Abstract
Objective
We aimed to assess whether oxidative stress is a predictor of mortality in HIV-infected patients.
Methods
We conducted a nested case-control study in CoRIS, a contemporary, multicentre cohort of HIV-infected patients, antiretroviral-naïve at entry, launched in 2004. Cases were patients who died with available stored plasma samples collected. Two age and sex-matched controls for each case were selected. We measured F2-isoprostanes (F2-IsoPs) and malondialdehyde (MDA) plasma levels in the first blood sample obtained after cohort engagement.
Results
54 cases and 93 controls were included. Median F2-IsoPs and MDA levels were significantly higher in cases than in controls. When adjustment was performed for age, HIV-transmission category, CD4 cell count and HIV viral load at cohort entry, and subclinical inflammation measured with highly-sensitive C-reactive protein (hsCRP), the association of F2-IsoPs with mortality remained significant (adjusted OR per 1 log10 increase, 2.34 [1.23–4.47], P = 0.009). The association of MDA with mortality was attenuated after adjustment: adjusted OR (95% CI) per 1 log10 increase, 2.05 [0.91–4.59], P = 0.080. Median hsCRP was also higher in cases, and it also proved to be an independent predictor of mortality in the adjusted analysis: OR (95% CI) per 1 log10 increase, 1.39 (1.01–1.91), P = 0.043; and OR (95% CI) per 1 log10 increase, 1.46 (1.07–1.99), P = 0.014, respectively, when adjustment included F2-IsoPs and MDA.
Conclusion
Oxidative stress is a predictor of all-cause mortality in HIV-infected patients. For plasma F2-IsoPs, this association is independent of HIV-related factors and subclinical inflammation.
Introduction
HIV infection is characterized by a progressive depletion of CD4+ T-cell populations and a state of chronic inflammation and immune activation [1, 2]. A related mechanism implicated in the pathogenesis of HIV disease and its complications is a pro-oxidative status associated with the infection and with antiretroviral therapy (ART) [1–5]. HIV induces the generation of reactive oxygen species (ROS) through the regulatory protein Tat and the envelope glycoprotein gp120 [3]. HIV-activated macrophages via TNF-α release, and activated polymorphonuclear leukocytes, also contribute to the generation and accumulation of ROS [4]. As a consequence, there is a deficiency in the antioxidant capacity of the organism, due in part to excessive consumption of antioxidant molecules in order to protect cells against ROS-induced damage [5], which contributes to further enhance the pro-oxidative status.
Numerous in vitro studies have linked oxidative stress with many aspects of HIV pathogenesis, including stimulation of HIV replication, numerical and functional impairment of CD4+ T cells, altered immune response, and toxicity of antiretrovirals [6–9]. It has also shown to play a central role in certain HIV-associated diseases, like HIV dementia [10]. Besides the HIV-related effects, oxidative stress has been associated with aging and with the development of several chronic diseases [11].
Increased oxidative stress biomarkers have been documented in HIV-infected and in AIDS patients compared to HIV-uninfected controls [5], and in patients receiving ART, with most studies being conducted in the era prior to currently recommended antiretroviral regimens [6, 9]. Despite the theoretical etiopathogenic role of oxidative stress in HIV disease, evidence from clinical studies is sparse. Oxidative stress biomarkers were found to be increased in patients with lipodystrophy and symptomatic hyperlactatemia in two cross sectional studies [12, 13], and to be associated with traditional and non-traditional cardiovascular risk factors [14], but did not predict peripheral neuropathy development in a longitudinal study of patients starting ART [15]. In addition, limited information is available about the association of oxidative stress with mortality in HIV patients.
F2-isoprostanes (F2-IsoPs) and malondialdehyde (MDA) are free radical-induced peroxidation products. Measurement of F2-IsoPs constitutes the most reliable approach to assess oxidative stress status in vivo [16]. MDA is also widely used as indicator of cellular injury [17]. We aimed to assess the role of plasma levels of F2-IsoPs and MDA as predictors of mortality in a contemporary cohort of HIV-infected patients.
Methods
Design, setting and study subjects
We conducted a nested case-control study in the ongoing open cohort of adults with HIV infection of the Spanish AIDS Research Network (CoRIS). This is a prospective, multicentre cohort of adult subjects with confirmed HIV infection, and naïve to ART at study entry. The cohort is linked to a centralized BioBank, where patients’ blood samples are processed, cryopreserved and stored. Participating centres are encouraged to obtain a first blood sample at engagement in the cohort, preferentially before starting ART, and follow-up samples preferentially annually, or at least biannually, thereafter. The BioBank has obtained the UNE-EN-ISO 9001:2008 Systems of Quality Management Requirements. Approval from each hospital’s Ethics Committee, and written informed consents from the patients, including the specific consent for the BioBank were obtained. Detailed description of CoRIS and the BioBank have been previously reported [18, 19].
Eligible subjects were all patients with available blood samples at the BioBank from cohort launching date (January 01, 2004) to administrative censoring date (October 31, 2010). Cases were all patients who died during the study period. For each case, two age (± 5 years) and sex individually-matched controls among those alive during the study period were randomly selected to increase the study efficiency. Due to insufficient plasma samples in selected controls, 15 of the cases could only be matched to one control each.
Date and causes of death were reported by the investigators to the coordinating center. Death due to an AIDS-defining event was defined as death attributable to a category C disease listed by the CDCs [20]. Death due to a non AIDS event was classified according to a revised version of the ‘Coding Death in HIV’ (CoDe) classification system [21].
The proportion of losses to follow-up in the cohort, defined as no information provided during the last year and no evidence of patients’ death, was below 20% [18].
Variables, data sources and measurements
Blood samples were kindly provided by the BioBank. The first patients’ blood samples available after engagement in care were analysed. Malondialdehyde (MDA) was measured in plasma with a commercial high performance liquid chromatography (HPLC) kit (CHROMSYSTEMS, Gräfelfing/Germany). Plasma levels of 8-isoprostane were measured with a commercial EIA kit (Cayman Chemycal, Michigan 48108, USA). Both biomarkers have shown to be stable at -80°C for 6 months [22, 23]. Highly-sensitive C-reactive protein (hsCRP) was measured with a chemiluminescent immunometric assay (Immulite 2000, Siemens).
Statistical analyses
Statistical analyses of the data were performed in R, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org/). Median values were compared with the Mann-Whitney or Wilcoxon tests, where appropriate, and chi-square was used to compare proportions. F2-IsoPs, MDA and hsCRP biomarkers values were logarithmically transformed. Conditional logistic regression analysis incorporating the case-control matching factors was used to study the associations of baseline values of F2-IsoPs and MDA with all-cause mortality. Effects are quantified in terms of the odds ratio (OR) per 1 log10 change for each biomarker. Adjusted analyses controlled for age, injection drug use (IDU) versus other HIV-transmission categories, CD4 cell count and HIV-RNA at cohort entry, and log10 hsCRP.
Results
Fifty four patients who died during the study period and 93 controls with available stored serum samples were identified. The causes of death were AIDS conditions (49.1%), non-AIDS events (38.2%), and unknown (12.7%). Most patients (91.9%) were male, and median (interquartile range, IQR) age at cohort entry was 46.7 (40.1–51.1) years. Other baseline characteristics of cases and controls are shown in Table 1.
Table 1. Baseline characteristics of the patients.
| Variable | All | Cases | Controls | p value¶ | |||
|---|---|---|---|---|---|---|---|
| Patients, no. | 147 | 54 | 93 | - | |||
| Female, no. (%) | 12 | (8.1) | 5 | (9.2) | 7 | (7.5) | 0.955 |
| Age at cohort entry, median years (IQR) | 46.7 | (40.1–51.1) | 47.8 | (42.1–52.4) | 46.1 | (40.0–51.0) | 0.377 |
| HIV transmission groups, no. (%) | 0.435 | ||||||
| IDU | 24 | (16.3) | 11 | (20.4) | 13 | (14.0) | |
| Non-IDU | 123 | (83.7) | 43 | (79.6) | 80 | (86.0) | |
| Education level*, no. (%) | 0.005 | ||||||
| Low | 68 | (46.2) | 32 | (59.2) | 36 | (38.7) | |
| Medium | 33 | (22.4) | 7 | (12.9) | 26 | (27.9) | |
| High | 30 | (20.4) | 6 | (11.1) | 24 | (25.8) | |
| Unknown | 16 | (10.8) | 9 | (16.6) | 7 | (7.5) | |
| Country of origin, no. (%) | 0.122 | ||||||
| Spain | 143 | (97.3) | 54 | (100) | 89 | (95.7) | |
| Other | 4# | (2.7) | 0 | (0) | 4 | (4.3) | |
| AIDS diagnosis at cohort entry, no. (%) | 21 | (14.2) | 13 | (24.0) | 8 | (8.6) | 0.012 |
| CD4 (cells/μL) at cohort entry$, no. (IQR) | 252 | (69–475) | 86 | (29–247) | 360 | (160–555) | 0.001 |
| Plasma HIV viral load (log10, copies/mL) at cohort entry$, median (IQR) | 4.43 | (3.56–5.25) | 4.77 | (2.73–5.37) | 4.39 | (3.68–5.16) | 0.168 |
| Patients with virological suppression&, no. (%) | 28 | (19.0) | 12 | (22.2) | 16 | (17.2) | 0.596 |
| Patients on treatment, no. (%), | 34 | (23.1) | 18 | (33.3) | 16 | (17.2) | 0.042 |
| Hepatitis C virus coinfection, no. (%) | 30 | (20.4) | 15 | (27.7) | 15 | (16.1) | 0.028 |
| Follow-up¥, median years (IQR) | 2.1 | (0.70–4.48) | 0.79 | (0.25–2.39) | 2.98 | (1.43–5.07) | 0.001 |
IQR, interquartile range; IDU, injection drug users.
¶p value between cases and control groups: Wilcoxon or Chi-squared tests were used where appropriate.
* Education level definition was based on the level of education completed at cohort entry, and subjects were classified into three levels: low, individuals with no education or with primary education; medium, individuals who completed secondary education; and high, individuals who completed university education.
$Median (IQR) difference of days between cohort inclusion to CD4/viral load measurements was 0 (0–5) days
&Virological suppression was defined as an HIV RNA < 200 copies/ml in the nearest determination to the biomarkers measurement.
#The four patients were born in Latin America.
¥, Years from cohort inclusion to which happened first: death, lost of follow-up or administrative censoring
Biomarkers of oxidative stress and subclinical inflammation
Median (interquartile range, [IQR]) F2-IsoPs and MDA levels are shown in Table 1. The majority of patients were ART naive when the first blood sample was collected, although 23% of patients had initiated ART, and 19% patients were virologically suppressed (HIV RNA < 200 copies/ml) at the time of the first available sample at the BioBank. Median (IQR) number of days from cohort enrollment to first blood sample used in the biomarkers determination was 23 (3–166) days; 22 (5.5–159) days for cases and 23 (2.5–179) days for controls, P = 0.082.
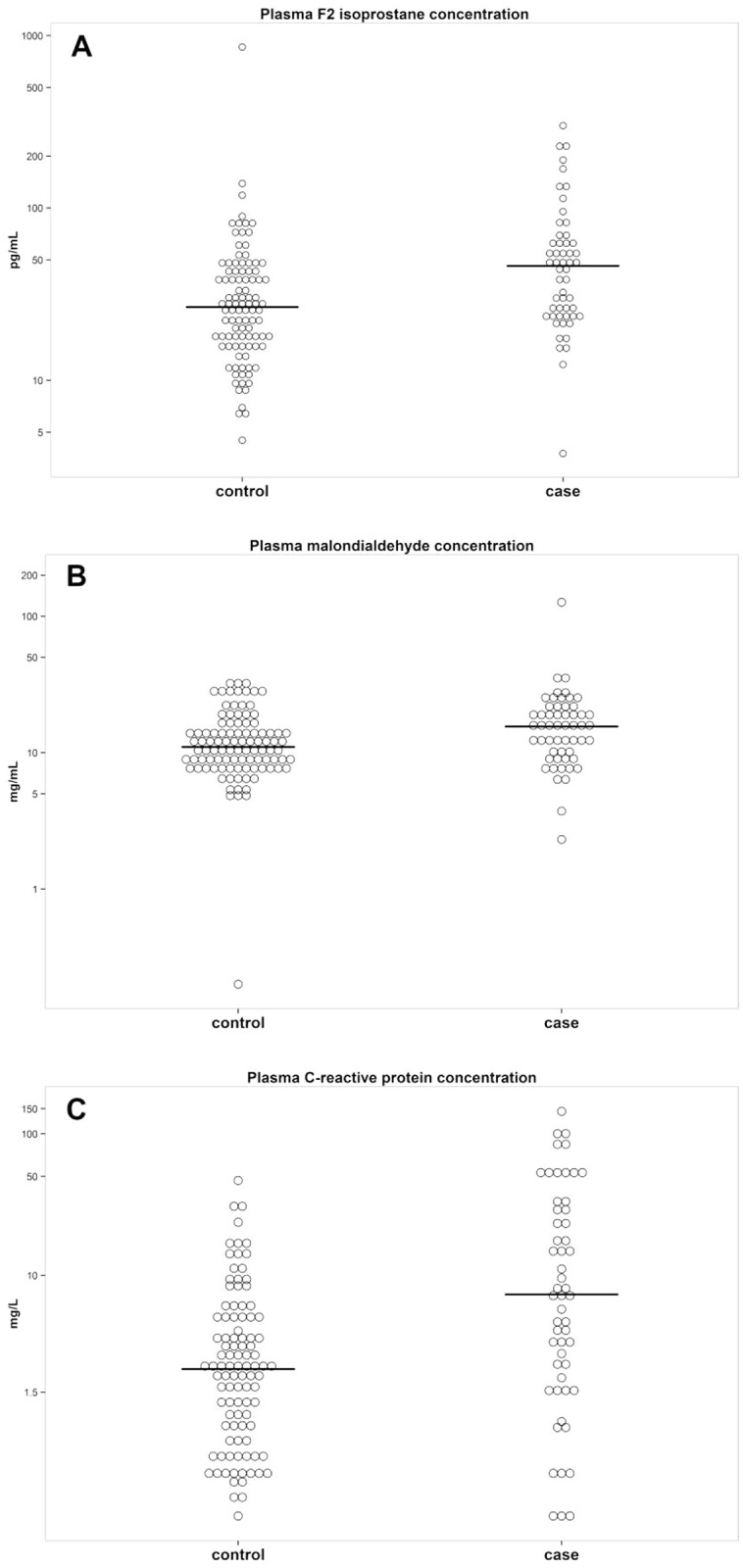
Median (IQR) levels of F2-IsoPs and MDA were higher in patients who died as compared to their matched controls: 46.20 (24.06, 64.68) pg/mL vs 26.64 (17.32–42.40) pg/mL, respectively, for F2-IsoPs, P = 0.001; and 15.56 (9.84, 20.49) mg/mL vs 11.01 (8.16, 14.41) mg/mL, respectively, for MDA, P = 0.008 (Table 1, Fig 1).
Fig 1. Plasma levels of F2-isoprostanes, malondialdehyde and C-reactive protein in cases and controls.
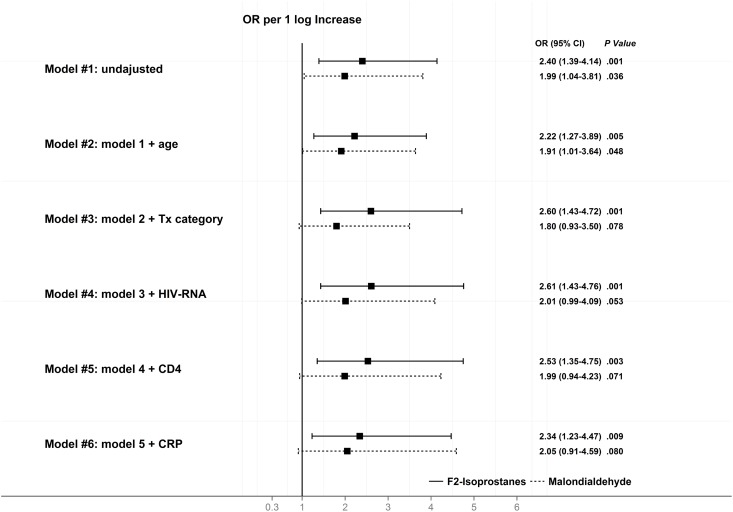
The odds ratio (OR) for death per 1 logarithm increase in the biomarkers levels in the unadjusted and adjusted analyses are shown in Fig 2.
Fig 2. Unadjusted and adjusted by conditional logistic regression odds ratios for death for plasma F2-isoprostanes and malondialdehyde levels.
When adjustment was performed for age, HIV transmission category (IDU versus non-IDU), CD4 cell count and HIV-RNA at cohort entry, the association of F2-IsoPs with mortality remained significant (adjusted OR [95% CI] per 1 log10 increase for F2-IsoPs level was 2.53 [1.35–4.75], P = 0.003), and there was a mild attenuation of the relationship of mortality with MDA, with a close to significant association (adjusted OR [95% CI] per 1 log10 increase for MDA was 1.99 [0.94–4.23], P = 0.071). The model was not adjusted for hepatitis C virus, because of collinearity with IDU. Adding hsCRP to the adjusted model did not alter significantly the relationship of the biomarkers with mortality: adjusted OR (95% CI) per 1 log10 increase for F2-IsoPs level was 2.34 (1.23–4.47), P = 0.009; adjusted OR (95% CI) per 1 log10 increase for MDA was 2.05 (0.91–4.59), P = 0.080.
Median levels of hsCRP were also higher in cases than in controls (Fig 1). When adjustment was performed for age, HIV transmission category, CD4 cell count and HIV-RNA at cohort entry, and the oxidative stress biomarkers levels, hsCRP showed to be an independent predictor of mortality: OR (95% CI) per 1 log10 increase, 1.39 (1.01–1.91), P = 0.043, and OR (95% CI) per 1 log10 increase, 1.46 (1.07–1.99), P = 0.014, respectively, when adjustment included F2-IsoPs and MDA.
Discussion
The oxidative stress biomarkers F2-IsoPs and MDA predict all-cause mortality in HIV-infected patients. For F2-IsoPs, this association is independent of the HIV transmission category, CD4 cell count, HIV viral load, and subclinical inflammation measured with hsCRP.
This is, as far as we know, the first cohort study to show an independent association of oxidative stress with death in HIV-infected patients. Our results indicate that oxidative stress constitutes an additional predictor of mortality, independent of established HIV-associated predictors such as CD4 cell count and HIV viral load, and also of inflammation. Likewise, the association was independent of the HIV transmission group. Injection drug use has been linked with increased all-cause mortality in the HIV population, including AIDS and non-AIDS events [24], and with increased oxidative stress in animal models and in clinical studies in HIV patients [25]. Adjusting for IDU did not alter the relationship of oxidative stress with mortality in our cohort.
Oxidative stress has been implicated in the pathogenesis of HIV disease, and it’s considered to play an important role in the progression from the asymptomatic stage to the development of AIDS [26]. Reactive oxygen species activate the NF-κ B transcription factor, that induces the expression and replication of HIV in human T cells [27]. NF-κ B also acts as a transcription factor for many inflammatory cytokines, like TNF-α, which further activates HIV replication [28]. Oxidative stress has shown to induce as well an abnormal immune response through functional impairment of T cells and DNA damage and apoptosis of CD4+ T lymphocytes, leading to CD4+ cell depletion [1, 8]. AIDS was a frequent cause of mortality in our cohort, which supports the relationship of oxidative stress with advanced disease and with disease progression. Noteworthy, an important proportion of these patients died relatively soon after cohort entry, probably representing delayed diagnoses. Apart from AIDS conditions, a high proportion of patients died as a consequence of non-AIDS events, as we had previously stated [29]. Oxidative stress has been implicated in cellular senescence and aging, and in the development of several chronic diseases including cancer, non-alcoholic liver disease, neurodegenerative disorders, or cardiovascular disease, among others [10, 30–32]. In animal studies, increased oxidative stress has been linked with shorter life expectancy [33]. However, data about the relationship of oxidative stress biomarkers with mortality in humans are limited. Recently, an association has been described with all-cause mortality in HIV-uninfected older adults [34]. Studies on specific oxidative biomarkers are limited to circumscribed clinical scenarios [35, 36].
We found that the relationship of oxidative stress biomarkers with mortality was independent of the HIV viral load at the time of engagement to care in the cohort. Because the proportion of patients under ART at study baseline was low, we could not separately evaluate the role of oxidative stress as a mortality predictor in virologically-suppressed patients. Available information to date regarding the influence of ART on oxidative stress is contradictory. While an improvement has been described with ART [8], there are a high number of studies linking ART with the induction of oxidative stress [6, 9]. Most of the main antiretroviral families, and even particular antiretrovirals, have been implicate; however, clinical studies addressing this unfavourable association generally included older ART regimens, frequently based on thymidine analogues, which might have contributed through mitochondrial toxicity to a pro-oxidizing status. The influence of currently used antiretroviral regimens on the oxidative balance remains to be defined. Alternative measures to ART, including supplementation with micronutrients containing antioxidants, have been explored in the HIV population, with benefits shown in morbi-mortality in African pregnant women and children [37]. A large randomized controlled clinical trial is currently being conducted comparing high-dose micronutrient and anti-oxidant supplementation versus recommended daily allowance vitamins to slow HIV immune deficiency progression in ART-naïve people with HIV infection [38].
The association of F2-IsoPs, and more marginally of MDA levels, with mortality was also independent of subclinical inflammation measured with hsCRP levels at study entry. Moreover, our study found that, in addition to the oxidative stress biomarkers, hsCRP is an independent predictor of mortality in HIV-infected patients as well. This supports the pathogenic role of inflammation in the development of complications and fatal outcome of the patients, as previously stated [39, 40]. Our results also suggest that F2-IsoPs, and to a lesser extent MDA levels, may point to a pathogenic pathway acting beyond inflammation that leads to tissue damage and death. Reactive oxygen species have been associated with aging and with lower life span by inducing structural damage on various macromolecules although, more recently, a functional impairment of the redox-regulated signaling mechanisms as a consequence of a pro-oxidizing shift in the cells has been postulated as a more likely hypothesis [41]. In addition, increased oxidative stress has been associated with accelerated telomere shortening [42], a mechanism underlying cellular aging and contributing to mortality. In HIV-infected patients, an inverse relationship has been described between telomere length and progression of immunosuppression [43] or immunological recovery despite a successful virological response [44].
A limitation of the study is the insufficient number of follow-up samples to verify that results were equivalent to those obtained with the first available samples. Another limitation consists on the potential bias introduced by the patients who were lost to follow-up, in whom the vital status is unknown. The limited number of patients precluded us from adjusting for additional relevant factors, including cardiovascular risk factors, and this could result in an over or underestimation of results. By contrast, information about covariates of interest was equally available for cases and controls. Some of the limitations inherent to case-control designs, such as the ascertainment of exposure, have been overcome given the availability of a biobank linked to the cohort. Unfortunately, due to low numbers some of our estimates are imprecise but are, nevertheless, extremely consistent. The association of hsCRP with mortality is also consistent with the results found in large cohorts [39, 40].
In conclusion, oxidative stress predicts all-cause mortality in HIV-infected patients. For plasma F2-IsoPs, this association is independent of HIV-related variables and subclinical inflammation. Our results support the pathogenic role of oxidative stress in HIV disease identified in experimental studies, and may suggest additional measures to ART to improve health status of HIV-infected patients.
Acknowledgments
The authors particularly acknowledge the patients in this study for their participation and the HIV Biobank integrated in the RIS and collaborating centers for the generous gifts of clinical samples used in this work. This study would not have been possible without the collaboration of all the patients, medical and nursery staff, and data managers who have taken part in the project.
The authors wish to thank Catalina Robledano for their excellent laboratory support.
Centers and investigators participating in CoRIS, Biobanco
Coordinating committee: Juan Berenguer, Julia del Amo, Federico García, Félix Gutiérrez, Pablo Labarga, Santiago Moreno y María Ángeles Muñoz.
Field work, data Management and analysis: Paz Sobrino Vegas, Victoria Hernando Sebastián, Belén Alejos Ferreras, Débora Álvarez, Susana Monge, Inmaculada Jarrín, Adela Castelló.
BioBanco: M Ángeles Muñoz-Fernández, Isabel García-Merino, Coral Gómez Rico, Jorge Gallego de la Fuente y Almudena García Torre.
Participating centers:
Hospital General Universitario de Alicante (Alicante): Joaquín Portilla Sogorb, Esperanza Merino de Lucas, Sergio Reus Bañuls, Vicente Boix Martínez, Livia Giner Oncina, Carmen Gadea Pastor, Irene Portilla Tamarit, Patricia Arcaina Toledo.
Hospital Universitario de Canarias (Santa Cruz de Tenerife): Juan Luis Gómez Sirvent, Patricia Rodríguez Fortúnez, María Remedios Alemán Valls, María del Mar Alonso Socas, Ana María López Lirola, María Inmaculada Hernández Hernández, Felicitas Díaz-Flores.
Hospital Carlos III (Madrid): Vicente Soriano, Pablo Labarga, Pablo Barreiro, Pablo Rivas, Francisco Blanco, Luz Martín Carbonero, Eugenia Vispo, Carmen Solera.
Hospital Universitario Central de Asturias (Oviedo): Victor Asensi, Eulalia Valle, José Antonio Cartón
Hospital Clinic (Barcelona): José M. Miró, María López-Dieguez, Christian Manzardo, Laura Zamora, Iñaki Pérez, Mª Teresa García, Carmen Ligero, José Luis Blanco, Felipe García-Alcaide, Esteban Martínez, Josep Mallolas, José M. Gatell
Hospital Doce de Octubre (Madrid): Rafael Rubio, Federico Pulido, Silvana Fiorante, Jara Llenas, Violeta Rodríguez, Mariano Matarranz.
Hospital Donostia (San Sebastián): José Antonio Iribarren, Julio Arrizabalaga, María José Aramburu, Xabier Camino, Francisco Rodríguez-Arrondo, Miguel Ángel von Wichmann, Lidia Pascual Tomé, Miguel Ángel Goenaga, Mª Jesús Bustinduy, Harkaitz Azkune Galparsoro
Hospital General Universitario de Elche (Elche): Félix Gutiérrez, Mar Masiá, José Manuel Ramos, Sergio Padilla, Andrés Navarro, Fernando Montolio, Yolanda Peral, Catalina Robledano, Joan Gregori
Hospital Germans Trías i Pujol (Badalona): Bonaventura Clotet, Cristina Tural, Lidia Ruiz, Cristina Miranda, Roberto Muga, Jordi Tor, Arantza Sanvisens
Hospital General Universitario Gregorio Marañón (Madrid): Juan Berenguer, Juan Carlos López Bernaldo de Quirós, Pilar Miralles, Jaime Cosín Ochaíta, Isabel Gutiérrez Cuellar, Margarita Ramírez Schacke, Belén Padilla Ortega, Paloma Gijón Vidaurreta, Ana Carrero Gras, Teresa Aldamiz-Echevarría Lois y Francisco Tejerina Picado.
Hospital Universitari de Tarragona Joan XXIII, IISPV, Universitat Rovira i Virgili (Tarragona): Francesc Vidal, Joaquín Peraire, Consuelo Viladés, Sergio Veloso, Montserrat Vargas, Miguel López-Dupla, Montserrat Olona, Alba Aguilar, Joan Josep Sirvent, Verónica Alba, Olga Calavia.
Hospital Universitario La Fe (Valencia): José López Aldeguer, Marino Blanes Juliá, José Lacruz Rodrigo, Miguel Salavert, Marta Montero, Eva Calabuig, Sandra Cuéllar.
Hospital Universitário La Paz (Madrid): Juan González García, Ignacio Bernardino de la Serna, José María Peña Sánchez de Rivera, José Ramón Arribas López, María Luisa Montes Ramírez, José Francisco Pascual Pareja, Blanca Arribas, Juan Miguel Castro, Fco Javier Zamora Vargas, Ignacio Pérez Valero, Miriam Estebanez, Raphael Mohr y Francisco Arnalich Fernández.
Hospital de la Princesa (Madrid): Ignacio de los Santos, Jesús Sanz Sanz, Johana Rodríguez, Ana Salas Aparicio, Cristina Sarriá Cepeda.
Hospital San Pedro-CIBIR (Logroño): José Antonio Oteo, José Ramón Blanco, Valvanera Ibarra, Luis Metola, Mercedes Sanz, Laura Pérez-Martínez
Hospital San Pedro II (Logroño): Javier Pinilla Moraza
Hospital de Navarra (Pamplona): Julio Sola Boneta, Javier Uriz, Jesús Castiello, Jesús Reparaz, María Jesús Arraiza, Carmen Irigoyen, David Mozas,
Hospital Ramón y Cajal (Madrid): Santiago Moreno, José Luis Casado, Fernando Dronda, Ana Moreno, María Jesús Pérez Elías, Dolores López, Carolina Gutiérrez, Beatriz Hernández, María Pumares, Paloma Martí.
Hospital Reina Sofía (Murcia): Alfredo Cano Sánchez, Enrique Bernal Morell, Ángeles Muñoz Pérez
Hospital San Cecilio (Granada): Federico García García, José Hernández Quero, Alejandro Peña Monje, Leopoldo Muñoz Medina, Jorge Parra Ruiz.
Centro Sanitario Sandoval (Madrid): Jorge Del Romero Guerrero, Carmen Rodríguez Martín, Teresa Puerta López, Juan Carlos Carrió Montiel, Cristina González, Mar Vera.
Hospital Universitario Santiago de Compostela (Santiago de Compostela): Antonio Antela, Arturo Prieto, Elena Losada
Hospital Son Espases (Palma de Mallorca): Melchor Riera, Javier Murillas, Maria Peñaranda, Maria Leyes, Mª Angels Ribas, Antoni Campins, Concepcion Villalonga, Carmen Vidal.
Hospital Universitario de Valme (Sevilla): Juan Antonio Pineda, Eva Recio Sánchez, Fernando Lozano de León, Juan Macías, José del Valle, Jesús Gómez-Mateos, Rosario Mata.
Hospital Virgen de la Victoria (Málaga): Jesús Santos González, Manuel Márquez Solero, Isabel Viciana Ramos, Rosario Palacios Muñoz
Hospital Universitario Virgen del Rocío (Sevilla): Pompeyo Viciana, Manuel Leal, Luis Fernando López-Cortés, Mónica Trastoy.
Data Availability
Due to ethical restrictions, minimal data used in the study are made available upon request from the corresponding author.
Funding Statement
This work has been (partially) funded by the RD12/0017/0023 project as part of the Plan Nacional R + D + I and cofinanced by ISCIII- Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER), FIS (PI08/893), FIS (PI13/02256), FISABIO UGP-14-197, and Contrato de Intensificación de la Actividad Investigadora INT 14/00207. The HIV Biobank, integrated in the RIS, is also supported by the ISCIII (RD06/0006/0035) and FIPSE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64 10.1111/imr.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241 [DOI] [PubMed] [Google Scholar]
- 3.Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free RadicBiol Med. 2010;48:1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson JM. Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol. 2009;131:465–469 10.1007/s00418-009-0565-5 [DOI] [PubMed] [Google Scholar]
- 5.Suresh DR, Annam V, Pratibha K, Prasad BV. Total antioxidant capacity—a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci. 2009;16:61 10.1186/1423-0127-16-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulgan T, Morrow J, D'Aquila RT, Raffanti S, Morgan M, Rebeiro P, et al. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1711–1717. [DOI] [PubMed] [Google Scholar]
- 7.Gendron K, Ferbeyre G, Heveker N, Brakier-Gingras L. The activity of the HIV-1 IRES is stimulated by oxidative stress and controlled by a negative regulatory element. Nucleic Acids Res. 2011;39:902–912 10.1093/nar/gkq885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aukrust P, Luna L, Ueland T, Johansen RF, Müller F, Frøland SS, et al. Impaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patients. Blood. 2005;105:4730–4735. [DOI] [PubMed] [Google Scholar]
- 9.Manda KR, Banerjee A, Banks WA, Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic Biol Med. 2011;50:801–810 10.1016/j.freeradbiomed.2010.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, et al. Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal. 2006;8:2089–2100. [DOI] [PubMed] [Google Scholar]
- 11.Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69:2999–3013 10.1007/s00018-012-0962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McComsey GA, Morrow JD. Oxidative markers are significantly increased in lipoatrophy but not in sustained asymptomatic hyperlactatemia. J Acquir Immune Defic Syndr. 2003;34:45–49 [DOI] [PubMed] [Google Scholar]
- 13.Vassimon HS, Deminice R, Machado AA, Monteiro JP, Jordao AA. The association of lipodystrophy and oxidative stress biomarkers in HIV-infected men. Curr HIV Res. 2010;8:364–369 [DOI] [PubMed] [Google Scholar]
- 14.Masiá M, Padilla S, Bernal E, Almenar MV, Molina J, Hernández I, et al. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: a prospective cross-sectional study in HIV-infected patients. Clin Ther. 2007;29:1448–1455 [DOI] [PubMed] [Google Scholar]
- 15.Hulgan T, Hughes M, Sun X, Smeaton LM, Terry E, Robbins GK, et al. Oxidant stress and peripheral neuropathy during antiretroviral therapy: an AIDS clinical trials group study. J Acquir Immune Defic Syndr. 2006;42:450–454. [DOI] [PubMed] [Google Scholar]
- 16.Montuschi P, Barnes P, Roberts LJ. Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–717 [DOI] [PubMed] [Google Scholar]
- 17.Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal. 2007;43:619–624 [DOI] [PubMed] [Google Scholar]
- 18.Sobrino-Vegas P, Gutiérrez F, Berenguer J, Labarga P, García F, Alejos-Ferreras B, et al. The Cohort of the Spanish HIV Research Network (CoRIS) and its associated biobank; organizational issues, main findings and losses to follow-up. Enferm Infecc Microbiol Clin. 2011;29:645–653 10.1016/j.eimc.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 19.García-Merino I, De las Cuevas N, Jiménez JL, Gallego J, Gómez C, Prieto C, et al. The Spanish HIV BioBank: a model of cooperative HIV research. Retrovirology. 2009;6:27 10.1186/1742-4690-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 21.Hernando V, Sobrino-Vegas P, Burriel MC, Berenguer J, Navarro G, Santos I, et al. Differences in the causes of death of HIV-positive subjects in a cohort study by data sources and coding algorithms. AIDS 2012; 26:1829–1834. [DOI] [PubMed] [Google Scholar]
- 22.Chehne F, Oguogho A, Lupattelli G, Budinsky AC, Palumbo B, Sinzinger H. Increase of isoprostane 8-epi-PGF(2alpha)after restarting smoking. Prostaglandins Leukot Essent Fatty Acids. 2001; 64:307–310 [DOI] [PubMed] [Google Scholar]
- 23.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clinical Chem. 1997; 43:1209–1214 [PubMed] [Google Scholar]
- 24.Ingle SM, May MT, Gill MJ, Mugavero MJ, Lewden C, Abgrall S, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–297 10.1093/cid/ciu261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang AM, Smit E. Oxidative stress in HIV-1-infected injection drug users. J Acquir Immune Defic Syndr. 2000;25 Suppl 1:S12–18. [DOI] [PubMed] [Google Scholar]
- 26.Sharma B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res. 2014;12:13–21 [DOI] [PubMed] [Google Scholar]
- 27.Pyo CW, Yang YL, Yoo NK, Choi SY. Reactive oxygen species activate HIV long terminal repeat via post-translational control of NF-kappaB. Biochem Biophys Res Commun. 2008;376:180–185 10.1016/j.bbrc.2008.08.114 [DOI] [PubMed] [Google Scholar]
- 28.Herbein G, Varin A, Larbi A, Fortin C, Mahlknecht U, Fulop T, et al. Nef and TNFalpha are coplayers that favorHIV-1 replication in monocytic cells and primary macrophages. Curr HIV Res. 2008;6:117–129 [DOI] [PubMed] [Google Scholar]
- 29.Masiá M, Padilla S, Álvarez D, López JC, Santos I, Soriano V, et al. Risk, predictors, and mortality associated with non-AIDS events in newly diagnosed HIV-infected patients: role of antiretroviral therapy. AIDS. 2013;27:181–189 10.1097/QAD.0b013e32835a1156 [DOI] [PubMed] [Google Scholar]
- 30.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003; 3:276–285. [DOI] [PubMed] [Google Scholar]
- 31.Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989; 320:915–924. [DOI] [PubMed] [Google Scholar]
- 33.Sinha JK, Ghosh S, Swain U, Giridharan NV, Raghunath M. Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging. Neuroscience. 2014;269:256–264 10.1016/j.neuroscience.2014.03.040 [DOI] [PubMed] [Google Scholar]
- 34.Schöttker B, Saum KU, Jansen EH, Boffetta P, Trichopoulou A, Holleczek B, et al. Oxidative Stress Markers and All-Cause Mortality at Older Age: A Population-Based Cohort Study. J Gerontol A Biol Sci Med Sci. 2014; 70; 518–524 10.1093/gerona/glu111 [DOI] [PubMed] [Google Scholar]
- 35.Lorente L, Martín MM, Abreu-González P, Domínguez-Rodriguez A, Labarta L, Díaz C, et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit Care 2013;17:R290 10.1186/cc13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roest M, Voorbij HA, Van der Schouw YT, Peeters PH, Teerlink T, Scheffer PG. High levels of urinary F2-isoprostanes predict cardiovascular mortality in postmenopausal women. J Clin Lipidol. 2008;2:298–303 10.1016/j.jacl.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 37.Irlam JH, Visser MM, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst Rev. 2010;12:CD003650 10.1002/14651858.CD003650.pub3 [DOI] [PubMed] [Google Scholar]
- 38.Balfour L, Spaans JN, Fergusson D, Huff H, Mills EJ, la Porte CJ, et al. Micronutrient deficiency and treatment adherence in a randomized controlled trial of micronutrient supplementation in ART-naïve persons with HIV. PLoS One. 2014;9:e85607 10.1371/journal.pone.0085607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–22 10.1097/QAI.0b013e3181e66216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masiá M, Bernal E, Padilla S, Graells ML, Jarrín I, Almenar MV, et al. The role of C-reactive protein as a marker for cardiovascular risk associated with antiretroviral therapy in HIV-infected patients. Atherosclerosis. 2007;195:167–171 [DOI] [PubMed] [Google Scholar]
- 41.Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–355 10.1016/j.freeradbiomed.2011.10.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawanishi S, Oikawa S. Mechanism of telomere shor tening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–284 [DOI] [PubMed] [Google Scholar]
- 43.Bestilny LJ, Gill MJ, Mody CH, Riabowol KT. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS. 2000; 14:771–780. [DOI] [PubMed] [Google Scholar]
- 44.Blanco JR, Jarrin I, Martinez A, Siles E, Larrayoz IM, Cañuelo A, et al. Shorter Telomere Length Predicts Poorer Immunological Recovery in Virologically Suppressed HIV-1-Infected Patients Treated With Combined Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;68:21–29 10.1097/QAI.0000000000000398 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions, minimal data used in the study are made available upon request from the corresponding author.