Abstract
Background
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related death in the United States; however, it remains poorly characterized in surgical populations. To better inform perioperative transfusion practice, and to help mitigate perioperative TRALI, the authors aimed to better define its epidemiology before and after TRALI mitigation strategies were introduced.
Methods
This retrospective cohort study examined outcomes of adult patients undergoing noncardiac surgery with general anesthesia who received intraoperative transfusions during 2004 (n = 1,817) and 2011 (n = 1,562). The demographics and clinical characteristics of transfusion recipients, blood transfusion descriptors, and combined TRALI/possible TRALI incidence rates were evaluated. Univariate analyses were used to compare associations between patient characteristics, transfusion details, and TRALI mitigation strategies with TRALI/possible TRALI incidence rates in a before-and-after study design.
Results
The incidence of TRALI/possible TRALI was 1.3% (23 of 1,613) in 2004 versus 1.4% (22 of 1,562) in 2011 (P = 0.72), with comparable overall rates in males versus females (1.4% [23 of 1,613] vs. 1.2% [22 of 1,766]) (P = 0.65). Overall, thoracic (3.0% [4 of 133]), vascular (2.7% [10 of 375]), and transplant surgeries (2.2% [4 of 178]) carried the highest rates of TRALI/possible TRALI. Obstetric and gynecologic surgical patients had no TRALI episodes. TRALI/possible TRALI incidence increased with larger volumes of blood product transfused (P < 0.001).
Conclusions
Perioperative TRALI/possible TRALI is more common than previously reported and its risk increases with greater volumes of blood component therapies. No significant reduction in the combined incidence of TRALI/possible TRALI occurred between 2004 and 2011, despite the introduction of TRALI mitigation strategies. Future efforts to identify specific risk factors for TRALI/possible TRALI in surgical populations may reduce the burden of this life-threatening complication.
For the past decade, transfusion-related acute lung injury (TRALI) has consistently been the leading cause of transfusion-related fatalities reported to the U.S. Food and Drug Administration.1 Indeed, recent estimates note that TRALI was implicated in 38% of all fatalities.1 Perhaps more concerning is the fact that passive reporting has repeatedly been shown to significantly underestimate the true burden of this syndrome.2,3 For example, Kopko et al.2 noted that only 13.3% of cases with symptoms suggestive of TRALI were reported to the blood bank by the clinical service as a possible transfusion reaction. These findings were replicated in a recent study carried out by this investigative group, in which just 14.1% of cases had been reported by the responsible clinical team.3 In addition to the potential impact on the delivery of appropriate care, this lack of recognition and reporting of TRALI may result in the failure to prevent subsequent reactions to blood products of implicated donors who were not identified after the initial TRALI episode. Moreover, the poorly defined epidemiology of TRALI has undoubtedly contributed to the lack of studies testing potential preventive or treatment strategies, as well as to uncertainty regarding the attributable burden of this syndrome on patient-important outcomes and healthcare resource utilization.
Previous estimates of the incidence of TRALI have ranged from 0.04 to 8.0% per transfused patient.4–8 However, many of these studies used passive reporting strategies, and thus are believed to dramatically underestimate the true incidence of TRALI. Additionally, most studies investigating the epidemiology of TRALI have focused on patients in the critical care setting. Although these investigations have provided valuable information about TRALI in critically ill patients, substantial differences frequently exist in patient characteristics and baseline risk factors for lung injury when the noncardiac surgical population is compared with the critically ill population. Therefore, the available data on the critically ill population may not be generalizable to surgical cohorts. Furthermore, the paucity of studies focusing on surgical patients has precluded the identification of potentially modifiable perioperative risk factors specific to this unique population.
Importantly, approximately 50% of all blood product transfusions take place in the perioperative environment.9 Consequently, anesthesiologists are well positioned to make meaningful contributions to improving transfusion-related outcomes for surgical patients. To this end, a critical first step is to establish a clear understanding of the epidemiology of transfusion-related complications. Therefore, the overall goal of this investigation was to clearly define the epidemiology of TRALI after intraoperative blood product administration in a large cohort of patients undergoing noncardiac surgery with general anesthesia. Of note, previous reports have shown a decline in the incidence of TRALI after the introduction of universal leukoreduction10 and the maleonly donor policy for plasma11 (occurring in 2005 and 2008, respectively, at our institution). Therefore, in order to evaluate the impact of these interventions in surgical patients, we conducted a before-and-after study, evaluating the outcomes of all patients transfused intraoperatively during the calendar years 2004 and 2011.
Materials and Methods
After obtaining Mayo Clinic Institutional Review Board approval, we conducted a retrospective cohort study evaluating the incidence of TRALI/possible TRALI in all transfused patients undergoing noncardiac surgery with general anesthesia during the calendar years 2004 and 2011. All patients had given previous consent for the use of their medical records in research. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were followed in the conduct of this study and in the reporting of its results.12
Study Population
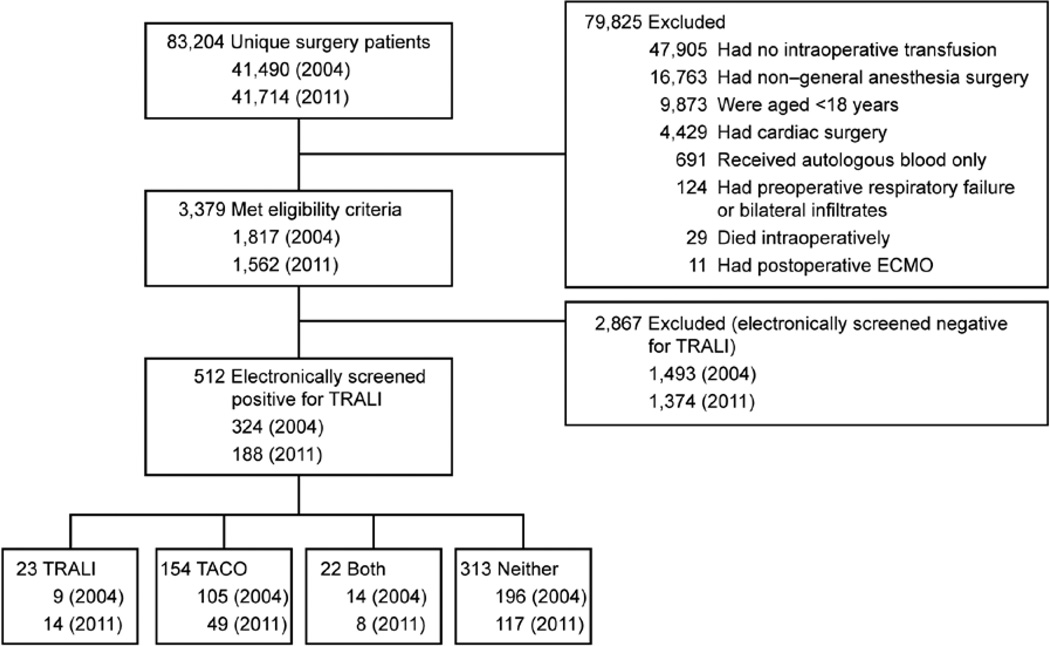
All noncardiac surgical patients receiving general anesthesia were identified from an institutional database (the perioperative data mart).13 This database captures high-resolution, near real-time data from all monitored care environments within our institution. Specifically, details relating to vital signs, laboratory tests, electrocardiogram monitoring, ventilator settings, fluid therapies, radiology results, medications, transfusion therapies, and procedures are stored within an open-access connectivity database accessed via JMP statistical software (SAS Institute Inc., Cary, NC). All noncardiac surgical patients receiving general anesthesia and allogeneic blood products in the operating room during the calendar years 2004 and 2011 were eligible for inclusion. Criteria for exclusion were (1) absence of research authorization, (2) age younger than 18 yr, (3) inclusion in study already, (4) preoperative respiratory failure, (5) preoperative diffuse bilateral infiltrates on chest radiograph, (6) intraoperative death, (7) postoperative requirement for extracorporeal membrane oxygenation, and (8) receipt of only autologous blood products (fig. 1). Patients who received only autologous blood products were not considered at risk for TRALI and were therefore not included in this investigation.
Fig. 1.
Patient flowchart. ECMO = extracorporeal membrane oxygenation; TACO = transfusion-associated circulatory overload; TRALI = transfusion-related acute lung injury.
Outcome Adjudication
In the current study, cases adjudicated as TRALI or as possible TRALI according to the 2004 Canadian consensus criteria14 (table 1) were considered as a single composite outcome. The rationale for combining these two outcomes is that many “high-risk” surgeries (e.g., aortic vascular, lung/esophageal resection) are known to be associated with a high rate of postoperative lung injury, thereby necessitating the adjudication of possible TRALI.15,16 Excluding this group from our analyses would therefore have resulted in the exclusion of a large number of potential cases with important deleterious outcomes. Thus, for purposes of this study, we refer to this composite outcome as TRALI.
Table 1.
TRALI and Possible TRALI Definitions from the 2004 Canadian Consensus Statement
|
Adapted, with permission, from Kleinman et al. Transfusion 2004; 44: 1774–89.14 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Data evaluated to inform decisions regarding the presence or absence of left atrial hypertension/fluid overload (when available) included: fluid balance, patient weight, echocardiographic data, pulmonary artery catheter hemodynamic data, central venous pressures, and the clinical context (comorbidities and acute physiology).
ALI = acute lung injury; Fio2 = fraction of inspired oxygen; PaO2 = partial pressure of arterial oxygen; SpO2 = oxygen saturation; TRALI = transfusion-related acute lung injury.
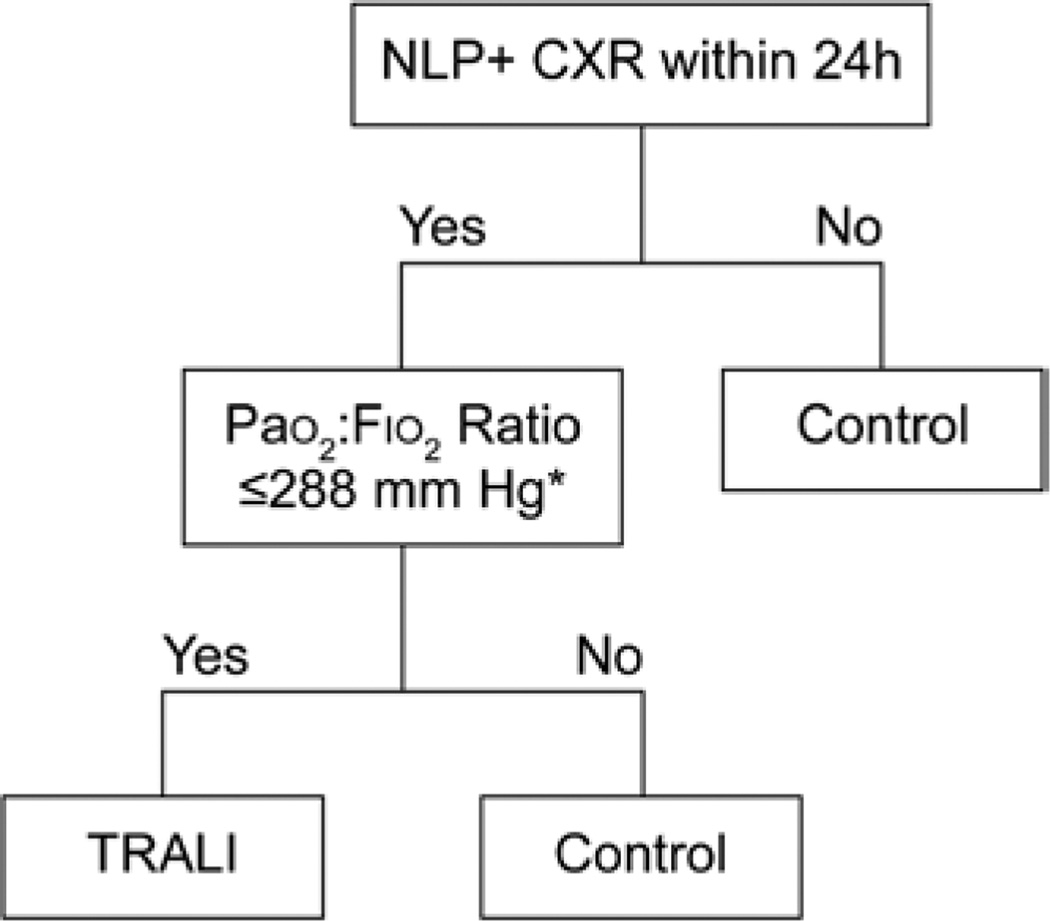
To identify cases of TRALI in this population, we used a three-step approach. First, to enhance the feasibility of this large study, and to facilitate the accurate detection of TRALI cases, we utilized our recently developed natural language processing (NLP)–based electronic screening algorithm (fig. 2).17 This algorithm was developed in an alternate cohort of patients that included subjects known to have had a TRALI episode, as well as in complication-free transfused controls. The algorithm variables and their cutoff thresholds were developed using classification and regression tree analysis, as previously described.17 In brief, this algorithm evaluates chest radiograph reports for the presence of terms and phrases believed to be consistent with a diagnosis of TRALI (table 2). In total, there were 4 explicit terms, 8 descriptive terms, 16 location terms, and 128 term combinations that were considered for NLP chest radiograph case identification using the open-source clinical text analysis and knowledge extraction system.18 In addition, vital status data stored within the electronic health record (including partial pressure of arterial oxygen [PaO2], PaO2 to fraction of inspired oxygen [Fio2] ratio [PaO2:Fio2], and oxygen saturation [SpO2]) were evaluated to identify potential TRALI cases. We have previously documented the diagnostic accuracy of this TRALI case detection algorithm with a sensitivity and specificity of 92.5% (95% CI, 84.6 to 96.7%) and 93.6% (95% CI, 85.0 to 97.6%), respectively.17
Fig. 2.
Screening algorithm. Natural language processing (NLP)–based transfusion-related acute lung injury (TRALI) screening algorithm. Asterisk indicates that, in the absence of a PaO2 :Fio2 ratio, surrogate markers of hypoxemia used in order of availability were PaO2 ≤117 mmHg followed by SpO2 ≤97%. CXR = chest radiograph; plus sign (+) = positive.
Table 2.
Variation in Descriptive Terms Corresponding to a Diagnosis of TRALI Included in Natural Language Processing
| Location Terms | Description Terms | Explicit Terms |
|---|---|---|
| Alveolar | Opacification | Edema |
| Perihilar | Opacity | ALI |
| Bilateral | Opacities | ARDS |
| Bilaterally | Infiltrate | CHF |
| Basilar | Infiltrates | |
| Bibasilar | Hazy | |
| Bibasilar | Fluffy | |
| Diffuse | Patchy | |
| Extensive | ||
| Airspace | ||
| Air space | ||
| Air-space | ||
| Parenchymal | ||
| Pulmonary | ||
| Lung | ||
| Lungs |
ALI = acute lung injury; ARDS = acute respiratory distress syndrome; CHF = congestive heart failure; TRALI = transfusion-related acute lung injury.
Second, two independent physicians (L.C. and Q.J.) manually reviewed the electronic health records of all patients who screened positive for TRALI and allocated a diagnosis based upon the 2004 Consensus Criteria (table 1).14 Due to our interest in the epidemiology of TRALI after intraoperative blood product administration, we limited our outcome assessment to 6 h after the last intraoperative blood product transfusion. Third, in situations in which these two physicians disagreed, a panel of three senior critical care physicians reviewed each case to adjudicate a final outcome. Physicians were able to adjudicate a diagnosis of TRALI using the 2004 Consensus Criteria, as described in table 1; of transfusion-associated circulatory overload (TACO) based on the 2014 National Healthcare Safety Network criteria* (table 3); of both TRALI and TACO (if there was evidence for both TRALI and TACO but neither was thought to fully explain the clinical picture); or of neither TRALI nor TACO (where neither the TRALI nor the TACO case definitions were met). Since the primary aim of the current investigation was to better describe the incidence of TRALI in a surgical population, data from patients with a diagnosis of only TACO were not analyzed as part of this investigation (fig. 1).
Table 3.
TACO from the CDC National Healthcare Safety Network Biovigilance Component 2013
| New onset or exacerbation of three or more of the following within 6 h of cessation of transfusion: |
| Acute respiratory distress (dyspnea, orthopnea, cough) |
| Increased BNP |
| Increased CVP |
| Evidence of left heart failure |
| Evidence of positive fluid balance |
| Radiographic evidence of pulmonary edema |
Adapted from the U.S. Centers for Disease Control, The National Healthcare Safety Network (NHSN) Manual: Biovigilance Component Protocol [Internet]. Atlanta (GA): Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention; January 2014 [cited 2014 Apr 15]. http://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf.
BNP = brain type natriuretic peptide; CDC = Centers for Disease Control; CVP = central venous pressure; TACO = transfusion-associated circulatory overload.
Data Sources and Collection
Data required to apply our electronic algorithm fit into two main categories: 1) structured data (e.g., vital signs) and 2) unstructured data (e.g., radiology reports processed using NLP). The required structured data points included PaO2; PaO2:Fio2 ratio; and SpO2 (fig. 2). To ensure completeness, we collected these data from three existing databases: the perioperative data mart (as described above in the Study Population subsection of the Materials and Methods section); the intensive care unit (ICU) data mart, a near real-time database similar to the perioperative data mart that captures multiple data points directly from the electronic health record for patients in the ICU13; and the Mayo Clinic Life Sciences System,19 a sophisticated data warehouse that contains a normalized replica of the electronic health record derived from multiple original clinical data sources, including vital signs for all patients. For the current study, the Mayo Clinic Life Sciences System was used to obtain structured data for patients located in nonmonitored care environments such as the general surgical floor. Baseline demographic data were also extracted from these three databases.
Extensive efforts have been undertaken to ensure the validity and reliability of these databases, and these efforts have been described in detail previously,13,19 To maximize the sensitivity of our algorithm, we identified the worst or the most extreme values for each patient within the time period of interest after blood product transfusion. For example, we used the lowest PaO2:Fio2 ratio. Errors were minimized by manually verifying the most extreme values in the electronic health record. Unstructured data used in this algorithm (e.g., chest radiograph reports) were obtained from the enterprise data trust, an existing database of clinical, research, and administrative data.19 NLP was applied to the reports to identify terms consistent with a diagnosis of TRALI as described previously.17
Detailed transfusion data were collected using the perioperative information tool. This user interface application was developed using Microsoft.NET (Microsoft Corp., Redmond, WA) technology and contains detailed information pertaining to all transfusions, including exact transfusion times, blood products, and volumes, and it allows users to retrieve multiple perioperative patient data points in an efficient manner. Data sources for this application include direct transmission from the perioperative data mart, as well as from the Amalga Unified Intelligence System (Microsoft Corp.).20 This latter resource captures a broader range of near real-time patient data. The automated nature of these complex databases ensures accuracy of data to the same extent that the original medical record is accurate. Considerable effort has been exerted to ensure the validity of these databases. Indeed, previously developed screening algorithms for other critical care syndromes (using this same technology) at our institution have proven highly successful.21–24
Statistics
In the current study, we included all eligible patients who underwent operations during the index years 2004 and 2011. Baseline characteristics are summarized by frequency (percentage) or median (interquartile range [IQR]) for categorical and continuous variables, respectively. Comparison of characteristics between calendar years was carried out using the t test and the chi-square test test, respectively. We evaluated the frequency of TRALI after intraoperative blood product transfusion by calculating event rates both overall and separately for the calendar years 2004 and 2011. Thereafter, age and transfusion volume were categorized by quintiles, and age-specific and transfusion volume–specific rates were calculated and tested statistically using the Cochran-Armitage trend test. Sex-specific rates were calculated and tested using the chi-square test, and surgical specialty–specific rates were calculated and tested using the Monte-Carlo estimate of the exact test. Agreement between physicians adjudicating outcome was assessed using κ coefficients. Finally, an unadjusted comparison of outcomes was carried out within this cohort between patients determined to have TRALI versus complication-free transfused patients (no evidence of TRALI or TACO). Specifically, ICU length of stay and hospital length of stay (median, IQR in days) were compared using the t test. In-hospital mortality (frequency [percentage]) was compared using the chi-square test, and an odds ratio (95% CI) of in-hospital death was generated. All statistical analyses were carried out using SAS version 9.1 (SAS Institute Inc.), with P values < 0.05 considered significant.
Results
A total of 83,204 unique patients underwent surgical procedures at our institution during the index calendar years (41,490 in 2004 and 41,714 in 2011). Of these, 3,379 adult patients undergoing noncardiac surgery with general anesthesia were eligible for inclusion in this study (1,817 in 2004 and 1,562 in 2011) (fig. 1). Baseline characteristics are summarized in table 4. In brief, the median age for this transfused cohort was 66 yr (range, 54 to 76 yr), with patients being marginally older in 2004. About half of the patients (1,614 [47.8%]) were male. The median intraoperative transfusion volume was 660 ml. Both age and median transfusion volume were comparable between calendar years. Overall, most patients underwent abdominal surgeries (944 of 3,379 [27.9%]), followed by orthopedic surgeries (673 of 3,379 [19.9%]), with the fewest patients undergoing neurologic surgery (99 of 3,379 [2.9%]). There was a statistically significant difference in the distribution of patients among surgical specialties between 2004 and 2011 (P < 0.001).
Table 4.
Baseline Characteristics of Transfusion Recipients by Age, Sex, Transfusion Volume, and Surgical Specialty in 2004 and 2011
| Characteristics | Overall (N = 3,379) |
2004 (n = 1,817) |
2011 (n = 1,562) |
P Value* |
|---|---|---|---|---|
| Age, median (IQR), yr* | 66 (54 to 76) | 67 (54 to 76) | 65 (54 to 75) | .004 |
| Male sex* | 1,614 (47.8) | 858 (47.2) | 756 (48.4) | .049 |
| Intraoperative transfusion volume, median (IQR), ml |
660 (350 to 1,200) | 639 (350 to 1,050) | 660 (330 to 1,028) | .070 |
| Surgical specialty | <0.001 | |||
| Abdominal | 994 (27.9) | 481 (26.5) | 463 (29.6) | |
| OB/GYN | 293 (8.7) | 157 (8.6) | 136 (8.7) | |
| Neurologic | 99 (2.9) | 45 (2.5) | 54 (3.5) | |
| Orthopedic | 673 (19.9) | 401 (22.1) | 272 (17.4) | |
| Spine | 249 (7.4) | 114 (6.3) | 135 (8.6) | |
| Thoracic | 133 (3.9) | 59 (3.2) | 74 (4.7) | |
| Transplant | 178 (5.3) | 81 (4.5) | 97 (6.2) | |
| Urology | 279 (8.3) | 154 (8.5) | 125 (8.0) | |
| Vascular | 375 (11.1) | 248 (13.6) | 127 (8.1) | |
| Other | 156 (4.6) | 77 (4.2) | 79 (5.1) |
Values are numbers (percentage) unless indicated otherwise.
P values compare medians and frequencies between 2004 and 2011 using the t test and the chi-square test, respectively.
IQR = interquartile range; OB/GYN = obstetrics and gynecology.
Overall, 512 (15.2%) patients electronically screened positive for TRALI, 324 in 2004 and 188 in 2011. Manual review showed that 45 of these 512 screen-positive patients (8.8%) had experienced TRALI/possible TRALI (agreement by κ statistic, 0.22). The overall rate of TRALI occurring within 6 h of the last intraoperative blood product transfusion was therefore 1.3% (95% CI, 1.0 to 1.8%) (45 of 3,379) (table 5). Specifically, 13 cases (0.4% [95% CI, 0.2 to 0.7%]) were deemed to be TRALI, whereas 32 cases (0.9% [95% CI, 0.7 to 1.3%]) were deemed to be possible TRALI due to the presence of alternate acute lung injury risk factors (table 5).14 Of the 13 true TRALI cases, 5 occurred in 2004 (0.3% [95% CI, 0.1 to 0.6%]) and 8 occurred in 2011 (0.5% [95% CI, 0.3%-1.0%]). Of the 32 possible TRALI cases, 18 occurred in 2004 (1.0% [95% CI, 0.6 to 1.6%]) and 14 occurred in 2011 (0.9% [95% CI, 0.5 to 1.5%]). Product-specific rates of TRALI and possible TRALI are also shown in table 6. The composite rate of TRALI did not change significantly between 2004 (23 of 1,817) and 2011 (22 of 1,562): 1.3% (95% CI, 0.8 to 1.9%) versus 1.4% (95% CI, 0.9 to 2.1%) (P = 0.72) (table 5).
Table 5.
Incidence Rates of Perioperative Transfusion-related Acute Lung Injury Overall and by Age, Sex, Transfusion Volume, and Surgical Specialty in 2004 and 2011
| Variable | 2004 No./Total (%) | 2011 No./Total (%) | Overall No./Total (%) |
P Value |
|---|---|---|---|---|
| Overall | 23/1,817 (1.3) | 22/1,562 (1.4) | 45/3,379 (1.3) | 0.72 |
| Age, yr | 0.06 | |||
| ≤49 | 3/318 (0.9) | 3/277 (1.1) | 6/595 (1.0) | |
| 50 to 59 | 2/296 (0.7) | 3/288 (1.0) | 5/584 (0.8) | |
| 60 to 69 | 4/414 (1.0) | 6/421 (1.4) | 10/835 (1.2) | |
| 70 to 79 | 8/486 (1.6) | 5/370 (1.4) | 13/856 (1.5) | |
| ≥80 | 6/303 (2.0) | 5/206 (2.4) | 11/509 (2.2) | |
| Sex | 0.65 | |||
| Male | 12/857 (1.4) | 11/756 (1.5) | 23/1,613 (1.4) | |
| Female | 11/960 (1.1) | 11/806 (1.4) | 22/1,766 (1.2) | |
| Surgical specialty | 0.07 | |||
| Abdominal | 5/481 (1.0) | 6/463 (1.3) | 11/944 (1.2) | |
| OB/GYN | 0/157 (0.0) | 0/136 (0.0) | 0/293 (0.0) | |
| Neurologic | 1/45 (2.2) | 1/54 (1.9) | 2/99 (2.0) | |
| Orthopedic | 4/401 (1.0) | 3/272 (1.1) | 7/673 (1.0) | |
| Spine | 1/114 (0.9) | 1/135 (0.7) | 2/249 (0.8) | |
| Thoracic | 2/59 (3.4) | 2/74 (2.7) | 4/133 (3.0) | |
| Transplant | 0/81 (0.0) | 4/97 (4.1) | 4/178 (2.2) | |
| Urology | 1/154 (0.6) | 1/125 (0.8) | 2/279 (0.7) | |
| Vascular | 7/248 (2.8) | 3/127 (2.4) | 10/375 (2.7) | |
| Other | 2/77 (2.6) | 1/79 (1.3) | 3/156 (1.9) | |
| Transfusion volume, ml | <0.001 | |||
| ≤350 | 2/606 (0.3) | 4/512 (0.8) | 6/1,118 (0.5) | |
| 351 to 700 | 2/635 (0.3) | 5/485 (1.0) | 7/1,120 (0.6) | |
| 701 to 1,050 | 3/200 (1.5) | 2/187 (1.1) | 5/387 (1.3) | |
| 1,051 to 1,400 | 3/130 (2.3) | 2/101 (2.0) | 5/231 (2.2) | |
| ≥1,401 | 13/246 (5.3) | 9/277 (3.2) | 22/523 (4.2) |
OB/GYN = obstetrics and gynecology.
Table 6.
Rates of TRALI and Possible TRALI by Type of Transfusion Product for 2004 and 2011
| TRALI, No. | Possible TRALI, No.* |
|||
|---|---|---|---|---|
| Product | 2004 (n = 5) |
2011 (n = 8) |
2004 (n = 18) |
2011 (n = 14) |
| Erythrocyte only | 1 | 3 | 6 | 5 |
| FFP only | 0 | 0 | 0 | 2 |
| Platelets only | 0 | 0 | 0 | 1 |
| Mixed | 4 | 5 | 12 | 6 |
Thirty-two cases were deemed as possible TRALI due to the presence of sepsis (n = 7), trauma (n = 6), shock (n = 6), aspiration (n = 3), pneumonia (n = 1), or surgical procedures with high risk for ALI/ARDS (n = 9). Some patients fit into multiple categories.
FFP = fresh frozen plasma; TRALI = transfusion-related acute lung injury.
The incidence of TRALI was comparable among male and female patients in both calendar years (1.4 vs. 1.2%) (P = 0.65) (table 5). Overall, thoracic (2 of 45 [3.0%]), vascular (10 of 45 [2.7%]), and transplant surgeries (4 of 45 [2.2%]) carried the highest rates of TRALI/possible TRALI. In fact, the rate of TRALI in thoracic surgical patients was approximately three times that of patients undergoing abdominal or orthopedic surgeries, and more than three times the risk observed in patients undergoing spinal or urologic surgeries. Obstetric and gynecologic surgical patients had no TRALI episodes during either study year. This variability in the rate of TRALI across surgical specialties failed to reach statistical significance (P = 0.07). There was some increased incidence of TRALI/possible TRALI with increasing age that was not significant (P = 0.06). Specifically, the rate of TRALI in patients aged ≥80 yr was almost twice the rate observed in all groups aged ≤ 69 yr, and almost 1.5 times the rate observed in patients aged 70 to 79 yr. The rate of TRALI also increased with greater volumes of transfused product (P < 0.001; table 5).
Importantly, of the 45 cases of TRALI identified during these two calendar years, none were reported to the transfusion medicine service. However, our comparison of outcomes for patients identified as having TRALI with outcomes for complication-free transfused patients revealed a number of important differences. Namely, for the 2,073 patients who were admitted to the ICU postoperatively (61.3% of the study cohort), the median length of ICU stay for patients with TRALI was 11.2 days (IQR, 5.7 to 27.9 days), compared with 5.9 days (IQR, 2.7 to 15.2 days) for transfused controls (P < 0.001). Patients with TRALI also had a significantly longer median hospital length of stay at 12.3 days (IQR, 6.1 to 23.0 days), compared with 6.6 days (IQR, 4.5 to 10.0 days) for complication-free transfused patients (P < 0.001). Finally, the rate of in-hospital mortality for the 45 TRALI cases was 28.9% (n = 13), compared with 2.5% (n = 81) for the 3,193 complication-free transfused patients. This resulted in an odds ratio of 15.6 (95% CI, 7.9 to 30.8) for death in TRALI cases, compared with transfused controls (P < 0.001).
Discussion
This large-scale cohort study of noncardiac surgical patients undergoing general anesthesia offers insights into the incidence of TRALI after intraoperative blood product transfusion. Using a novel NLP-based algorithm, followed by a rigorous two-phase manual review strategy, we conducted a detailed epidemiologic analysis of postoperative TRALI. Our findings confirmed that TRALI is dramatically underrecognized and underreported in clinical practice; specifically, its 1.3% incidence is greater than many previous estimates. Notably, increasing transfusion volume was a potent predictor of postoperative TRALI, whereas increasing incidence with age fell just short of statistical significance. Importantly, the median duration of hospitalization was markedly prolonged for patients with TRALI versus controls (ICU, 11.2 vs. 5.9 days; hospital overall, 12.3 vs. 6.6 days). Furthermore, in-hospital mortality was augmented (odds ratio, 15.6) in TRALI patients versus complication-free transfused controls. Despite targeted mitigation strategies, TRALI incidence was unchanged between 2004 and 2011 (1.3% vs. 1.4%) (P = 0.72).
Despite its underrecognition,2,3,25,26 TRALI remains the leading cause of transfusion-related fatalities in developed countries.1 Beyond the prognostic impact on transfusion recipients, accurate reporting of TRALI is critical for identification—and possible exclusion—of implicated, potentially alloimmunized donors. As highlighted by Kopko et al.,2 unrecognized TRALI may lead to the administration of blood products from “risky” donors, precipitating additional TRALI episodes in vulnerable recipients.
In this study, we determined rates of TRALI specific to surgical patients transfused intraoperatively. A large proportion of blood product transfusions (about 50%) takes place in operating rooms,9 and a substantial proportion (41%) of TRALI is experienced after intraoperative transfusion.3 Nonetheless, most studies investigating TRALI rates have focused on critically ill patients, with an incidence historically estimated at 0.02 to 0.05% per unit transfused5,26 and at 2.2 to 8.2% per patient transfused in the ICU.4,27,28
Although the importance of TRALI in surgical populations has been recognized since the 1990s,29 data are sparse concerning its occurrence. Early investigations of perioperative TRALI were limited by passive reporting, markedly underestimating its true incidence.30 Several recent isolated studies have focused on high-risk surgical populations, which, coupled with differences in methodology and outcome adjudication, limits generalizability to the wider surgical population.31,32
In 2012, Tsai et al.33 conducted a large retrospective study of postoperative TRALI in which they identified only 15 cases in 14,441 patients (0.1%). Notably, they retrospectively identified previously adjudicated TRALI diagnoses from an existing database that defined TRALI as hypoxemia (PaO2:Fio2 ratio <300 mmHg), with evidence of pulmonary edema. However, the absence of an available PaO2:Fio2 ratio in these patients precluded the diagnosis of TRALI. The authors acknowledged that inadequate retrospective documentation may have discounted less severe forms of TRALI. We overcame this limitation by including other parameters of hypoxemia (SpO2 and PaO2 [fig. 2]).
Tsai et al’s33 exclusion of patients with other risk factors for acute lung injury would also have excluded all cases of possible TRALI. In contrast, we grouped TRALI and possible TRALI as a single outcome, likely accounting for much of the difference in our incidence rates. Tsai et al.33 also excluded patients undergoing thoracic surgery, whereas we included them and found them to be at highest risk for postoperative TRALI (3.0%). Finally, Tsai et al.33 included patients receiving exclusively regional anesthesia (none experienced TRALI), whereas we evaluated postoperative TRALI in patients undergoing general anesthesia only.
Undoubtedly, uncertainty remains about the true incidence of TRALI; however, numerous explanations may account for the variable previous estimates. Before the 2004 consensus criteria for TRALI,14 its definition in the medical literature was inconsistent. Since then, explicit criteria applied under study conditions have varied.34,35 Furthermore, while some authors reported TRALI rates,33,36 others grouped TRALI and possible TRALI37 (as we did).
Of equal importance are the different methods by which cases are identified. Some investigators have used manual retrospective chart review35; others have conducted prospective active surveillance36; but few have used electronic algorithms in case identification.38 As demonstrated previously, passive identification strategies are prone to underrecognition, particularly of less severe cases. Additionally, the populations in each study range from all transfused patients36 to the critically ill4 and, rarely, to surgical subsets,31,32 with differing underlying risks for TRALI, thus limiting comparability. Finally, compounding these study design differences is the impact of the passage of time. Not only has TRALI recognition improved, but there have also been changes in blood management and donor procurement strategies that may have reduced the rate of TRALI.10,11,37 Although our study did not demonstrate this hypothesized decline in incidence after TRALI mitigation strategies were introduced (as discussed in the next paragraph of this Discussion), a substantial and growing body of medical literature supports this effect.11,37 Consequently, we believe that early data before the mid-2000s are unlikely to be representative of the current syndrome burden.
Interestingly, we did not observe the expected decline in TRALI rates from 2004 to 2011, with the introduction of TRALI mitigation strategies, perhaps because we grouped TRALI and possible TRALI as a combined outcome. Although previous reports indicate that TRALI mitigation strategies have resulted in a significant decline in the rate of true TRALI events, their impact on the occurrence of possible TRALI remains unclear.11 We believe this previously observed decline in TRALI incidence with TRALI mitigation strategies to be a true effect, but our detailed breakdown of results by year, implicated product, and TRALI versus possible TRALI (table 6) does not support this hypothesis. However, given the low overall event rate (45 cases in 2 yr), our study was not sufficiently powered to detect such a difference.
Another noteworthy observation is the association between TRALI incidence and transfusion volume. Indeed, a positive association between “dose” of transfusion and TRALI incidence has been reported.36 Although TRALI is believed to be largely immune mediated, the risk of receiving an alloimmunized donor unit undoubtedly increases with more transfusions. Indeed, patients experiencing TRALI have often received a proportionally greater number of units than their complication-free transfused counterparts. In addition, in the current study, physician reviewers adjudicated cases as TRALI, TACO, both, or neither. Patients who had both TRALI and TACO (n = 23) were those in whom the evidence of volume overload did not fully explain the extent of respiratory distress. These patients were included in the current analyses, which may partly explain the increased average volume transfused in TRALI patients.
Notably, several study limitations merit discussion. First, in light of the excellent sensitivity of our electronic screening algorithm, we limited manual review to screen-positive transfusion recipients. Hence, we may have overlooked some cases that screened false-negative. Without manual review of all screen-negative patients, we are unable to corroborate this possibility. However, we again stress that the algorithm achieved a sensitivity of 92.5% (95% CI, 84.6 to 96.7%) in our derivation cohort.17 Although deficiencies may still exist, our NLP-based algorithm has been shown to significantly improve upon manual detection alone,3 as well as upon non-NLP–based electronic algorithms.17 For a sensitivity analysis, we tested the assumption that our algorithm may have performed at the lower boundary of the CI of its previously demonstrated sensitivity (84.6%). In this circumstance, we determined that there could have been up to 53 cases of TRALI in this population of 3,379 patients as opposed to the 45 identified in our primary analyses. The rate of TRALI would therefore marginally increase to 1.6% as opposed to the 1.3% reported in the primary analyses. We also emphasize that there were no cases of TRALI reported to the blood bank during our study period among the screen-negative population. This further supports the accuracy of the TRALI screening algorithm. Second, the nature of the study population at our single-center, tertiary-care referral center limits the generalizability of our results. We surmise that patients may have had a greater prevalence of comorbid conditions and may have been more acutely unwell than surgical populations elsewhere. Thus, the incidence of TRALI in our practice may be above average. Finally, our investigation was also limited by well-recognized difficulties pertaining to making a TRALI diagnosis. Indeed, diagnosing TRALI, TACO, or acute lung injury can be challenging in the best clinical settings. This diagnostic dilemma was further pronounced in our attempt to retrospectively identify cases of TRALI in a postoperative cohort that frequently experiences atelectasis. However, to overcome this dilemma, we implemented robust methodology whereby patients were first electronically screened. Thereafter, two independent physicians with previous training manually reviewed cases. When discrepancies arose, their review was followed by a panel review by three senior critical care physicians. We believe that these procedures represent a marked improvement over those used in past studies aiming to characterize TRALI epidemiology using only passive reporting or single-reviewer outcome adjudication.
In conclusion, our findings document a contemporary rate of TRALI/possible TRALI of 1.4% after intraoperative transfusion for noncardiac surgery. This rate was consistent for the 2 yr investigated (2004 and 2011) and was not meaningfully impacted by TRALI mitigation strategies. These results should help increase awareness among perioperative healthcare providers, and thereby improve case recognition, reporting, and, ultimately, transfusion practice. Future efforts should focus on identification of pertinent and potentially modifiable risk factors for postoperative TRALI.
What We Already Know about This Topic
Transfusion-related acute lung injury is the leading cause of transfusion-related death in the United States
What This Article Tells Us That Is New
A retrospective cohort analysis from one institution documented that perioperative transfusion-related acute lung injury occurs approximately 1.4 to 3% in surgical patients, with higher rates in patients who received larger volumes of blood component therapies
Acknowledgments
This study was performed with the support of funds from the Mayo Clinic Center for Translational Science Activities High-Impact, Pilot and Feasibility Award (HIPFA-2012—Dr. Pathak; 94164003), Rochester, Minnesota, and of funds from the Department of Critical Care Independent Multidisciplinary Program, Mayo Clinic, Rochester, Minnesota.
Footnotes
Presented at the American Society of Anesthesiology Annual Congress, San Francisco, California, October 13, 2013.
CDC. The National Healthcare Safety Network (NHSN) Manual: Biovigilance Component Protocol [Internet]. Atlanta, GA: Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention; January 2014 [cited April 15, 2014]. Available at: www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf. Accessed November 13, 2014.
Competing Interests
The authors declare no competing interests.
This article may be accessed for personal use at no charge through the Journal Web site, www.anesthesiology.org.
References
- 1.Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual summary for fiscal year 2013: 2013 US Department of Health and Human Services. Rockville (MD: US Food and Drug Administration; [Google Scholar]
- 2.Kopko PM, Marshall CS, MacKenzie MR, Holland PV, Popovsky MA. Transfusion-related acute lung injury: Report of a clinical look-back investigation. JAMA. 2002;287:1968–1971. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 3.Clifford L, Singh A, Wilson GA, Toy P, Gajic O, Malinchoc M, Herasevich V, Pathak J, Kor DJ. Electronic health record surveillance algorithms facilitate the detection of transfusion-related pulmonary complications. Transfusion. 2013;53:1205–1216. doi: 10.1111/j.1537-2995.2012.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: Prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 6.Gajic O, Moore SB. Transfusion-related acute lung injury. Mayo Clin Proc. 2005;80:766–770. doi: 10.1016/S0025-6196(11)61531-0. [DOI] [PubMed] [Google Scholar]
- 7.Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G, Ambruso DR. Transfusion-related acute lung injury: Epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 8.Wallis JP, Lubenko A, Wells AW, Chapman CE. Single hospital experience of TRALI. Transfusion. 2003;43:1053–1059. doi: 10.1046/j.1537-2995.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Dijk PM, Kleine JW. The transfusion reaction in anesthesiological practice. Acta Anaesthesiol Belg. 1976;27:247–254. [PubMed] [Google Scholar]
- 10.Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, Kirkley SA, Fialkow LB. An association between decreased cardiopulmonary complications (transfusionrelated acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50:2738–2744. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersum-Osselton JC, Middelburg RA, Beckers EA, van Tilborgh AJ, Zijlker-Jansen PY, Brand A, van der Bom JG, Schipperus MR. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion. 2011;51:1278–1283. doi: 10.1111/j.1537-2995.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 13.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: A non-iT approach A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(42):44–45. [PubMed] [Google Scholar]
- 14.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusionrelated acute lung injury: Statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 15.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, III, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M. U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS): Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kor DJ, Warner DO, Alsara A, Fernández-Pérez ER, Malinchoc M, Kashyap R, Li G, Gajic O. Derivation and diagnostic accuracy of the surgical lung injury prediction model. ANESTHESIOLOGY. 2011;115:117–128. doi: 10.1097/ALN.0b013e31821b5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford L, Wilson GA, Gajic O, Toy P, Herasevich V, Murphy S, Pathak J, Kor DJ. Natural language processing of chest radiograph reports improves the identification of transfusion-related pulmonary complications [abstract] Am J Respir Crit Care Med. 2013;187:A2218. [Google Scholar]
- 18.Savova GK, Masanz JJ, Ogren PV, Zheng J, Sohn S, Kipper-Schuler KC, Chute CG. Mayo clinical Text Analysis and Knowledge Extraction System (cTAKES): architecture, component evaluation and applications. J Am Med Inform Assoc. 2010;17:507–513. doi: 10.1136/jamia.2009.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chute CG, Beck SA, Fisk TB, Mohr DN. The Enterprise Data Trust at Mayo Clinic: A semantically integrated warehouse of biomedical data. J Am Med Inform Assoc. 2010;17:131–135. doi: 10.1136/jamia.2009.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaisant C, Lam S, Shneiderman B, Smith MS, Roseman D, Marchand G, Gillam M, Feied C, Handler J, Rappaport H. Searching electronic health records for temporal patterns in patient histories: A case study with Microsoft amalga. AMIA Annu Symp Proc. 2008:601–605. [PMC free article] [PubMed] [Google Scholar]
- 21.Alsara A, Warner DO, Li G, Herasevich V, Gajic O, Kor DJ. Derivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injury. Mayo Clin Proc. 2011;86:382–388. doi: 10.4065/mcp.2010.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartin-Ceba R, Kojicic M, Li G, Kor DJ, Poulose J, Herasevich V, Kashyap R, Trillo-Alvarez C, Cabello-Garza J, Hubmayr R, Seferian EG, Gajic O. Epidemiology of critical care syndromes, organ failures, and life-support interventions in a suburban US community. Chest. 2011;140:1447–1455. doi: 10.1378/chest.11-1197. [DOI] [PubMed] [Google Scholar]
- 23.Schmickl CN, Li M, Li G, Wetzstein MM, Herasevich V, Gajic O, Benzo RP. The accuracy and efficiency of electronic screening for recruitment into a clinical trial on COPD. Respir Med. 2011;105:1501–1506. doi: 10.1016/j.rmed.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh B, Singh A, Ahmed A, Wilson GA, Pickering BW, Herasevich V, Gajic O, Li G. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87:817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallis JP. Transfusion-related acute lung injury (TRALI)- Under-diagnosed and under-reported. Br J Anaesth. 2003;90:573–576. doi: 10.1093/bja/aeg101. [DOI] [PubMed] [Google Scholar]
- 26.Popovsky MA, Chaplin HC, Jr, Moore SB. Transfusion-related acute lung injury: A neglected, serious complication of hemotherapy. Transfusion. 1992;32:589–592. doi: 10.1046/j.1537-2995.1992.32692367207.x. [DOI] [PubMed] [Google Scholar]
- 27.Wallis JP. Transfusion-related acute lung injury (TRALI): Presentation, epidemiology and treatment. Intensive Care Med. 2007;33(suppl 1):S12–S16. doi: 10.1007/s00134-007-2874-2. [DOI] [PubMed] [Google Scholar]
- 28.Palf M, Berg S, Ernerudh J, Berlin G. A randomized controlled trial oftransfusion-related acute lung injury: Is plasma from multiparous blood donors dangerous? Transfusion. 2001;41:317–322. doi: 10.1046/j.1537-2995.2001.41030317.x. [DOI] [PubMed] [Google Scholar]
- 29.Florell SR, Velasco SE, Fine PG. Perioperative recognition, management, and pathologic diagnosis of transfusion-related acute lung injury. ANESTHESIOLOGY. 1994;81:508–510. doi: 10.1097/00000542-199408000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Weber JG, Warner MA, Moore SB. What is the incidence of perioperative transfusion-related acute lung injury? ANESTHESIOLOGY. 1995;82:789. doi: 10.1097/00000542-199503000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Wright SE, Snowden CP, Athey SC, Leaver AA, Clarkson JM, Chapman CE, Roberts DR, Wallis JP. Acute lung injury after ruptured abdominal aortic aneurysm repair: The effect of excluding donations from females from the production of fresh frozen plasma. Crit Care Med. 2008;36:1796–1802. doi: 10.1097/CCM.0b013e3181743c6e. [DOI] [PubMed] [Google Scholar]
- 32.Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Kulik W, Korevaar J, de Mol BA, Koopman MM, Porcelijn L, Binnekade JM, Vroom MB, Schultz MJ, Juffermans NP. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: A prospective nested case-control study. Blood. 2011;117:4218–4225. doi: 10.1182/blood-2010-10-313973. [DOI] [PubMed] [Google Scholar]
- 33.Tsai HI, Chou AH, Yang MW. Perioperative transfusion-related acute lung injury: A retrospective analysis. Acta Anaesthesiol Taiwan. 2012;50:96–100. doi: 10.1016/j.aat.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Eder AF, Herron R, strupp A, DY B, Notari EP, Chambers LA, Dodd RY, Benjamin RJ. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 35.Ozier Y, Muller JY, Mertes PM, Renaudier P, Aguilon P, Canivet N, Fabrigli P, Rebibo D, Tazerout M, Trophilme C, Willaert B, Caldani C. Transfusion-related acute lung injury: Reports to the French Hemovigilance Network 2007 through 2008. Transfusion. 2011;51:2102–2110. doi: 10.1111/j.1537-2995.2011.03073.x. [DOI] [PubMed] [Google Scholar]
- 36.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, Lowell CA, Norris PJ, Murphy EL, Weiskopf RB, Wilson G, Koenigsberg M, Lee D, Schuller R, Wu P, Grimes B, Gandhi MJ, Winters JL, Mair D, Hirschler N, sanchez Rosen R, Matthay MA. TRALI Study Group: Transfusion-related acute lung injury: Incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arinsburg SA, skerrett DL, Karp JK, Ness PM, Jhang J, Padmanabhan A, Gibble J, Schwartz J, King KE, Cushing MM. Conversion to low transfusion-related acute lung injury (TRALI)-risk plasma significantly reduces TRALI. Transfusion. 2012;52:946–952. doi: 10.1111/j.1537-2995.2011.03403.x. [DOI] [PubMed] [Google Scholar]
- 38.Finlay HE, Cassorla L, Feiner J, Toy P. Designing and testing a computer-based screening system for transfusion-related acute lung injury. Am J Clin Pathol. 2005;124:601–609. doi: 10.1309/1XKQKFF83CBU4D6H. [DOI] [PubMed] [Google Scholar]