Abstract
AIM
To compare the efficacy of single-session 360-degree selective laser trabeculoplasty (SLT) for reduction of intraocular pressure (IOP) in patients with pseudoexfoliative glaucoma (PXFG) and primary open angle glaucoma (POAG).
METHODS
This is a single-center, prospective, nonrandomized comparative study. Patients older than 18 years of age with uncontrolled PXFG or POAG eyes requiring additional therapy while on maximally tolerated IOP-lowering medications were included. The primary outcome measure changed in IOP from baseline. Success was defined as IOP reduction ≥20% from baseline without any additional IOP-lowering medication. All patients were examined at 1d, 1wk, 1, 3, 6, 9, 12mo after SLT.
RESULTS
Nineteen patients (20 eyes) with PXFG and 27 patients (28 eyes) with POAG were included in the study. In the visual fields mean deviation was -2.88 (±1.67) in the POAG and -3.1 (±1.69) in the PXFG groups (P=0.3). The mean (±SD) IOP was 22.9 (±3.7) mm Hg in the POAG group and 25.7 (±4.4) mm Hg in the PXFG group at baseline and decreased to 18.4 (±3.2) and 18.0 (±3.9) mm Hg in the POAG group (P<0.001 and P=0.02), and to 17.9 (±4.0) and 21.0 (±6.6) mm Hg in the PXFG group (P<0.001 and P=0.47) at 6 and 12mo, respectively. The number of medications was 2.6 (±0.8) in the POAG group and 2.5 (±0.8) in the PXFG group at baseline, and did not change at all follow-up visits in both groups (P=0.16 in POAG and 0.57 in PXFG). Based on Kaplan-Meier survival analysis, the success rate was 75% in the POAG group compared to 94.1% in the PXFG group (P=0.08; log rank test) at 6mo, and 29.1% and 25.0% at 12mo, respectively (P=0.9; log rank).
CONCLUSION
The 360-degree SLT is an effective and well-tolerated therapeutic modality in patients with POAG and PXFG by reducing IOP without any change in number of medications. The response was more pronounced early in the postoperative period in patients with PXFG whereas there was no statistically significant difference at 12-month follow-up.
Keywords: primary open angle glaucoma, pseudoexfoliative glaucoma, selective laser trabeculoplasty
INTRODUCTION
Selective laser trabeculoplasty (SLT) was introduced by Latina and Park in 1995 as an option for the treatment of open angle glaucoma[1]. In this method, a Q-switched, frequency doubled laser with a wavelength of 532 nm and pulse duration of 3ns affects only the pigmented trabecular meshwork cells while the non-pigmented meshwork cells remain intact[2]–[3]. SLT has been widely adopted for treatment of glaucoma and is used by some clinicians early in the course of treatment of the disease[4]–[5]. Results of different studies have shown that SLT reduces the intraocular pressure (IOP) between 11% to 40% in various types of glaucoma in short to intermediate-term period[6]. The exact mechanism remains uncertain by which SLT reduces IOP but in particular, cytokine secretion, matrix metalloproteinase induction, increased cell division, repopulation of burn sites and macrophage recruitment are responsible for IOP reduction[7].
Although the efficacy of SLT in patients with primary open angle glaucoma (POAG) has been well documented, there are few studies that have investigated its efficacy in other types of glaucoma including pigmentary and pseudoexfoliative glaucoma (PXFG)[8]–[9]. In this prospective study, we compared the efficacy of 360-degree SLT treatment in patients with PXFG to those with POAG.
SUBJECTS AND METHODS
Study Subjects
This single-center, prospective, nonrandomized comparative study was performed between March 2010 and March 2013 at the Hazrat Rasoul Akram Hospital, Tehran, Iran. The Ethics Committee at Iran University of Medical Sciences, Tehran, Iran, approved the study protocol. Written informed consents were obtained from all patients and the study was carried out in accordance with the principles of Declaration of Helsinki.
Patients aged 18 years or older with uncontrolled PXFG or POAG requiring additional therapy while on maximally tolerated IOP-lowering medications were enrolled. Exclusion criteria were eyes with prior history of laser or incisional surgery such as phacoemulsification, glaucoma procedures or argon laser trabeculoplasty; ocular trauma or any other preexisting corneal disease precluding the angle evaluation; or if the trabecular meshwork could not be viewed for 360 degrees. Preoperative exams included slit-lamp biomicroscopy, IOP measurement using Goldmann applanation tonometry, gonioscopy, dilated fundoscopic examination with a 90 D lens, and standard automated perimetry with 24-2 Swedish interactive threshold Algorithm (SITA; Carl Zeiss Meditec Inc. Dublin, CA, USA).
Outcome Measures
The primary outcome measure was IOP (mm Hg) before and after 360-degree SLT in both groups at 1d, 1wk and then every 3mo after surgery. All of IOP measurements performed at morning in sitting position. Other outcome measures included success rate, changes in the number of IOP-lowering medications, and complications. Success was defined as IOP reduction ≥20% from the baseline without additional IOP-lowering medications. Failure was defined as IOP reduction <20% from the baseline at two consecutive visits and/or addition of IOP-lowering medications.
Laser Procedure
All the study eyes underwent 360-degree SLT for the first time. All the procedures were performed by two surgeons (Miraftabi A or Nilforushan N) with the same protocol, which included Q-switched frequency-doubled 532 nm Nd:YAG laser (Ellex Tango SLT/Photodistruptor Combination Laser, Ellex Medical Lasers Ltd., Adelaide, Australia) with pulse duration of 3 ns and a spot size of 400 µm for 360 degrees. No pretreatment with IOP-lowering medications was used. After topical anesthesia with 0.5% tetracaine hydrochloride, a laser goniolens (Ocular Latina SLT Gonio Laser Lens, Ocular Instruments, USA) was placed on the eye and a series of 70-90 non-overlapping shots was evenly carried out for 360 degrees in mid-trabecular meshwork. The laser energy was initially selected based on the intensity of angle pigmentation: 0.4 mJ for eyes with densely pigmented trabecular meshwork, 0.6 mJ for eyes with moderate pigmentation, and 0.8 mJ for lower grades of trabecular meshwork pigmentation, and increased by 0.1-0.2 mJ until small “champagne bubbles” were observed and then decreased by 0.1 mJ. All patients were given 0.5% bethametasone drops 4 times daily for 7d after the procedure, and instructed to continue routine IOP-lowering medications. The medications were modified at follow-up visits according to IOP measurements as needed.
Statistical Analysis
The sample size was calculated based on a difference of at least 1.3 mm Hg in IOP between two groups, standard deviation of 3 mm Hg and a confidence interval of 95%. Kolmogorov-Smirnov test and Q-Q plots were used to evaluate the normality of IOP measurements. To compare the two study groups, we used t-test, Mann-Whitney test, Chi-square and Fisher exact test, whenever appropriate. Log-rank test was used to compare survival curves. Univariate and multivariate Cox's regression analyses were used to assess factors associated with time to failure. All statistical analyses were performed with SPSS (version 21.0, Chicago, IL, USA). P-values less than 0.05 were considered statistically significant.
RESULTS
Forty-eight eyes of 46 patients (27 females and 19 males) who met the eligibility criteria were consecutively enrolled in the study. There were 27 patients with POAG and 19 patients with PXFG. In each group, one patient had both eyes included in the study. The baseline characteristics of the study subjects are described in Table 1. There were no significant differences between the two study groups in baseline characteristics except for gender (with a higher ratio of female in the POAG group), and IOP, which was higher in the PXFG group (P=0.025). In the visual fields the average mean deviation (MD) was -2.88 (±1.67) in the POAG and -3.1 (±1.69) in the PXFG groups (P=0.3). The number of patients at each follow-up visit, and changes in IOP and number of medications from baseline in each study group are described in Tables 2 and 3.
Table 1. The baseline characteristics of the study patients.
| Variables | Total | POAG | PXFG | P |
| No. of eyes | 48 | 28 | 20 | |
| No. of patients | 46 | 27 | 19 | |
| Age (a) | 64.0±9.0 | 62.2±8.9 | 68.6±7.5 | 0.2 |
| Sex | ||||
| Male | 19 (41.7) | 6 | 13 | 0.01 |
| Female | 27 (58.3) | 21 | 6 | 0.01 |
| Laterality | ||||
| Right eye | 20 (41.7) | 13 (46.4) | 7 (35.0) | |
| Left eye | 28 (58.3) | 15 (53.6) | 13 (65.0) | |
| Diabetes mellitus | ||||
| No | 35 (72.9) | 20 (71.4) | 15 (75) | |
| Yes | 13 (27.1) | 8 (28.6) | 5 (25) | |
| Central corneal thickness | 537±31 | 531.6±31.84 | 537.6 ±29.9 | 0.5 |
| Cup to disc ratio | 0.6±0.2 | 0.570±0.14 | 0.591±0.17 | 0.78 |
| Baseline IOP (mm Hg) | 24±4.2 | 22.9±3.7 | 25.7±4.4 | 0.025 |
| Baseline number of medications | 3.0±0.8 | 2.6±0.8 | 2.5±0.8 | 0.84 |
| Baseline MD (dB) | -3.0 ±1.67 | -2.88±1.67 | -3.1±1.69 | 0.3 |
POAG: Primary open angle glaucoma; PXFG: Pseudoexfoliative glaucoma.
x±s, n (%)
Table 2. IOP measurements in the study groups at different follow-up visits.
| Time | POAG | PXFG | 1P |
| Baseline | |||
| No. of eyes | 28 | 20 | |
| Value | 22.9±3.7 | 25.7±4.4 | 0.02 |
| 1d | |||
| No. of eyes | 28 | 20 | |
| Value | 17.8±5.1 | 14.7±4.3 | 0.03 |
| Change | -5.2±5.0 | -11±5.3 | <0.001 |
| Change % | -22±20 | -42±18 | <0.001 |
| 1wk | |||
| No. of eyes | 23 | 20 | |
| Value | 19.8±3.6 | 18.7±4.2 | 0.33 |
| Change | -3.2±3.2 | -7.1±6.2 | 0.01 |
| Change % | -13±13 | -26±21 | 0.02 |
| 1mo | |||
| No. of eyes | 27 | 20 | |
| Value | 18.4±4.9 | 17.7±4.3 | 0.61 |
| Change | -4.5±4.0 | -7.9±5.6 | 0.02 |
| Change % | -20±18 | -30±19 | 0.04 |
| 3mo | |||
| No. of eyes | 28 | 20 | |
| Value | 18.1±2.7 | 17.0±3.4 | 0.22 |
| Change | -4.8±3.0 | -8.7±5.8 | 0.005 |
| Change % | -20±11 | -32±19 | 0.009 |
| 6mo | |||
| No. of eyes | 28 | 20 | |
| Value | 18.4±3.2 | 17.9±4.0 | 0.62 |
| Change | -4.6±3.0 | -7.8±5.5 | 0.01 |
| Change % | -19±11 | -29±17 | 0.02 |
| 9mo | |||
| No. of eyes | 23 | 17 | |
| Value | 17.9±3.3 | 16.3±1.0 | 0.96 |
| Change | -4.9±3.5 | -6.7±2.9 | 0.30 |
| Change % | -21±13 | -28±11 | 0.22 |
| 12mo | |||
| No. of eyes | 18 | 15 | |
| Value | 18.0±3.9 | 21.0±6.6 | 0.32 |
| Change | -3.5±4.2 | -4.7±9.3 | 0.75 |
| Change % | -16±17 | -16±32 | 0.9 |
POAG: Primary open angle glaucoma; PXFG: Pseudoexfoliative glaucoma. 1t-test.
x±s
Table 3. Number of IOP-lowering medications in the study groups at different follow-up visits.
| Time | POAG | PXFG | 1P |
| Baseline | 2.6±0.8 | 2.5±0.8 | 0.84 |
| 1d | 2.5±0.8 | 2.3±0.9 | 0.49 |
| 1wk | 3.0±3.0 | 2.3±0.7 | 0.41 |
| 1mo | 3.0±2.3 | 2.3±0.8 | 0.10 |
| 3mo | 2.4±0.8 | 2.3±0.7 | 0.72 |
| 6mo | 2.4±0.7T | 2.4±0.8 | 1.00 |
| 9mo | 2.6±0.9 | 2.5±1.0 | 0.75 |
| 12mo | 2.4±1.1 | 2.7±0.6 | 0.74 |
POAG: Primary open angle glaucoma; PXFG: Pseudoexfoliative glaucoma. 1Mann-Whitney test.
x±s
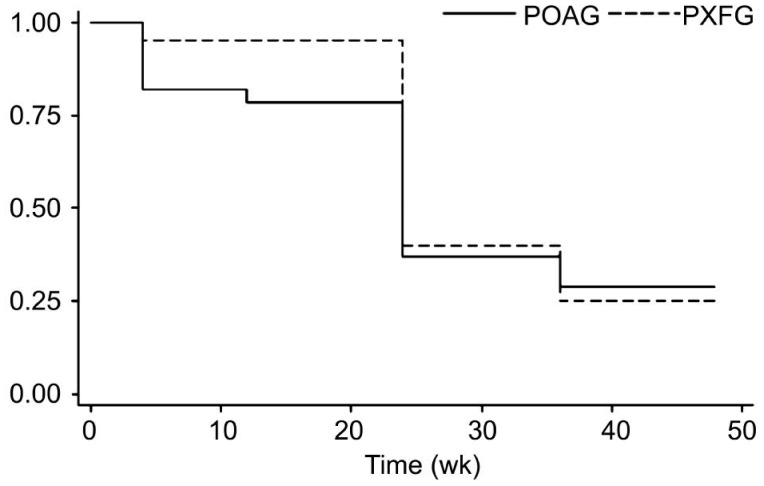
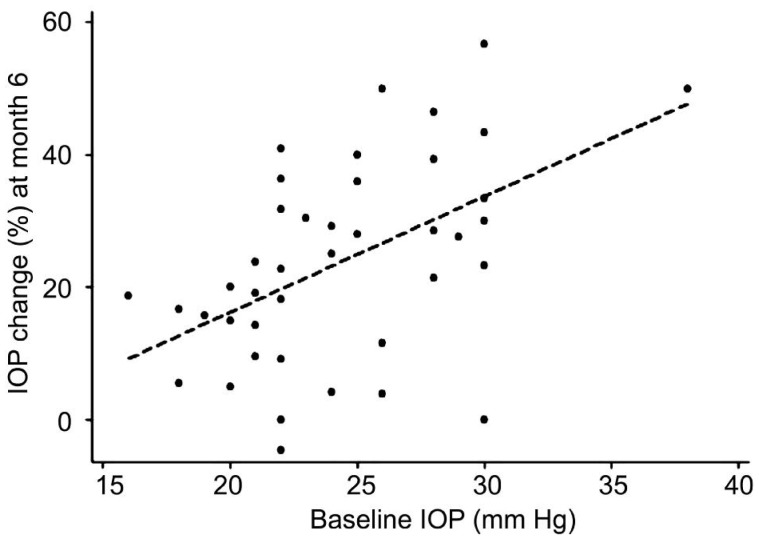
The mean±SD IOP at baseline and the last visit was 22.9±3.7 and 18.0±3.9 mm Hg in the POAG group (P=0.02), and 25.7±4.4 and 21.0±6.6 mm Hg in the PXFG group (P=0.47), respectively. Mean number of IOP-lowering medications at baseline was 2.6±0.8 in the POAG group and 2.5±0.8 in the PXFG group, which decreased to 2.4±1.1 and increased to 2.7±0.6 at the last follow-up visit, respectively. The differences were not statistically significant in both groups (P=0.16, 0.57). On Kaplan-Meier survival analysis, the success rate was 75.0% in the POAG group compared to 94.1% in the PXFG group (P=0.08; Log-rank test) at 6mo, and 29.1% in the POAG group and 25.0% in the PXFG group at 12mo (P=0.9; Log-rank test; Figure 1). The median time to failure was 6mo in both groups. The percentage of IOP reduction was statistically significantly (P=0.02) in the PXFG group during the first 6mo of follow-up (Table 2) that might be related to higher baseline IOP in PXFG group (Figure 2).
Figure 1. Kaplan-Meier survival curve analysis of event-free rates between the POAG and PXFG groups.
Figure 2. Graph showing the relationship between baseline IOP and changes of IOP at month 6 from baseline.
On univariate analyses, there was no statistically significant association between time to failure and the following factors: age, gender, glaucoma type, laterality, diabetes, central corneal thickness, cup to disc ratio, preoperative IOP and number of medications, and the percentage of IOP reduction at postoperative week 1. The only observed complication was redness without intraocular inflammation in one eye in each group one day after SLT, which resolved spontaneously after 3d.
DISCUSSION
In this prospective comparative study, we investigated intermediate-term outcomes of 360-degree SLT in patients with mild to moderate POAG and PXFG with uncontrolled IOP despite maximally tolerated IOP-lowering medications. Although the absolute post-laser IOP measurements were similar in both study groups at all follow-up visits (except 1d), the percent IOP reduction was statistically significantly more pronounced in the PXFG group during the first 6mo of follow-up; this might be attributed to higher baseline IOP in the PXFG group. The mean number of IOP-lowering medications was statistically similar in both groups at baseline and SLT did not significantly reduce the number of medications at any of the follow-up visits in both groups. In a retrospective study, Kara et al [10] similarly reported that the PXFG patients had significantly higher percentage of IOP reduction compared to POAG patients over one year of follow-up[10]. They also found that SLT did not reduce the number of medications at any follow-up visits in both POAG and PXFG groups[10]. In another prospective study, Ayala and Chen[11] found that SLT reduced IOP by about 6-7 mm Hg in both POAG and PXFG patients one month after the procedure. Although the reduction was slightly better in their PXFG patients, the difference was not statistically significant[11]. In our study, the efficacy of SLT decreased over time in both groups, and this was more pronounced in the PXFG group. Our results are similar to Gracner findings in PXFG patients[12]. This study showed that SLT is an effective procedure for lowering IOP in PXFG and POAG eyes, although the effect seems to last less in PXFG eyes. In another study, Kara et al [10] showed that SLT was more effective in reducing IOP in PXFG compared to POAG, and the effect did not decrease over one year of follow-up[10]. They found that IOP significantly decreased by 4.4±2.1 mm Hg in the POAG group and by 6.1±3.6 mm Hg in the PXFG group at the 12-month follow up[10].
A recent Meta-analysis showed that IOP reduction at 12mo post-SLT in 35 studies ranged from 6.9% to 35.9%[13]. Although few studies have included other types of glaucoma than POAG, the efficacy of SLT appears to vary according to type of glaucoma[8],[14]. Overall, it seems that the IOP-lowering effect of SLT is less in patients with normal tension glaucoma (range: 14% to 16%), and relatively greater in PXFG patients (range: 11% to 32%)[13],[15]–[16]. The result of this Meta-analysis also showed that medications reduced IOP more than SLT (0.9 mm Hg more with multi therapy and 0.6 mm Hg more with mono-therapy); however, the difference was not statistically significant[13]. The effect of SLT on reducing the number of medications varies in different studies. While some studies have shown that the number of medications did not change after SLT, others have reported that SLT reduced the number for medications[13],[15],[17]–[18]. In our study, the mean number of IOP-lowering medications was statistically similar in both groups at baseline and SLT did not significantly reduce the number of medications at any of the follow-up visits in both groups. Lee et al[19] studied different predictors of success/failure of SLT in 111 eyes (65 patients) with normal tension glaucoma and POAG[19]. They reported that higher baseline IOP, use of carbonic anhydrase inhibitors, thinner retinal nerve fiber layer, and lower day 1 IOP were predictors of success[19]. Their definition for success was IOP reduction ≥20%. Martow et al[20] also found that the only predictive factor of SLT success was the pretreatment IOP and that glaucoma type including PXF was not a predictor[20].
Tzimis et al [21] showed that only baseline IOP was the most powerful variable in predicting outcome of SLT[21]. In contrast, no baseline factors were found to be a predictor for failure after SLT in our study. This may be a factor of the fairly small sample size of our study since the sample size was determined for a different outcome measure.
The most common side effects of SLT are IOP spike, anterior chamber inflammation, eye pain or discomfort, photophobia, and conjunctival hyperemia[13]. These side effects are generally transient and minor. The reported incidence of IOP spike varied from 0 to 62% in different studies[13]. In studies where prophylactic IOP-lowering medications were used, the reported incidence was lower (between 0 and 29%)[13]. In our study, we did not observe any significant complications during and after the procedure although we did not use any prophylactic medication.
This study has some shortcomings. The fairly small number of patients in our study limited the power of our study for finding predictors of failure after SLT as mentioned above. Furthermore, the degree of angle pigmentation was not recorded in our study and therefore, the potential role of this variable could not be explored.
In conclusion, we found that 360-degree SLT is an effective and well-tolerated therapeutic modality in patients with POAG and PXFG. We found that SLT was more effective early in the postoperative period in patients with PXFG compared with patients with POAG. However, the efficacy of SLT decreased over time and this was more pronounced in PXFG patients.
Acknowledgments
Conflicts of Interest: Miraftabi A, None; Nilforushan N, None; Nassiri N, None; Nouri-Mahdavi K, None.
REFERENCES
- 1.Park CH, Latina MA, Schuman JS. Developments in laser trabeculoplasty. Ophthalmic Surg Lasers. 2000;31(4):315–322. [PubMed] [Google Scholar]
- 2.Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology. 2001;108(4):773–779. doi: 10.1016/s0161-6420(00)00660-6. [DOI] [PubMed] [Google Scholar]
- 3.Cvenkel B, Hvala A, Drnovsek-Olup B, Gale N. Acute ultrastructural changes of the trabecular meshwork after selective laser trabeculoplasty and low power argon laser trabeculoplasty. Lasers Surg Med. 2003;33(3):204–208. doi: 10.1002/lsm.10203. [DOI] [PubMed] [Google Scholar]
- 4.Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol. 2003;121(7):957–960. doi: 10.1001/archopht.121.7.957. [DOI] [PubMed] [Google Scholar]
- 5.Samples JR, Singh K, Lin SC, Francis BA, Hodapp E, Jampel HD, Smith SD. Laser trabeculoplasty for open-angle glaucoma: a report by the american academy of ophthalmology. Ophthalmology. 2011;118(11):2296–2302. doi: 10.1016/j.ophtha.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Stein JD, Challa P. Mechanisms of action and efficacy of argon laser trabeculoplasty and selective laser trabeculoplasty. Curr Opin Ophthalmol. 2007;18(2):140–145. doi: 10.1097/ICU.0b013e328086aebf. [DOI] [PubMed] [Google Scholar]
- 7.Kagan DB, Gorfinkel NS, Hutnik CM. Mechanisms of selective laser trabeculoplasty: a review. Clin Experiment Ophthalmol. 2014;42(7):675–681. doi: 10.1111/ceo.12281. [DOI] [PubMed] [Google Scholar]
- 8.Ayala M. Long-term outcomes of selective laser trabeculoplasty (SLT) treatment in pigmentary glaucoma patients. J Glaucoma. 2014;23(9):616–619. doi: 10.1097/IJG.0b013e318287abb7. [DOI] [PubMed] [Google Scholar]
- 9.Shazly TA, Smith J, Latina MA. Long-term safety and efficacy of selective laser trabeculoplasty as primary therapy for the treatment of pseudoexfoliation glaucoma compared with primary open-angle glaucoma. Clin Ophthalmol. 2011;5:5–10. doi: 10.2147/OPTH.S15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kara N, Altan C, Yuksel K, Tetikoglu M. Comparison of the efficacy and safety of selective laser trabeculoplasty in cases with primary open-angle glaucoma and pseudoexfoliative glaucoma. Kaohsiung J Med Sci. 2013;29(9):500–504. doi: 10.1016/j.kjms.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Ayala M, Chen E. Comparison of selective laser trabeculoplasty (SLT) in primary open angle glaucoma and pseudoexfoliation glaucoma. Clin Ophthalmol. 2011;5:1469–1473. doi: 10.2147/OPTH.S25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grancner T. Intraocular pressure response of capsular glaucoma and primary open-angle glaucoma to selective Nd:YAG laser trabeculoplasty: a prospective, comparative clinical trial. Eur J Ophthalmol. 2002;12(4):287–292. doi: 10.1177/112067210201200406. [DOI] [PubMed] [Google Scholar]
- 13.Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol. 2015;60(1):36–50. doi: 10.1016/j.survophthal.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Tokuda N, Inoue J, Yamazaki I, Matsuzawa A, Munemasa Y, Kitaoka Y, Takagi H, Ueno S. Effects of selective laser trabeculoplasty treatment in steroid-induced glaucoma. Nippon Ganka Gakkai Zasshi. 2012;116(8):751–757. [PubMed] [Google Scholar]
- 15.Lee JW, Ho WL, Chan JC, Lai JS. Efficacy of selective laser trabeculoplsty for normal tension glaucoma: 1 year results. BMC Ophthalmol. 2015;15:1. doi: 10.1186/1471-2415-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenfeld M, Geyer O, Segev E, Kaplan-Messas A, Melamed S. Selective laser trabeculoplasty in uncontrolled pseudoexfoliation glaucoma. Ophthalmic Surg Lasers Imaging. 2011;42(5):390–393. doi: 10.3928/15428877-20110630-01. [DOI] [PubMed] [Google Scholar]
- 17.Katz LJ, Steinmann WC, Kabir A, Molineaux J, Wizov SS, Marcellino G, SLT/Med Study Group Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460–468. doi: 10.1097/IJG.0b013e318218287f. [DOI] [PubMed] [Google Scholar]
- 18.Abdelrahman AM, Eltanamly RM. Selective laser trabeculoplasty in egyptian patients with primary open-angle glaucoma. Middle East Afr J Ophthalmol. 2012;19(3):299–303. doi: 10.4103/0974-9233.97930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JW, Liu CC, Chan JC, Lai JS. Predictors of success in selective laser trabeculoplasty for Chinese open-angle glaucoma. J Glaucoma. 2014;23(5):321–325. doi: 10.1097/IJG.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 20.Martow E, Hutnik CM, Mao A. SLT and adjunctive medical therapy: a prediction rule analysis. J Glaucoma. 2011;20(4):266–270. doi: 10.1097/IJG.0b013e3181e3d2c1. [DOI] [PubMed] [Google Scholar]
- 21.Tzimis V, Tze L, Ganesh J, Muhsen S, Kiss A, Kranemann C, Birth CM. Laser trabeculoplasty: an investigation into factors that might influence outcomes. Can J Ophthalmol. 2011;46(4):305–309. doi: 10.1016/j.jcjo.2011.06.005. [DOI] [PubMed] [Google Scholar]