Abstract
Background
Topically applied opioids promote angiogenesis and healing of ischemic wounds in rats. We examined if topical fentanyl stimulates wound healing in diabetic rats by stimulating growth-promoting signaling, angiogenesis, lymphangiogenesis and nerve regeneration.
Methods
We used Zucker diabetic fatty rats that develop obesity and diabetes on a high fat diet due to a mutation in the Leptin receptor. Fentanyl blended with hydrocream was applied topically on ischemic wounds twice daily, and wound closure was analyzed regularly. Wound histology was analyzed by hematoxylin and eosin staining. Angiogenesis, lymphangiogenesis, nerve fibers and phospho-PDGFR-β were visualized by CD31-, lymphatic vessel endothelium-1, protein gene product 9.5- and anti-phospho PDGFR-β-immunoreactivity, respectively. Nitric oxide synthase (NOS) and PDGFR-β signaling were analyzed using Western immunoblotting.
Results
Fentanyl significantly promoted wound closure as compared to PBS. Histology scores were significantly higher in fentanyl-treated wounds, indicative of increased granulation tissue formation, reduced edema and inflammation, and increased matrix deposition. Fentanyl treatment resulted in increased wound angiogenesis, lymphatic vasculature, nerve fibers, nitric oxide, NOS and PDGFR-β signaling as compared to PBS. Phospho PDGFR-β co-localized with CD31 co-staining for vasculature.
Conclusions
Topically applied fentanyl promotes closure of ischemic wounds in diabetic rats. Increased angiogenesis, lymphangiogenesis, peripheral nerve regeneration, NO and PDGFR-β signaling are associated with fentanyl-induced tissue remodeling and wound healing.
Keywords: opioid, opioid receptor, fentanyl, pain, wound, nerve fiber, angiogenesis, lymphangiogenesis
Introduction
Leg ulcers are a serious complication of diabetes.1 Half of all lower extremity amputations in hospitalized patients occur in diabetics.2,3 No satisfactory therapy exists for this severely debilitating handicap, and the mechanisms underlying ulceration and wound healing are not clearly understood.
Diabetes is accompanied by long-term microvascular and neurologic complications, mediated by destructive inflammatory processes stemming from glycation of native proteins.4,5 Microangiopathy in diabetes may prevent the adequate transfer of nutrients and oxygen to wounded tissue, thereby interfering with the normal healing process6 and triggering nerve dysfunction.7,8 Blood vessels have been shown to contribute to the nerve fiber thickness in the retina,9 suggestive of the dependence of peripheral nerves on the local vasculature. Agents that stimulate angiogenesis also promote wound healing.10,11 Angiogenesis is often followed by lymphangiogenesis.
Growth factors including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and platelet derived growth factor (PDGF) have shown promise in the healing of acute and chronic wounds, but lack appropriate supportive clinical data and show limited success.12-16 These growth factors act via their respective receptor tyrosine kinases (RTK) on epithelial, vascular, lymphatic and fibroblast cells, which are integral to tissue regeneration. Opioids co-activate RTKs for VEGFR2, PDGFR-β and EGFR.17-21 Opioids also stimulate lymphangiogenesis22 and promote pericyte recruitment to vasculature via phosphorylation of PDGFR-β in tumors in mice.19 Topically applied opioids including fentanyl stimulate angiogenesis in ischemic wounds in rats, and promote wound closure/healing via opioid receptor-mediated signaling.23
Opioids interact with the nervous system via mu-, delta- and kappa-opioid receptors (MOP/R, DOP/R and KOP/R, respectively), but provide analgesia via MOP/R.24 These opioid receptors (OP/Rs) are also expressed on the endothelium, inflammatory cells and epithelial cells in the skin.25-27 MOP/R also stimulates NO in vascular endothelium.28 Lower NO has been reported in diabetes and sickle cell disease patients as compared to normal healthy subjects and may contribute to leg ulceration and poor healing.29-32 NO-based therapies have been shown to be effective in the healing of wounds.30,33-35 Thus, opiods have the ability to promote the regenerative process by inducing growth-promoting signaling and NO at a multicellular level. Indeed, when applied topically fentanyl, morphine, and hydromorphone, were effective in healing ischemic wounds in Fischer 344 rats.23 In this study, fentanyl was more effective than the other two opioids. Therefore, we examined if topically applied fentanyl promotes healing of ischemic wounds in Zucker diabetic fatty (ZDF) rats via PDGFR and NO mediated signaling.
METHODS
All animal studies were performed with approval from the Institutional Animal Care and Use Committee at the University of Minnesota.
Animal model of diabetes
ZDF Rats (Charles River, Indianapolis, IN) a model of type II diabetes that demonstrates both hyperglycemia and insulin resistance was used.36 ZDF rats are Leptin receptor mutants, and develop obesity and diabetes on a high fat diet (Purina diet #5008, Purina Mills, Richmond, IL) after 11 weeks of age, with blood glucose rising to 400 mg/dL. Healing of perforated tympanic membranes is significantly delayed in these rats as compared to normal control Sprague-Dawley rats and streptozotocin-induced diabetes in rats.37 Blood glucose was measured using glucostrips twice a week and rats with blood glucose levels of 400 ± 57 mg/dL were used.
Excisional Ischemic Open Wound Model
We used the delayed excisional wound-healing model, which mimics the physiologic and molecular abnormalities of chronic wounds in humans as described previously.23 We created 2 full thickness wounds per animal, 8 mm in diameter, within a narrow bipedicled flap raised on the dorsum of rat, by incisions on the two sides, under isofluorane anesthesia. In this model the blood supply to the wounds is mostly impaired, resulting in ischemia and slower healing.
Topical Fentanyl Application and Wound Measurement
Pharmacological grade fentanyl (Baxter Esilerderle Mfd. Healthcare Corporation, Cherry Hill, NJ) was obtained from the Boynton Pharmacy at the University of Minnesota. Fentanyl, 5 μg/g hydrocream (Paddock Labs, Minneapolis, MN) or phosphate buffered saline (PBS) were blended with hydrocream. Wounds were cleaned gently with saline prior to each application. Fentanyl or control PBS cream was then applied topically in a thin film (approx. 1 mm thick) covering the entire wound twice a day. Gross observations were made on each wound daily. Wound area was measured in two different ways: (i) tracing twice onto clear plastic sheets every 2 days and (ii) recording digital images using a Nikon Coolpix 5700 digital camera once a week. Wound area was calculated using Adobe Photoshop and NIH Image as described.23 The initial (Day 1) wound area following the creation of wound was used to calculate % wound closure for each wound on any given day.38,39 The percentage of wound area covered by new granulation tissue = ([Areai - Arean]/Areai) × 100, where Ai is the initial area and An is the area at day n. At the experimental end point, rats were euthanized by CO2 and wound scars were collected for analysis.
Wound Histology
Sections of formalin-fixed wound scars were stained with hematoxylin and eosin (H&E) and scored for edema, congestion, hemorrhage, thrombosis, granulation tissue thickness, dermal and epidermal organization and inflammatory cells as described.23 Briefly, in a double blind manner histological scores were determined on a scale of 1 – 4, where 1 is the least and 4 is the most organized and dense dermal organization and granulation tissue. Another measure of tissue remodeling, collagen staining, was evaluated by Masson’s trichrome staining as described.23
Immunofluorescence Staining
Sections of cryopreserved scars were stained with rat endothelial cell-specific antibody (Ab) a-RECA-1 (Serotec Ltd., Oxford, UK) and lymphatic vessel endothelium-specific anti-LYVE-1 (Upstate Cell Signaling Solutions, Charlottesville, VA), followed by secondary Abs, Rhodamine Red-conjugated goat anti-mouse Ab and FITC-conjugated anti-rabbit Ab, respectively (Jackson Immunoresearch Laboratories Inc., West Grove, PA). Sections were co-stained with 4’,6-Diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) for nuclear co-localization as described.22,23 Another set of cryosections was co-stained with anti-RECA-1 and PDGFR-β Ab (Upstate Cell Signaling Solutions, Charlottesville, VA), followed by Rhodamine Red and FITC-conjugated secondary antibodies as described above. For peripheral nerve localization, 10-micron thick cryosections were fixed in 4% paraformaldehyde and permeabilized with 0.3% TritonX100, followed by staining with a-protein gene product 9.5 (PGP 9.5; Calbiotech, Spring Valley, CA) and secondary conjugated Ab Cy-5 (Jackson). Nerve bundles were quantified in a double blind manner in each field of view by enumerating their numbers from the immunofluorescent images.
Microvessel Density Analysis
Morphometric analysis of wound angiogenesis, was performed on RECA-1 positive images following their binarization and skeletonization using Adobe Photoshop and the Image Processing Tool-Kit Plug-in Functions for Adobe Photoshop (Reindeer Games, Asheville, NC). Immunoreactive pixels and total length of vessels were quantified as described by us previously.19
Nitric Oxide Assay
Total NO was analyzed in the skin/wound lysates by colorimetric determination of nitrite by Greiss reaction following the enzymatic conversion of nitrate to nitrite by nitrate reductase using the Total Nitric Oxide and Nitrate/Nitrite Assay kit from R&D Systems, Minneapolis, MN. The optical density (OD) of the color produced was measured in a microplate reader set at 540 nm with wavelength correction at 690 nm.
Western Blot Analysis
Lysates of wound scars were resolved on a 3-15% gradient SDS-PAGE gel and transferred to a PVDF membrane (Immobilon; Millipore, Bedford, MA). Membranes were probed with antibodies, α-RECA 1 (Serotec Inc., Raleigh, NC); α-phospho- and α-total-PDGFR-β (Upstate Cell Signaling, Charlottesville, VA), α-i- and α-e-NOS (BD Transduction Laboratories, San Diego, CA), α-PCNA and α–β Actin (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive protein bands were visualized with species-specific secondary Abs conjugated with alkaline phosphatase and ECF Western Blotting system (Amersham Life Sciences, Buckinghamshire, UK). Chemiluminescent signals of protein bands were acquired using a Storm Phosphorimager (Molecular Dynamics, Sunnyvale, CA) as described.21
Statistical Analysis
Student’s t-test was used to compare data between PBS and fentanyl treatment. Data are presented as mean ± SEM, and a p-value of < 0.05 was considered significant. All statistical analysis was performed using Prism software (v 5.0a, GraphPad Prism Inc., San Diego, CA).
RESULTS
Topical fentanyl promotes wound closure in diabetic rats
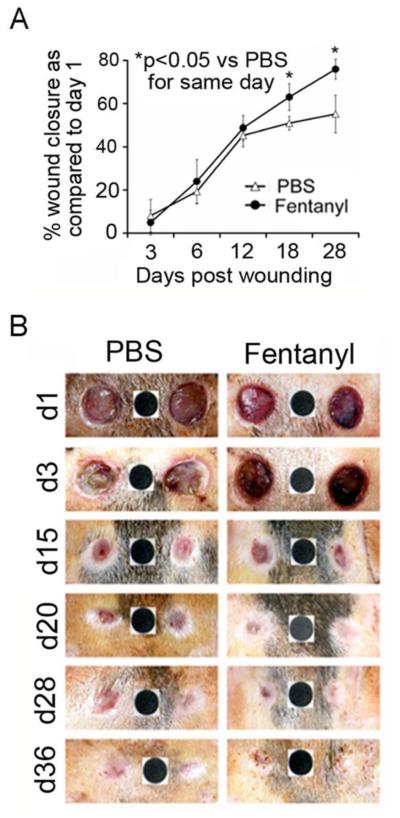
We analyzed wound closure over a period of time following PBS or fentanyl treatment applied topically. Wound area was calculated on day one upon wounding for each wound and was used for calculating % wound closure for each respective wound on any given day. Fentanyl consistently promoted wound healing after day 12 over time, resulting in complete healing of all wounds between days 28 and 36 (Fig. 1 A, B). PBS treated wounds showed swelling and exudate formation at early time points (Day 1 and 3). Fentanyl-treated wounds did not show swelling, but appeared more vascular (Fig. 1B). PBS-treated wounds started healing but stalled after day 12 (Fig. 1A), and wounds appeared larger on day 28 (left wound) as compared to day 20 (Fig. 1B). In contrast, wound closure continued to progress in fentanyl-treated wounds, showing normal pigmentation of skin on day 36 (Fig. 1B). Thus, fentanyl accelerates closure of ischemic wounds in diabetic rats.
Figure 1. Topical fentanyl accelerates healing of ischemic wounds in Zucker diabetic fatty rats.
(A) Each point shows the mean ± SEM of % wound area healed relative to day 1 following fentanyl (solid circles) or PBS (open triangles) treatment. *p<0.05 Vs same day treatment between fentanyl and PBS. (B) Representative images of wound appearance over a period of time with PBS or fentanyl. Black dot (6 mm in diameter) shows the standard area that was used for calculating wound area in (A). Wound area for each time point was compared to the area of wound created upon wounding on day 1. N = 8 separate wounds per treatment from 4 different animals.
Fentanyl treatment influences wound histology and collagen deposition
On day 28, PBS-treated wounds showed poor dermal and epidermal organization and thin granulation tissue in wound scars stained with H&E (Fig. 2). In contrast, fentanyl treatment resulted in complete re-epithelialization and dense granulation tissue in the wound scars on day 36 (Fig 2). Overall, fentanyl-treated wounds demonstrated significantly higher histological scores as compared to PBS treatment (Table 1). Masson’s trichrome staining demonstrated increased collagen content in fentanyl- as compared to PBS-treated wounds (Fig. 2).
Figure 2. Effect of fentanyl on wound histology and collagen formation.
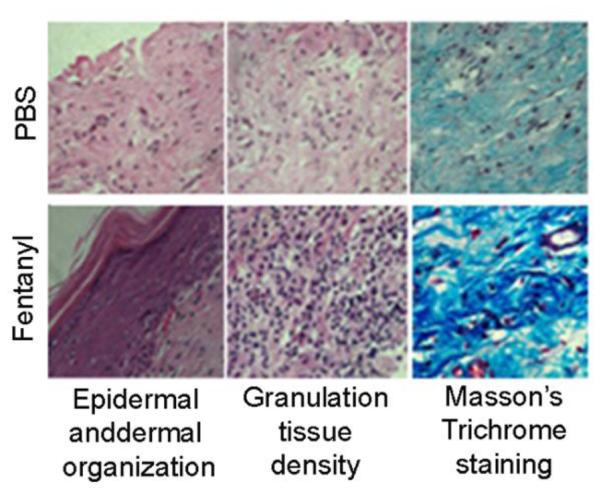
Wound scars from ZDF rats were analyzed for dermal and epidermal organization using H&E stained sections after 36 days post-wounding and for collagen formation using Masson’s trichrome staining. Original magnification x100. Each figure represents 12 sections stained from 4 different animals per treatment.
Table 1.
Changes in wound histology induced by topical fentanyl
| Group |
Histologic
score |
Epidermal
and dermal regeneration |
Granulation
tissue |
Vasculature |
|---|---|---|---|---|
| PBS | 1.2 | Little epidermal and dermal regeneration |
Thin granulation tissue |
Altered angiogenesis (1-2 vessels per site). These vessels have a high degree of edema, hemorrhage with occasional congestion and thrombosis |
| Fentanyl | 3.6 | Complete epidermal and dermal regeneration |
Thick granulation tissue |
Newly formed capillaries (5-6 per site). There is moderate degree of perivascular and interstitial edema with congestion. There is no thrombosis or congestion. There is also the presence of slight perivascular edema |
Histological scores on a scale of 1–4, where 1 represents little epidermal organization/thin granulation and 4 represents complete dermal organization/thick granulation.
Fentanyl stimulates angiogenesis, lymphangiogenesis and nerve fiber formation
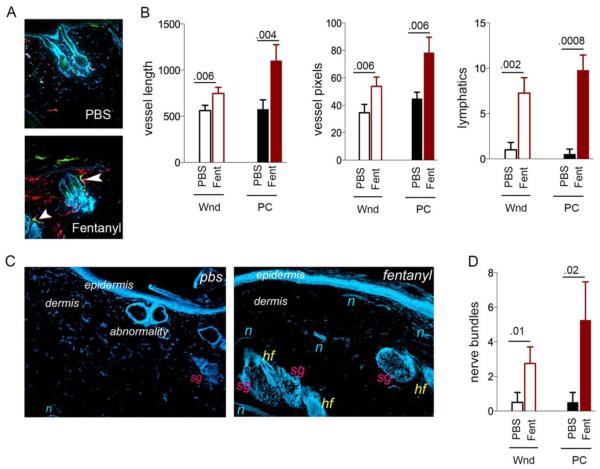
Fentanyl treatment markedly increased both angiogenesis (red vessels) and lymphangiogenesis (yellow-green and green vessels) on day 36 (Fig. 3A). The intense red staining pattern in fentanyl-treated wounds indicates increased vasculature (Fig. 3A). Some red vessels are also seen surrounding hair follicles, suggestive of normal architectural organization of vasculature in addition to increased density. In the intact unwounded skin of ZDF rats, lymphatic vessels are scarce and display defective orientation (right panel, Fig. 3A): unlike in normal skin, where lymphatics run perpendicular to the epidermis and parallel to hair follicles, lymphatics in ZDF rat skin are parallel to the epidermis and do not robustly parallel hair follicles. Fentanyl stimulated lymphangiogenesis, with lymphatics running perpendicular to the epidermis and some aligned parallel to hair follicle (arrows, Fig. 3A with fentanyl). Quantitatively the blood vessels and lymphatics are significantly increased in the wounds and in the healed skin scars post-closure following fentanyl treatment as compared to PBS (Fig. 3B). Thus, fentanyl treatment stimulates angiogenesis and lymphangiogenesis, and also normalizes the vascular and lymphatic architecture during the healing process.
Figure 3. Vascular, lymphatic and nerve fiber modulation by topical fentanyl.
(A) Representative merged images of CD31-ir blood vessels (red), LYVE-1-ir lymphatics (green) and DAPI-stained nuclei (blue) in PBS or fentanyl treated wound scars post closure from ZDF rats. N=4 different rats/treatment. White arrows show lymphatics. Original magnification, x150. (B) Blood vessel pixels and length and number of lymphatics in each field of view of images in (A) are shown. Each bar shows the mean±SD from 4 separate experiments. P values are indicated above the bars. (C) Protein gene product 9.5-ir nerve fibers in the PBS or fentanyl treated wound scars post closure of wounds. Original magnification, x100. Each image represents 12 sections from 4 different animals per treatment. (D) Number of nerve bundles in each field of view shown in (C). Each bar shows the mean±SD from 4 separate experiments. P values are indicated above the bars. Abbreviations: Wnd, wound on day 20; PC, post closure on day 36; PBS, phosphate buffered saline; Fent, fentanyl; hf = hair follicle, sg = sebaceous gland, n = nerve fibers.
Fentanyl-treated wounds also showed significantly increased PGP 9.5-immunoreactive nerve fiber density, with different layers of nerve fibers running parallel to the epidermis in the sub-epidermal and dermal regions (Fig. 3C,D). Nerve fibers are barely visible in PBS-treated wounds. Together, these data demonstrate that fentanyl treatment supports vascular, lymphatic and nerve fiber density and architecture, consistent with the promotion of wound healing.
Fentanyl stimulates phosphorylation of PDGFR-β in a time-dependent manner
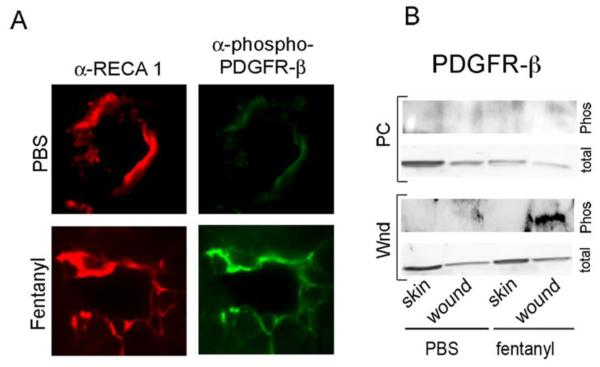
We detected robust activation of PDGFR-β that co-localized with the vascular endothelium after 20 days of fentanyl application (Fig. 4A and B). PDGFR-β was not activated by fentanyl at 36 days, or in unwounded or PBS-treated skin at any time (Fig 4B). Therefore, it is likely that fentanyl stimulates the transactivation of PDGFR-β only in the wounded skin during the healing process and not in normal skin.
Figure 4. Fentanyl stimulates the endothelial-cell specific phosphorylation of platelet-derived growth factor-beta receptor in a time-dependent manner in wounds.
(A) Immunofluorescent microscopy of wound scars 20 days post-treatment costained with α-RECA1 for vessel endothelium (red) and a-phospho-PDGFR-β (green). Original magnification x600. Each figure represents images from 4 different animals per treatment. (B) Protein bands for phospho PDGFR-β and total PDGFR-β on days 20 and 36 in the wound scars post-wounding and in the intact unwounded skin. Blots represent images from 3 reproducible experiments from tissues obtained from 3 different animals per time point for each treatment. Abbreviations: Wnd, wound day 20; PC, wound post closure on d 36; α-RECA 1, rat endothelial cell antigen I; PDGFR-β, platelet-derived growth factor-β
Fentanyl stimulates NO/NOS signaling and correlative PCNA expression
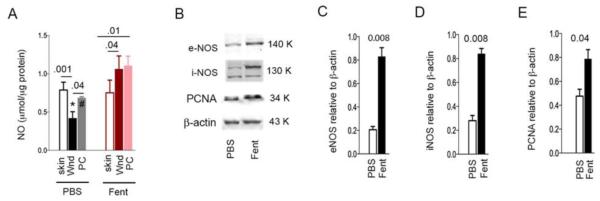
We found that NO was significantly reduced in the PBS-treated wounds as compared to the intact skin (Fig 5A). Conversely, in fentanyl treated wounds NO was significantly increased in the wounds as compared to the intact skin and remained consistently increased following wound closure. NO levels were significantly increased in wounds treated with fentanyl as compared to PBS during the process of healing and at the time of wound closure. Consistent with increased NO, fentanyl treatment led to a significant increase in e- and i-NOS (Fig. 5B-D). PCNA, the marker of cell proliferation was also significantly increased in the fentanyl treated wounds (Fig 5E). Together, these data suggest that fentanyl-induced NO signaling may promote cell proliferation in the wounds.
Figure 5. Fentanyl stimulates nitric oxide signaling and cellular proliferation.
(A) Intact skin removed to create wounds, wounds on day 20 and wound scars post-closure were analyzed for NO concentration. *p=0.002 Vs. fentanyl treated wound; #p=0.007 Vs. fentanyl treated post-closure scar. Other p values are indicated above the bars. Each bar shows the mean±SD from 4 separate experiments. (B) Representative images of 4 different Western blots of wound scars on day 20 analyzed for i- and e-NOS, PCNA and β-Actin. (C-E) Densitoimetric values of each protein band weighed against the respective β -Actin band of the Western blotting in (B) are shown. Each bar represents mean±SD from 4 different rat’s Western blots. P values are indicated above each set of bars. Abbreviations: NO, nitric oxide; PBS, phosphate buffered saline; Fent, fentanyl; Wnd, wound scar 20 days post-wounding; PC, wound scar post closure on day 36.
DISCUSSION
Diabetic wounds continue to pose major therapeutic challenges, and the mechanisms underlying impaired wound healing in diabetes remain unclear. We demonstrate that an inter-dependent triad of vascular, lymphatic and peripheral nervous system changes are integral to the healing process. Fentanyl, a MOP/R agonist, can interact with the nervous, vascular and lymphatic systems. We show that fentanyl promotes healing of ischemic wounds in diabetic rats by acting on each of these three systems and activating PDGFR-β signaling and NO. Our data are consistent with prior reports of the contribution of opioids and opioid receptors to wound healing.23,25-27
A comprehensive review of published clinical reports concluded that topically applied opioids significantly reduced pain in chronic wounds without any adverse effects, whereas systemic delivery had minimal effect on pain.26 The effect of opioids on wound healing was not reported, but it was noted that exudate may impair drug penetration and may even dilute drug concentration, thus impairing opioid efficacy.26 Cleaning of the wound prior to drug application is essential to enable drugs to penetrate tissue.40 We cleaned wounds with saline prior to each application, which perhaps increased the efficacy of fentanyl in our study.
MOP/R signaling has a cytoprotective effect that can lead to the healing of intestinal injury in mice.41,42 Morphine induced healing via DOP/R-mediated MAPK/ERK signaling in primary human mucosal epithelial cell scratch wound assays.43 Morphine has also been shown to be cardioprotective in ischemic injury in rodents via MOP/R, DOP/R and KOP/R.44,45 The MOP/R agonist remifentanil reduced the markers of cardiac injury following coronary artery bypass surgery in a double blind, randomized clinical trial.46 Taken together, these data suggest that opioid signaling through OP/Rs has a cytoprotective effect in ischemic injury and potentiates healing.
In the present study, fentanyl-induced wound closure was accompanied by normal dermal and epidermal organization, with more collagen formation and denser granulation tissue than PBS-treated wounds. These granulation and epidermal changes may be direct results of fentanyl and/or secondary to increased angiogenesis. In acute wounds, β-endorphin and MOP/R are expressed at a balanced level, but in chronic wounds the ligand is highly expressed while MOP/R is downregulated.47 Dalargin, a leu-enkephalin analog, accelerates healing by promoting macrophage-fibroblast interaction, capillary growth, fibroblast proliferation, granulation tissue maturation and epithelialization,48 similar to opioid-induced wound healing in rats observed by us previously23 and in the current study. Furthermore, β-endorphin plays an important role in limb generation in non-mammalian vertebrates.49 On the other hand, deletion of MOP/R leads to thinner epidermis in mouse skin, suggesting that MOP/R is required for epithelialization.50 These data suggest that MOP/R and its agonists such as fentanyl may play critical roles in multiple processes required for skin regeneration. Furthermore, additional OP/Rs may also regulate the normal structural organization of the skin and facilitate the healing process. For example, DOP/R knockout mice show impaired skin wound healing by influencing keratinocyte adhesion and migration.51
The OP/R antagonist naltrexone as well as opioid growth factor (OGF) has been studied in the setting of wound healing, and lead to the promotion and inhibition of corneal re-epithelialization, respectively.52,53 Naltrexone inhibits corneal neovascularization,54 consistent with our observations that OP/R-agonists promote neovascularization while OP/R-antagonists inhibit it.55 OGF is an endogenous peptide that acts via an OGF receptor, unlike exogenously applied OP/R agonists that act via classical OP/Rs. Moreover, neovascularization is required for healing of skin wounds but it has a negative effect on corneal healing due to the avascular nature of the cornea. These findings suggest that corneal and skin wounds may have different underlying pathophysiology and mechanisms of regeneration. Thus, it is critical to use highly specific models to study wound healing such as the ZDF rat skin ischemic wound model used by us herein.
We found that impaired angiogenesis in diabetic rat wounds is accompanied by poorly developed lymphatics and peripheral nerve fibers, which are restored with fentanyl treatment. Fentanyl-treated wounds showed a well-formed epidermis with mature sub-epidermal and dermal regions, including restoration of peripheral nerves in these tissues. The role of peripheral nerve destruction is well described in the development of diabetic neuropathy, and the degeneration of nerves and lymphatics may preclude ulcer healing. It is likely that increased angiogenesis stimulated by fentanyl leads to subsequent effects on lymphatics and nerve fibers, but a direct effect of fentanyl on these structures is also possible. We previously found that morphine promoted lymphangiogenesis in a murine breast cancer model,22 but the mechanistic role of OP/R agonists in lymphangiogenesis remains unclear.
The lymphatic system consists of a network of thin-walled capillaries of endothelial cells that drain protein-rich lymph from the extracellular space, maintain normal pressure, and mediate the immune response.56-59 The time course and extent of newly formed lymphatics in circumferential wounds of the rabbit ear, including lymphatic bridging through newly formed scar tissue, was documented in early studies.60,61 Lymphatic insufficiency may be responsible for the characteristic impairment of lymphatic drainage seen on clinical lymphangiography of individuals with lymphedema-distichiasis, a disabling condition characterized by swelling of extremities.47,62 Notably, half of these individuals also show venous insufficiency. This is likely of significance in non-healing venous ulcers. Therefore, the excessive swelling and exudates observed in non-healing ulcers could in part be due to disrupted lymphangiogenesis. Prevention of edema and exudate formation in fentanyl-treated wounds suggests that lymphatics drain wound fluid, which may otherwise interfere with the normal healing process. We hypothesize that this fluid imbalance and inflammation due to the absence and dysfunction of lymphatics may also contribute to diabetic wound pathobiology.
Opioids stimulate pro-angiogenic signaling directly and via co-activation of RTKs, which leads to the promotion of angiogenesis.17,19,20,55,56 Growth-promoting signaling pathways including mitogen activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and Stat3, activated by EGFR, VEGFR2 and PDGFRs are key mediators of the normal healing process.61 Importantly, opioids co-activate PDGFR, EGFR and VEGFR2.17-21,24 Fentanyl-induced activation of PDGFR-β in wound endothelium is consistent with opioid-induced activation of RTKs. We detected robust activation of PDGFR-β that co-localized with the vascular endothelium after 20 days of fentanyl application. This signal was absent on day 36 when wounds had healed completely, and also in unwounded skin. Fentanyl may thus stimulate the transactivation of PDGFR-β only in wounded skin during the process of angiogenesis, and not in normal skin where angiogenesis is not actively occurring. Furthermore, MOP/R downregulation in healed and normal skin may attenuate fentanyl signaling. We have observed that MOP/R protein and RNA were upregulated in the wounds but were barely detectable in the unwounded skin of C57BL/6 mice (unpublished observations).
The activation of PDGFR-β is critical with respect to concomitant increase in NO and NOS in fentanyl stimulated wounds. Activation of PDGFR-β is known to induce vascular and lymphatic dilatation via a nitric oxide mediated mechanism.63,64 It is likely that activation of PDGFR-β by fentanyl leads to increased vascular and lymphatic relaxation via a NO mediated mechanism. Together, these physiological effects of PDGFR-β activation lead to increased wound oxygenation and reduced edema, thus promoting a microenvironment conducive to wound healing.
NO perhaps plays a critical role in fentanyl-induced wound healing by promoting angiogenesis. Like PDGFR-β, angiopoietin-like 4 stimulates NO via iNOS and STAT-3 activation and promotes wound healing in diabetic mice.34 In the present study fentanyl stimulated NO, i-NOS and e-NOS in the wounds of ZDF rats. PCNA expression was also increased in these wounds, suggestive of cell proliferation via NO mediated signaling which may contribute to the promotion of healing observed by us. NO releasing nanoparticles have been shown to accelerate wound healing in mice.33,35 NO-nanoparticle treatment led to increased angiogenesis, cell migration and collagen formation in the wounds. Fentanyl-induced angiogenesis, collagen formation and acceleration of wounds in diabetic rats observed by us is likely due to fentanyl-induced increase in NO in the wounds. Higher expression of both e- and i-NOS in fentanyl treated wounds as compared to PBS-treated wounds contributes to increased NO. Furthermore, NO-based therapies appear to be promising in healing of diabetic wounds.30 MOP/R mediates the release of NO in vascular endothelium.28 Thus, fentanyl promotes the healing of wounds by stimulating NO via MOP/R and activation of PDGFR-β. Since leg ulcers are often painful, the added advantage of using MOP/R agonists such as fentanyl is that they can also ameliorate pain.
Many patients such as those with sickle cell disease are on chronic opioid therapy but still develop painful non-healing leg ulcers.31,65 Since, these wounds are ischemic, it is likely that vasculopathy and microangiopathy that occur in sickle cell disease or diabetes preclude delivery of systemic therapy to the wound when applied away from the wound bed. These wounds may thus require local, topically applied therapy such as that used by us in this study.
Taken together, our observations demonstrate that topical opioid treatment of ischemic wounds may be potentially beneficial in the healing of diabetic wounds, augmenting their known analgesic effect. Additionally, these data provide insight into the underlying signaling mechanisms and requirement of normal vascular, lymphatic and nerve architecture in the skin for the regenerative process.
ACKNOWLEDGEMENTS
The authors are thankful to Niroop S. Ammbashankar for technical help with peripheral nerve analysis, and Susan T. Thompson for preparation of graphics. This work was supported by NIH RO1 HL68802 and UO1 HL117664-01.
Footnotes
The significant finding of the study: Topical application of fentanyl accelerates the healing of ischemic open wounds in diabetic rats.
This study adds: Mu opioid receptor agonists show the potential to stimulate angiogenesis, lymphangiogenesis and nerve fiber expansion in association with nitric oxide and PDGFR-β signaling in the wounds, and may even reduce pain.
DISCLOSURE
None declared.
REFERENCES
- 1.Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22(3):382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Boyko EJ, Ahroni JH, et al. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22(7):1029–35. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 3.Connor H. Diabetic foot disease--where is the evidence? Diabet Med. 1999;16(10):799–800. doi: 10.1046/j.1464-5491.1999.0139a.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23(2):117–45. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 5.McNeely MJ, Boyko EJ, Ahroni JH, et al. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. How great are the risks? Diabetes Care. 1995;18(2):216–9. doi: 10.2337/diacare.18.2.216. [DOI] [PubMed] [Google Scholar]
- 6.Shyng YC, Devlin H, Sloan P. The effect of streptozotocin-induced experimental diabetes mellitus on calvarial defect healing and bone turnover in the rat. Int J Oral Maxillofac Surg. 2001;30(1):70–4. doi: 10.1054/ijom.2000.0004. [DOI] [PubMed] [Google Scholar]
- 7.Flynn MD, Tooke JE. Aetiology of diabetic foot ulceration: a role for the microcirculation? Diabetes Med. 1992;9(4):320–9. doi: 10.1111/j.1464-5491.1992.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 8.Stevens MJ, Obrosova I, Cao X, et al. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49(6):1006–15. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 9.Hood DC, Fortune B, Arthur SN, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. 2008;17(7):519–28. doi: 10.1097/IJG.0b013e3181629a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galeano M, Deodato B, Altavilla D, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46(4):546–55. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
- 11.Galeano M, Torre V, Deodato B, et al. Raxofelast, a hydrophilic vitamin E-like antioxidant, stimulates wound healing in genetically diabetic mice. Surgery. 2001;129(4):467–77. doi: 10.1067/msy.2001.112072. [DOI] [PubMed] [Google Scholar]
- 12.Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J. 2004;18(10):1111–3. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 13.Kunz D, Walker G, Bedoucha M, et al. Expression profiling and Ingenuity biological function analyses of interleukin-6- versus nerve growth factor-stimulated PC12 cells. BMC Genomics. 2009;10:90. doi: 10.1186/1471-2164-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rees RS, Robson MC, Smiell JM, et al. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound repair and regeneration. 1999;7(3):141–7. doi: 10.1046/j.1524-475x.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsang MW, Wong WK, Hung CS, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003;26(6):1856–61. doi: 10.2337/diacare.26.6.1856. [DOI] [PubMed] [Google Scholar]
- 16.Wietecha MS, Dipietro LA. Therapeutic approaches to the regulation of wound angiogenesis. Adv Wound Care. 2013;2(3):81–86. doi: 10.1089/wound.2011.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Farooqui M, Gupta K. Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res. 2006;3(3):171–80. doi: 10.2174/156720206778018767. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka N, Nguyen J, Chen C, et al. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg. 2011;113(6):1353–64. doi: 10.1213/ANE.0b013e318232b35a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luk K, Boatman S, Johnson KN, et al. Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy. J Oncol. 20122012:458385. doi: 10.1155/2012/458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singleton PA, Lingen MW, Fekete MJ, et al. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72(1-2):3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Weber ML, Chen C, Li Y, et al. Morphine stimulates platelet-derived growth factor receptor-beta signalling in mesangial cells in vitro and transgenic sickle mouse kidney in vivo. Br J Anaesth. 2013;111(6):1004–12. doi: 10.1093/bja/aet221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen J, Luk K, Vang D, et al. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014 doi: 10.1093/bja/aeu090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poonawala T, Levay-Young BK, Hebbel RP, et al. Opioids heal ischemic wounds in the rat. Wound Repair Regen. 2005;13(2):165–74. doi: 10.1111/j.1067-1927.2005.130207.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta M, Li Y, Gupta K. Maragoudakis ME, Papadimitriou E, editors. Opioids as promoters and regulators of angiogenesis. Angiogenesis: Basic Science and Clinical Applications: Transworld Research Network. 2007:303–18. [Google Scholar]
- 25.Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin--where do we stand? Exp Dermatol. 2009;18(5):424–30. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 26.Farley P. Should topical opioid analgesics be regarded as effective and safe when applied to chronic cutaneous lesions? J Pharm Pharmacol. 2011;63(6):747–56. doi: 10.1111/j.2042-7158.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- 27.Stein C, Kuchler S. Targeting inflammation and wound healing by opioids. Trends Pharmacol Sci. 2013;34(6):303–12. doi: 10.1016/j.tips.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Stefano GB, Hartman A, Bilfinger TV, et al. Presence of the mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J Biol Chem. 1995;270(51):30290–3. doi: 10.1074/jbc.270.51.30290. [DOI] [PubMed] [Google Scholar]
- 29.Delaney KM, Axelrod KC, Buscetta A, et al. Leg ulcers in sickle cell disease: current patterns and practices. Hemoglobin. 2013;37(4):325–32. doi: 10.3109/03630269.2013.789968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikaili P, Moloudizargari M, Aghajanshakeri S. Treatment with topical nitroglycerine may promote the healing process of diabetic foot ulcers. Med Hypotheses. 2014;83(2):172–4. doi: 10.1016/j.mehy.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Minniti CP, Delaney KM, Gorbach AM, et al. Vasculopathy, inflammation, and blood flow in leg ulcers of patients with sickle cell anemia. Am J Hematol. 2014;89(1):1–6. doi: 10.1002/ajh.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaffer MR, Tantry U, Efron PA, et al. Diabetes-impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery. 1997;121(5):513–9. doi: 10.1016/s0039-6060(97)90105-7. [DOI] [PubMed] [Google Scholar]
- 33.Blecher K, Martinez LR, Tuckman-Vernon C, et al. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine. 2012;8(8):1364–71. doi: 10.1016/j.nano.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Chong HC, Chan JS, Goh CQ, et al. Angiopoietin-like 4 Stimulates STAT3-mediated iNOS Expression and Enhances Angiogenesis to Accelerate Wound Healing in Diabetic Mice. Mol Ther. 2014 doi: 10.1038/mt.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han G, Nguyen LN, Macherla C, et al. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol. 2012;180(4):1465–73. doi: 10.1016/j.ajpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Shafrir E. Animal models of non-insulin-dependent diabetes. Diabetes Metab Rev. 1992;8(3):179–208. doi: 10.1002/dmr.5610080302. [DOI] [PubMed] [Google Scholar]
- 37.Vrabec JT. Tympanic membrane perforations in the diabetic rat: a model of impaired wound healing. Otolaryngol Head Neck Surg. 1998;118(3):304–8. doi: 10.1016/S0194-59989870305-5. Pt 1. [DOI] [PubMed] [Google Scholar]
- 38.Chesnoy S, Lee PY, Huang L. Intradermal injection of transforming growth factor-beta1 gene enhances wound healing in genetically diabetic mice. Pharm Res. 2003;20(3):345–50. doi: 10.1023/a:1022635600479. [DOI] [PubMed] [Google Scholar]
- 39.Kirchner LM, Meerbaum SO, Gruber BS, et al. Effects of vascular endothelial growth factor on wound closure rates in the genetically diabetic mouse model. Wound Repair Regen. 2003;11(2):127–31. doi: 10.1046/j.1524-475x.2003.11208.x. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher RE, Arndt DR, Hunt KL. Analgesic effects of topical methadone: a report of four cases. Clin J Pain. 2005;21(2):190–2. doi: 10.1097/00002508-200503000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Goldsmith JR, Perez-Chanona E, Yadav PN, et al. Intestinal epithelial cell-derived mu-opioid signaling protects against ischemia reperfusion injury through PI3K signaling. Am J Pathol. 2013;182(3):776–85. doi: 10.1016/j.ajpath.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldsmith JR, Uronis JM, Jobin C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving Stat3 signaling. American journal of pathology. 2011;179(2):673–83. doi: 10.1016/j.ajpath.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charbaji N, Schafer-Korting M, Kuchler S. Morphine stimulates cell migration of oral epithelial cells by delta-opioid receptor activation. PLoS One. 2012;7(8):e42616. doi: 10.1371/journal.pone.0042616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol. 2006;291(2):H827–34. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 45.Li R, Wong GT, Wong TM, et al. Intrathecal morphine preconditioning induces cardioprotection via activation of delta, kappa, and mu opioid receptors in rats. Anesthesia and analgesia. 2009;108(1):23–9. doi: 10.1213/ane.0b013e3181884ba6. [DOI] [PubMed] [Google Scholar]
- 46.Wong GT, Huang Z, Ji S, et al. Remifentanil reduces the release of biochemical markers of myocardial damage after coronary artery bypass surgery: a randomized trial. J Cardiothorac Vasc Anesth. 2010;24(5):790–6. doi: 10.1053/j.jvca.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Petrova TV, Karpanen T, Norrmen C, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10(9):974–81. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 48.Sehgal PB. Interleukin-6: molecular pathophysiology. J Invest Dermatol. 1990;94(6 Suppl):2S–6S. doi: 10.1111/1523-1747.ep12874963. [DOI] [PubMed] [Google Scholar]
- 49.Goodman L, Stein GH. Basal and induced amounts of interleukin-6 mRNA decline progressively with age in human fibroblasts. Journal of biological chemistry. 1994;269(30):19250–5. [PubMed] [Google Scholar]
- 50.Penkowa M, Camats J, Hadberg H, et al. Astrocyte-targeted expression of interleukin-6 protects the central nervous system during neuroglial degeneration induced by 6-aminonicotinamide. J Neurosci Res. 2003;73(4):481–96. doi: 10.1002/jnr.10681. [DOI] [PubMed] [Google Scholar]
- 51.Bigliardi PL, Neumann C, Teo YL, et al. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. Br J Pharmacol. 2014 doi: 10.1111/bph.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the rabbit cornea is regulated by opioid growth factor. Brain Res. 1998;803(1-2):61–8. doi: 10.1016/s0006-8993(98)00610-6. [DOI] [PubMed] [Google Scholar]
- 53.Zagon IS, Sassani JW, Myers RL, et al. Naltrexone accelerates healing without compromise of adhesion complexes in normal and diabetic corneal epithelium. Brain Res Bull. 2007;72(1):18–24. doi: 10.1016/j.brainresbull.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Zagon IS, Klocek MS, Griffith JW, et al. Prevention of exuberant granulation tissue and neovascularization in the rat cornea by naltrexone. Arch Ophthalmol. 2008;126(4):501–6. doi: 10.1001/archopht.126.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62(15):4491–8. [PubMed] [Google Scholar]
- 56.Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4(1):35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 57.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16(7):773–83. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 58.Scavelli C, Weber E, Agliano M, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat. 2004;204(6):433–49. doi: 10.1111/j.0021-8782.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witte MH, Bernas MJ, Martin CP, et al. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55(2):122–45. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 60.Bellman S, Oden B. Regeneration of surgically divided lymph vessels; an experimental study on the rabbit's ear. Acta Chir Scand. 1959;116(2):99–117. [PubMed] [Google Scholar]
- 61.Oden B. A micro-lymphangiographic study of experimental wounds healing by second intention. Acta Chir Scand. 1960;120:100–14. [PubMed] [Google Scholar]
- 62.Brice G, Mansour S, Bell R, et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet. 2002;39(7):478–83. doi: 10.1136/jmg.39.7.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cunningham LD, Brecher P, Cohen RA. Platelet-derived growth factor receptors on macrovascular endothelial cells mediate relaxation via nitric oxide in rat aorta. The Journal of clinical investigation. 1992;89(3):878–82. doi: 10.1172/JCI115667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maejima D, Kawai Y, Ajima K, et al. Platelet-derived growth factor (PDGF)-BB produces NO-mediated relaxation and PDGF receptor beta-dependent tonic contraction in murine iliac lymph vessels. Microcirculation. 2011;18(6):474–86. doi: 10.1111/j.1549-8719.2011.00108.x. [DOI] [PubMed] [Google Scholar]
- 65.Halabi-Tawil M, Lionnet F, Girot R, et al. Sickle cell leg ulcers: a frequently disabling complication and a marker of severity. British journal of dermatology. 2008;158(2):339–44. doi: 10.1111/j.1365-2133.2007.08323.x. [DOI] [PubMed] [Google Scholar]