition. When activated, this current counteracts inhibition, and because of its slow deactivation, Ih causes rebound excitation after inhibition. The presence of Ih helps neurons to escape from inhibition and promote phase transition. IA is usually inactivated at resting membrane potential. When inhibition removes the inactivation of IA and the cell is excited, activation of IA counteracts neuronal activation and delays phase transitions.
Ionic currents that promote burst termination are sodium- and calcium-activated potassium currents (IKCa and IKNa, respectively), which cause prolonged post-activation inhibition that functions as a burst-terminating mechanism.
IT, Ih, IA, IKCa and IKNa are all found in variable amounts in many spinal neurons and are subject to neuromodulation14.
Acknowledgements
The work in the Kiehn laboratory is supported by the Swedish Research Council, The European Research Council advanced grant, Ragnar and Torsten Söderbergs Foundation, Karolinska Institutet, NIH and Hjärnfonden. The author thanks colleagues for many inspiring discussions regarding themes discussed in this Review. The author thanks K. Dougherty for reading a previous version of this article.
Glossary
- Gait
A description of the pattern of limb movements. Different gaits have different patterns of movements and are often expressed as a function of the speed of locomotion.
- Mesencephalic locomotor region
(MLR). A region in the midbrain where electrical stimulation initiates locomotion. The strength of stimulation regulates the speed of locomotion.
- Commissural neurons
(CNs). Excitatory or inhibitory neurons that have axons crossing between the left side and the right side of the nervous system.
- Transcription factor
A protein that binds to DNA and controls the transcription of DNA to RNA. Expressed in specific populations of neurons during development.
- Monosynaptically restricted trans-synaptic labelling
An anatomical viral-based method in which a fluorescently labelled virus jumps one synapse from a target population of neurons to their immediate presynaptic partners. Used for detailed connectivity studies.
- Rhythm-generating neurons
Excitatory neurons that are primarily involved in rhythm generation.
- Pacemaker properties
Neuronal membrane properties that endow cells with the capability to produce endogenous bursting.
- Renshaw cells
Inhibitory neurons that are excited via recurrent collaterals from motor neurons. They project back to motor neurons and inhibit them. They also inhibit reciprocal Ia interneurons.
- Recurrent inhibition
Inhibitory cells that are activated by excitatory cells and that provide inhibition of other cells occasionally including the cell that provided the excitation.
- Golgi tendon organs
(GTOs). Force-activated receptors in tendons.
- Proprioception
The awareness of body and limb position. Mediated by proprioceptive movement-activated receptors in muscles, tendons and joints.
Footnotes
Competing interests statement The author declares no competing interests.
References
- 1.Drew T, Marigold DS. Taking the next step: cortical contributions to the control of locomotion. Curr. Opin. Neurobiol. 2015;33:25–33. doi: 10.1016/j.conb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov. Disord. 2013;28:1483–1491. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Rill E. The basal ganglia and the locomotor regions. Brain Res. 1986;396:47–63. [PubMed] [Google Scholar]
- 4.Grillner S, Robertson B. The basal ganglia downstream control of brainstem motor centres — an evolutionarily conserved strategy. Curr. Opin. Neurobiol. 2015;33:47–52. doi: 10.1016/j.conb.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E. The physiology of the pedunculopontine nucleus: implications for deep brain stimulation. J. Neural Transmission. 2015;122:225–235. doi: 10.1007/s00702-014-1243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. Curr. Pharm. Design. 2013;19:4448–4470. doi: 10.2174/1381612811319240011. [DOI] [PubMed] [Google Scholar]
- 7.Dubuc R, et al. Initiation of locomotion in lampreys. Brain Res. Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. From Mollusc to Man. Oxford Univ. Press; 1998. [Google Scholar]
- 9.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 11.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 12.Brown T. The intrinsic factors in the act of progression in mammals. Proc. R. Soc. Lond. B. 1911;84:308–319. [Google Scholar]
- 13.Grillner S. In: Hanbook of Physiology. Brooks V, editor. American Physiological Society; 1981. pp. 1179–1236. [Google Scholar]
- 14.Harris-Warrick RM. Neuromodulation and flexibility in Central Pattern Generator networks. Curr. Opin. Neurobiol. 2011;21:685–692. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sillar KT, Combes D, Simmers J. Neuromodulation in developing motor microcircuits. Curr. Opin. Neurobiol. 2014;29:73–81. doi: 10.1016/j.conb.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front. Neural Circuits. 2014;8:55. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson KG. Generating the walking gait: role of sensory feedback. Prog. Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- 18.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 19.Stuart DG, Hultborn H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920–), and the neural control of stepping. Brain Res. Rev. 2008;59:74–95. doi: 10.1016/j.brainresrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr. Opin. Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res. Rev. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan JT. Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog. Neurobiol. 2001;63:441–466. doi: 10.1016/s0301-0082(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 24.Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res. Rev. 2008;57:22–28. doi: 10.1016/j.brainresrev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 25.El Manira A. Dynamics and plasticity of spinal locomotor circuits. Curr. Opin. Neurobiol. 2014;29:133–141. doi: 10.1016/j.conb.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: an outline. Front. Behav. Neurosci. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiehn O. Development and functional organization of spinal locomotor circuits. Curr. Opin. Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res. Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean DL, Dougherty KJ. Peeling back the layers of locomotor control in the spinal cord. Curr. Opin. Neurobiol. 2015;33:63–70. doi: 10.1016/j.conb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein PS. Molecular, genetic, cellular, and network functions in the spinal cord and brainstem. Ann. NY Acad. Sci. 2013;1279:1–12. doi: 10.1111/nyas.12083. [DOI] [PubMed] [Google Scholar]
- 32.Gordon IT, Whelan PJ. Deciphering the organization and modulation of spinal locomotor central pattern generators. J. Exp. Biol. 2006;209:2007–2014. doi: 10.1242/jeb.02213. [DOI] [PubMed] [Google Scholar]
- 33.O'Donovan MJ, et al. Mechanisms of excitation of spinal networks by stimulation of the ventral roots. Ann. NY Acad. Sci. 2010;1198:63–71. doi: 10.1111/j.1749-6632.2010.05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez FJ, Benito-Gonzalez A, Siembab VC. Principles of interneuron development learned from Renshaw cells and the motoneuron recurrent inhibitory circuit. Ann. NY Acad. Sci. 2013;1279:22–31. doi: 10.1111/nyas.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res. Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimaru H, Kakizaki M. The role of inhibitory neurotransmission in locomotor circuits of the developing mammalian spinal cord. Acta Physiol. (Oxf.) 2009;197:83–97. doi: 10.1111/j.1748-1716.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 37.Stepien AE, Arber S. Probing the locomotor conundrum: descending the `V' interneuron ladder. Neuron. 2008;60:1–4. doi: 10.1016/j.neuron.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Fetcho JR. The utility of zebrafish for studies of the comparative biology of motor systems. J. Exp. Zool. B Mol. Dev. Evol. 2007;308:550–562. doi: 10.1002/jez.b.21127. [DOI] [PubMed] [Google Scholar]
- 39.Nissen UV, Mochida H, Glover JC. Development of projection-specific interneurons and projection neurons in the embryonic mouse and rat spinal cord. J. Comp. Neurol. 2005;483:30–47. doi: 10.1002/cne.20435. [DOI] [PubMed] [Google Scholar]
- 40.Matsuyama K, Kobayashi S, Aoki M. Projection patterns of lamina VIII commissural neurons in the lumbar spinal cord of the adult cat: an anterograde neural tracing study. Neuroscience. 2006;140:203–218. doi: 10.1016/j.neuroscience.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J. Comp. Neurol. 2002;446:349–359. doi: 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]
- 42.Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur. J. Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber I, Veress G, Szucs P, Antal M, Birinyi A. Neurotransmitter systems of commissural interneurons in the lumbar spinal cord of neonatal rats. Brain Res. 2007;1178:65–72. doi: 10.1016/j.brainres.2007.06.109. [DOI] [PubMed] [Google Scholar]
- 44.Restrepo CE, et al. Transmitter-phenotypes of commissural interneurons in the lumbar spinal cord of newborn mice. J. Comp. Neurol. 2009;517:177–192. doi: 10.1002/cne.22144. [DOI] [PubMed] [Google Scholar]
- 45.Jankowska E, Krutki P, Matsuyama K. Relative contribution of Ia inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J. Physiol. 2005;568:617–628. doi: 10.1113/jphysiol.2005.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- 47.Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J. Neurosci. 2007;27:6521–6530. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierani A, et al. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 49.Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]; The first study to genetically manipulate a transcription-defined population of spinal neurons in the mouse spinal cord.
- 50.Talpalar AE, et al. Dual-mode operation of neuronal networks involved in left–right alternation. Nature. 2013;500:85–88. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]; Using mouse genetics in a behavioural context, this study shows that two separate neuronal populations — which are characterized by the expression of specific molecular markers — control alternating gait. These separate circuits are necessary for alternation at slow and fast speeds of locomotion.
- 51.Bellardita C, Kiehn O. Phenotypic characterization of speed-aasociated gait changes in mice reveals modular organization of locomotor networks. Curr. Biol. 2015;25:1426–1436. doi: 10.1016/j.cub.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive characterization of speed-associated gait changes in mice and the loss of specific gaits after genetic ablation of commissural neurons.
- 52.Serradj N, Jamon M. The adaptation of limb kinematics to increasing walking speeds in freely moving mice 129/Sv and C57BL/6. Behav. Brain Res. 2009;201:59–65. doi: 10.1016/j.bbr.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 53.Shevtsova NA, et al. Organization of left–right coordination of neuronal activity in the mammalian spinal cord: insights from computational modelling. J. Physiol. 2015;593:2403–2426. doi: 10.1113/JP270121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borowska J, et al. Functional subpopulations of V3 interneurons in the mature mouse spinal cord. J. Neurosci. 2013;33:18553–18565. doi: 10.1523/JNEUROSCI.2005-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallstedt A, Kullander K. Dorsally derived spinal interneurons in locomotor circuits. Ann. NY Acad. Sci. 2013;1279:32–42. doi: 10.1111/j.1749-6632.2012.06801.x. [DOI] [PubMed] [Google Scholar]
- 57.Andersson LS, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts A, Li WC, Soffe SR. Roles for inhibition: studies on networks controlling swimming in young frog tadpoles. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2008;194:185–193. doi: 10.1007/s00359-007-0273-3. [DOI] [PubMed] [Google Scholar]
- 59.Li WC, Soffe SR, Roberts A. The spinal interneurons and properties of glutamatergic synapses in a primitive vertebrate cutaneous flexion reflex. J. Neurosci. 2003;23:9068–9077. doi: 10.1523/JNEUROSCI.23-27-09068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li WC, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J. Neurosci. 2007;27:12267–12276. doi: 10.1523/JNEUROSCI.3694-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahmood R, Restrepo CE, El Manira A. Transmitter phenotypes of commissural interneurons in the lamprey spinal cord. Neuroscience. 2009;164:1057–1067. doi: 10.1016/j.neuroscience.2009.08.069. [DOI] [PubMed] [Google Scholar]
- 62.Satou C, Kimura Y, Higashijima S. Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J. Neurosci. 2012;32:1771–1783. doi: 10.1523/JNEUROSCI.5500-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat. Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows speed-related neuronal switching.
- 64.McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]; This study demonstrated speed-related recruitment pattern of spinal interneurons and the specific role of ventral excitatory neurons in controlling slow swimming.
- 65.Goetz C, Pivetta C, Arber S. Distinct limb and trunk premotor circuits establish laterality in the spinal cord. Neuron. 2015;85:131–144. doi: 10.1016/j.neuron.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 66.Buchanan JT, Grillner S. Newly identified `glutamate interneurons' and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]; The first demonstration of excitatory locomotor-related neurons in the lamprey spinal cord.
- 67.Li WC, Roberts A, Soffe SR. Locomotor rhythm maintenance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J. Physiol. 2009;587:1677–1693. doi: 10.1113/jphysiol.2008.166942. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the presence and connectivity of a network of excitatory neurons in the brainstem and spinal cord underlying rhythm generation in young tadpoles.
- 68.Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J. Neurosci. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dale N, Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J. Physiol. 1985;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]; Electrophysiological demonstration of excitatory last-order neurons in the young tadpole spinal cord.
- 70.Parker D, Grillner S. The activity-dependent plasticity of segmental and intersegmental synaptic connections in the lamprey spinal cord. Eur. J. Neurosci. 2000;12:2135–2146. doi: 10.1046/j.1460-9568.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 71.Moult PR, Cottrell GA, Li WC. Fast silencing reveals a lost role for reciprocal inhibition in locomotion. Neuron. 2013;77:129–140. doi: 10.1016/j.neuron.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR–Cas9. Nat. Rev. Genet. 2015;16:299–311. doi: 10.1038/nrg3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 2010;13:246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]; By using optogenetics, this was the first direct demonstration that activation of glutamatergic neurons in the mammalian spinal cord can evoke locomotor-like activity.
- 74.Hagglund M, et al. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc. Natl Acad. Sci. USA. 2013;110:11589–11594. doi: 10.1073/pnas.1304365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cazalets JR, Bertrand S. Ubiquity of motor networks in the spinal cord of vertebrates. Brain Res. Bull. 2000;53:627–634. doi: 10.1016/s0361-9230(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 76.Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J. Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhong G, Shevtsova N, Rybak I, Harris-Warrick R. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor CPG organization. J. Physiol. 2012;590:4735–4759. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J. Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- 79.Grillner S, Zangger P. The effect of dorsal root transection on the efferent motor pattern in the cat's hindlimb during locomotion. Acta Physiol. Scand. 1984;120:393–405. doi: 10.1111/j.1748-1716.1984.tb07400.x. [DOI] [PubMed] [Google Scholar]
- 80.Machado TA, Pnevmatikakis E, Paninski L, Jessell TM, Miri A. Primacy of flexor locomotor pattern revealed by ancestral reversion of motor neuron identity. Cell. 2015;162:338–350. doi: 10.1016/j.cell.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dominici N, et al. Locomotor primitives in newborn babies and their development. Science. 2011;334:997–999. doi: 10.1126/science.1210617. [DOI] [PubMed] [Google Scholar]
- 82.Hinckley CA, et al. Spinal locomotor circuits develop using hierarchical rules based on motorneuron position and identity. Neuron. 2015;87:1008–1021. doi: 10.1016/j.neuron.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crone SA, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]; Comprehensive review that outline the early molecular code for spinal neurons.
- 85.Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur. J. Neurosci. 2007;26:3003–3015. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- 86.Lundfald L, et al. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur. J. Neurosci. 2007;26:2989–3002. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 87.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J. Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study along with reference 83 show that the lack of excitatory V2a neurons in mice leads to changes in the left–right coordination without affecting the rhythm generation. V2a neurons regulate left–right alteration in a speed-dependent manner.
- 88.Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]; Study that describes the use of the monosynaptically restricted trans-synaptic labelling technique to reveal premotor networks in the spinal cord.
- 89.Dougherty KJ, Kiehn O. Firing and cellular properties of V2a interneurons in the rodent spinal cord. J. Neurosci. 2010;30:24–37. doi: 10.1523/JNEUROSCI.4821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong G, et al. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J. Neurosci. 2010;30:170–182. doi: 10.1523/JNEUROSCI.4849-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nat. Commun. 2011;2:274. doi: 10.1038/ncomms1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dougherty KJ, et al. Locomotor rhythm generation linked to the output of spinal Shox2 excitatory interneurons. Neuron. 2013;80:920–933. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]; Through the use of various genetic techniques to identify, chronically silence and optogenetically control populations of glutamatergic interneurons, this study identified glutamatergic SHOX2+ neurons as constituent members of the locomotor rhythm generator in mammals.
- 93.Wilson JM, et al. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J. Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J. Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- 95.Ziskind-Conhaim L, Hinckley CA. Hb9 versus type 2 interneurons. J. Neurophysiol. 2008;99:1044–1046. doi: 10.1152/jn.01163.2007. [DOI] [PubMed] [Google Scholar]
- 96.Brocard F, Tazerart S, Vinay L. Do pacemakers drive the central pattern generator for locomotion in mammals? Neuroscientist. 2010;16:139–155. doi: 10.1177/1073858409346339. [DOI] [PubMed] [Google Scholar]
- 97.Bui TV, et al. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron. 2013;78:191–204. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J. Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eklof-Ljunggren E, et al. Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc. Natl Acad. Sci. USA. 2012;109:5511–5516. doi: 10.1073/pnas.1115377109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using laser ablation of neurons in the larval zebrafish, this study show that the V2a neurons in zebrafish larvae are involved in producing swimming.
- 100.Ljunggren EE, Haupt S, Ausborn J, Ampatzis K, El Manira A. Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J. Neurosci. 2014;34:134–139. doi: 10.1523/JNEUROSCI.4087-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science. 2014;343:197–200. doi: 10.1126/science.1245629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ampatzis K, Song J, Ausborn J, El Manira A. Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron. 2014;83:934–943. doi: 10.1016/j.neuron.2014.07.018. [DOI] [PubMed] [Google Scholar]; This study shows that separate excitatory neurons that are directly presynaptic to motor neurons are recruited in a speed-dependent manner, which matches motor neuron recruitment.
- 103.Ampatzis K, Song J, Ausborn J, El Manira A. Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish. J. Neurosci. 2013;33:10875–10886. doi: 10.1523/JNEUROSCI.0896-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr. Biol. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 105.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing, so near, yet so far. Annu. Rev. Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiehn O, Johnson BR, Raastad M. Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience. 1996;75:263–273. doi: 10.1016/0306-4522(96)00250-3. [DOI] [PubMed] [Google Scholar]
- 107.Hochman S, McCrea DA. Effects of chronic spinalization on ankle extensor motoneurons. III. Composite Ia EPSPs in motoneurons separated into motor unit types. J. Neurophysiol. 1994;71:1480–1490. doi: 10.1152/jn.1994.71.4.1480. [DOI] [PubMed] [Google Scholar]
- 108.Reith CA, Sillar KT. A role for slow NMDA receptor-mediated, intrinsic neuronal oscillations in the control of fast fictive swimming in Xenopus laevis larvae. Eur. J. Neurosci. 1998;10:1329–1340. doi: 10.1046/j.1460-9568.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 109.Wallen P, Grillner S. N-methyl-D-aspartate receptor-induced, inherent oscillatory activity in neurons active during fictive locomotion in the lamprey. J. Neurosci. 1987;7:2745–2755. doi: 10.1523/JNEUROSCI.07-09-02745.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li WC, Roberts A, Soffe SR. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J. Neurosci. 2010;30:16609–16620. doi: 10.1523/JNEUROSCI.3695-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cowley KC, Zaporozhets E, Maclean JN, Schmidt BJ. Is NMDA receptor activation essential for the production of locomotor-like activity in the neonatal rat spinal cord? J. Neurophysiol. 2005;94:3805–3814. doi: 10.1152/jn.00016.2005. [DOI] [PubMed] [Google Scholar]
- 112.Beato M, Bracci E, Nistri A. Contribution of NMDA and non-NMDA glutamate receptors to locomotor pattern generation in the neonatal rat spinal cord. Proc. Biol. Sci. 1997;264:877–884. doi: 10.1098/rspb.1997.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dai Y, Jordan LM, Fedirchuk B. Modulation of transient and persistent inward currents by activation of protein kinase C in spinal ventral neurons of the neonatal rat. J. Neurophysiol. 2008;101:112–128. doi: 10.1152/jn.01373.2007. [DOI] [PubMed] [Google Scholar]
- 114.Tazerart S, Viemari JC, Darbon P, Vinay L, Brocard F. Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. J. Neurophysiol. 2007;98:613–628. doi: 10.1152/jn.00316.2007. [DOI] [PubMed] [Google Scholar]
- 115.Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J. Neurosci. 2007;27:4507–4518. doi: 10.1523/JNEUROSCI.0124-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ziskind-Conhaim L, Wu L, Wiesner EP. Persistent sodium current contributes to induced voltage oscillations in locomotor-related hb9 interneurons in the mouse spinal cord. J. Neurophysiol. 2008;100:2254–2264. doi: 10.1152/jn.90437.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brocard F, et al. Activity-dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron. 2013;77:1047–1054. doi: 10.1016/j.neuron.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Locomotor-like activity in the isolated neonatal rodent spinal cord reduces the level of extracellular calcium and increases the extracellular potassium concentration as a consequence of neuronal activity. The study shows that these changes trigger persistent sodium-dependent pacemaker activities in interneurons located in the region of the locomotor network and suggest that such properties are dynamically modulated during locomotion.
- 118.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology. 2014;29:58–71. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Endo T, Kiehn O. Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J. Neurophysiol. 2008;100:3043–3054. doi: 10.1152/jn.90729.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hochman S, Schmidt BJ. Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord. J. Neurophysiol. 1998;79:743–752. doi: 10.1152/jn.1998.79.2.743. [DOI] [PubMed] [Google Scholar]
- 121.Hultborn H. Transmission in the pathway of reciprocal Ia inhibition to motoneurones and its control during the tonic stretch reflex. Prog. Brain Res. 1976;44:235–255. doi: 10.1016/S0079-6123(08)60736-0. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, Li L, Goulding M, Frank E. Early postnatal development of reciprocal Ia inhibition in the murine spinal cord. J. Neurophysiol. 2008;100:185–196. doi: 10.1152/jn.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Talpalar AE, et al. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron. 2011;71:1071–1084. doi: 10.1016/j.neuron.2011.07.011. [DOI] [PubMed] [Google Scholar]; This study identified a minimal network composed of inhibitory reciprocally connected Ia interneurons as responsible for out-of-phase activation of antagonistic muscles across a joint.
- 124.Geertsen SS, Stecina K, Meehan CF, Nielsen JB, Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. J. Physiol. 2011;589:119–134. doi: 10.1113/jphysiol.2010.199125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pratt CA, Jordan LM. Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J. Neurophysiol. 1987;57:56–71. doi: 10.1152/jn.1987.57.1.56. [DOI] [PubMed] [Google Scholar]
- 126.Deliagina TG, Orlovsky GN. Activity of Ia inhibitory interneurons during fictitious scratch reflex in the cat. Brain Res. 1980;193:439–447. doi: 10.1016/0006-8993(80)90176-6. [DOI] [PubMed] [Google Scholar]
- 127.Wilson JM, Blagovechtchenski E, Brownstone RM. Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion. J. Neurosci. 2010;30:1137–1148. doi: 10.1523/JNEUROSCI.1401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479:61–66. doi: 10.1038/nature10538. [DOI] [PubMed] [Google Scholar]
- 129.Angel MJ, Jankowska E, McCrea DA. Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J. Physiol. 2005;563:597–610. doi: 10.1113/jphysiol.2004.076034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bannatyne BA, et al. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J. Physiol. 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moran-Rivard L, et al. Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron. 2001;29:385–399. doi: 10.1016/s0896-6273(01)00213-6. [DOI] [PubMed] [Google Scholar]
- 132.Zhou Y, Yamamoto M, Engel JD. GATA2 is required for the generation of V2 interneurons. Development. 2000;127:3829–3838. doi: 10.1242/dev.127.17.3829. [DOI] [PubMed] [Google Scholar]
- 133.Gosgnach S, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J, et al. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron. 2014;82:138–150. doi: 10.1016/j.neuron.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using mouse genetics and physiology, this study showed that flexor–extensor alternation in rodents depends on the combined activity of inhibitory V1 and V2b neurons, two molecularly defined groups of neurons in the mammalian spinal cord that include Renshaw cells and Ia-INs.
- 135.Britz O, et al. A genetically defined asymmetry underlies the inhibitory control of flexor-extensor locomotor movements. eLife. 2015;4:e04718. doi: 10.7554/eLife.04718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Higashijima S, Masino MA, Mandel G, Fetcho JR. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J. Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li WC, Higashijima S, Parry DM. Roberts, A. & Soffe, S. R. Primitive roles for inhibitory interneurons in developing frog spinal cord. J. Neurosci. 2004;24:5840–5848. doi: 10.1523/JNEUROSCI.1633-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peng CY, et al. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–827. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grillner S, Williams T, Lagerbäck PA. The edge cell, a possible intraspinal mechanoreceptor. Science. 1984;223:500–503. doi: 10.1126/science.6691161. [DOI] [PubMed] [Google Scholar]
- 140.Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]; This study demonstrates that movement-activated receptors in the hip are important for initiating the swing phase during locomotion.
- 141.Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J. Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- 142.Hultborn H, et al. How do we approach the locomotor network in the mammalian spinal cord? Ann. NY Acad. Sci. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- 143.Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp. Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- 144.Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc. Natl Acad. Sci. USA. 2014;111:16877–16882. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that mouse locomotor patterns are significantly changed after genetic elimination of proprioceptive feedback from muscle spindles and GTOs. Activity in muscle spindles alone affects swing-stance transition and the combination of muscle spindles and GTOs affects stance-swing phase transitions.
- 145.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]; This study shows that locomotor recovery after spinal cord injury is promoted by sensory feedback originating in muscle spindles. Mice that lack the muscle spindle sensory feedback exhibit disturbed descending circuits during recovery. The findings suggest that muscle spindle feedback facilitaties circuit reorganization after spinal cord injury.
- 146.Ausborn J, Mahmood R, El Manira A. Decoding the rules of recruitment of excitatory interneurons in the adult zebrafish locomotor network. Proc. Natl Acad. Sci. USA. 2012;109:E3631–E3639. doi: 10.1073/pnas.1216256110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35:373–381. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 149.Zagoraiou L, et al. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perry S, et al. Firing properties of Renshaw cells defined by Chrna2 are modulated by hyperpolarizing and small conductance ion currents Ih and ISK. Eur. J. Neurosci. 2015;41:889–900. doi: 10.1111/ejn.12852. [DOI] [PubMed] [Google Scholar]
- 151.Zeisel A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 152.Ray RS, et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508:357–363. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- 156.Caggiano V, Sur M, Bizzi E. Rostro-caudal inhibition of hindlimb movements in the spinal cord of mice. PLoS ONE. 2014;9:e100865. doi: 10.1371/journal.pone.0100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fetcho JR. Imaging neuronal activity with calcium indicators in larval zebrafish. CSH Protoc. 2007 doi: 10.1101/pdb.prot4781. http://dx.doi.org/10.1101/pdb.prot4781. [DOI] [PubMed]
- 158.Hamel EJ, Grewe BF, Parker JG, Schnitzer MJ. Cellular level brain imaging in behaving mammals: an engineering approach. Neuron. 2015;86:140–159. doi: 10.1016/j.neuron.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feierstein CE, Portugues R, Orger MB. Seeing the whole picture: A comprehensive imaging approach to functional mapping of circuits in behaving zebrafish. Neuroscience. 2015;296:26–38. doi: 10.1016/j.neuroscience.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 160.Harris-Warrick RM. Voltage-sensitive ion channels in rhythmic motor systems. Curr. Opin. Neurobiol. 2002;12:646–651. doi: 10.1016/s0959-4388(02)00377-x. [DOI] [PubMed] [Google Scholar]
- 161.Dale N, Kuenzi F. Ionic currents, transmitters and models of motor pattern generators. Curr. Opin. Neurobiol. 1997;7:790–796. doi: 10.1016/s0959-4388(97)80137-7. [DOI] [PubMed] [Google Scholar]
- 162.Grillner S, Wallen P, Hill R, Cangiano L, El Manira A. Ion channels of importance for the locomotor pattern generation in the lamprey brainstem-spinal cord. J. Physiol. 2001;533:23–30. doi: 10.1111/j.1469-7793.2001.0023b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res. Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- 164.El Manira A, Kyriakatos A, Nanou E. Beyond connectivity of locomotor circuitry-ionic and modulatory mechanisms. Prog. Brain Res. 2010;187:99–110. doi: 10.1016/B978-0-444-53613-6.00007-1. [DOI] [PubMed] [Google Scholar]
- 165.Kiehn O, Dougherty K. In: Neuroscience in the 21st Century. Pfaff DW, editor. Springer; 2013. pp. 1209–1237. [Google Scholar]
- 166.Alaynick WA, Jessell TM, Pfaff SL. SnapShot: spinal cord development. Cell. 2011;146:178–178.e1. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]