Abstract
Background
Several studies have shown Drug Eluting Stents (DES) to be better compared to Bare Metal Stents (BMS) in patients with type 2 Diabetes Mellitus (T2DM). Since, the adverse clinical outcomes in patients with Insulin-Treated Type 2 Diabetes Mellitus (ITDM) implanted with DES and BMS have not been previously studied, we aim to compare the clinical outcomes in similar patients with cardiovascular diseases, treated with DES and BMS.
Methods
Randomized Controlled Trials (RCTs) comparing patients treated with DES and BMS were searched from PubMed and EMBASE databases. Outcome data for the patients with ITDM were carefully extracted. Major Adverse Cardiac Events (MACEs), mortality, Target Vessel Revascularization (TVR), Target Lesion Revascularization (TLR), Myocardial Infarction (MI) and Stent Thrombosis (ST) were considered as the clinical endpoints for this analysis. Odds ratios (OR) with 95% confidence intervals (CIs) were calculated and the pooled analyses were performed with RevMan 5.3 software.
Results
Ten RCTs consisting of 830 patients with ITDM (477 patients in the DES group and 353 patients in the BMS group) from a total number of 9,141 patients were included in this analysis. During a follow-up period from one month to one year, MACEs were not increased with the use of DES in these patients with ITDM. At 9 months, MACEs were significantly lower in the DES group with OR: 0.40, 95% CI: 0.23–0.72; P = 0.002 with no increase in mortality. TVR and TLR also favored the DES group with OR: 0.44, 95% CI: 0.22–0.88, P = 0.02 and OR: 0.28, 95% CI: 0.14–0.53; P = 0.0001 respectively at 9 months, and OR: 0.46, 95% CI: 0.23–0.94, P = 0.03 and OR: 0.28, 95% CI: 0.14–0.55; P = 0.0003 respectively at one year. Results for MI, and ST were not statistically significant.
Conclusion
Compared to BMS, DES were associated with a significantly lower rate of repeated revascularization, without any increase in MACEs or mortality in these patients with ITDM during a follow up period of one year. However, due to the very small population size, further studies with a larger number of randomized patients are required to completely solve this issue.
Introduction
Today, an estimated 171 million people suffer from Diabetes Mellitus (DM) all over the world and the prevalence of this chronic disease is expected to double over the next two decades [1]. DM is associated with a higher rate of repeated revascularization compared to non-DM [2]. Even if studies have shown that type 2 Diabetes Mellitus (T2DM) is independently associated with significantly higher adverse clinical outcomes whether with Drug Eluting Stents (DES) or Bare Metal Stents (BMS) [3], compared to non-T2DM, other studies have shown DES to have been associated with better outcomes compared to BMS in patients with T2DM. For example, the pooled analysis of seven Randomized Controlled Trials (RCTs) by Lijima et al showed that DES significantly improved adverse outcomes compared to BMS, in patients with T2DM who underwent Percutaneous Coronary Intervention (PCI) for Acute Myocardial Infarction (AMI) [4]. However, because of contraindications to the use of DES, BMS is still considered equally important in several subgroups of patients. T2DM can be further divided into Insulin-Treated Diabetes Mellitus (ITDM) (requiring insulin therapy with or without oral hypoglycemic medications as treatment) and non-ITDM (require only diet control and oral hypoglycemic agents as treatment). Since insulin therapy is associated with increased adverse cardiovascular outcomes after PCI [5] and because the adverse clinical outcomes observed with the use of DES and BMS have not been previously studied in patients with ITDM, we aim to solve this issue using data only from RCTs.
Methods
Search Strategy
RCTs comparing DES with BMS were searched from Medline and EMBASE by typing the words ‘drug eluting stents, bare metal stents, and/or percutaneous coronary intervention’. Abbreviations (DES, BMS and/or PCI) were also used. To widen the search or simply to be more specific, the word ‘diabetes mellitus’ was also included. No language restriction was applied.
Inclusion and Exclusion Criteria
Studies were included if:
They were RCTs.
They compared DES with BMS.
They included data for patients with ITDM.
They reported adverse outcomes as their clinical endpoints.
Studies were excluded if:
They were non-RCTs for example observational studies, case studies or meta-analyses.
They did not include patients with ITDM.
Adverse outcomes were not reported among their clinical endpoints.
Defining outcomes and follow-up period
The reported outcomes were:
Major Adverse Cardiac Events (MACEs)
Mortality
Myocardial Infarction (MI)
Repeated revascularization including Target Vessel Revascularization (TVR) and Target Lesion Revascularization (TLR)
Stent Thrombosis (ST)
The follow-up period varied from one month to one year. The reported outcomes and their corresponding follow up periods have been listed in Table 1.
Table 1. The adverse outcomes reported in the included trials.
Abbreviations: MACEs: major adverse cardiac events, MI: myocardial infarction, TLR: target lesion revascularization, TVR: target vessel revascularization, ST: stent thrombosis.
| Trials | Reported Outcomes | Follow up period (months) |
|---|---|---|
| SCORPIUS | Mortality, MI and TLR | 1, 8 and 12 |
| PASEO | Mortality, MI, TLR, MACEs, ST | 12 |
| DIABETES | MACEs, revascularization | 1 and 9 |
| HORIZONS-AMI | Mortality, MACEs, TLR, TVR, ST, MI | 12 |
| PRODIGY | TVR, TLR, ST | 24 |
| TAXUS VI | Mortality, MACEs, TVR, TLR, MI | 1 and 9 |
| TAXUS IV | MACEs, TVR, TLR | 12 |
| SIRIUS | MACEs, TLR | 9 |
| STONE2005 | Mortality, MACEs, TLR, TVR, MI, ST | 1 and 9 |
| STONE2004 | Mortality, MI, ST, TVR, TLR, MACEs | 9 |
Data Extraction and Analysis
Three authors (P.K.B, A.B and M.Z.S.S) independently reviewed the data and each of them assessed whether the trials were eligible for this study. The methodological quality of all the trials were also assessed. First of all, outcome data for the patients with ITDM were carefully extracted. Then information regarding the study type, the author names, the year of publications and the patient characteristics as well as the clinical outcomes reported and the follow up periods was systematically extracted. Disagreements raised were discussed among the authors, and then a decision was made. However, if the authors could not reach a consensus, disagreements were resolved by the fourth author (M.Y.L). Risk of bias among the trials was assessed in accordance to the components recommended by the Cochrane Collaboration [6].
Statistical Analysis
Recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement were considered [7]. Heterogeneity across trials was assessed using the Cochrane Q-statistic (whereby P ≤0.05 was considered statistically significant whereas P > 0.05 was considered statistically insignificant) and also using the I2-statistic whereby I2 described the percentage of total variation across studies (a low value of I2 indicated lower heterogeneity whereas a larger value indicated increased heterogeneity). A fixed effect model was used if I2 was <50%. However, if I2 was >50%, a random effect model was used. Publication bias, which was also taken into consideration in our study, was visually estimated by assessing the funnel plots. We calculated odds ratios (OR) and 95% confidence intervals (CIs) for categorical variables and the pooled analyses of data from our included studies were performed with RevMan 5.3 software.
Ethics
Ethical approval was not necessary for Systematic Review and Meta-Analysis.
Results
Study selection
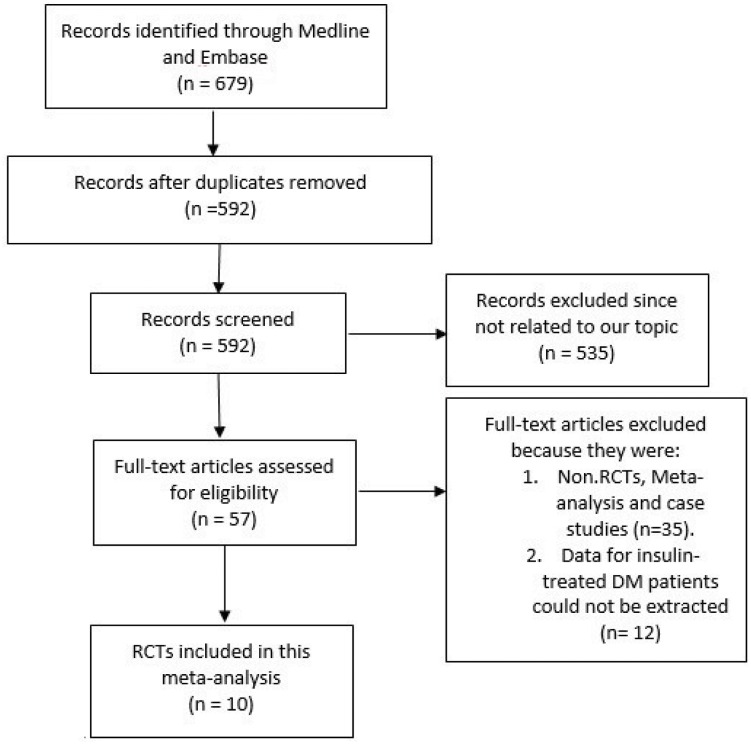
679 articles were searched from Medline and EMBASE databases. After eliminating the duplicate studies and those studies not related to our topic, 57 full text articles were assessed for eligibility. After another careful screening with strict considering to the inclusion and exclusion criteria of our meta-analysis, 35 more articles were eliminated because they were either: meta-analyses, non-RCTs and case studies. Further 12 articles were eliminated because data for patients with ITDM could not be extracted. Finally, 10 RCTs were selected for this meta-analysis [8–17]. Fig 1 represents the flow chart for the study selection.
Fig 1. The flow diagram for the study selection.
A total number of 830 patients with ITDM (including 477 patients from the DES group and 353 patients from the BMS group) were extracted from a total number of 9141 patients (including 5855 patients from the DES group and 3286 patients from the BMS group). Table 2 lists the number of patients with ITDM which were extracted from the DES and BMS groups, as well as the types of DES used in those trials.
Table 2. The total number of patients included from each trials.
Abbreviations: DES: drug eluting stents, BMS: bare metal stents, PES: paclitaxel eluting stents, SES: sirolimus eluting stents, ZES: zotarolimus eluting stents, EES: everolimus eluting stents, ITDM: insulin-treated diabetes mellitus.
| Trials | Type of DES | Total no of patients treated with DES (n) | ITDM patients in the DES group (n) | Total no of patients treated with BMS (n) | ITDM patients in the BMS group (n) |
|---|---|---|---|---|---|
| SCORPIUS | SES | 94 | 40 | 96 | 39 |
| PASEO | PES, SES | 180 | 15 | 90 | 8 |
| DIABETES | SES | 80 | 26 | 80 | 27 |
| HORIZONS-AMI | PES | 2256 | 98 | 749 | 31 |
| PRODIGY | ZES, PES, EES | 1501 | 94 | 502 | 23 |
| TAXUS VI | PES | 219 | 15 | 227 | 20 |
| TAXUS IV | PES | 155 | 51 | 163 | 54 |
| SIRIUS | SES | 131 | 38 | 148 | 44 |
| STONE2005 | PES | 577 | 49 | 579 | 53 |
| STONE2004 | PES | 662 | 51 | 652 | 54 |
Baseline characteristics
The baseline features of these included trials have been summarized in Table 3. On average, more than 60% of the population were males with a mean age above 60 years old. No significant differences have been observed in the baseline features among patients classified under the DES and BMS groups. Further details are listed in Table 3.
Table 3. The baseline features reported within the included trials.
Abbreviations: DES: drug eluting stents, BMS: bare metal stents.
| Trials | Age (years) | Males | Hypertension | Dyslipidemia | Smoker |
|---|---|---|---|---|---|
| DES/BMS | DES/BMS | DES/BMS | DES/BMS | DES/BMS | |
| SCORPIUS | 66.0/66.0 | 66.0/62.0 | 93.0/94.0 | 82.0/80.0 | 20.0/18.0 |
| PASEO | 62.5/62.0 | 70.0/71.1 | 27.3/24.4 | - | 24.4/26.7 |
| DIABETES | 65.2/67.9 | 62.5/62.5 | 66.3/66.3 | 61.3/61.3 | 45.0/50.0 |
| HORIZONS-AMI | 59.9/59.3 | 77.0/76.0 | 51.2/51.9 | 42.2/41.1 | 46.3/51.9 |
| PRODIGY | 68.0/69.0 | 77.3/74.0 | 71.0/75.0 | 56.0/51.0 | 23.3/25.0 |
| TAXUS VI | 61.8/63.4 | 76.3/76.2 | 57.5/58.1 | 70.3/73.4 | 22.5/23.9 |
| TAXUS IV | 62.6/61.8 | 59.4/67.5 | 79.4/82.8 | 77.0/66.0 | - |
| STONE2005 | 62.9/62.8 | 70.2/68.7 | 76.4/73.6 | 72.3/73.9 | 21.1/19.9 |
| STONE2004 | 62.8/62.1 | 71.8/72.4 | 70.5/69.0 | 65.0/65.6 | 23.4/20.1 |
Percentage (%) was used to represent the baseline characteristics listed in Table 3.
Baseline feature for the SIRIUS trial was not provided in the original manuscript and hence has been omitted in our study.
Main result of this study
Results of this meta-analysis have been summarized in Table 4.
Table 4. Shows the results of this analysis.
Abbreviations: OR: odds ratio, MACEs: major adverse cardiac events, MI: myocardial infarction, TVR: target vessel revascularization, TLR: target lesion revascularization, ST: stent thrombosis.
| Outcomes | Number of participants (n) | OR with 95% CI | P value | I2 (%) |
|---|---|---|---|---|
| One month | ||||
| MACEs | 190 | 0.70 [0.18–2.74] | 0.61 | 0 |
| MI | 216 | 0.86 [0.21–3.55] | 0.83 | 0 |
| Nine months | ||||
| MACEs | 377 | 0.40 [0.23–0.72] | 0.002 | 0 |
| Mortality | 321 | 1.38 [0.30–6.32] | 0.67 | 0 |
| MI | 321 | 1.24 [0.44–3.51] | 0.68 | 0 |
| TVR | 324 | 0.44 [0.22–0.88] | 0.02 | 0 |
| TLR | 456 | 0.28 [0.14–0.53] | 0.0001 | 0 |
| 12 months | ||||
| MACEs | 374 | 0.65 [0.36–1.19] | 0.16 | 0 |
| Mortality | 336 | 0.74 [0.22–2.42] | 0.62 | 0 |
| MI | 453 | 0.50 [0.22–1.13] | 0.10 | 0 |
| TVR | 351 | 0.46 [0.23–0.94] | 0.03 | 0 |
| TLR | 453 | 0.28 [0.14–0.55] | 0.0003 | 0 |
| ST | 269 | 0.71 [0.13–3.72] | 0.68 | 0 |
Adverse clinical outcomes at one month follow up
At one month, 130 patients from the DES group and 139 patients from the BMS group, with a total number of 269 patients classified under the ITDM subgroup were analyzed.
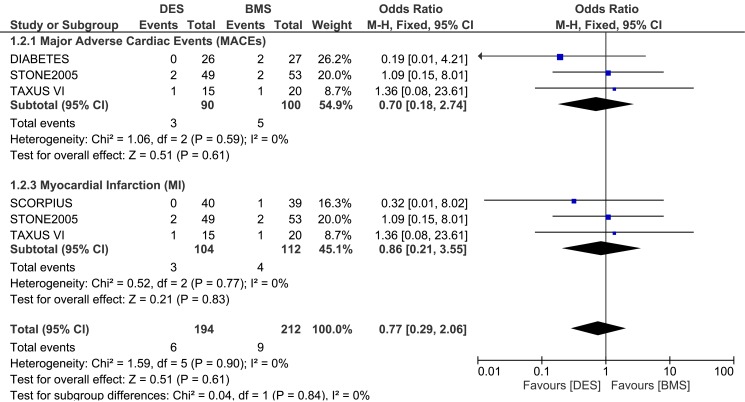
The pooled analysis showed that MACEs were not increased with the use of DES in these patients with ITDM, OR: 0.70, 95% CI: 0.18–2.74; P = 0.61. Results for MI was also not statistically significant between DES and BMS with OR: 0.86, 95% CI: 0.21–3.55; P = 0.83. This result has been illustrated in Fig 2.
Fig 2. Forest plot comparing the adverse clinical outcomes between DES and BMS in patients with insulin-treated diabetes mellitus during a one month follow up.
Adverse clinical outcomes at nine months follow up
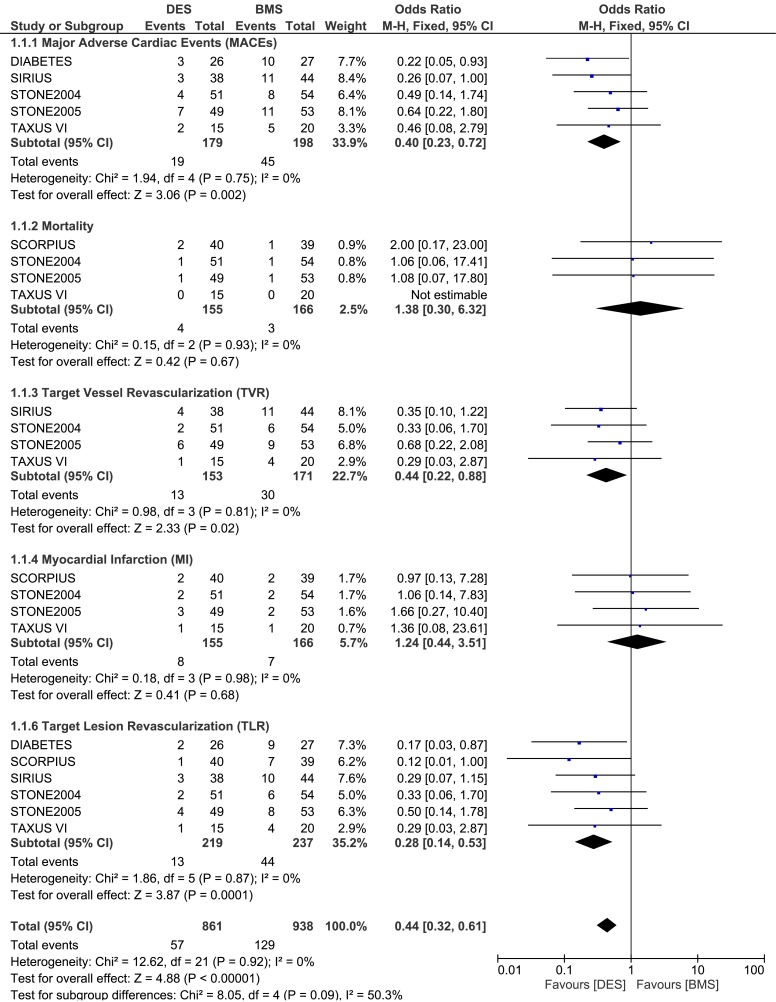
During a follow up of 9 months, whereby 219 patients from the DES group and 237 patients from the BMS group were analyzed, MACEs were significantly lower in the DES group with OR: 0.40, 95% CI: 0.23–0.72, P = 0.002. Revascularization including TVR and TLR were also significantly lower in the DES group with OR: 0.44, 95% CI: 0.22–0.88; P = 0.02 and OR: 0.28, 95% CI: 0.14–0.53, P = 0.0001 respectively. However, results for mortality and MI were not statistically significant. Result for the 9 months follow up period has been illustrated in Fig 3.
Fig 3. Forest plot comparing the adverse clinical outcomes between DES and BMS in patients with insulin-treated diabetes mellitus during a follow up period of nine months.
Adverse clinical outcomes at one year follow up
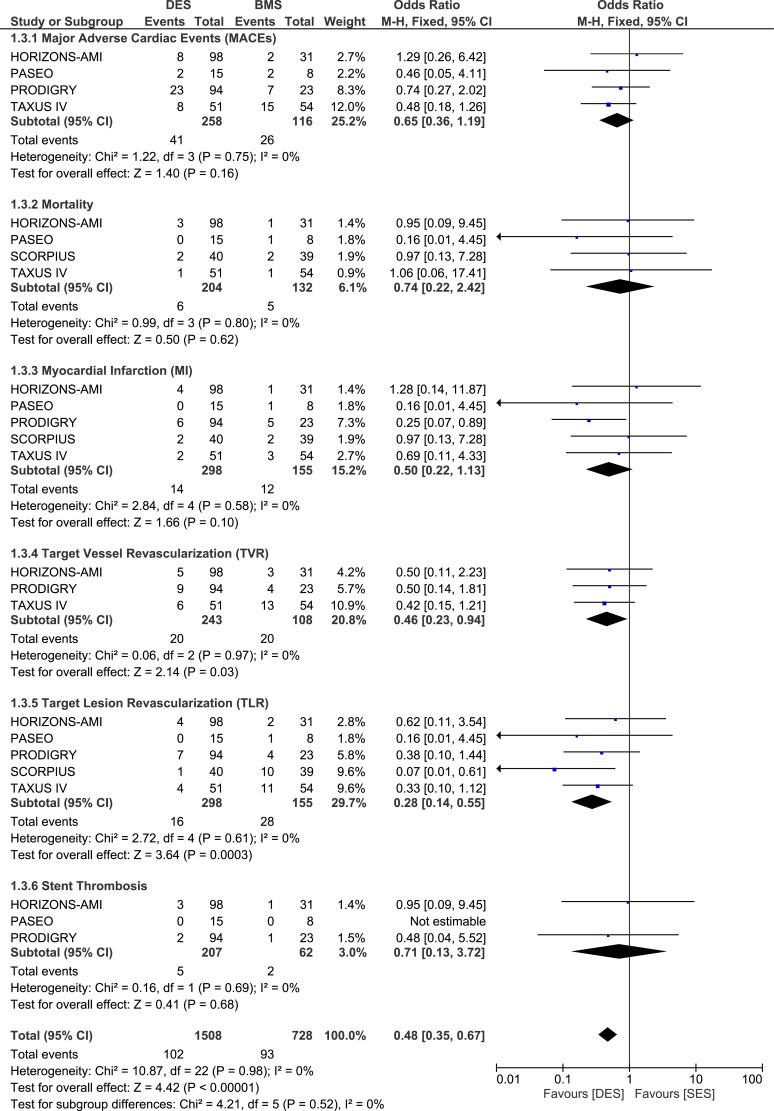
During the one year follow up, whereby 298 patients with ITDM in the DES group and 155 patients with ITDM in the BMS group were analyzed, MACEs did not increase with the use of DES with OR: 0.65, 95% CI: 0.36–1.19; P = 0.16. Similarly, mortality rate did not increase. TVR and TLR were still significantly reduced in the DES group with OR: 0.46, 95% CI: 0.23–0.94; P = 0.03 and OR: 0.28, 95% CI: 0.14–0.55, P = 0.0003 respectively. MI was lower in the DES group but the result was not statistically significant. No increase in ST was noted. These results have been illustrated in Fig 4.
Fig 4. Forest plot comparing the adverse clinical outcomes between DES and BMS in patients with insulin-treated diabetes mellitus during a one year follow up.
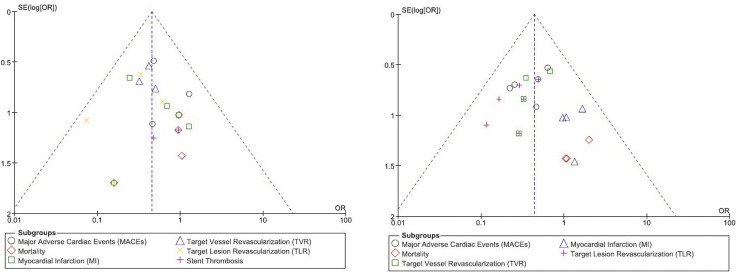
For all of the above analyses, sensitivity analyses yielded consistent results. Based on a visual inspection of the funnel plots, there has been no evidence of publication bias observed among the included trials that assessed all clinical endpoints in this study. The funnel plots have been illustrated in (Fig 5A and 5B).
Fig 5. (A and B): funnel plots representing the sensitivity analysis.
Discussion
Nowadays DES are the most widely used stents in PCI centers. Several studies have shown DES to be better compared to BMS. Patients with T2DM have higher risks of MACEs and repeated revascularization after PCI, and DES have shown to be beneficial to this particular subgroup of patients as well. T2DM can further be classified into ITDM and non-ITDM. Because insulin therapy is expected to be associated with significantly higher cardiovascular outcomes compared to NITDM after PCI [5] and since DES and BMS have not been previously compared in patients with ITDM, we aim to compare the adverse outcomes between DES and BMS in similar patients.
Our results showed that during a follow up period from one month to one year, MACEs and mortality did not increase with the use of DES in these patients with ITDM. TVR and TLR were significantly lower in the DES group indicating that DES are associated with lower repeated revascularization rates compared to BMS. Results for MI and ST were not statistically significant in our study.
Normally, after coronary revascularization, patients with T2DM have an increased risk of Target Vessel Failure (TVF) and repeated revascularization due to a faster restenosis rate compared to patients without DM [18]. Several mechanisms have been suggested to increase the risk of plaque formation and aggravate coronary artery disease [19–21]. Hyperglycemia can result in altered inflammatory pathways, resistance to insulin, and altered free fatty acid metabolism thus causing endothelial dysfunction, increased platelet production, monocyte activation, foam cell transformation and altered smooth muscle cell migration. The increased production of pro-inflammatory cytokines such as tumor necrosis factor and interleukins 6 in these patients with T2DM could contribute to thrombogenesis. Whether patients with T2DM are treated with insulin or not, all patients are at risk of repeated revascularization but however, studies have shown a higher risk of repeated revascularization among patients within the ITDM group indicating that insulin therapy could partly be responsible or could act as an adjuvant thus contributing to such outcomes.
However, compared to BMS, DES are associated with a decreased rate of restenosis and repeated revascularization whether in patients with DM or non-DM [22]. Our results showed DES to significantly reduce the rate of TVR and TLR in these patients with ITDM but however, revascularization rate is expected to be higher in those patients with T2DM who require insulin therapy compared to those who do not require insulin as treatment. Patients with ITDM have the highest rate of restenosis regardless of stent types. Further studies are needed to clarify on the exact reasons for such a result and its association with insulin therapy.
The meta-analysis published by Patti et al comparing DES with BMS in patients with T2DM using data from nine trials, showed patients receiving DES to have lower rate of in-stent restenosis and TLR compared to those patients treated with BMS [23]. A decrease in in-stent restenosis was clearly observed in both, the insulin-treated and non-insulin treated subgroups. DES also significantly reduced the rate of MI without increasing mortality or ST in these patients with T2DM.
Similar to our meta-analysis, the pooled analysis by Lijima et al comparing DES and BMS in patients with T2DM suffering from AMI also showed DES to significantly reduce the revascularization rate without increasing the mortality rate or the rate of MI [4]. Another result from the PRISON II study showed that after three years, TLR was significantly reduced from 27% in the BMS group compared to only 7% in the DES group. Reported TVR was 30% in the BMS group whereas it was only 11% in the DES group [24]. In their study, MACEs were also significantly reduced in the DES group whereby only 10% were observed compared to 34% in the BMS group. However, similar to our result, the rates of mortality, MI and ST were not significant increased between these two groups. Their study compared DES and BMS in patients with total coronary occlusion and had a longer follow up period. However, our study compared DES and BMS in patients with ITDM having a shorter follow up period compared to their study.
Another result from the same trial showed that during a follow up period between 6 months and 1 year, TVR and TLR were significantly lower in the DES group [25]. However, similar to our study, the result for MACEs was not statistically significant. This PRISON II study included only 4% of patients with ITDM in the BMS group. According to two different follow up periods from the same trial, MACEs were still not increased with the use of DES and repeated revascularization was still significantly lower with the use of DES.
The meta-analysis by Kirtane et al comparing the safety and effectiveness of DES and BMS showed a significantly lower revascularization rate associated with the use of DES [26]. However, a similar rate of mortality or MI were observed in the general population analyzed. Moreover, the meta-analysis conducted by Kastrati et al comparing SES and BMS in the general population (involving 14 trials) showed that during a long-term follow up period, SES did not have a significant effect on the overall mortality rate or MI. ST was indifferent to BMS [27]. However, the revascularization rate was significantly reduced with the use of this type of DES. The study by Mauri et al comparing ZES with BMS and the study published by Caixeta et al also showed similar results with our study [28–29].
In addition, the study by Uthamalingum et al comparing the outcomes in octogenarians treated with either DES or BMS showed that at one year, BMS had an increased risk of MACEs compared to DES [30]. The result for death was not statistically significant. Their study included 21.6% of patients with T2DM in the BMS group and 27.5% of patients with T2DM in the DES group. However, the number of patients treated with insulin was unknown.
Our study did not show any significant difference in ST between DES and BMS in these patients with ITDM. Nevertheless, the collaborative network meta-analysis comparing DES and BMS in patients with and without T2DM showed a significant difference in ST between SES and BMS [31]. But Bangalore et al found that SES were not superior to BMS. As a direct corollary, Bangalore et al carried out a large-scale meta-analysis that included 10 714 patients with T2DM, which compared BMS with different types of DES [18]. As this is the most recent study, it is likely that these results are the most reliable.
Another meta-analysis comparing DES and BMS in unprotected left coronary artery stenosis showed results which differed partly from our meta-analysis [32]. Results from that meta-analysis showed a significantly lower revascularization and MACEs during a follow up period of less than one year, an additional significantly lower rate of death at 2 years, and an another addition of significantly lower MI after 3 years in these patients. However, the study included only 85 patients with ITDM in the DES group and 63 patients with ITDM in the BMS group among the 8861 patients with T2DM analyzed.
A comprehensive network meta-analysis by Palmerini et al demonstrated DES to be superior to BMS after a median follow up period of 3.8 years [33]. In this study, cobalt-chromium everolimus eluting stents (EES) were associated with a significantly lower rate of MI, definite ST and death compared to BMS. Revascularization was also significantly lower in the DES group. However, our study comparing DES and BMS in patients with ITDM, did not show any significant decrease in death or MI in the DES group. Our follow up period was also not longer than one year.
DES have proved to be beneficial in T2DM (including both ITDM and NITDM patients). However, controversies still exist about the efficacy of the different types of DES in patients with T2DM. It is now clear than DES are the preferred stents in patients with T2DM including both ITDM and NITDM, but questions are raised about the different types of DES, which have different levels of benefits among these patients with T2DM. For example, when PES, SES and BMS were compared in 3852 patients with T2DM, SES showed to be more beneficial than PES for having a longer event free period and a lower ST at 1 year in these patients with T2DM [30]. Moreover, studies showed EES to be associated with decreased neointima formation, lumen loss and vessel narrowing in patients with T2DM when compared to PES [34]. When EES were compared to SES, similar safety outcomes have been observed. However, EES were associated with a decreased ST and restenosis rate compared to SES [35]. Therefore, not only choosing DES instead of BMS is important, but selecting the correct and most suitable types of DES should also be considered.
Even though BMS were widely used before, they are not commonly preferable to DES. However, even if DES are widely used in this new era, advance research shows the development of carbo and bioresorbable stents to overcome some of the drawbacks of DES. Only future studies can show these differences.
Limitations
This study also has several limitations. First of all, due to the very small number of patients with ITDM used in this study, this analysis may not generate the required or expected results. Secondly, several trials did not report all-cause mortality among their clinical outcomes. Those studies included: STONE2004, STONE2005 and TAXUS IV and VI. In those studies, cardiac death was considered. Moreover, when comparing DES with BMS during a 9 months follow up period, one study had a follow up period of 8 months and has been included in the comparison. Also, another study had a follow up period of 2 years and because of a limited number of studies with a follow up above one year, we included this study in the one year follow up category for the comparison of outcomes. Trials analyzed for the 9 months follow up included: DIABETES, SIRUS, STONE2004, STONE2005, SCORPIUS and TAXUS VI whereas trials analyzed for the one year follow up included: HORIZONS-AMI, PASEO, PRODIGRY, TAXUS IV and SCORPIUS.
Conclusion
Compared to BMS, DES were associated with a significantly lower rate of repeated revascularization, without any increase in MACEs or mortality in these patients with ITDM. However, due to the very small population size, further research with a larger number of randomized patients and with a longer follow up period are required to generate a more reliable result.
Supporting Information
(DOCX)
(DOC)
(DOC)
Acknowledgments
This work was supported by Health self-raising foundation of Guangxi (No. Z2015528). No writing assistance was required for this research.
Abbreviations
- T2DM
type 2 diabetes mellitus
- ITDM
insulin-treated diabetes mellitus
- DES
drug eluting stents
- BMS
bare metal stents
- MACEs
major adverse cardiac events
- PCI
percutaneous coronary intervention
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Health self-raising foundation of Guangxi (No. Z2015528).
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 2.Kugelmass AD, Cohen DJ, Houser F, Mack M, Simon AW, Battaglia SL, et al. The influence of diabetes mellitus on the practice and outcomes of percutaneous coronary intervention in thecommunity: a report from the HCA database. J Invasive Cardiol. 2003; 15(10):568–74. [PubMed] [Google Scholar]
- 3.Akin I, Bufe A, Schneider S, Reinecke H, Eckardt L, Richardt G, et al. Clinical outcomes in diabetic and non-diabetic patients with drug-eluting stents: results from the first phase of the prospective multicenter German DES.DE registry. Clin Res Cardiol. 2010; 99(6):393–400. 10.1007/s00392-010-0136-8 [DOI] [PubMed] [Google Scholar]
- 4.Iijima R, Byrne RA, Dibra A, Ndrepepa G, Spaulding C, Laarman GJ, et al. Drug-eluting stents versus bare-metal stents in diabetic patients with ST-segment elevation acute myocardial infarction: a pooled analysis of individual patient data from seven randomized trials. Rev Esp Cardiol. 2009; 62(4):354–64. [DOI] [PubMed] [Google Scholar]
- 5.Bundhun PK, Li N, Chen MH. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol. 2015; 14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JPT, Altman DG. Assessing risk of bias in included studies In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Wiley, 2008:187–241. [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcareinterventions: explanation and elaboration. BMJ. 2009; 339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgart D, Klauss V, Baer F, Hartmann F, Drexler H, Motz W, et al. ; SCORPIUS Study Investigators. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J Am Coll Cardiol. 2007; 50(17):1627–34. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo E, De Luca G, Sauro R, Varricchio A, Capasso M, Lanzillo T, et al. The PASEO (PaclitAxel or Sirolimus-Eluting Stent Versus Bare Metal Stent in Primary Angioplasty) RandomizedTrial. JACC Cardiovasc Interv. 2009; 2(6):515–23. [DOI] [PubMed] [Google Scholar]
- 10.Sabaté M, Jiménez-Quevedo P, Angiolillo DJ, Gómez-Hospital JA, Alfonso F, Hernández-Antolín R, et al. ; DIABETES Investigators. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronaryrevascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005; 112(14):2175–83. [DOI] [PubMed] [Google Scholar]
- 11.Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, et al. ; HORIZONS-AMI Trial Investigators. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009; 360(19):1946–59. 10.1056/NEJMoa0810116 [DOI] [PubMed] [Google Scholar]
- 12.Valgimigli M, Tebaldi M, Borghesi M, Vranckx P, Campo G, Tumscitz C, et al. ; PRODIGY Investigators. Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comerpatients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY). JACC Cardiovasc Interv. 2014; 7(1):20–8. 10.1016/j.jcin.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Dawkins KD, Grube E, Guagliumi G, Banning AP, Zmudka K, Colombo A, et al. ; TAXUS VI Investigators. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005; 112(21):3306–13. [DOI] [PubMed] [Google Scholar]
- 14.Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, et al. ; TAXUS-IV Investigators. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005; 45(8):1172–9. [DOI] [PubMed] [Google Scholar]
- 15.Moussa I, Leon MB, Baim DS, O'Neill WW, Popma JJ, Buchbinder M, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004; 109(19):2273–8. [DOI] [PubMed] [Google Scholar]
- 16.Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. ; TAXUS V Investigators. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005; 294(10):1215–23. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. ; TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 350(3):221–31. [DOI] [PubMed] [Google Scholar]
- 18.Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ. 2012; 345:e5170 10.1136/bmj.e5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal Ca(2+) homeostasis in diabetes mellitus. Am J Physiol Heart Circ Physiol. 2001; 280:H1480–H1489. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulindependent diabetes mellitus. Circulation. 1993; 88: 2510–2516. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997; 100:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirtane AJ, Ellis SG, Dawkins KD, Colombo A, Grube E, Popma JJ, et al. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008; 51:708–715. [DOI] [PubMed] [Google Scholar]
- 23.Patti G, Nusca A, Di Sciascio G. Meta-analysis comparison (nine trials) of outcomes with drug eluting stents versus bare metal stents in patientswith diabetes mellitus. Am J Cardiol. 2008. November 15;102(10):1328–34. 10.1016/j.amjcard.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 24.Rahel BM, Laarman GJ, Kelder JC, Ten Berg JM, Suttorp MJ. Three-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomizedcomparison of bare-metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (Primary Stenting of Totally Occluded Native Coronary Arteries [PRISON] II study). Am Heart J. 2009; 157(1):149–55. 10.1016/j.ahj.2008.08.025 [DOI] [PubMed] [Google Scholar]
- 25.Suttorp MJ, Laarman GJ, Rahel BM, Kelder JC, Bosschaert MA, Kiemeneij F, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006; 114(9):921–8. [DOI] [PubMed] [Google Scholar]
- 26.Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009; 119(25):3198–206. 10.1161/CIRCULATIONAHA.108.826479 [DOI] [PubMed] [Google Scholar]
- 27.Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007; 356(10):1030–9. [DOI] [PubMed] [Google Scholar]
- 28.Mauri L, Massaro JM, Jiang S, Meredith I, Wijns W, Fajadet J, et al. Long-term clinical outcomes with zotarolimus-eluting versus bare-metal coronary stents. JACC Cardiovasc Interv. 2010; 3(12):1240–9. [DOI] [PubMed] [Google Scholar]
- 29.Caixeta A, Leon MB, Lansky AJ, Nikolsky E, Aoki J, Moses JW, et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J Am Coll Cardiol. 2009; 54(10):894–902. [DOI] [PubMed] [Google Scholar]
- 30.Uthamalingam S, Ahmado I, Selvaraj V, Dewey R, Flynn J. Long term outcomes in octogenarians undergoing percutaneous coronary intervention: comparison of baremetal versus drug eluting stent. Int J Cardiol. 2015; 179:385–9. 10.1016/j.ijcard.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 31.Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. 2008; 337:a1331 10.1136/bmj.a1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandya SB, Kim YH, Meyers SN, Davidson CJ, Flaherty JD, Park DW, et al. Drug-eluting versus bare-metal stents in unprotected left main coronary artery stenosis a meta-analysis. JACC Cardiovasc Interv. 2010; 3(6):602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Valgimigli M, et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013; 62(6): 496–504. [DOI] [PubMed] [Google Scholar]
- 34.Otake H, Ako J, Yamasaki M, Tsujino I, Shimohama T, Hasegawa T, et al. Comparison of everolimus- versus paclitaxel-eluting stents implanted in patients with diabetes mellitus as evaluated by three-dimensional intravascular ultrasound analysis. Am J Cardiol. 2010; 106:492–497. 10.1016/j.amjcard.2010.03.059 [DOI] [PubMed] [Google Scholar]
- 35.Kim WJ, Lee SW, Park SW, Kim YH, Yun SC, Lee JY, et al. ; ESSENCE-DIABETES Study Investigators. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE-DIABETES): results from the ESSENCE-DIABETES trial. Circulation. 2011; 124:886–892. 10.1161/CIRCULATIONAHA.110.015453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.