Abstract
AIM
To study the effects of cytokeratin 17 (CK17) on sodium iodate (NaIO3) induced rat retinal pigment epithelium (RPE) degeneration, laser induced rat choroidal neovascularization (CNV), and oxidative stress of human retinal pigment epithelium cells (ARPE-19) and human umbilical vein endothelial cell (HUVEC).
METHODS
Thirty 8-week-old male Brown Norway rats were randomly divided into 3 groups, 10 rats in control group treated with solvent alone; 10 rats in NaIO3 group treated with solvent and 35 mg/kg NaIO3 injection through hypoglossal vein and 10 rats in CK17+NaIO3 group treated with 1% CK17 eye drop 3 times a day for 1wk before and 4wk after NaIO3 injection. RPE function was measured with c-wave of electroretinogram (ERG). Another 20 rats were randomly divided into 2 groups. Of them 10 rats in CK17 group were anesthetized to receive Nd:YAG laser and given 1% CK17 eye drop before same as above; 10 rats in control were received Nd:YAG and treated with solvent. The development of choroidal neovascularization (CNV) was determined by fundus fluorescein angiography (FFA) performed on 4wk after laser. Methylthiazoly tetrazolium (MTT) assay was used to study effect of CK17 on various oxidants induced injury in ARPE-19 and HUVEC in vitro.
RESULTS
Four weeks after NaIO3 injection, the c-wave amplitude of ERG was 0.393±0.02 V in the control group, 0.184±0.018 V in NaIO3 group and 0.3±0.01 V in CK17+NaIO3 group. There was a significant reversal of the c-wave by CK17 as compared to NaIO3 group (P<0.01). Four weeks after laser, the size of the CNV lesion was 2.57±0.27 mm2 in control group and 1.64±0.08 mm2 in CK17 group. The lesion size significantly diminished in CK17 group (P<0.01). The in vitro results showed CK17 also reversed the various oxidants induced injuries in ARPE-19 at the dose of 100 µg/mL and enhanced the injury in HUVECs at different concentrations.
CONCLUSION
CK17 can significantly protect RPE from NaIO3 induced degeneration in vivo and in vitro and also could reverse the various oxidants induced injuries in vitro. It inhibits the development of CNV in rat model, interfered with vascular endothelial cell proliferation in vitro.
Keywords: cytokeratin 17, age-related macular degeneration, choroidal neovascularization, retinal pigment epithelium, human umbilical vein endothelial cells
INTRODUCTION
Retinal degenerative disease can lead to blindness. Age-related macular degeneration (AMD) is the most common retinal degenerative diseases[1]. A century ago, the AMD has been described on the medical literature, but until the last century 70's, there has been a description of the treatment of AMD. About 30% of adults aged 75y or older have some signs of maculopathy, and 6% to 8% of these individuals are afflicted with the advanced stages of AMD[2]. According to reports, in the United States AMD accounted for 54% of the current blindness cases among the Caucasian[3].
Single layer of retinal pigment epithelium (RPE) separates the retinal nerve sensory layer and choroid. Its main function is to provide nutrition and phagocytose the outer segments of adjacent photoreceptors[4]. The RPE disable to remove the metabolic waste results in the accumulation of drusen. RPE dysfunction causes the breakdown of the Bruch's membrane. The breaks in Bruch's membrane under the detached RPE serve as an entrance for new and immature choroidal vessels into the subretinal space that lead to the formation of choroidal neovascularization (CNV). Furthermore, RPE loss may cause loss of choriocapillaris[5]. Therefore, RPE can be used as a target for improving photoreceptor survival and treatment related ocular diseases[6].
Cytokeratin has been used as a marker for epithelial cells. They help in providing architecture and a definite cellular organisation to the tissue as a whole, by controlling morphogenetic migration of cells[7]. Cytokeratin abnormal expression will result in cell dysfunction. This study is to observe the effects of 1% cytokeratin 17 (CK17) on sodium iodate (NaIO3) induced RPE degeneration, laser induced choroidal neovascularization, and oxidative stress of RPE cells and human umbilical vein endothelial cells (HUVECs).
MATERIALS AND METHODS
Retinal Pigment Epithelium Degeneration Model in Rat Eyes
All animals used in this study were bred in a colony at the University of Utah, and maintained under a 12h light/dark cycle. They were housed and handled with the authorization and supervision of the Institutional Animal Care and Use Committee from the Texas A&M Health Science Center.
A total of 30 8-week-old male Brown-Norway rats were randomly divided into 3 groups, 10 rats in control group, 10 rats in NaIO3 group and 10 rats in CK17+NaIO3 group. Control group was treated with solvent (Dulbecco's phosphate buffered saline; DPBS) eye drop alone throughout the trial without NaIO3 (Sigma-Aldrich Chemical Co., MO, USA) injection. NaIO3 group was treated with solvent eye drop and 35 mg/kg NaIO3 hypoglossal vein injection, whereas CK17+NaIO3 group was treated with 1% CK17 (Sigma-Aldrich Chemical Co., MO, USA) eye drop and NaIO3 injection. All eye drops were instilled both eyes with 1 drop for 3 times a day for 1wk before and 4wk after NaIO3 injection. At the end of 4wk, all rats were measured with c-wave of electroretinogram (ERG).
Rats were dark adapted for 2h, and then anesthetized with ketamine 35 mg/kg plus xylazine 5 mg/kg intramuscular injection. Half of the initial dose was given each 1h thereafter. Pupils of all rats were dilated with one drop of 1% atropine and 2.5% phenylephrine. ERG recording methods developed by Peachey et al[8] were followed. Briefly, Silver chloride electrode is arranged on the surface of the binocular cornea, and the stainless steel needle electrode was used as the reference electrode and the ground electrode, which were respectively arranged on the same side of the cheek and the tail. Responses were amplified (dc-100Hz; gain 1000×; DP-31, Warner Instruments, Hamden, CT, USA). Data were analyzed by iWORX LabScribe Data Recording Software (iWorxoCB Sciences, Dover, NH, USA).
Choroidal Neovascularization Model in Rat Eyes
A total of 20 8-week-old male Brown-Norway rats were randomly divided into 2 groups, 10 rats in control group, 10 rats in CK17 group. CK17 group was instilled with 1% CK17 eye drops and control group was treated with solvent (DPBS) eye drop. Both eyes of all rats were instilled with 1 drop for 3 times a day for 1wk before and 4wk after laser.
Rats were anesthetized and the pupils were dilated as mentioned above. The fundus was visualized with a VOLK super pupil XL biomicroscopy lens (Keeler Instrument Inc., Broomall, PA, USA). A double-frequency Nd:YAG laser (Laserex LP3532; Lumenis Inc., Salt Lake City, UT, USA) was used at 532 nm wavelength. Laser parameters used were 100 µm spot size, 0.15s exposure and l50-200 mw powers. Five laser spots were made to the ocular fundus at approximately equal distances around the optic nerves. Acute vapor bubbles suggested rupture of Bruch's membrane[9]. Only laser spots with bubble formation were included in the study. Lesions with subretinal hemorrhage were excluded.
Fundus fluorescein angiography (FFA) was performed 4wk after laser treatment with a digital fundus camera (TRC-50EX; TOPCON, Tokyo, Japan). Ten percent fluorescein sodium salt was injected through hypoglossal vein at 0.5 mL/kg. Both early (under 2min) and late (over 7min) fluorescein phases were captured. Ten percent fluorescein isothiocyanate-dextran was injected through hypoglossal vein at l.4 mg/kg after 3d of fluorescein sodium salt injection. Fluorescein pictures were taken within 20min. The clearest pictures were chosen for the areas of CNV formation measurement by IMAGEnet2000 digital imaging system (Topcon Medical Systems, Inc., Paramus, NJ, USA).
Cell Culture
Human retinal pigment epithelium cells (ARPE-19; ATCC, Manassas, VA, USA) were grown in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12, 1:1; Sigma-Aldrich Chemical Co., MO, USA) supplemented with 10% fetal bovine serum (FBS; ATCC, Manassas, VA, USA), 100 units/mL penicillin G, and 100 µg/mL streptomycin sulfate. HUVECs (ATCC, Manassas, VA, USA) were grown in vascular cell basal medium supplemented with endothelial cell growth kit (ATCC, Manassas, VA, USA). Cells were incubated in a humidified incubator at 37°C under 5% CO2 and 95% air.
Cells were allowed to attach overnight, and then exposed to CK17 or vehicle solution under hypoxic condition for 72h. Hypoxic conditions (1% O2, 5% CO2 and 94% N2) were maintained by using a temperature and humidity controlled environmental C-chamber by O2 and CO2 controllers (Proox Model 110 and Pro CO2 Model 120, Bio Spherix Ltd., Redfield, NY, USA) with N2 and CO2 gas sources. Thiazolyl blue tetrazolium bromide (MTT) assay (Sigma-Aldrich Chemical Co., MO, USA) was used to measure the viability of ARPE-19 and HUVECs. Cells (1×105) were seeded in 96-well plates (100 µL/well) and allowed to grow overnight. Negative control was prepared by adding 100 µL medium without cells. The cells were then treated with fresh medium with CK17 [CK17 was dissolved in 30% 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), the final concentration of HP-β-CD in cells is less than 0.3%] and/or oxidizing agents (NaIO3/H2O2/NaN3/t-BHP) at the same time for 12, 24, or 72h (200 µL/well). The vehicle control group was treated with 30% HP-β-CD with fresh medium (the final concentration of HP-β-CD in cells is less than 0.3%). MTT (5 mg/mL; 20 µL) was added to wells, and incubated for another 4h. After incubation, the medium was discarded and 100 µL DMSO was added to solubilize formazan produced from MTT by the viable cells. Absorbance was measured at 570 nm using a microplate reader (Bio-Rad Laboratories, Inc., CA, USA). Cells viability was calculated according to the following formula: Viability of cells (%)=(absorbance in tested sample-absorbance in negative control)/(absorbance in vehicle control-absorbance in negative control)×100%.
Statistical Analysis
All data were presented as mean±SEM. A nonpaired Student's t-test was perfonned to analyze the significance between two groups at a certain time point. Analysis of variance for comparison between three groups and more. The differences were considered significant at P<0.05.
RESULTS
Electroretinogram Recordings
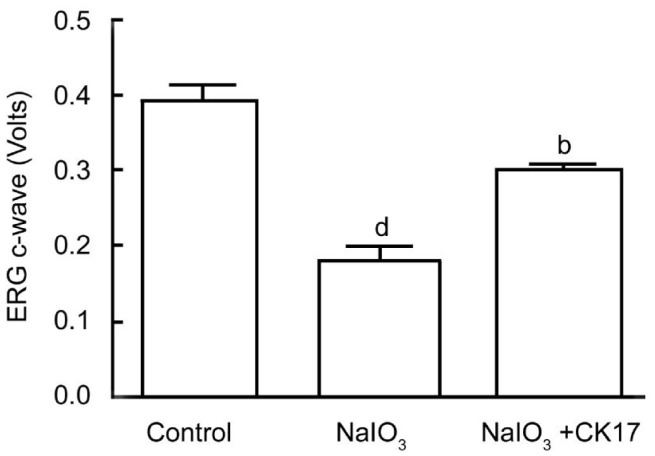
Four weeks after NaIO3 injection, the amplitude of ERG c-wave was 0.393±0.02 V in the control group, 0.184±0.018 V in the NaIO3 group, 0.3±0.01 V in the CK17+NaIO3 group. There was a significant reversal of the ERG c-wave by CK17 as compared to NaIO3 group (P<0.01) (Figure 1).
Figure 1. ERG outcomes of CK17 on NaIO3 induced RPE degeneration in rat eyes.
Data were expressed as mean±SEM. dP<0.01 vs control group; bP<0.01 vs NaIO3 group.
Fundus Fluorescein Angiography
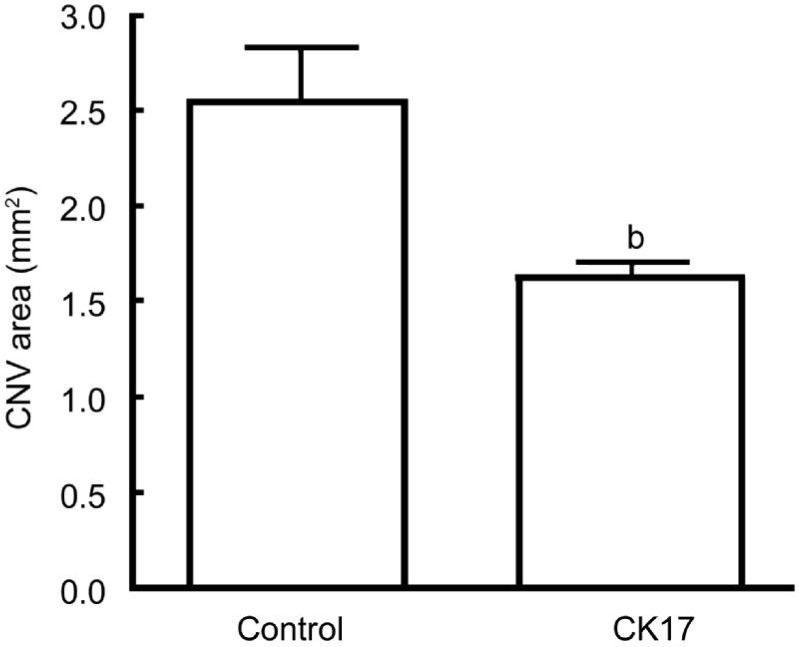
The angiograms showed diminished lesion size in CK17 group 4wk after laser. The size of the CNV lesion was 2.57±0.27 mm2 in control group, 1.64±0.08 mm2 in CK17 group (P<0.01) (Figure 2).
Figure 2. Effect of CK17 on laser induced CNV rat model.
Data were expressed as mean±SEM. bP<0.01 vs control group.
Cytotoxicity of Cytokeratin 17 on Human Retinal Pigment Epithelium and Umbilical Vein Endothelial Cells
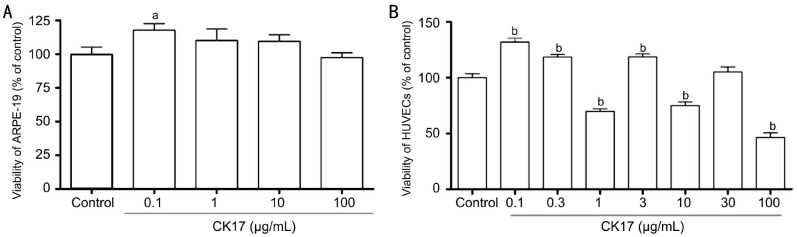
The results showed that CK17 had no effect on ARPE-19 cells except at the concentration of 0.1 µg/mL it significantly increased the proliferation of ARPE-19 (P<0.05, Figure 3A). At the concentration of 0.1, 0.3 and 3 µg/mL, CK17 significantly increased the proliferation of HUVECs (P<0.01, Figure 3B). However, CK17 inhibited the proliferation of HUVECs at the concentration of 1, 10 and 100 µg/mL (P<0.01, Figure 3B).
Figure 3. Cytotoxicity of CK17 on ARPE-19 and HUVECs.
A: Effect of CK17 on proliferation of ARPE-19; B: Effect of CK17 on proliferation of HUVECs. Data were expressed as means±SEM; n=6. aP<0.05, bP<0.01 vs control group.
Effect of Cytokeratin 17 on Hypoxia-induced Damage in Human Retinal Pigment Epithelium and Umbilical Vein Endothelial Cells
CK17 significantly increased the viability of ARPE-19 cells by 16% in hypoxic condition at concentration of 100 µg/mL (P<0.01, Figure 4A). It also could increase the viability of HUVECs by 15% in hypoxic condition at concentration of 0.1 µg/mL (P<0.05, Figure 4B). However, the other concentration of CK17 had no effect on both ARPE-19 and HUVECs.
Figure 4. Effect of CK17 on hypoxia-induced damage in ARPE-19 and HUVECs.
A: Effect of CK17 on hypoxia-induced injury in ARPE-19; B: Effect of CK17 on hypoxia-induced injury in HUVECs. Data were expressed as means±SEM; n=6. aP<0.05 and bP<0.01 vs model group.
Effect of Cytokeratin 17 on NaIO3-induced Injury in Human Retinal Pigment Epithelium and Umbilical Vein Endothelial Cells
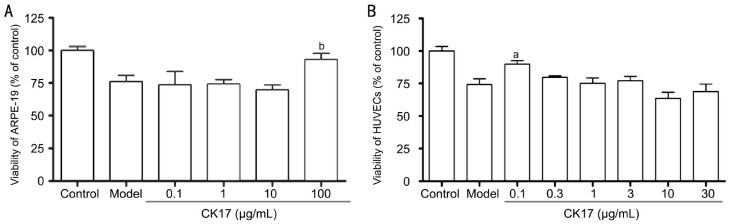
At the concentration of 100 µg/mL, CK17 significantly increased the viability of 30, 100 and 300 µg/mL NaIO3-induced injury by 23%, 15% and 27% in ARPE-19 cells, respectively (All P<0.05, Figure 5A). However, all concentration of CK17 couldn't reverse NaIO3-induced injury in HUVECs. On the contrary, it enhanced NaIO3-induced injury in HUVECs especially the concentrations 1 and 10µg/mL (P<0.01, Figure 5B).
Figure 5. Effect of CK17 on NaIO3-induced injury in ARPE-19 and HUVECs.
A: Effect of CK17 on NaIO3-induced injury in ARPE-19; B: Effect of CK17 on NaIO3-induced injury in HUVECs. Data were expressed as means±SEM; n=6. aP<0.05 and bP<0.01 vs NaIO3 group.
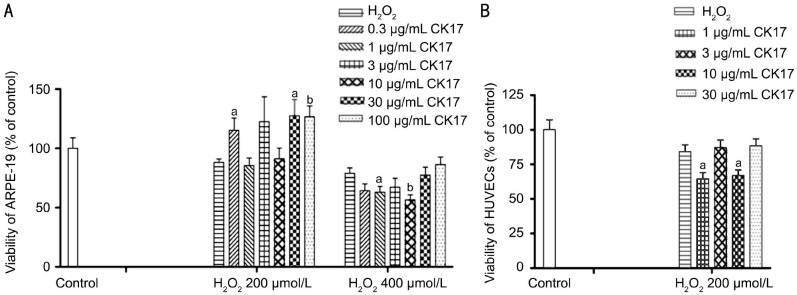
Effect of Cytokeratin 17 on H2O2-induced Injury in Human Retinal Pigment Epithelium and Umbilical Vein Endothelial Cells
CK17 with 0.3, 30 and 100 µg/mL reversed 200 µmol/L H2O2-induced injury by 27%, 39% and 27% in ARPE-19, respectively. However, 1 and 10 µg/mL CK17 significantly enhanced 400 µmol/L H2O2-induced injury in ARPE-19 (Figure 6A). CK17 with 1 and 10 µg/mL significantly enhanced 200 µmol/L H2O2-induced injury in HUVECs (Figure 6B).
Figure 6. Effect of CK17 on H2O2-induced injury in ARPE-19 and HUVECs.
A: Effect of CK17 on H2O2-induced injury in ARPE-19; B: Effect of CK17 on H2O2-induced injury in HUVECs. Data were expressed as means±SEM; n=6. aP<0.05, bP<0.01 vs H2O2 group.
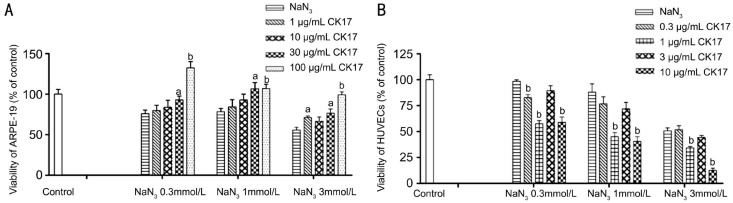
Effect of Cytokeratin 17 on NaN3-induced Injury in Human Retinal Pigment Epithelium and Umbilical Vein Endothelial Cells
CK17 reversed 0.3, 1 and 3 mmol/L NaN3-induced injury in ARPE-19 at the concentration of 30 and 100 µg/mL (Figure 7A). On the contrary, 1 and 10 µg/mL CK17 significantly enhanced 0.3, 1 and 3 mmol/L NaN3-induced injury in HUVECs (P<0.01, Figure 7B).
Figure 7. Effect of CK17 on NaN3-induced injury in ARPE-19 and HUVECs.
A: Effect of CK17 on NaN3-induced injury in ARPE-19; B: Effect of CK17 on NaN3-induced injury in HUVECs. Data were expressed as means±SEM; n=6. aP<0.05 and bP<0.01 vs NaN3 group.
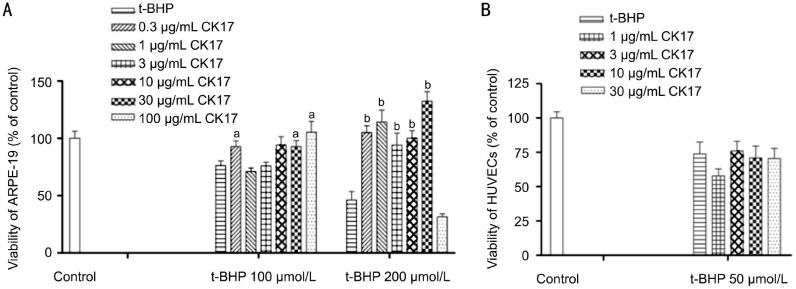
Effect of Cytokeratin 17 on t-BHP-induced Injury in Human Retinal Pigment Epithelium and Umbilical Vein Endothelial Cells
CK17 reversed the viability of 100 and 200 µmol/L t-BHP-induced injury in ARPE-19 at the different concentration (Figure 8A). CK17 had no effect on 50 µmol/L t-BHP-induced injury in HUVECs (Figure 8B).
Figure 8. Effect of CK17 on t-BHP-induced injury in ARPE-19 and HUVECs.
A: Effect of CK17 on t-BHP-induced injury in ARPE-19; B: Effect of CK17 on t-BHP -induced injury in HUVECs. Data were expressed as means±SEM; n=6. aP<0.05, bP<0.01 vs t-BHP group.
DISCUSSION
There are two clinical types of AMD: non-exudative or atrophic AMD (dry-AMD), which is characterized by the degeneration of choriocapillaries, RPE and neurosensoty retina; and neovascular or exudative AMD (wet-AMD), which is characterized by the development of serious RPE detachments and/or choroidal neovascularization that can lead to bleeding, exudation, and eventual scar formation. Although the form of wet-AMD only accounts for 10%-20% of the overall incidence of AMD, it is responsible for over 90% of cases with severe visual loss[10]–[11]. Most of optical treatments are for the wet-AMD, not for dry-AMD and for now there is no effective treatment for the most prevalent dry-AMD[12]. Dry-AMD is triggered by abnormalities in RPE that lies beneath the photoreceptor cells and normally provides critical metabolic support to these light-sensing cells. Secondary to RPE dysfunction, macular rods and cones degenerate leading to the irreversible loss of vision. Oxidative stress, formation of drusen, accumulation of lipofuscin, local inflammation and reactive gliosis represent the pathologic processes implicated in pathogenesis of dry-AMD. The direct toxic effect of NaIO3 on RPE cells with secondary effects on photoreceptors and the choriocapillaries in vivo is well known[13].The mechanisms of the toxicity of NaIO3 to RPE cells are as follows: first, NaIO3 can increase the ability of melanin to convert glycine into glyoxylate, a potential cell toxic compound[14]; second, NaIO3 could denaturant retinal proteins by changes of -SH levels in retina[15]; third, NaIO3 could cause considerable structure changes by breakdown of RPE diffusion barrier or by reduction of adhesion between RPE and photoreceptor cells [16]–[19]; finally, NaIO3 inhibits various enzyme activities, such as noice phosphate dehydragenase, succinodehydrogenase and lactate dehydrogenase[18],[20].
The ERG results showed that CK17 can significantly reverse NaIO3-induced injury in RPE cells. CK17 showed protective effect against NaIO3-induced RPE degeneration in rat eyes. The NaIO3 intoxication causes death of RPE cells and photoreceptor damage followed by marked phagocytic activity of proliferating de-differentiated pigmental cells. Further morphological study is needed to reveal the CK17 role in RPE protection. FFA diagraphs showed that CK17 could significantly decrease the intensity of fluorescein leakage from the photocoagulated lesions and the size of CNV induced by laser treatment on Brown Norway rats, and interfered with vascular endothelial cell proliferation in vitro. Endothelial cells played an important role in the process of CNV development. CNV is the result of angiogenesis, which include endothelial cell proliferation, migration, and adhesion. Certain concentration of CK17 could obviously inhibit the growth of HUVECs. So appropriate dose of CK17 may inhibit the development of CNV through regulation of the behavior of endothelial cells directly.
Mitochondria are the powerhouse of the cell, and their primary function is to generate ATP through oxidative phosphorylation via the electron transport chain[21]. Any kind of oxidative stress can be inhibitor of cytochrome oxidase and catalase, and will downregulate electron transport and O2 consumption to cause the death of cells[22]. Our experiments showed the protection of CK17 on oxidative stress.
With the change of the concentration of CK, the biological effects are obviously different. And the different degree of damage can also lead to the different role of CK. For the next study, we will focus on finding suitable CK treatment concentrations for different injury options.
In conclusion, CK17 might slow the oxidative process of RPE cell layer which leads to RPE degeneration. CK17 might also prevent the formation of CNV. According to the rational of the AMD, the RPE abnonnalities or degeneration is the key point of both dry- and wet-AMD, so CK17 could be used to prevent and treat both dry- and wet-AMD in the future.
Acknowledgments
Conflicts of Interest: Shen Y, None; Zhuang P, None; Xiao T, None; Chiou GCY, None.
REFERENCES
- 1.Lin TC, Chang HM, Hsu CC, Hung KH, Chen YT, Chen SY, Chen SJ. Retinal prostheses in degenerative retinal diseases. J Chin Med Assoc. 2015;78(9):501–505. doi: 10.1016/j.jcma.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Complications of Age-Related Macular Degeneration Prevention Trial Study Group The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clin Trials. 2004;1(1):91–107. doi: 10.1191/1740774504cn007xx. [DOI] [PubMed] [Google Scholar]
- 3.Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Engelmann K, Valtink M. RPE cell cultivation. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):65–67. doi: 10.1007/s00417-003-0811-9. [DOI] [PubMed] [Google Scholar]
- 5.Liu CF, Lin CH, Chen CF, Huang TC, Lin SC. Antioxidative effects of tetramethylpyrazine on acute ethanol-induced lipid peroxidation. Am J Chin Med. 2005;33(6):981–988. doi: 10.1142/S0192415X05003570. [DOI] [PubMed] [Google Scholar]
- 6.Ohtaka K, Machida S, Ohzeki T, Tanaka M, Kurosaka D, Masuda T, Ishii T. Protective effect of hepatocyte growth factor against degeneration of the retinal pigment epithelium and photoreceptor in sodium iodate-injected rats. Curr Eye Res. 2006;31(4):347–355. doi: 10.1080/02713680600629797. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Gupta PD. Tampering with cytokeratin expression results in cell dysfunction. Epithelial Cell Biol. 1994;3(2):79–83. [PubMed] [Google Scholar]
- 8.Peachey NS, Stanton JB, Marmorstein AD. Noninvasive recording and response characteristics of the rat dc-electroretinogram. Vis Neurosci. 2002;19(6):694–701. doi: 10.1017/s0952523802196015. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Xu X, Chiou GC. Effect of interleukin-1 blockers, CK112, and CK116 on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. J Ocul Pharmacol Ther. 2006;22(1):19–25. doi: 10.1089/jop.2006.22.19. [DOI] [PubMed] [Google Scholar]
- 10.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthahnol. 1988;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 11.Votruba M, Gregor Z. Neovascnlar age-related macular degeneration: present and future treatment options. Eye (Lond) 2001;15(Pt 3):424–429. doi: 10.1038/eye.2001.147. [DOI] [PubMed] [Google Scholar]
- 12.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr Eye Res. 2002;25(6):373–379. doi: 10.1076/ceyr.25.6.373.14227. [DOI] [PubMed] [Google Scholar]
- 14.Baich A, Ziegler M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment Cell Res. 1992;5(6):394–395. doi: 10.1111/j.1600-0749.1992.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 15.Sorsby A, Reading HW. Experimental degeneration of the retina. XI. The effect of sodium iodate on retinal -SH levels. Vision Res. 1964;4(10):511–514. doi: 10.1016/0042-6989(64)90057-4. [DOI] [PubMed] [Google Scholar]
- 16.Flage T, Ringvold A. The retinal pigment epithelium diffusion barrier in the rabbit eye after sodium iodate injection. A light and electron microscopic study using horseradish peroxidase as a tracer. Exp Eye Res. 1982;34(6):933–940. doi: 10.1016/0014-4835(82)90072-0. [DOI] [PubMed] [Google Scholar]
- 17.Sen HA, Berkowitz BA, Ando N, de Juan E., Jr In vivo imaging of breakdown of the inner and outer blood-retinal barriers. Invest Ophthalmol Vis Sci. 1992;33(13):3507–3512. [PubMed] [Google Scholar]
- 18.Ashbum FS, Jr, Pilkerton AR, Rao NA, Marak GE. The effects of iodate and iodoacetate on the retinal adhesion. Invest Ophthalmol Vis Sci. 1980;19(12):1427–1432. [PubMed] [Google Scholar]
- 19.Stern WH, Ernest JT, Steinberg RH, Miller SS. Interrelationships between the retinal pigment epithelium and the neurosensory retina. Aust J Ophthalmol. 1980;8(4):281–288. doi: 10.1111/j.1442-9071.1980.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, McCall MA. Behavioral and anatomical abnonnalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82(3):441–448. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZJ, Liang CL, Li GM, Yu CY, Yin M. Stearic acid protects primaty cultured cortical neurons against oxidative stress. Acta Pharmacol Sin. 2007;28(3):315–326. doi: 10.1111/j.1745-7254.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118(1):47–55. [PubMed] [Google Scholar]