Abstract
Meal ingestion is followed by release of numerous hormones from enteroendocrine cells interspersed among the epithelial cells lining the intestine. Recently, the de-orphanization of G protein-coupled receptor (GPCR)-type nutrient receptors, expressed on the apical membranes of enteroendocrine cells, has suggested a plausible mechanism whereby luminal nutrients trigger the release of gut hormones. Activation of nutrient receptors triggers intracellular signaling mechanisms that promote exocytosis of hormone-containing granules into the submucosal space. Hormones released by foregut enteroendocrine cells include the glucagon-like peptides (GLP) affecting glycemic control (GLP-1) and releasing pro-proliferative, hypertrophy-inducing growth factors (GLP-2). The foregut mucosa, being exposed to pulses of concentrated HCl, is protected by a system of defense mechanisms, which includes epithelial bicarbonate and mucus secretion and augmentation of mucosal blood flow. We have reported that luminal co-perfusion of AA with nucleotides in anesthetized rats releases GLP-2 into the portal vein, associated with increased bicarbonate and mucus secretion and mucosal blood flow. The GLP-2 increases bicarbonate secretion via release of vasoactive intestinal peptide (VIP) from myenteric nerves. Luminal bile acids also release gut hormones due to activation of the bile-acid receptor known as G Protein-Coupled Receptor (GPR) 131, G Protein Bile Acid Receptor (GPBAR) 1, or Takeda G Protein-Coupled Receptor (TGR) 5, also expressed on enteroendocrine cells. The GLP are metabolized by dipeptidyl peptidase IV (DPPIV), an enzyme of particular interest to pharmaceutical, because its inhibition increases plasma concentrations of GLP-1 to treat diabetes. We have also reported that DPPIV inhibition enhances the secretory effects of nutrient-evoked GLP-2. Understanding the release mechanism and the metabolic pathways of gut hormones is of potential utility to the formulation of feedstuff additives that, by increasing nutrient absorption due to increased mucosal mass, can increase yields.
Keywords: anion secretion, bicarbonate, duodenum, enteroendocrine cells, glucagon-like peptides, serotonin
INTRODUCTION
All animals have an intestine, even some that do not have brains, and grow up by absorbing necessary elements from the intestinal lumen. The luminal environment of the intestine includes nutrient compounds from the diet. Dietary nutrients when present in the intestinal lumen evoke the release of numerous hormones that affect gut motility, hunger, discomfort, and satiation, all common postprandial responses. We will develop the hypothesis that the intestinal epithelium, which is an interface between the luminal (external) and internal environment, contains specialized cells expressing chemosensors that serve as “sentinels” of the intestinal content.
OUTLOOK
Role of Epithelial Cells in Mucosal Integrity
Epithelial barrier functions are provided by physical (intercellular junction) and chemical (ion and protective peptide/protein secretion) factors. Renewing every 3 to 7 d, the intestinal epithelium is a monolayer constituted of distinct functional cells that differentiate from the stem cells located near the crypt base (van der Flier and Clevers, 2009; Gerbe et al., 2011).
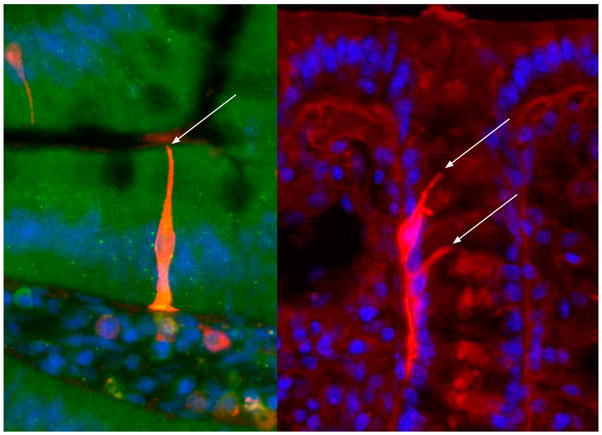
Enteroendocrine cells constitute 1 to 5% of epithelial cells throughout the intestine with most concentrated in the proximal duodenum and rectum in human (Sjölund et al., 1983). At least 20 subpopulations are defined based on their granule contents that release distinct peptides or aromatic amines. The turnover time of enteroendocrine cells is similar to enterocytes and goblet cells in the small intestine (Cheng, 1974) although the turnover time of colonic enteroendocrine cells is much longer (e.g., 23 d in mice (Tsubouchi and Leblond, 1979), suggesting their unique regulatory mechanisms of maturation and migration. The enteroendocrine cells have distinct shapes (Fig. 1); the majority in small intestine has a flask-like shape whereas some in the colon has long basal processes, but almost of their apical membrane reaches lumen in a small area.
Figure 1.
Representative enteroendocrine cell morphology in the rat duodenum (left) and colon (right). Arrows indicate the where the apical membrane of each enteroendocrine cell projects to the lumen. See online version for figure in color.
Numerous chemical receptors are expressed in 5-hydroxytryptophan-containing enterochromaffin (EC) cells isolated from duodenum and EC cell lines (Braun et al., 2007; Kidd et al., 2008). L cells have large granules that containing glucagon-like peptide (GLP)-1, GLP-2, and peptide tyrosine-tyrosine (PYY), and also express several nutrient receptors (Rozengurt et al., 2006; Reimann et al., 2008). These observations suggest the important role of enteroendocrine cells in the gut chemosensing.
Brush cells (also called as tuft or caveolated cells) are first revealed by electron microscopy and distinguished by the unique morphology; they have long rootlet and sparse microvilli. The secretory granules have not been identified in the brush cells, but some immunohistochemical studies, which use cytokeratin-18 or transient receptor potential (TRP) M5 as a brush cell marker, suggest that the brush cells are the source of endogenous opioids and PG in the intestinal epithelia (Bezençon et al., 2008; Kokrashvili et al., 2009; Gerbe et al., 2011). This rare (<1%) population of epithelial cells have been considered as an individual secretory cell linage based on the distinct differentiation markers (Gerbe et al., 2011; Bjerknes et al., 2012). The expression of taste signaling proteins, including gustducin, type 1 taste receptors (T1R), and TRPM5 in the brush cells, is implicated in their chemosensory nature (Hofer et al., 1996; Bezençon et al., 2007; Hass et al., 2010).
CHEMICAL SENSING IN DUODENAL DEFENSE MECHANISM
The duodenum, comprising the first part of the intestine, has specialized acid sensing mechanisms that detect gastric acid, which serves the putative physiological function of killing ingested microbes but also is highly injurious to the foregut mucosa. We and others have already reported that enhancement of duodenal “defense mechanisms” (i.e., physiological functions that prevent acid-induced injury) are timed to coincide with peak acid entry into the duodenal lumen, which occurs after a meal. Postprandial luminal pH is decreased to 2 by antrally propelled gastric acid and rapidly raised to 7 by neutralization in the duodenum. Duodenal defense mechanisms include premucosal, mucosal, and submucosal factors. Membrane carbonic anhydrase facilitates the conversion of luminal acid (H+) and secreted bicarbonate to CO2, which diffuses into the epithelial cells. Intracellular CO2 is converted by cytosolic carbonic anhydrase to H+ and bicarbonate, the latter secreted into the lumen. Thus, bicarbonate secretion and CO2 absorption are equivalent to net H+ absorption into the mucosal epithelial cells. The absorbed H+ acidifies the epithelial cells and then is basolaterally excreted by the Na+/H+ exchanger-1 (NHE1). Subepithelial H+ activates TRPV1 on afferent nerves followed by release of vasoactive mediators, with consequent increase of mucosal blood flow. Increased blood flow supplies bicarbonate to the epithelial cells, enhancing bicarbonate and mucus secretion (Akiba et al., 2009). Thus, highly caustic luminal HCl is converted to the acid equivalent CO2, which can be safely absorbed by the duodenal mucosa, triggering protective responses such as the increase in mucosal blood flow, which delivers bicarbonate and removes acid form the mucosa, and bicarbonate secretion into the lumen, which locally neutralizes HCl.
We have developed a measurement technique for these defense factors, including the rate of mucus secretion (premucosal), in vivo measurement of intracellular pH (pHi; mucosal) and mucosal blood flow (submucosal) simultaneously using in vivo microscopy, and bicarbonate secretion in a perfused duodenal loop (Akiba et al., 1999, 2000; Akiba and Kaunitz, 1999). Combined studies using these techniques have enabled us to investigate the integrated regulation of mucosal defense factors in response to luminal chemicals of dietary substances in rat duodenum.
AMINO ACID SENSING IN DUODENAL BARRIER FUNCTION
L-glutamate (L-glu) is one of predominant free AA in foodstuffs, the proposed main energy source of epithelial cells in small intestine. L-glutamate activates several receptors, such as metabotropic glutamate receptors (mGluR), calcium sensing receptor (CaSR), and umami (proteinaceous) taste receptor (heterodimer of T1R1 and T1R3), that are expressed in the intestinal mucosa (Chattopadhyay et al., 1998; Mace et al., 2007; Margolskee et al., 2007; San Gabriel et al., 2007). We hypothesized that luminal nutrients such as L-Glu may, in addition to luminal acid, enhance foregut defense mechanisms in the postprandial state. Luminal perfusion of L-Glu (10 mM) increased pHi and mucus secretion through PG synthesis and afferent neural pathways in rats (Akiba et al., 2009). These effects were mimicked by mGluR4 agonists and were inhibited by mGluR4 antagonists, indicating that the effects of L-Glu on pHi and mucus secretion are mainly mediated by mGluR4. The lack of effects of D-Glu, L-Asp, L-Ala, or L-Leu on pHi and mucus secretion further suggests the specific function of L-Glu/mGluR4 signaling in the control of mucosal defense factors. On the other hand, luminal perfusion of CaSR agonists such as high Ca2+ (4 mM) solution or spermine (1 mM) decreased pHi but increased mucus secretion, bicarbonate secretion, and blood flow. These responses resemble those to acid perfusion but differ from those to L-Glu perfusion, suggesting that CaSR is unlikely to contribute to the protective effects of luminal L-Glu. Interestingly, although L-Glu alone has no effect, co-perfusion of L-Glu (10 mM) with the purine 5′-inosine monophosphate (IMP; 0.1 mM) increased duodenal bicarbonate secretion (Akiba et al., 2009). The combination of L-Glu/IMP synergistically increased duodenal bicarbonate secretion (Wang et al., 2011). Moreover, L-Asp and L-Leu alone minimally increased the rate of bicarbonate secretion, which was markedly enhanced by IMP co-perfusion (Akiba et al., 2009). These results suggest that synergistic activation of the umami receptor T1R1/T1R3 enhances duodenal mucosal protective factors through increasing the rate of bicarbonate secretion.
Luminal carbohydrate releases gut hormones such as GLP-1, GLP-2, and glucose-dependent insulinotropic polypeptide (GIP) from enteroendocrine cells. Bicarbonate secretion evoked by luminal L-Glu/ IMP was inhibited by the GLP-2 receptor antagonist GLP-2(3–33) whereas the GIP receptor antagonist Pro3GIP or the GLP-1 receptor antagonist Ex-3(9–39) had no effect, supporting the hypothesis that GLP-2 mediates L-Glu/IMP-evoked secretory response (Wang et al., 2011). Furthermore, L-Glu/IMP co-perfusion significantly increased GLP-2 release followed by GLP-1 release but did not alter GIP release (Wang et al., 2011). These data suggest that T1R1/T1R3-induced bicarbonate secretion is mediated by GLP-2. The GLP-2 receptors are expressed on enteric neurons (Guan et al., 2006). The L-Glu/IMP-evoked bicarbonate secretion was inhibited by the vasoactive intestinal peptide (VIP) receptor antagonist VIP6–28 and the NO synthase (NOS) inhibitor L-NAME but not by atropine or de-afferentation by high dose capsaicin. Luminal L-Glu/IMP co-perfusion increased portal venous concentrations of VIP and luminal nitrite and nitrate (NOx) release, both inhibited by GLP-2(3–33). These results are consistent with the hypothesis that submucosal VIP and NOS-positive neurons are activated by local GLP-2 release, independent of capsaicin-sensitive afferent nerves (Wang et al., 2011). Because VIP is a well-established activator of duodenocyte bicarbonate secretion via epithelial VIP receptors, the GLP-2/VIP pathway is consistent with available data.
The T1R1/T1R3 are localized in duodenal L cells in mice and humans (Reimann et al., 2008; Theodorakis et al., 2006), but in rat jejunum T1R1/T1R3 are co-expressed in brush cell-like solitary cells and the apical membrane of enterocytes (Mace et al., 2007). In rat duodenum, the immunoreactivity for T1R1 and T1R3 is detected in solitary epithelial cells that morphologically resemble enteroendocrine cells in duodenal villi (Wang et al., 2011), indicating the segmental heterogeneity in chemosensory function.
In conclusion, GLP-2 is one of important signaling molecules for the regulation of duodenal mucosal defenses in rat duodenum, even though duodenum has relatively fewer L cells than are present in the lower intestine. Duodenal nutrient sensing may serve an important function in the regulation of defense mechanisms and also in the control of glucose metabolism via the release of the incretin GLP-1. Furthermore, the T1R1/T1R3 heterodimer is functionally present in all mammalian genomes available (except giant panda bear), in addition to chicken, cat, and fish although the latter species lack the T1R2 component of the sweet taste receptor (Roura et al., 2008; Shi and Zhang, 2006). A broad distribution across vertebrate species indicates that the savory T1R1/T1R3 signal is an essential and highly conserved mechanism.
BILE ACID SENSING IN DUODENAL BARRIER FUNCTION
Bile acids are also major luminal components in the postprandial duodenum. Because luminal taurocholic acid promptly induces GLP-1 release in the isolated vascularly perfused rat ileum (Dumoulin et al., 1998), a surface bile acid sensor was hypothesized. In addition to the nuclear receptor farnesoid X receptor (FXR), the transmembrane bile acid receptor GPBAR1 or GPR131 was recently identified as a candidate for the bile acid surface sensor. To test the hypothesis that luminal bile acids induce GLP release, we perfused GPR131 ligands into the duodenal lumen, using bicarbonate secretion and hormone release into the portal vein as readouts. Due to the detergent properties of bile acids in physiological concentrations, we used high-affinity GPR131 agonists, betulinic acid (BTA) and a nonsterol type agonist 3-(2-chlorophenyl)-N-(4-chlorophenyl)-N,5-dimethylisoxazole-4-carboxamide (CCDC). When perfused alone, both compounds minimally increased the rate of bicarbonate secretion or GLP-2 release. Nevertheless, both BTA and CCDC significantly enhanced L-Glu/IMP-evoked bicarbonate secretion and GLP-2 release (Inoue et al., 2012). Because GPR131 activation increases intracellular cyclic adenosine monophosphate (cAMP) (Kawamata et al., 2003) and enhances glucose-induced GLP-1 release (Thomas et al., 2009), luminal bile acids may amplify nutrient sensing signals in L cells via a complementary cAMP pathway.
Released GLP-2, GLP-1, GIP, and other bioactive peptides are rapidly degraded by the serine protease dipeptidyl peptidase IV (DPPIV). The DPPIV exists in two forms, an extracellular membrane-bound enzyme and a secreted protein present in increased concentrations in plasma (Lambeir et al., 2003). Intravenous administration of the DPPIV inhibitor NVP728 6-{2-[2-((S)-5-Cyano-pyrazolidin-1-yl)-2-oxo-ethylamino]-ethylamino}-nicotinonitrile enhanced L-Glu/IMP- and GPR131 agonist-evoked bicarbonate secretion, abolished by a GLP-2 receptor antagonist (Inoue et al., 2012). These results indicate that the DPPIV inhibition potentiates the protective effect of luminal L-Glu/IMP and GPR131 agonists via a GLP-2 signaling pathway. Using a fluorogenic DPPIV substrate, DPPIV activity was visualized in situ in frozen sections (Inoue et al., 2012). Strong activity was detected in submucosal interstitial cells, suggesting that released hormones are rapidly degraded in the submucosa. Interestingly, DPPIV activity was also predominant in villus brush border whereas luminal perfusion of NVP728 has no effect on either basal or L-Glu/IMP-evoked duodenal bicarbonate secretion in vivo (Inoue et al., 2012). Because DPPIV has a broad substrate specificity, brush border DPPIV may be an important component of the digestive process.
These studies strongly suggest the protective role of GLP-2 that is released by luminal chemicals corresponding via G protein-coupled receptor (GPCR) activation (Fig. 2) and that DPPIV inhibitor modulates mucosal defense mechanisms in the duodenum.
Figure 2.
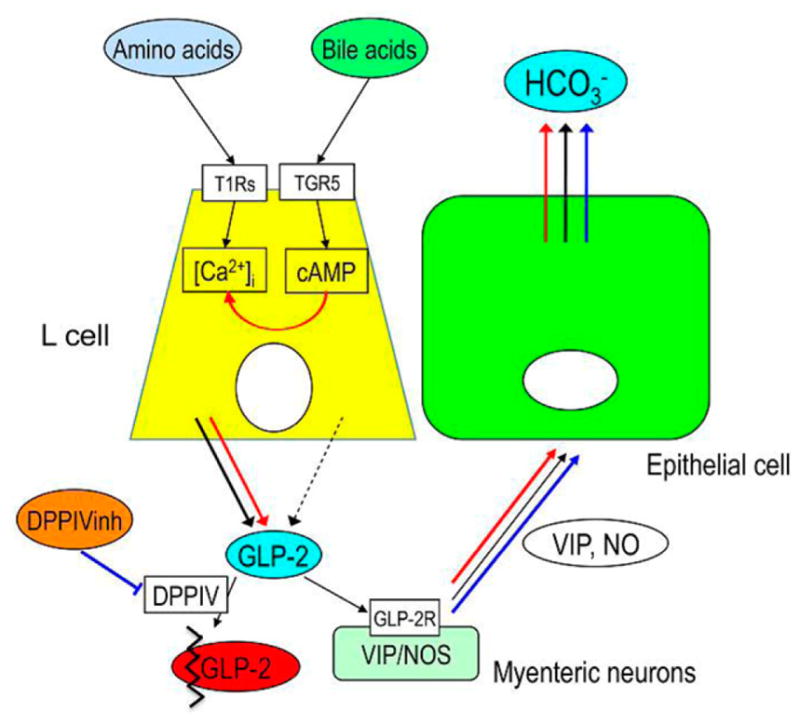
Schema of proposed mechanisms of type 1 taste receptors (T1R)- and the transmembrane bile acid receptor Takeda G Protein-Coupled Receptor (TGR) 5, also referred to as G Protein Bile Acid Receptor (GPBAR)1 and G Protein-Coupled Receptor (GPR)131-mediated glucagon-like peptide (GLP-2) release and bicarbonate secretion. Luminal AA may activate T1R on L cells, releasing GLP-2 via increased intracellular Ca2+ concentration ([Ca2+]i) followed by activation of GLP-2 receptors (GLP-2R) on myenteric neurons containing vasoactive intestinal peptide (VIP) or NO synthase (NOS), increasing VIP and NO release and then increasing bicarbonate (HCO3−) secretion (black arrows). Luminal bile acids may activate TGR5 on L cells, increasing intracellular cyclic adenosine monophosphate (cAMP), but have little effect on GLP-2 release (black dashed arrows). The activation of TGR5 may amplify the effect of amino acid-T1Rs-[Ca2+]i pathway and then enhance GLP-2 release, resulting the enhanced HCO3− secretion (red arrows). Inhibition of dipeptidyl peptidase IV (DPPIV) impairs GLP-2 degradation, enhancing the effect of GLP-2 on HCO3− secretion (blue arrows). Modified and adapted from Inoue et al. (2012). See online version for figure in color.
SUMMARY AND CONCLUSIONS
Luminal chemosensing is an important epithelial function that enables the gut mucosa to continuously sense the luminal milieu. The recent discovery of nutrient-sensing GPCR expressed on enteroendocrine cells that signal the release of hormones has improved understanding of the mechanism of luminal nutrientinduced hormone release. Hormones, such as GLP-2, are important to the regulation of key duodenal defense mechanisms such as bicarbonate secretion, through intermediary release of VIP. Dysfunction of chemosensing system may be implicated in the pathogenesis of foregut mucosal injury due to acid-pepsin secretion. Therefore, understanding of mucosal host defense mechanisms maintained by luminal chemosensory receptors provides potentials for therapeutic targets and prevention of gastrointestinal disorders. Furthermore, understanding of nutrient-induced gut hormone release may be useful to formulate feedstuff additives that can increase yields by increasing nutrient absorption due to increased mucosal mass.
Footnotes
Based on a presentation at the preconference symposium titled “Gut Chemosensing: Integrating nutrition, gut function and metabolism in pigs” preceding the 12th International Symposium on Digestive Physiology of Pigs in Keystone, Colorado, May 29–June 1, 2012, with publication sponsored by Lucta S.A. (Barcelona, Spain), the American Society of Animal Science, and the Journal of Animal Science.
We thank Bea Palileo for her assistance with manuscript preparation. Supported by funding from a Grant-in-Aid for Japan Society for the Promotion of Science Fellow (I. Kaji), a research grant from Ajinomoto, Japan (Y. Akiba), Department of Veterans Affairs Merit Review Award (J.D. Kaunitz), and NIH-NIDDK R01 DK54221 (J.D. Kaunitz).
LITERATURE CITED
- Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G437–G447. doi: 10.1152/ajpgi.2000.279.2.G437. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Kaunitz JD. Regulation of intracellular pH and blood flow in rat duodenal epithelium in vivo. Am J Physiol Gastrointest Liver Physiol. 1999;276:G293–G302. doi: 10.1152/ajpgi.1999.276.1.G293. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G781–G791. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezençon C, Furholz A, Raymond F, Mansourian R, Metairon S, le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Khandanpour C, Moroy T, Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martin MG, Cheng H. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol. 2012;362:194–218. doi: 10.1016/j.ydbio.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: Sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am J Physiol. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV Paneth cells. Am J Anat. 1974;141:521–535. doi: 10.1002/aja.1001410406. [DOI] [PubMed] [Google Scholar]
- Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780–3786. doi: 10.1210/endo.139.9.6202. [DOI] [PubMed] [Google Scholar]
- Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010;339:493–504. doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- Kidd M, I, Modlin M, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. 2008;295:G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology. 2009;137:598–606. doi: 10.1053/j.gastro.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura E, Humphrey B, Tedo G, Ipharraguerre I. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can J Anim Sci. 2008;88:535–558. [Google Scholar]
- Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett. 2007;581:1119–1123. doi: 10.1016/j.febslet.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol Biol Evol. 2006;23:292–300. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- Sjölund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: An immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: Source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi S, Leblond CP. Migration and turnover of entero-endocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of 3H-thymidine into mice. Am J Anat. 1979;156:431–451. doi: 10.1002/aja.1001560403. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–73. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]