Abstract
Diabetic retinopathy (DR) occurs to some extent in most people with at least 20 years duration of diabetes mellitus. Progression of DR to its sight threatening stages is usually associated with worsening of underlying retinal vascular dysfunction and disease. The plasma kallikrein kinin system (KKS) is activated during vascular injury, where it mediates important functions in innate inflammation, blood flow, and coagulation. Recent findings from human vitreous proteomics and experimental studies on diabetic animal models have implicated the KKS in contributing to DR. Vitreous fluid from people with advanced stages of DR contains increased levels of plasma KKS components, including plasma kallikrein (PK), coagulation Factor XII, and high molecular weight kininogen. Both bradykinin B1 (B1R) and B2 (B2R) receptors isoforms are expressed in human retina, and retinal B1R levels are increased in diabetic rodents. Activation of the intraocular KKS induces retinal vascular permeability, vasodilation, and retinal thickening, and these responses are exacerbated in diabetic rats. Preclinical studies have shown that administration of PK inhibitors and B1R antagonists to diabetic rats ameliorates retinal vascular hyperpermeability and inflammation. These findings suggest that components of plasma KKS are potential therapeutic targets for diabetic macular edema.
Keywords: Plasma kallikrein kinin system, diabetic retinopathy, bradykinin receptor, retina, vascular permeability
Introduction
Diabetic retinopathy is one of the most prevalent microvascular complications of diabetes mellitus and a leading cause of vision loss in working-aged adults (Mohamed et al., 2007; Prokofyeva and Zrenner, 2012). A recent meta-analysis of data from nearly 23,000 subjects has shown that for people with ≥20 years duration of diabetes, the prevalence of sight-threatening stages of DR including diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR) is about 20% and 32%, respectively, although a variety of factors can influence these rates (Yau et al., 2012). While there has been important progress in reducing the onset of advanced stages of DR and managing the disease once it occurs (Mohamed et al., 2007; Antonetti et al., 2012), there remains a tremendous clinical need for additional effective treatments for patients who are refractory or who do not fully respond to current therapeutic options. Moreover, this unmet clinical need to prevent and treat DR is projected to increase with the rise in incidence and earlier onset on diabetes (Ko et al., 2012).
Emerging clinical and experimental findings have implicated the plasma KKS in contributing to DR. Recent proteomic analysis of vitreous from people with advanced stages of DR has revealed that vitreous fluid contains components of the KKS (Gao et al., 2007; Kim et al., 2007; Gao et al., 2008). Increased levels of KKS components in the retina also occur in diabetic animal models, which display increased retinal vascular responses to exogenously administered PK and des-Arg9 bradykinin (DABK) (Abdouh et al., 2008; Clermont et al., 2011). Moreover, preclinical studies have shown that both PK inhibitors and bradykinin (BK) receptor antagonists ameliorate retinal functional abnormalities caused by diabetes (Lawson et al., 2005; Abdouh et al., 2008; Clermont et al., 2011). This review examines the regulation and mechanisms of KKS action in the retina and discusses potential strategies for targeting this pathway as a new therapeutic approach for treating DR.
1. Stages and Progression of Diabetic Retinopathy
DR is a chronic disease that is classified into multiple stages and involves combinations of pathophysiological processes, which can lead to retinal neovascularization, edema, and vision loss. Since it is unlikely that a single pathway or system contributes to all phases of this disease, a brief description of the pathogenesis of DR can facilitate the discussion of the stages and processes that could be most affected by the KKS system. DR is classified into multiple levels of nonproliferative diabetic retinopathy (NPDR), including mild, moderate, and severe NPDR, and PDR according to the type and severity of retinal abnormalities. The earliest vascular changes in NPDR involve alterations in retinal hemodyamics, changes in retinal vessel diameters, increases in leakage across the blood-retinal barrier (BRB), and the appearance of microaneurysms, retinal hemorrhage, hard exudates, and cotton wool spots (Cheung et al., 2010). Later changes in NPDR include the worsening of these early changes and development of intraretinal microvascular abnormalities and venous beading. Although these changes alone usually do not cause visual impairment, the progression and accumulation of this vascular injury in NPDR can cause capillary non-perfusion, leading to ischemia and hypoxia-stimulated neovascularization. This sight-threatening stage, termed PDR, involves the formation of disorganized and fragile preretinal vessels that are prone to bleed and can lead to fibrosis and tractional retinal detachment (Antonetti et al., 2012). DR can also cause intraretinal fluid accumulation and thickening of the macula, termed DME. This vision threatening condition can occur at any stage of DR, although its prevalence is increased in patients with greater underlying diabetic retinal disease. The breakdown of BRB function in advanced stages of DR is also associated with marked alterations in the biochemical composition of intraocular fluids (Gao et al., 2007; Yu et al., 2008; Kim et al., 2010). The clinical characteristics of hyperpermeability, hemorrhage, ischemia, and vasogenic edema are biological processes that have been associated with the activation of the KKS in a variety of organs and diseases, including hereditary angioedema and stroke (Cicardi et al., 2010a; Cicardi et al., 2010b; Liu et al., 2010b; Langhauser et al., 2012), suggesting areas of potential physiological interaction between the KKS and DR.
2. Upregulation and activation of the KKS in DR
2.1 The kallikrein-kinin system
The plasma KKS mediates important functions in blood flow, coagulation, innate inflammation, pain, and blood pressure regulation. The effects of this system are mediated by two serine protease zymogens, coagulation Factor XII (FXII) and plasma prekallikrein (PPK), and its substrate, high molecular weight kininogen (HK) (Fig.1). Although the physiological mechanisms responsible for activation of the KKS are not fully understood, a key initiating step appears to involve interactions of FXII with an activating surface, which results in the zymogen activation of FXII to FXIIa (Sainz et al., 2007). The primary substrates of FXIIa include FXI, leading to activation of intrinsic coagulation, and PPK, resulting in the generation of PK. This proteolytically active PK can feed back to activate additional FXII and also cleave HK to liberate the nonapeptide BK. BK activates the G-protein-coupled BK 2 receptor (B2R). Subsequent cleavage of BK by carboxypeptidases generates DABK, which activates the B1 receptor (B1R). In addition to the well-established effects of PK that are mediated by HK cleavage and BK receptor activation (Joseph and Kaplan, 2005; Sainz et al., 2007), this serine protease has a number of other substrates that may mediate effects that are independent of BK receptors. For example, PK can proteolytically activate plasminogen (Selvarajan et al., 2001), and can also directly cleave collagen and a diverse group of secreted proteins (Liu et al., 2010b). While these findings suggest that PK may have a variety of substrates, the physiological significance of many of these interactions is not yet available. Recently, we have shown that PK can also mediate effects via its collagen binding properties, which interferes with collagen-induced platelet activation and exacerbates intracerebral hematoma expansion via a BK-independent mechanism (Liu et al., 2011). Collectively, these data suggest that the KKS mediates its effects via both kinin-dependent and kinin-independent mechanisms.
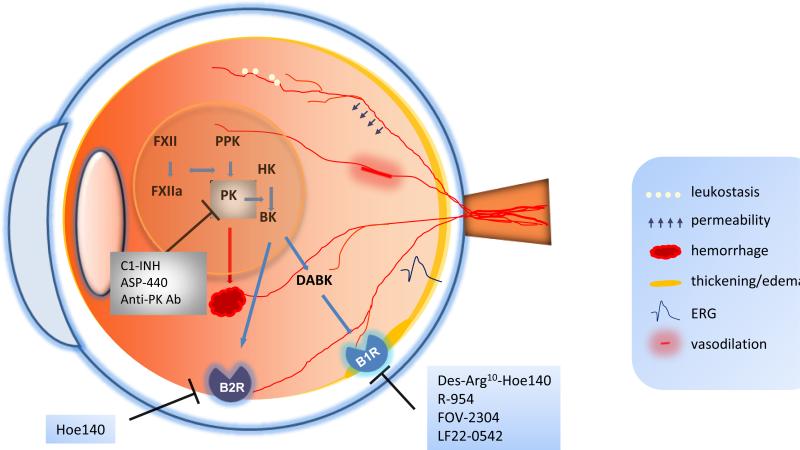
Figure 1.
Schematic of the eye illustrating the involvement of the components of the KKS in diabetic retinopathy. During the activation of the KKS system, FXII and PPK are proteolytically activated to FXIIa and PK, respectively. PK then cleaves HK to release BK, which can be subsequently cleaved to des-Arg9 BK (DABK). BK and DABK bind to B2R and B1R, respectively, thereby changing retinal vascular permeability, vasodilation, leukostasis, electroretinogram (ERG) activity, and retinal thickness. PK may also contribute to retinal hemorrhage. The activity of PK can be inhibited by C1-INH, PK inhibitor ASP-440, and neutralizing anti-PK antibody. The antagonists of B1R include Des-Arg10 Hoe140, R-954, FOV-2304 and LF22-0542, and an antagonist of B2R is Hoe-140. KKS, kallikrein–kinin system; FXII, factor XII; FXIIa, factor XIIa; PPK, prekallikrein; PK, plasma kallikrein; HK: high molecular weight kininogen; BK: bradykinin; B1R, bradykinin 1 receptor; B2R, bradykinin 2 receptor; C1-INH, C1 inhibitor; DABK: des-Arg9-bradykinin.
2.2 KKS components in the DR vitreous
Recent proteomic analyses have identified components of the plasma KKS, including PK, FXII, and HK in the vitreous fluid obtained from patients with advanced DR undergoing pars plana vitrectomy (Kim et al., 2007; Gao et al., 2008). Moreover, vitreous HK and FXII levels detected by mass spectrometry are increased in PDR patients compared with non-diabetic group. Immunoblot analysis has shown that a large fraction of PPK and FXII was proteolytically cleaved to active PK and FXIIa (Gao et al., 2007). Increased levels of PK and PK activity in the retina also occur in diabetic rats compared with nondiabetic controls (Clermont et al., 2011; Catanzaro et al., 2012). Consistent with plasma extravasation in DR, we also observed a 2-fold increase in C1 inhibitor (C1-INH), the primary physiologic inhibitor of PK, in the vitreous from PDR subjects compared with vitreous from nondiabetic subjects (Gao et al., 2007). However, although C1-INH is detected in the vitreous it is not known if concentrations are sufficient to effectively inhibit the KKS.
2.3 Retinal bradykinin receptor expression
Both B1R and B2R are expressed at high levels in human retina, choroid and ciliary body, and relatively low levels in the optic nerve (Ma et al., 1996). Although an in-depth analysis of BK receptors expression in retinal cell types is not yet available, these receptors are widely expressed in neuronal, glial, and vascular cell lineages. Within the retina, prominent B2R expression is observed in the ganglion cell layer, inner nuclear layer, outer nuclear layer, and vascular endothelium (Yasuyoshi et al., 2000). Diabetes has been shown to increase retinal B1R expression in rats (Abdouh et al., 2008; Pouliot et al., 2012). However, the effects of diabetes and DR on retinal B1R expression in humans have not yet been reported.
2.4 Intraocular activation of KKS
Multiple mechanisms can regulate KKS activation, however the contributions of these mechanisms to ocular KKS activity in DR are not fully understood. Increased levels of contact system components (PPK, FXII, and HK) in the eye in DR are likely the result of plasma extravasations, intraocular hemorrhage, and binding interactions that retain components in the vitreous matrix and retina. Intraocular KKS activation could occur following retinal or vitreous hemorrhage, which could increase vitreous contact system proteins along with factors that activate this system, including platelet polyphosphates, microparticles, heparin, and erythrocyte carbonic anhydrase I (CA-1) (Gao et al., 2007; Muller et al., 2009; Oschatz et al., 2011; Van Der Meijden et al., 2012). FXII in the retina and vitreous may also become activated following interactions with extracellular matrix proteins, including collagen (van der Meijden et al., 2009) and laminin (White-Adams et al., 2010), and misfolded or modified proteins (Maas et al., 2008). PPK activation can also involve FXII-independent mechanisms mediated by heat shock protein 90 (Joseph et al., 2002) and prolylcarboxypeptidase (Shariat-Madar et al., 2002). Moreover, activated PK can bind to collagen (Liu et al., 2011), which may retain activated PK in the vitreous and retinal extracellular matrix. Once activated, PK's actions in the eye may be prolonged due to the relative deficiency of C1-INH in the vitreous compared with plasma (Kita and Feener, Unpublished data). In addition to the activation of contact system components, increased retinal B1R expression, as described in the preceding section, may also potentiate KKS actions in DR.
3. KKS action in DR
The physiological actions of the plasma KKS have been extensively studied both in humans and in animal models. In humans, hyperactivity of PK due to genetic C1-INH deficiency has been shown to cause vasogenic edema (Longhurst and Cicardi, 2012). Experimental studies have demonstrated that activation of the KKS exerts a number of biological effects that also occur in DR, including increased vascular permeability and edema, changes in vascular diameter and hemodynamics, and a variety of effects on inflammation, angiogenesis, and neuronal functions. This section examines the role of the KKS in these retinal abnormalities associated with DR.
3.1 Retinal vascular permeability
The effects of the KKS on retinal vascular permeability (RVP) have been examined by intravitreal injection of active components of KKS and by local and systemic administration of PK inhibitors and BK receptor antagonists. Intraocular activation of the KKS by injection of CA-1 into the vitreous has been shown to increase RVP, and this response was inhibited by co-injection of C1-INH, neutralizing antibody against PK, and a small-molecule PK inhibitor, 1-benzyl-1H-pyrazole-4-carboxylic acid 4-carbamimidoyl-benzylamide (ASP-440) (Clermont et al., 2011). Intravitreal injection of PK increased RVP and retinal thickness by a greater extent in diabetic rats compared to similarly treated nondiabetic rats, suggesting that diabetes enhances retinal responses to intraocular KKS activation (Clermont et al., 2011). Systemic administration of ASP-440 decreased RVP both in diabetic rats and in rats subjected to angiotensin II-induced hypertension (Phipps et al., 2009; Clermont et al., 2011). Intravitreal injection of BK increased RVP in both diabetic and nondiabetic rats, whereas only diabetic rats demonstrated a RVP response to DABK (Abdouh et al., 2008; Phipps et al., 2009). Administration of B1R antagonist reduced RVP in streptozotocin (STZ)-induced diabetic rats (Lawson et al., 2005; Abdouh et al., 2008). These data suggest that the activation of the KKS in the circulation and/or locally in the retina and vitreous can increase RVP via both B1R and B2R and that diabetes appears to increase actions mediated via the B1R.
3.2 Retinal blood flow and vasodilation
The KKS can regulate retinal vessel diameters and hemodynamics. Intravitreal injection of BK acutely stimulated increases in retinal vessel diameters and blood flow in adult cats (Sogawa et al., 2010). Intravenous infusion of BK increases retinal vessel diameter in rats (Kojima et al., 2009), however this model also displays marked lowering of systemic blood pressure, which may contribute to its effects on retinal vessels. Ex vivo studies have shown that BK can induce dilation in vessel preparations from rat and porcine retina (Hardy et al., 1998; Abdouh et al., 2003). DABK increased vessels diameters in retinal vessels from diabetic rats but not in retinal vessels from nondiabetic controls (Abdouh et al., 2003). While these acute effects of BK show that the KKS can induce retinal vessel vasodilation, a two week systemic administration of a PK inhibitor to diabetic rats also increased retinal vessel diameters and increased retinal blood flow (Clermont et al., 2011). The mechanisms that mediate these chronic effects of the KKS on retinal hemodynamics are unknown.
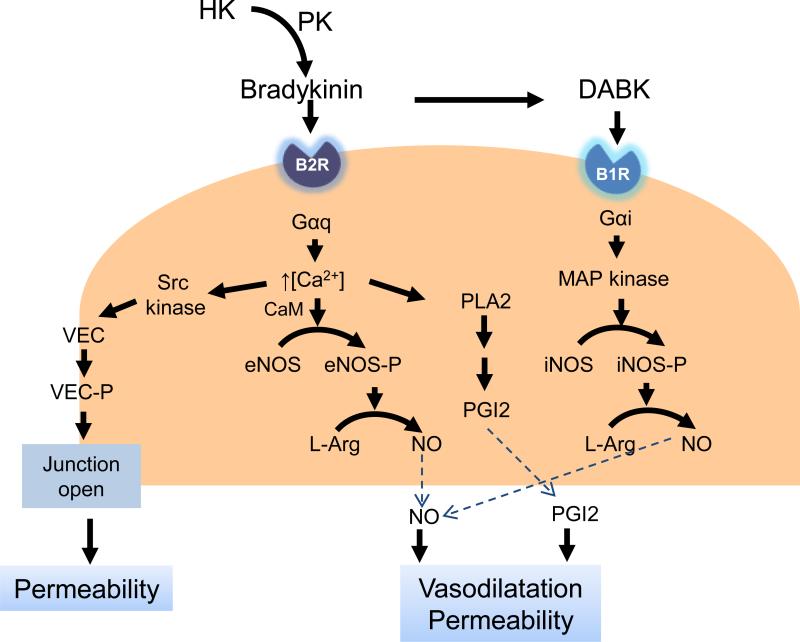
The effects of B1R and B2R on retinal vessel dilation have been mainly attributed to nitric oxide (NO) and prostaglandins (PGs) generation from vascular endothelial cells (Fig. 2). Pouliot et al. have shown that B1R blockade reduces a retinal expression of potential inflammatory mediators, including iNOS and COX-2 (Pouliot et al. 2012). In vitro, BK-induced vasodilation responses were inhibited by N (G)-nitro-L- arginine methyl ester (L-NAME), indomethacin, and the B2R antagonist Hoe140, suggesting that vasodilation induced by BK is mediated by NO and prostaglandins (Hardy et al., 1998; Jeppesen et al., 2002; Abdouh et al., 2003; Dalsgaard et al., 2010). BK and DABK stimulate increases in the intracellular concentrations of free calcium by coupling Gαq/11 or Gαi/o through the B2R or B1R, respectively (Busse and Fleming, 1996; Kuhr et al., 2010). Ca2+-induced stimulation of phospholipase A2 liberated arachidonic acid from membrane phospholipids, which can lead to the synthesis of prostacyclin (PGI2) (Kolte et al., 2011). B2R stimulates eNOS phosphorylation via Ca2+-calmodulin-dependent activation, while under inflammatory conditions, B1R stimulation results in much higher and prolonged NO production via Gαi activation of the MAP kinase pathway leading to activation of iNOS (Kuhr et al., 2010; Brovkovych et al., 2011). Activation of eNOS and iNOS can independently and additively increase NO production (Yayama and Okamoto, 2008; Kuhr et al., 2010). BK also activates Src kinases and the subsequent vascular endothelial cadherin phosphorylation, leading to the quick and reversible opening of endothelial cell junctions and plasma leakage (Orsenigo et al., 2012). NO and PGI2 activates the downstream signaling cascade in vascular smooth muscle cells to produce vasodilatation (Giles et al., 2012).
Figure 2.
The molecular pathways involved in the KKS induced vascular permeability. Bradykinin and DABK bind to B2R and B1R on endothelial cells. The activation of B1R and B2R increases the release of NO and PGI2 and VE-cadherin phosphorylation. PLA2: phospholipase A2; PGI2: prostacyclin; L-Arg: L-arginine; VEC: vascular endothelial cadherin.
3.3 Inflammation and angiogenesis
Recruitment of inflammatory cells to the retina and local release of proinflammatory cytokines have been implicated in contributing to the pathogenesis of DR (Joussen et al., 2004; Huang et al., 2011). Although increased retinal inflammation alone is not sufficient to cause DR, the additive effects of inflammation on vascular permeability, capillary degeneration, reactive oxygen species production, and cytokine actions on the metabolic effects of diabetes may accelerate or worsen DR progression (Talahalli et al., 2012). The pro-inflammatory effects of the KKS on the retina have been primarily attributed to the B1R, which is increased in the diabetic retina (Abdouh et al., 2008; Pouliot et al., 2012). Although the effects of B1R activation on the retina are not fully understood, B1R activation has been shown to mediate a host of inflammatory effects in other systems, including the recruitment and activation of neutrophils and microglia (Ehrenfeld et al., 2006; Ifuku et al., 2007). Pouliot et al. have reported that B1R antagonism reduced retinal leukostasis and levels of inflammatory mediators including iNOS, COX-2, and Il-1β in diabetic rats (Pouliot et al., 2012). Interestingly, this report also showed that B1R blockade reduced retinal B1R levels, suggesting the B1R mediates positive feedback of its expression.
The development of capillary degeneration and closure in the retina can lead to areas of nonperfusion resulting in hypoxia-induced neovascularization. While the role of the KKS in the ischemic retina is not yet available, both PK and B1R have been implicated in contributing to ischemic injury of other tissues, including the brain (Storini et al., 2006; Austinat et al., 2009). A recent study has reported that tissue kallikrein could inhibit retinal neovascularization in mice via the cleavage of VEGF165 (Nakamura et al., 2011), however, the potential effects of the plasma KKS on retinal new vessel growth are still unknown. In other systems, both pro-angiogenic and anti-angiogenic effects of plasma KKS components have been described (Parenti et al., 2001; Guo and Colman, 2005; Krankel et al., 2008). Although the KKS could potentially influence both the ischemic injury leading to retinal neovascularization and processes contributing new vessel growth via multiple mechanisms in PDR, additional information in these areas is needed.
3.4 Neuroretinal functions
Diabetes can cause retinal neurodegeneration, which mainly involves the inner retinal layers including the ganglion cell layer (van Dijk et al., 2012; Yang et al., 2012). The effects of diabetes on retinal function has been demonstrated using electroretinography (ERG), which measures the responses of photoreceptors (a-wave) to incident light stimulation followed by the combined responses of bipolar, amacrine, ganglion, and Müller cells (b-wave). ERG studies in patients with diabetes have indicated inner retinal neural impairment (Lecleire-Collet et al., 2011). In the normal cat retina, ERG studies have suggested that BK influences inner retinal function by affecting b-wave implicit time without affecting its amplitude (Jacobi et al., 1996). BK has been shown to mediate a variety of activities within neuronal tissues, including stimulating the glutamate release from astrocytes and modulating activities of sensory ion channels (Parpura et al., 1994; Mizumura et al., 2009; Liu et al., 2010a). In addition, both B1R and B2R activation can regulate glutamate clearance by retinal pigmented epithelial cells through a PKC-Akt-COX-2 signaling cascade (Lim et al., 2009). The potential effects of intraocular activation of the KKS on the neuroretina are largely unknown. Analysis of BK receptor expression in the neuroretina has indicated B1R expression in the ganglion cell layer, outer nuclear layer, and inner nuclear layer (Ma et al., 1996; Takeda et al., 1999). In vitro, BK has also been shown to have protective effects on cultured rat retinal neurons from glutamate cytotoxicity via B2R by opening the mitochondrial adenosine triphosphate (ATP)-sensitive potassium channel (Yasuyoshi et al., 2000; Yamauchi et al., 2003). Since BK is a potent neuropeptide, it is possible that activation of the intraocular KKS may contribute to neuroretinal dysfunction in DR.
4. KKS inhibitors: novel therapeutic applications to diabetic retinopathy
Diabetes is associated with both intraocular and systemic contact system activation. In addition to increases in the intraocular KKS in DR described above, diabetes can also have systemic effects on the contact activation system. Increased fasting glucose and glycosylated hemoglobin A1c correlates with shortened activated partial thromboplastin time (APTT), which measures intrinsic and common coagulation cascades, including the contact system (Lippi et al., 2009; Zhao et al., 2011). Shortened APTT in patients with DR also correlates with increased PPK (Kedzierska et al., 2005). In both humans and animal models, genetic deficiency of PPK causes prolongation of APTT without causing an apparent prothrombotic phenotype or bleeding diathesis (Girolami et al., 2010; Bird et al., 2012). In experimental studies, APTT is shortened in STZ-induced diabetic rats and this decrease is reversed by systemic administration of a PK inhibitor (Liu et al., 2011). These results suggest that systemic PK activity contributes to APTT shortening in diabetes. Since increased activities of PK and BK receptors have been linked to vasogenic edema (Plesnila et al., 2001), which is a primary cause of DME, inhibitors of the KKS may provide therapeutic opportunities for this disease. Targeting the KKS could occur at multiple levels, including inhibition of contact system activation, selective inhibition of PK activity, and blockade of BK receptors.
4.1 Inhibiting contact system activation
Decreasing contact system activation may provide opportunities to reduce the effects of the KKS in DR. In our previous studies, we found that CA-1 is increased in the vitreous in PDR patients, intravitreal injection of CA-1 into rats increases RVP, and this response is blocked by co-injection with carbonic anhydrase inhibitors, a PK neutralizing antibody, BK receptor antagonists, and small molecule PK inhibitors (Gao et al., 2007; Clermont et al., 2011). These findings revealed that increased CA activity in the vitreous leads to KKS activation, and suggest that CA-1 inhibitors may reduce DME, in part, via reducing PK activity. Further elucidation of the mechanisms that contribute to intraocular KKS activation, including factors that activate FXII (described in Section 2.4), may suggest additional opportunities to regulate this pathway.
4.2 Plasma kallikrein inhibitors
PK inhibitors include endogenous inhibitors, engineered proteins, and small molecules. C1-INH is a primary physiological inhibitor of PK, FXIa, FXIIa, C1r, and C1s proteases. Both plasma-derived and recombinant forms of C1-INH are effective treatments for hereditary angioedema (Longhurst and Cicardi, 2012). We have shown that intravitreal injection of exogenous C1-INH reduced retinal vascular hyperpermeability induced by diabetes and by intravitreal CA-1 in rats (Gao et al., 2007; Clermont et al., 2011). Although C1-INH is detected in the vitreous, it is unknown whether intravitreal concentrations of this endogenous serpin protease inhibitor are sufficient to inhibit PK. Exogenously administered C1-INH into the vitreous may provide an opportunity to inhibit the KKS, as well as other proteases in the complement and intrinsic coagulation cascades.
Selective PK inhibition could provide increased efficacy and targeting of the inflammatory effects of the plasma KKS, while preserving the potential beneficial effects of the tissue kallikrein system. Previous studies have also shown that intravitreal injection of a neutralizing PK antibody in rats reduced CA-1 stimulated RVP (Gao et al., 2007). Administration of small molecule selective PK inhibitor, ASP-440, in diabetic rats normalized both RVP and retinal blood flow (Clermont et al., 2011). This small molecule inhibitor also reduced RVP induced by intravitreal CA-1 injection and by angiotensin II-induced hypertension, but did not affect VEGF-stimulated RVP (Phipps et al., 2009; Clermont et al., 2011). These reports suggest that both systemic and intravitreal administration of PK inhibitors are effective in reducing retinal vascular hyperpermeability in diabetes.
4.3 B1 receptor antagonists
The effects of the KKS are mediated in large part via the generation of BK peptides that activate B1 and B2 receptors, which are expressed in a variety of ocular cell types and tissues. Since both PK- and tissue kallikrein-mediated pathways activate BK receptors, antagonism of these receptors blocks the effects of both kallikrein systems. Although both B1 and B2 receptors can induce RVP, the B1R appears to mediate a greater effect on increased plasma extravasations in DR. The effects of several B1R antagonists on retinal vascular dysfunction in rodent models of diabetes have been described. The selective peptide B1R antagonist, R-954, reduced vascular permeability in a variety of tissues from STZ-induced diabetic rats, including the retina (Lawson et al., 2005). When STZ-induced diabetic rats were treated with R-954 for 5 days at the end of the 4 and 12 week periods of diabetes onset, NO, kallikrein activity, and capillary permeability were remarkably reduced and Na+/K+ ATPase activity in the retina was increased (Catanzaro et al., 2012). Treatment of diabetic rats with FOV-2304, a non-peptide B1R antagonist administered via an eye-drop formulation, reduced RVP, leukocyte adhesion and normalized retinal mRNA expression of inflammatory mediators (Pruneau et al., 2010). Pouliot et al. have reported that retinal plasma extravasation, leukostasis, and RVP were significantly increased in diabetic retina compared to control rats and these abnormalities were reversed to control levels when treated with one eye drop of the non-peptide B1R antagonist LF22-0542 twice a day for 7 days (Pouliot et al., 2012). These reports indicate that both local and systemic administrations of B1R antagonists are effective in ameliorating retinal vascular abnormalities in diabetic rodents, which are similar to findings observed using PK inhibitors.
Conclusion
A growing body of evidence has implicated the KKS in contributing to the development of DR. Recent studies have revealed that the KKS is increased in the vitreous in people with advanced DR and retinal B1R expression is increased in diabetic rodents. Activation of the KKS may contribute to the pathogenesis of DR by increasing RVP, leukostasis, retina hemorrhage, and retinal thickening and by altering retina blood flow and ERG responses. In experimental animal models, both systemic and local ocular delivery of PK inhibitors and B1R antagonists ameliorate retinal functional abnormalities caused by diabetes. Taken together, these findings suggest that inhibition of the plasma KKS may provide new opportunities for the treatment of diabetic retinopathy, especially DME.
Acknowledgements
This work was supported in part by the US National Institutes of Health (grants EY019029 & DK36836) and the JDRF grant 17-2011-251.
References
- Abdouh M, Khanjari A, Abdelazziz N, Ongali B, Couture R, Hassessian HM. Early upregulation of kinin B1 receptors in retinal microvessels of the streptozotocin-diabetic rat. Br J Pharmacol. 2003;140:33–40. doi: 10.1038/sj.bjp.0705210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdouh M, Talbot S, Couture R, Hassessian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. Br J Pharmacol. 2008;154:136–143. doi: 10.1038/bjp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, Renne T, Kleinschnitz C. Blockade of bradykinin receptor B1 but not bradykinin receptor B2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40:285–293. doi: 10.1161/STROKEAHA.108.526673. [DOI] [PubMed] [Google Scholar]
- Bird JE, Smith PL, Wang X, Schumacher WA, Barbera F, Revelli JP, Seiffert D. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: Murine Ortholog of the Fletcher Trait. Thromb Haemost. 2012;107:1141–1150. doi: 10.1160/th-11-10-0682. [DOI] [PubMed] [Google Scholar]
- Brovkovych V, Zhang Y, Brovkovych S, Minshall RD, Skidgel RA. A novel pathway for receptor-mediated post-translational activation of inducible nitric oxide synthase. Journal of Cellular and Molecular Medicine. 2011;15:258–269. doi: 10.1111/j.1582-4934.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Fleming I. Molecular responses of endothelial tissue to kinins. Diabetes 45 Suppl. 1996;1:S8–13. doi: 10.2337/diab.45.1.s8. [DOI] [PubMed] [Google Scholar]
- Catanzaro O, Labal E, Andornino A, Capponi JA, Di Martino I, Sirois P. Blockade of early and late retinal biochemical alterations associated with diabetes development by the selective bradykinin B1 receptor antagonist R-954. Peptides. 2012;34:349–352. doi: 10.1016/j.peptides.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Cicardi M, Banerji A, Bracho F, Malbran A, Rosenkranz B, Riedl M, Bork K, Lumry W, Aberer W, Bier H, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. The New England Journal of Medicine. 2010a;363:532–541. doi: 10.1056/NEJMoa0906393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicardi M, Levy RJ, McNeil DL, Li HH, Sheffer AL, Campion M, Horn PT, Pullman WE. Ecallantide for the treatment of acute attacks in hereditary angioedema. The New England Journal of Medicine. 2010b;363:523–531. doi: 10.1056/NEJMoa0905079. [DOI] [PubMed] [Google Scholar]
- Clermont A, Chilcote TJ, Kita T, Liu J, Riva P, Sinha S, Feener EP. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes. 2011;60:1590–1598. doi: 10.2337/db10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Misfeldt M, Bek T, Simonsen U. Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br J Pharmacol. 2010;160:1496–1508. doi: 10.1111/j.1476-5381.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, Bhoola KD, Figueroa CD. Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol. 2006;80:117–124. doi: 10.1189/jlb.1205744. [DOI] [PubMed] [Google Scholar]
- Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the Vitreous Proteome in Diabetes without Diabetic Retinopathy and Diabetes with Proliferative Diabetic Retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. Journal of clinical hypertension. 2012;14:198–205. doi: 10.1111/j.1751-7176.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolami A, Scarparo P, Candeo N, Lombardi AM. Congenital prekallikrein deficiency. Expert review of hematology. 2010;3:685–695. doi: 10.1586/ehm.10.69. [DOI] [PubMed] [Google Scholar]
- Guo YL, Colman RW. Two faces of high-molecular-weight kininogen (HK) in angiogenesis: bradykinin turns it on and cleaved HK (HKa) turns it off. J Thromb Haemost. 2005;3:670–676. doi: 10.1111/j.1538-7836.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- Hardy P, Abran D, Hou X, Lahaie I, Peri KG, Asselin P, Varma DR, Chemtob S. A major role for prostacyclin in nitric oxide-induced ocular vasorelaxation in the piglet. Circ Res. 1998;83:721–729. doi: 10.1161/01.res.83.7.721. [DOI] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, et al. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27:13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi PC, Osswald H, Zrenner E. Bradykinin receptor inhibition affects the rod b-wave in the cat electroretinogram. Vision Res. 1996;36:3843–3849. doi: 10.1016/0042-6989(96)00096-x. [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Aalkjaer C, Bek T. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2002;43:1891–1896. [PubMed] [Google Scholar]
- Joseph K, Kaplan AP. Formation of bradykinin: a major contributor to the innate inflammatory response. Adv Immunol. 2005;86:159–208. doi: 10.1016/S0065-2776(04)86005-X. [DOI] [PubMed] [Google Scholar]
- Joseph K, Tholanikunnel BG, Kaplan AP. Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII. Proc Natl Acad Sci U S A. 2002;99:896–900. doi: 10.1073/pnas.022626899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Ciechanowski K, Golembiewska E, Safranow K, Ciechanowicz A, Domanski L, Myslak M, Rozanski J. Plasma prekallikrein as a risk factor for diabetic retinopathy. Arch Med Res. 2005;36:539–543. doi: 10.1016/j.arcmed.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim SJ, Yu HG, Yu J, Park KS, Jang IJ, Kim Y. Verification of biomarkers for diabetic retinopathy by multiple reaction monitoring. J Proteome Res. 2010;9:689–699. doi: 10.1021/pr901013d. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SJ, Kim K, Kang UB, Lee C, Park KS, Yu HG, Kim Y. Profiling of vitreous proteomes from proliferative diabetic retinopathy and nondiabetic patients. Proteomics. 2007;7:4203–4215. doi: 10.1002/pmic.200700745. [DOI] [PubMed] [Google Scholar]
- Ko F, Vitale S, Chou CF, Cotch MF, Saaddine J, Friedman DS. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999-2002 and 2005-2008. JAMA. 2012;308:2361–2368. doi: 10.1001/jama.2012.85685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Saito M, Mori A, Sakamoto K, Nakahara T, Ishii K. Role of cyclooxygenase in vasodilation of retinal blood vessels induced by bradykinin in Brown Norway rats. Vascul Pharmacol. 2009;51:119–124. doi: 10.1016/j.vph.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Kolte D, Osman N, Yang J, Shariat-Madar Z. High molecular weight kininogen activates B2 receptor signaling pathway in human vascular endothelial cells. J Biol Chem. 2011;286:24561–24571. doi: 10.1074/jbc.M110.211557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krankel N, Katare RG, Siragusa M, Barcelos LS, Campagnolo P, Mangialardi G, Fortunato O, Spinetti G, Tran N, Zacharowski K, et al. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhauser F, Gob E, Kraft P, Geis C, Schmitt J, Brede M, Gobel K, Helluy X, Pham M, Bendszus M, et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain-barrier damage and inflammation. Blood. 2012 doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SR, Gabra BH, Guerin B, Neugebauer W, Nantel F, Battistini B, Sirois P. Enhanced dermal and retinal vascular permeability in streptozotocin-induced type 1 diabetes in Wistar rats: blockade with a selective bradykinin B1 receptor antagonist. Regul Pept. 2005;124:221–224. doi: 10.1016/j.regpep.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lecleire-Collet A, Audo I, Aout M, Girmens JF, Sofroni R, Erginay A, Le Gargasson JF, Mohand-Said S, Meas T, Guillausseau PJ, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52:2861–2867. doi: 10.1167/iovs.10-5960. [DOI] [PubMed] [Google Scholar]
- Lim SK, Han HJ, Kim KY, Park SH. Both B1R and B2R act as intermediate signaling molecules in high glucose-induced stimulation of glutamate uptake in ARPE cells. J Cell Physiol. 2009;221:677–687. doi: 10.1002/jcp.21906. [DOI] [PubMed] [Google Scholar]
- Lippi G, Franchini M, Targher G, Montagnana M, Salvagno GL, Guidi GC, Favaloro EJ. Epidemiological association between fasting plasma glucose and shortened APTT. Clin Biochem. 2009;42:118–120. doi: 10.1016/j.clinbiochem.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl-channels. J Clin Invest. 2010a;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao B-B, Feener E. Proteomic Identification of Novel Plasma Kallikrein Substrates in the Astrocyte Secretome. Translational Stroke Research. 2010b;1:276–286. doi: 10.1007/s12975-010-0039-z. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao BB, Clermont AC, Blair P, Chilcote TJ, Sinha S, Flaumenhaft R, Feener EP. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med. 2011;17:206–210. doi: 10.1038/nm.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhurst H, Cicardi M. Hereditary angio-oedema. Lancet. 2012;379:474–481. doi: 10.1016/S0140-6736(11)60935-5. [DOI] [PubMed] [Google Scholar]
- Ma JX, Song Q, Hatcher HC, Crouch RK, Chao L, Chao J. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp Eye Res. 1996;63:19–26. doi: 10.1006/exer.1996.0087. [DOI] [PubMed] [Google Scholar]
- Maas C, Govers-Riemslag JW, Bouma B, Schiks B, Hazenberg BP, Lokhorst HM, Hammarstrom P, ten Cate H, de Groot PG, Bouma BN, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Excitation and sensitization of nociceptors by bradykinin: what do we know? Exp Brain Res. 2009;196:53–65. doi: 10.1007/s00221-009-1814-5. [DOI] [PubMed] [Google Scholar]
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Morimoto N, Tsuruma K, Izuta H, Yasuda Y, Kato N, Ikeda T, Shimazawa M, Hara H. Tissue kallikrein inhibits retinal neovascularization via the cleavage of vascular endothelial growth factor-165. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1041–1048. doi: 10.1161/ATVBAHA.111.223594. [DOI] [PubMed] [Google Scholar]
- Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Young Koh G, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschatz C, Maas C, Lecher B, Jansen T, Bjorkqvist J, Tradler T, Sedlmeier R, Burfeind P, Cichon S, Hammerschmidt S, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34:258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Ledda F, Granger HJ, Ziche M. The bradykinin/B1 receptor promotes angiogenesis by up-regulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. FASEB J. 2001;15:1487–1489. [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Phipps JA, Clermont AC, Sinha S, Chilcote TJ, Bursell SE, Feener EP. Plasma kallikrein mediates angiotensin II type 1 receptor-stimulated retinal vascular permeability. Hypertension. 2009;53:175–181. doi: 10.1161/HYPERTENSIONAHA.108.117663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnila N, Schulz J, Stoffel M, Eriskat J, Pruneau D, Baethmann A. Role of bradykinin B2 receptors in the formation of vasogenic brain edema in rats. J Neurotrauma. 2001;18:1049–1058. doi: 10.1089/08977150152693746. [DOI] [PubMed] [Google Scholar]
- Pouliot M, Talbot S, Senecal J, Dotigny F, Vaucher E, Couture R. Ocular application of the kinin B1 receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One. 2012;7:e33864. doi: 10.1371/journal.pone.0033864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic research. 2012;47:171–188. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- Pruneau D, Belichard P, Sahel JA, Combal JP. Targeting the kallikrein-kinin system as a new therapeutic approach to diabetic retinopathy. Current opinion in investigational drugs. 2010;11:507–514. [PubMed] [Google Scholar]
- Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb Haemost. 2007;98:77–83. [PubMed] [Google Scholar]
- Selvarajan S, Lund LR, Takeuchi T, Craik CS, Werb Z. A plasma kallikrein-dependent plasminogen cascade required for adipocyte differentiation. Nat Cell Biol. 2001;3:267–275. doi: 10.1038/35060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- Sogawa K, Nagaoka T, Izumi N, Nakabayashi S, Yoshida A. Acute hyperglycemia-induced endothelial dysfunction in retinal arterioles in cats. Invest Ophthalmol Vis Sci. 2010;51:2648–2655. doi: 10.1167/iovs.09-4070. [DOI] [PubMed] [Google Scholar]
- Storini C, Bergamaschini L, Gesuete R, Rossi E, Maiocchi D, De Simoni MG. Selective inhibition of plasma kallikrein protects brain from reperfusion injury. J Pharmacol Exp Ther. 2006;318:849–854. doi: 10.1124/jpet.106.105064. [DOI] [PubMed] [Google Scholar]
- Takeda H, Kimura Y, Higashida H, Yokoyama S. Localization of B2 bradykinin receptor mRNA in the rat retina and sclerocornea. Immunopharmacology. 1999;45:51–55. doi: 10.1016/s0162-3109(99)00057-0. [DOI] [PubMed] [Google Scholar]
- Talahalli R, Zarini S, Tang J, Li G, Murphy R, Kern TS, Gubitosi-Klug RA. Leukocytes regulate retinal capillary degeneration in the diabetic mouse via generation of leukotrienes. J Leukoc Biol. 2012 doi: 10.1189/jlb.0112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden PE, Munnix IC, Auger JM, Govers-Riemslag JW, Cosemans JM, Kuijpers MJ, Spronk HM, Watson SP, Renne T, Heemskerk JW. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114:881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renne T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10:1355–1362. doi: 10.1111/j.1538-7836.2012.04758.x. [DOI] [PubMed] [Google Scholar]
- van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abramoff MD. Early neurodegeneration in the retina of type 2 diabetic patients. Investigative ophthalmology & visual science. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, McCarty OJ. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost. 2010;8:1295–1301. doi: 10.1111/j.1538-7836.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kashii S, Yasuyoshi H, Zhang S, Honda Y, Akaike A. Mitochondrial ATP-sensitive potassium channel: a novel site for neuroprotection. Invest Ophthalmol Vis Sci. 2003;44:2750–2756. doi: 10.1167/iovs.02-0815. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mao D, Chen X, Zhao L, Tian Q, Liu C, Zhou BL. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Molecular vision. 2012;18:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- Yasuyoshi H, Kashii S, Zhang S, Nishida A, Yamauchi T, Honda Y, Asano Y, Sato S, Akaike A. Protective effect of bradykinin against glutamate neurotoxicity in cultured rat retinal neurons. Invest Ophthalmol Vis Sci. 2000;41:2273–2278. [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayama K, Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: role of bradykinin and nitric oxide. Int Immunopharmacol. 2008;8:312–318. doi: 10.1016/j.intimp.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Yu J, Liu F, Cui SJ, Liu Y, Song ZY, Cao H, Chen FE, Wang WJ, Sun T, Wang F. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics. 2008;8:3667–3678. doi: 10.1002/pmic.200700824. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang J, Zhang J, Wu J. Diabetes mellitus is associated with shortened activated partial thromboplastin time and increased fibrinogen values. PLoS One. 2011;6:e16470. doi: 10.1371/journal.pone.0016470. [DOI] [PMC free article] [PubMed] [Google Scholar]