Abstract
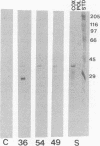
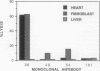
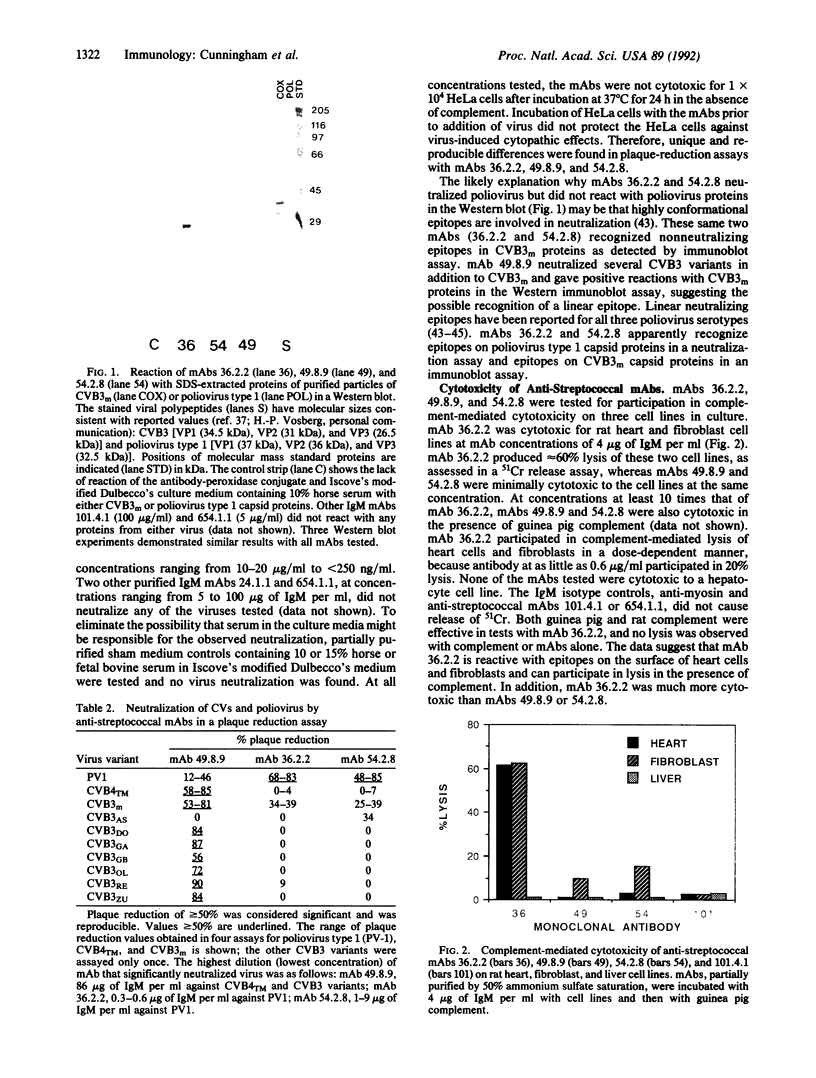
The development of autoimmunity in certain instances is related to infectious agents. In this report, cytotoxic monoclonal antibodies (mAbs) that recognize epitopes on both enteroviruses and the bacterium Streptococcus pyogenes are described. Murine anti-streptococcal mAbs that were crossreactive with streptococcal M protein, human cardiac myosin, and other alpha-helical coiled-coil molecules were found to neutralize coxsackieviruses B3 and B4 or poliovirus type 1. The viral-neutralizing anti-streptococcal mAbs were also cytotoxic for heart and fibroblast cell lines and reacted with viral capsid proteins on a Western immunoblot. Alignment of amino acid sequences shared between streptococcal M protein, coxsackie-virus B3 capsid protein VP1, and myosin revealed 40% identity in a 14- to 15-amino acid overlap. Synthetic peptides containing these sequences blocked mAb reactivity with streptococcal M protein. The data show that antibodies against alpha-helical structures of bacterial and viral antigens can lead to cytotoxic reactions and may be one mechanism to explain the origin of autoimmune heart disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez F. L., Neu N., Rose N. R., Craig S. W., Beisel K. W. Heart-specific autoantibodies induced by Coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987 Apr;43(1):129–139. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Dighiero G., Lymberi P., Guilbert B. Studies on natural antibodies and autoantibodies. Ann Immunol (Paris) 1983 Jul-Aug;134D(1):103–113. [PubMed] [Google Scholar]
- Beatrice S. T., Katze M. G., Zajac B. A., Crowell R. L. Induction of neutralizing antibodies by the coxsackievirus B3 virion polypeptide, VP2. Virology. 1980 Jul 30;104(2):426–438. doi: 10.1016/0042-6822(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Beisel K. W., Srinivasappa J., Olsen M. R., Stiff A. C., Essani K., Prabhakar B. S. A neutralizing monoclonal antibody against Coxsackievirus B4 cross-reacts with contractile muscle proteins. Microb Pathog. 1990 Feb;8(2):151–156. doi: 10.1016/0882-4010(90)90079-6. [DOI] [PubMed] [Google Scholar]
- Bessen D., Jones K. F., Fischetti V. A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989 Jan 1;169(1):269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P., Stafford D., Schwartz R. S., Stollar B. D. Murine monoclonal anti-DNA autoantibodies bind to endogenous bacteria. J Immunol. 1985 Aug;135(2):1086–1090. [PubMed] [Google Scholar]
- Chow M., Yabrov R., Bittle J., Hogle J., Baltimore D. Synthetic peptides from four separate regions of the poliovirus type 1 capsid protein VP1 induce neutralizing antibodies. Proc Natl Acad Sci U S A. 1985 Feb;82(3):910–914. doi: 10.1073/pnas.82.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Phillips G. N., Jr Spikes and fimbriae: alpha-helical proteins form surface projections on microorganisms. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5303–5304. doi: 10.1073/pnas.78.9.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Fenderson P. G., Ho M. K., Beachey E. H., Dale J. B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. 1989 Oct 15;143(8):2677–2683. [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Talaber L. R., Harley J. B., Ayoub E. M., Muneer R. S., Chun L. T., Reddy D. V. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J Immunol. 1988 Oct 15;141(8):2760–2766. [PubMed] [Google Scholar]
- Cunningham M. W., Swerlick R. A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986 Oct 1;164(4):998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1985 Aug 1;162(2):583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J Exp Med. 1985 Jan 1;161(1):113–122. doi: 10.1084/jem.161.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Wimmer E. Priming for and induction of anti-poliovirus neutralizing antibodies by synthetic peptides. Nature. 1983 Aug 25;304(5928):699–703. doi: 10.1038/304699a0. [DOI] [PubMed] [Google Scholar]
- Fenderson P. G., Fischetti V. A., Cunningham M. W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989 Apr 1;142(7):2475–2481. [PubMed] [Google Scholar]
- Fischetti V. A., Parry D. A., Trus B. L., Hollingshead S. K., Scott J. R., Manjula B. N. Conformational characteristics of the complete sequence of group A streptococcal M6 protein. Proteins. 1988;3(1):60–69. doi: 10.1002/prot.340030106. [DOI] [PubMed] [Google Scholar]
- Garzelli C., Taub F. E., Scharff J. E., Prabhakar B. S., Ginsberg-Fellner F., Notkins A. L. Epstein-Barr virus-transformed lymphocytes produce monoclonal autoantibodies that react with antigens in multiple organs. J Virol. 1984 Nov;52(2):722–725. doi: 10.1128/jvi.52.2.722-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J Immunol. 1986 Sep 1;137(5):1695–1702. [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Gulizia J. M., Cunningham M. W., McManus B. M. Immunoreactivity of anti-streptococcal monoclonal antibodies to human heart valves. Evidence for multiple cross-reactive epitopes. Am J Pathol. 1991 Feb;138(2):285–301. [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Horstmann R. D., Sievertsen H. J., Knobloch J., Fischetti V. A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke T., Diederich K. W., Haas W., Schleich J., Lichter P., Pfordt M., Bach A., Vosberg H. P. The complete sequence of the human beta-myosin heavy chain gene and a comparative analysis of its product. Genomics. 1990 Oct;8(2):194–206. doi: 10.1016/0888-7543(90)90272-v. [DOI] [PubMed] [Google Scholar]
- KAPLAN M. H., BOLANDE R., RAKITA L., BLAIR J. PRESENCE OF BOUND IMMUNOGLOBULINS AND COMPLEMENT IN THE MYOCARDIUM IN ACUTE RHEUMATIC FEVER. ASSOCIATION WITH CARDIAC FAILURE. N Engl J Med. 1964 Sep 24;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- Kraus W., Ohyama K., Snyder D. S., Beachey E. H. Autoimmune sequence of streptococcal M protein shared with the intermediate filament protein, vimentin. J Exp Med. 1989 Feb 1;169(2):481–492. doi: 10.1084/jem.169.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Seyer J. M., Beachey E. H. Vimentin-cross-reactive epitope of type 12 streptococcal M protein. Infect Immun. 1989 Aug;57(8):2457–2461. doi: 10.1128/iai.57.8.2457-2461.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher K., Cunningham M. W. Myosin: a link between streptococci and heart. Science. 1985 Jan 25;227(4685):413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959 Aug 1;110(2):271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch B. Immunologic regulator and effector functions in perimyocarditis, postmyocarditic heart muscle disease and dilated cardiomyopathy. Basic Res Cardiol. 1986;81 (Suppl 1):217–241. doi: 10.1007/978-3-662-11374-5_21. [DOI] [PubMed] [Google Scholar]
- Maisch B., Trostel-Soeder R., Stechemesser E., Berg P. A., Kochsiek K. Diagnostic relevance of humoral and cell-mediated immune reactions in patients with acute viral myocarditis. Clin Exp Immunol. 1982 Jun;48(3):533–545. [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem Biophys Res Commun. 1986 Oct 30;140(2):684–690. doi: 10.1016/0006-291x(86)90786-2. [DOI] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Page G. S., Mosser A. G., Hogle J. M., Filman D. J., Rueckert R. R., Chow M. Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol. 1988 May;62(5):1781–1794. doi: 10.1128/jvi.62.5.1781-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Uhlig J., Wiegers K., Dernick R. A new antigenic site of poliovirus recognized by an intertypic cross-neutralizing monoclonal antibody. Virology. 1990 Oct;178(2):606–610. doi: 10.1016/0042-6822(90)90363-v. [DOI] [PubMed] [Google Scholar]
- Wiley J. A., Brodeur B. R., Dimock K. D., Sattar S. A. Neutralizing monoclonal antibody against enterovirus-70 reacts with viral proteins 1C and 1D. Viral Immunol. 1990 Summer;3(2):137–146. doi: 10.1089/vim.1990.3.137. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Rose N. R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985 May 1;161(5):1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. Rheumatic fever: the interplay between host, genetics, and microbe. Lewis A. Conner memorial lecture. Circulation. 1985 Jun;71(6):1077–1086. doi: 10.1161/01.cir.71.6.1077. [DOI] [PubMed] [Google Scholar]