Abstract
Two major barriers in the immunotherapy of breast cancer include tumor-induced immune suppression and the establishment of long-lasting immune responses against the tumor. Recently, we demonstrated in an animal model of breast carcinoma that expanding and reprogramming tumor-sensitized lymphocytes, ex vivo, yielded T memory (Tm) cells as well as activated CD25+ NKT cells and NK cells. The presence of activated CD25+ NKT and NK cells rendered reprogrammed T cells resistant to MDSC-mediated suppression, and adoptive cellular therapy (ACT) of reprogrammed lymphocytes protected the host from tumor development and relapse. Here, we performed a pilot study to determine the clinical applicability of our protocol using peripheral blood mononuclear cells (PBMCs) of breast cancer patients, ex vivo. We show that bryostatin 1 and ionomycin (B/I) combined with IL-2, IL-7 and IL-15 can expand and reprogram tumor-sensitized PBMCs. Reprogrammed lymphocytes contained activated CD25+ NKT and NK cells as well as Tm cells and displayed enhanced reactivity against HER-2/neu in the presence of MDSCs. The presence of activated NKT cells was highly correlated with the rescue of anti-HER-2/neu immune responses from MDSC suppression. Ex vivo blockade experiments suggest that the NKG2D pathway may play an important role in overcoming MDSC suppression. Our results show the feasibility of reprogramming tumor-sensitized immune cells, ex vivo, and provide rationale for ACT of breast cancer patients.
Keywords: Cancer Immunotherapy, breast cancer, NKT cells, NK cells, myeloid-derived suppressor cells (MDSC)
Introduction
Conventional therapies, including surgery, chemotherapy, hormonal therapy, targeted agents and radiation, have significantly reduced the mortality associated with primary breast cancer. However, distant relapse and metastasis following conventional therapy remain the main cause of mortality in breast cancer patients. Therefore, biological therapies have been pursued as a means to induce anti-tumor immune responses that can eliminate residual tumor cells to prevent relapse and metastasis. It was initially thought that tumors evade immune recognition, and that breaking immunological tolerance could result in immune-mediated tumor rejection [1]. However, we have learned that cancer patients generate pre-existing immune responses specific for tumor antigens [2-4] which, nevertheless, fail to protect them. The failure of endogenous anti-tumor immune responses results, in part, from the increased activity of myeloid-derived suppressor cells (MDSC) [5]. The impairment of cancer immunotherapy resulting from MDSC suppression is well established [6, 7]. MDSCs consist of immature cells of myeloid origin which exert suppression of T cells by modulation of the local T cell extracellular environment, as well as via direct cell contact [8, 9]. Many attempts have been made to develop combinatorial therapies by depleting suppressor cells or blocking suppressor pathways while simultaneously actively inducing anti-tumor immune responses in vivo, or adoptively transferring tumor-specific T cells [10, 11]. In fact, the very limited success of immunotherapy of solid tumors to date has been achieved only against melanoma using adoptive T cell therapy or blockade of immune checkpoints [12-15], and against prostate cancer using a vaccine which improves patient survival but has no apparent inhibitory effect on disease progression [16]. We have recently developed an antigen-free protocol which utilizes the pharmacological agents bryostatin 1 and ionomycin (B/I), as well as common gamma chain (γ-c) cytokines IL-2, IL-7 and IL-15, to reprogram tumor-reactive lymphocytes of the innate (NKT cells and NK cells) and adaptive (CD4+ and CD8+ T cells) immune systems. Bryostatin 1 is a macrocyclic lactone derived from Bulgula neritina, a marine invertebrate. In culture, bryostatin 1 activates protein kinase C, while ionomycin increases intracellular calcium [17-19]. When combined, these pharmacological agents mimic signaling through the CD3/T cell receptor (TcR) complex and promote activation and proliferation of antigen-sensitized T cells [17, 18, 20, 21]. IL-2, IL-7 and IL-15 have been widely reported to support homeostatic T cell proliferation as well as enhancement of NK cell function and terminal NKT cell maturation [22-25]. IL-2 increases the size of the CD8+ memory pool if present at the time of initial T cell activation [26]. IL-2 has also been shown to elicit the production of both pro- and anti-inflammatory cytokines from NKT cells [27]. A recent study reports that human NKT cells are hyper-responsive in vitro to IL-7, leading to induced proliferation and increased frequency of cells expressing the terminal differentiation marker, CD161 [28]. IL-15 induces strong and selective expansion of memory-phenotype CD8+ cells and NK cells [29]. IL-15 has also been linked to delivering crucial signals in promoting NKT cell survival, developmental progression, and terminal differentiation associated with acquisition of effector functions [25].
We have reported that such reprogrammed cells render T cells resistant to MDSC-mediated suppression in the FVBN202 transgenic mouse model of breast carcinoma [30, 31]. Furthermore, we have shown that adoptive cellular therapy (ACT) utilizing these reprogrammed immune cells protected the animals from tumor challenge, and generated long lasting memory responses resulting in relapse-free survival [30]. Such an effective anti-tumor response exerted by the reprogrammed immune cells was dependent on the presence of activated NKT and/or NK cells, which rescued tumor-reactive T cells from MDSC suppression [30].
Here, we sought to determine the clinical applicability of our protocol in expanding tumor-sensitized Tm cells and reprogramming NKT and/or NK cells using PBMCs from breast cancer patients, as well as evaluating their resistance to MDSC suppression in vitro. We determined that expanding and reprogramming human PBMCs was feasible and yielded CD62L+ Tm cells as well as activated NKT cells, which were found to be the effector cells responsible for rescuing HER-2/neu-reactive T cells from MDSC suppression, possibly via an NKG2D-dependent pathway.
Materials and Methods
Patient characteristics
A total of 16 patients were enrolled into the study (Table 1). This study was conducted under Institutional Review Board (IRB) protocol# MCC-13740 at Virginia Commonwealth University. All patients had the capacity to give informed consent to participate in this research. Fourteen patients were at tumor stage IIb and below, while one patient had stage IV disease. Tumor size ranged from 0.5 to > 3.5 cm. Thirteen tumors were invasive ductal carcinoma (IDC) and ten also had a component of DCIS. Five patients had low grade tumor histology, five had high grade, two had intermediate grade and three were unknown. Eleven patients had ER+ tumors with six having HER-2/neu positive tumors. Two patients had positive sentinel lymph node biopsies (SLN) but went on to have negative completion axillary node dissections. Two patients had positive axillary node dissections, but the majority had node-negative breast cancer (n=12). Eleven patients underwent chemotherapy between visits and seven underwent hormone therapy. Two patients had surgery to remove their tumor between the first and second visit.
Table 1.
| Patients (n=16) | |
|---|---|
| Tumor stage | Number of patients |
| IA | 6 |
| IB | 0 |
| IIA | 7 |
| IIB | 1 |
| IIIA | 0 |
| IIIB | 0 |
| IV | 1 |
| Size | |
| <0.5 cm | 0 |
| 0.51-1.00 cm | 1 |
| 1.01-1.50 cm | 6 |
| 1.51-2.00 cm | 1 |
| 2.01-2.50 cm | 4 |
| 2.51-3.00 cm | 1 |
| 3.01-3.50 cm | 1 |
| >3.50 cm | 2 |
| Main Tumor Histology | |
| IDC | 13 |
| ILC | 3 |
| DCIS | 10 |
| LCIS | 3 |
| Lymph node status | |
| SLN + | 2 |
| Axillary dissection – | 12 |
| Axillary dissection + | 2 |
| Grade | |
| Low | 5 |
| Intermediate | 2 |
| High | 5 |
| Unknown | 3 |
| Hormone status | |
| ER+ | 11 |
| PR+ | 9 |
| HER2+ | 6 |
| Adjuvant therapy | |
| Chemotherapy | 11 |
| Hormone therapy | 7 |
Study design
Breast cancer patients enrolled in this study (n=16) provided two blood samples in two separate visits. During the first visit, monocyte-derived dendritic cells (DCs) were prepared as described below and cryopreserved for use in the second visit; PBMCs were either cryopreserved (65% of total) or expanded and reprogrammed ex vivo (B/I-Fresh) for use in phenotype analysis by flow cytometry and then cryopreserved. Six days before the second visit, cryopreserved PBMCs collected during the patient's first visit which had not been reprogrammed were quickly thawed at 37°C and washed 2x in complete medium (RPMI 1640 supplemented with 10% FBS, L-glutamine (2mM), 100 U/ml penicillin, and 100 μg/ml Streptomycin) pre-warmed to 37°C, and were then counted. Sixty percent of these PBMCs were cultured in IL-2 (40U/ml) for six days (IL-2) and 40% were reserved for reprogramming (Freeze-B/I) or treatment with cytokines without B/I stimulation (IL-2/7/15). One day before the second visit, lymphocytes previously frozen after reprogramming (B/I-Freeze) and DCs were thawed. DCs were then maintained in GM-CSF (100ng/ml) and IL-4 (50ng/ml) overnight, while the B/I-Freeze PBMCs were cultured in IL-2 (40U/ml) overnight. On the day of the second visit, MDSCs were sorted from peripheral blood. PBMCs from each condition were then cultured with recombinant HER-2/neu (intracellular domain (ICD)) pulsed DCs in the presence or absence of MDSCs. The maturation of MDSCs into DCs was determined via flow cytometry after an identical co-culture with reprogrammed PBMCs in which DCs were not present. Phenotype analysis was also performed on B/I-Freeze, Freeze-B/I and IL-2/7/15 PBMCs to compare the reprogramming efficacy of these conditions as well as to identify any phenotypic fluctuations as a result of the cryopreservation process.
Ex vivo reprogramming and expansion of lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated from breast cancer patients using Ficoll-Hypaque (GE Healthcare, Uppsala, Sweden), as described by our group [32]. After density gradient separation, PBMCs were cultured at 37°C for 2 hours; adherent cells were used for the generation of monocyte-derived DCs as previously described [32, 33] and were then placed in freezing medium (90% FBS, 10% DMSO) at 106cells/ml and cryopreserved in liquid nitrogen. Non-adherent cells were immediately reprogrammed (35% of total) as described below, or were cryopreserved (65% of total) for use in the patient's second visit. For reprogramming, lymphocytes (106 cells/ml) were cultured in complete medium and were stimulated with Bryostatin 1 (2nM) (Sigma, Saint Louis, MO), Ionomycin (1μM) (Calbiochem, San Diego, CA), and 80U/ml of IL-2 (Peprotech) for 16-18 hours. Lymphocytes were then washed three times and cultured at 106cells/ml in complete medium with IL-7 and IL-15 (20ng/ml, Peprotech, Rocky Hill, NJ). After 24 hours, 20 U/ml of IL-2 was added to the complete medium. The following day the cells were washed and cultured at 106 cells/ml in complete medium with 40 U/ml of IL-2. After 48 hrs, cells were washed and cultured at 106 cells/ml in complete medium with 40 U/ml of IL-2. Twenty-four hours later, lymphocytes were washed and cultured at 106 cells/ml in complete medium with 40 U/ml of IL-2. Lymphocytes were harvested 24hrs later on the sixth day and were then either used in vitro studies or were placed in freezing medium (106 cells/ml) and cryopreserved.
RNA extraction and RT reaction
RNA was extracted from CD3+ PBMC using TRIzol reagent according to manufacturer's protocol (Invitrogen, Carlsbad, CA). The cDNA was prepared as previously described [34].
High-throughput T cell receptor sequencing
Upon confirmation of the purity of the cDNA by running PCR product of GAPDH amplification, 1 μg to 119 μg (average, 55 μg) per sample of cDNA was sent to Adaptive Biotechnologies (Seattle, WA) for high-throughput sequencing of the TcR variable beta (Vβ) CDR3 region using the ImmunoSEQ assay, as previously described by our group [34].
Flow cytometry
Antibodies used for flow cytometry were purchased from Biolegend (San Diego, CA), (FITC-CD161 (HP-3G10); FITC-CD62L (DREG-56); PE-NKG2D (1D11); PECD44 (IM7); PE-HLA-DR (L243); PE/CY5-CD33 (WM53); Allophycocyanin-CD11b (ICRF44); PE/CY5-CD4 (OKT4); PE/CY5- and Allophycocyanin-CD3 (HIT3a); FITC- and PECD25 (BC96); FITC- and PE/CY5-CD56 (HCD56); PE- and Allophycocyanin-CD8α (HIT8a)). Antibodies were used at the manufacture's recommended concentration. Cellular staining was performed as previously described by our group [30, 33]. Multicolor data acquisition was performed using a Becton Dickinson FACSCanto II and analyzed using FlowJo software v10.0.5. (Tree Star, Inc., Ashland, OR).
MDSC sorting
To sort MDSCs from peripheral blood, erythrocytes were lysed from whole blood treated with Ammonium-Chloride-Potassium lysing buffer according to the manufacturer's procedure (Quality Biological, Inc., Gaithersburg, MD). Total leukocytes were then stained as described by our group [30, 33]. The cells were then washed with sterile PBS supplemented with 2% FBS and were then sorted into a CD33+ CD11b+ HLA-DRlo/− population, representative of MDSCs, into 100% FBS using a Becton Dickinson FACSAria III. Sorting efficiency was always greater than 94%.
Recombinant HER-2/neu ICD protein
Recombinant human HER-2/neu ICD was prepared as described by our group [32].
IFN-γ ELISA
HER-2/neu specific responses and susceptibility to MDSC suppression were determined for Reprogrammed and IL-2 PBMCs. These cells were cultured in complete medium at a 2:1 ratio with autologous DCs pulsed with recombinant HER-2/neu ICD protein (40μg/ml). Reprogrammed PBMCs were also cultured in complete medium at a 2:1 ratio with autologous DCs pulsed with recombinant HER-2/neu ICD protein in the presence or absence of sorted MDSCs (2:1) and in the presence or absence of anti-NKG2D blocking antibody (1D11, 10μg/ml) for 20 hours. Supernatants were then collected and stored at −80° C until use. IFN-γ was detected using a Human IFN-γ ELISA kit (BD Pharmingen, San Jose, California) according to the manufacturer's protocol.
Statistical analysis
Repeated measures analysis of variance was used to compare Day 6 from baseline and between conditions for expansion of PBMC, T memory (Tm) cells, activated CD25+ NK and NKT cells. Tukey's HSD was used to adjust for multiple pairwise comparisons. Data transformation has been made to satisfy the normality assumption as follows: natural logarithm of number of PBMC and fold expansion, and arcsine of square root of Tm%, activated CD25+ NK% and NKT%. The Pearson correlation coefficient was calculated between numbers of activated NK cells or NKT cells or NKT cells expressing NKG2D and response in rescuing reprogrammed PBMCs from MDSCs and a t-test was used for testing its significance. Paired two-tailed t-tests were performed unless otherwise denoted. P values ≤ 0.05 were considered significant.
Results
PBMCs expand and are reprogrammed following B/I activation and combined IL-7, IL15 and IL-2 treatment, ex vivo
In order to determine the clinical applicability of our pre-clinical studies of tumor-sensitized lymphocyte expansion and reprogramming, PBMCs of breast cancer patients underwent a 16-18 hour treatment with B/I followed by a 5-day culture in the γ-c cytokines IL-2, IL-7 and IL-15. Regardless of individual variations, the overall expansion of reprogrammed cells was found to be significant when compared to the total number of PBMCs before reprogramming (baseline) (Figure 1A). Cellular expansion was successful in 13 out of the 16 patients.
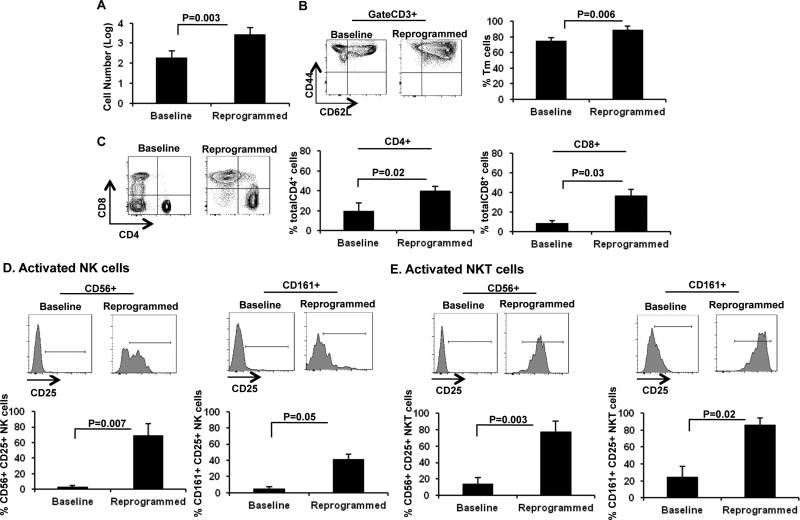
Figure 1. B/I and common γ-c cytokines expand and reprogram PBMCs.
A) Log number of PBMCs determined by trypan blue exclusion before (baseline) and after six days of ex vivo expansion (Reprogrammed; n=16). B) Frequency of Tm cells in baseline PBMCs as wells as reprogrammed PBMCs (n=5). C) Frequency of total CD4+ and CD8+ cells was determined in baseline as well as reprogrammed PBMCs (n=5). D) Frequency of CD25+ CD56+ NK cells (left panel, n=6) and CD25+ CD161+ NK cells (right panel, n=4) in baseline PBMCs or reprogrammed PBMCs. E) Frequency of CD25+ CD56+ NKT cells (left panel, n=6) and CD25+ CD161+ NKT cells (right panel, n=4) in baseline or reprogrammed PBMCs. Data represent Mean ± SEM.
Next, we examined the efficacy of our reprogramming procedure to enrich for specific cellular phenotypes and activate NKT and NK cells relative to baseline PBMCs. Similar to our observations in animal models, we detected the differentiation of T cells into a CD44+ CD62L+ Tm phenotype after treatment with B/I and γ-c cytokines when compared with baseline PBMCs (Figure 1B). Additional phenotypic analysis revealed the enrichment of both CD8+ T cells and CD4+ T cells (Figure 1C) after cellular reprogramming. Furthermore, expanded PBMCs yielded enriched populations of activated NK cells (Figure 1D: left panel, CD3-CD8-CD56+CD25+ NK cells; right panel, CD3-CD8-CD161+CD25+ NK cells) and activated NKT cells (Figure 1E: left panel, CD3+CD8-CD56+CD25+ NKT cells; right panel, CD3+CD8-CD161+CD25+ NKT cells). Therefore, given the success in overall expansion of PBMCs treated with B/I and γ-c cytokines, increased frequencies of these cellular subsets correlate with increased absolute cell numbers after expansion. Such data mimic our pre-clinical findings and therefore represent successful cellular expansion and reprogramming of PBMCs from tumor-bearing individuals.
In order to address logistical issues associated with ACT, the retention of tumor-reactive immune cells following cryopreservation was determined in three conditions: i) PBMCs that were frozen and then reprogrammed after thawing (Freeze-B/I), ii) PBMCs that were reprogrammed immediately after isolation (B/I-Fresh), and iii) control PBMCs that were cultured in IL-2 alone (IL-2). Due to logistical limitations, analysis of these conditions was not feasible for every patient. Compared to PBMCs cultured in IL-2 alone, B/I-Fresh PBMCs underwent significant expansion, as did Freeze-B/I PBMCs (Supplemental Figure 1A). Since total cellular expansion was not affected by cryopreservation, we then sought to determine if reprogrammed PBMCs may be sensitive to cryopreservation within specific cellular subsets. We compared three different conditions: i) Freeze-B/I, ii) B/I-Fresh, and iii) PBMCs that were reprogrammed and then subjected to freeze/thaw (B/I-Freeze). We observed a significantly increased frequency of activated CD56+ NK and NKT cells and activated CD161+ NK and NKT cells when compared with baseline, regardless of previous storage conditions (Supplemental Figure 1B-E). Additionally, we observed significant increases in the frequency of CD3+ T cells in all of the conditions monitored (Supplemental Figure 1F). However, impairment in the enrichment of Tm cells was observed only in B/I-Freeze PBMCs, when compared with baseline PBMCs (Supplemental Figure 1G). This suggests that fully differentiated Tm cells are cryosensitive. These data also suggest that the most effective reprogramming conditions for ACT, which would yield a predominant Tm phenotype, would be to expand and reprogram either fresh or stored PBMCs immediately prior to ACT.
Bryostatin 1 and Ionomycin are required for NKT cell activation and the cellular expansion of specific T cell clones
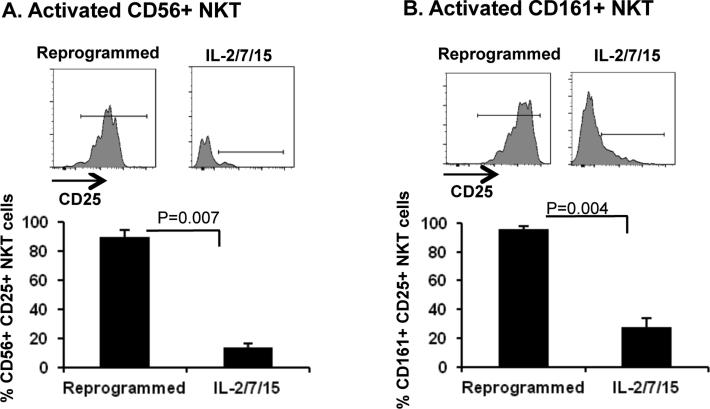
Studies were then performed to determine the necessity of B/I stimulation for effective reprogramming of PBMCs. The exclusion of B/I, thereby culturing PBMCs only in γ-c cytokines failed to expand the cells (data not shown). Importantly, we demonstrate in Figure 2 that culturing PBMC in γ-c cytokines without B/I stimulation (IL-2/7/15) exhibited a significantly reduced yield of activated CD56+ NKT (Figure 2A) and CD161+ NKT (Figure 2B) compared with PBMCs reprogrammed with B/I and γ-c cytokines. Conversely, we observed no significant difference in the frequency of CD3+ cells (Supplemental Figure 2A, p=0.2), Tm cells (Supplemental Figure 2B, p=0.2), CD56+ NK cells (Supplemental Figure 2C, p=0.12) or CD161+ NK cells (Supplemental Figure 2D, p=0.07). These data suggest that B/I is required to induce total PBMC expansion, as well as to activate NKT cells. However, our observation of equivalent frequencies of activated NK cells, CD3+ cells and Tm cells among reprogrammed PBMCs as well as PBMCs treated with γ-c cytokines without B/I suggest that enrichment and differentiation of these cells is supported by γ-c cytokines alone, but that total cellular expansion and increasing the absolute number of these cells is dependent on the activity of B/I.
Figure 2. Bryostatin 1 and Ionomycin are required for NKT cell activation.
The contribution of B/I to cellular activation of CD56+ and CD161+ NKT cells was determined using flow cytometry analysis to compare reprogrammed PBMCs, to those treated only with common γ-c cytokines ex vivo for six days (IL-2/7/15, n=3). Data represent Mean ± SEM
Spectratyping analysis of the TcR Vβ prior to and after reprogramming did not show a meaningful trend in T cell clonality (data not shown). Therefore, we performed high throughput sequencing of TcR Vβ in one patient prior to adjuvant therapy in order to delineate the contribution of B/I or γ-c cytokines to the expansion of specific T cell clones. Analysis of the VJ recombination was performed in which comparisons were drawn from untreated PBMCs (baseline) to those cultured only in γ-c cytokines (IL-2/7/15 PBMC), as well as reprogrammed PBMCs (B/I + γ-c cytokines), to determine the frequency of specific Vβ gene recombination events. This analysis revealed that certain clones demonstrating monoclonality at baseline experienced clone-specific expansion in reprogrammed PBMCs as demonstrated by Vβ9 J2-2, which experienced a ~10 fold increase over baseline and a 5-fold increase over IL2-7-15 PBMCs (Supplemental Figure 3 A). Similar results were obtained for Vβ7-6 J2-2, Vβ10-2 J2-2, Vβ11-2 J2-2, Vβ13 J1-6, Vβ15 J2-2, and Vβ27 J2-2 (data not shown), in which the dominant clone at baseline persisted after reprogramming with a 5-fold or higher increase in frequency. Furthermore, we show that the inclusion of B/I in the regimen selectively expands specific low-frequency VJ recombination events present at baseline. As shown in Supplemental Figure 3B, B/I activation promotes the emergence of monoclonality; Vβ5-7 J2-3 clones emerge from a very low frequency in reprogrammed PBMCs compared with baseline, while the frequency of other clones expressing Vβ5-7 in baseline and IL-2/7/15 PBMCs either decline or remain static. This phenomenon was also observed in Vβ6-7 J1-2, Vβ6-7 J1-4 andVβ6-8 J1-4 with each emergent peak having at least a 6-fold increase in frequency (data not shown). Interestingly, despite the retention of monoclonality, the Vβ11-3 J2-2 clone did not expand either in reprogrammed or in IL-2/7/15 PBMCs (Supplemental Figure 3C). Similar observations were made in Vβ7-1 J2-2, Vβ 7-3 J2-2, Vβ7-7 J2-2, Vβ10-3 J2-2, Vβ11-1 J2-2, Vβ14 J2-1, and Vβ23 J1-3 (data not shown). Such data suggest that B/I combined with γ-c cytokines results in the selective expansion of specific clones within the T cell repertoire.
Reprogrammed PBMCs exhibit enhanced anti-HER-2/neu reactivity
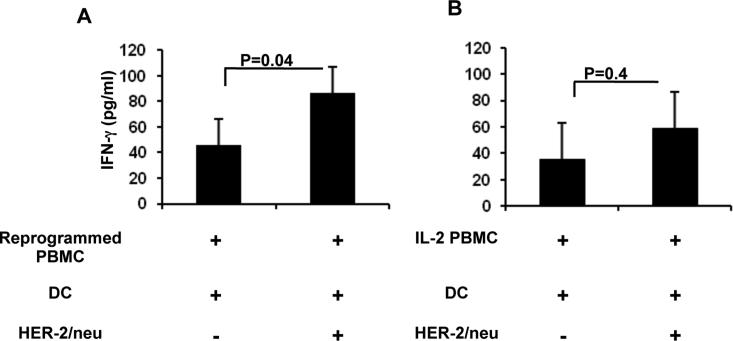
Based on our previous report in an animal model of breast carcinoma, we anticipated that reprogrammed PBMCs would exhibit a more potent anti-HER-2/neu response than PBMCs cultured in IL-2 alone. We used recombinant ICD of HER-2/neu to quantify IFN-γ release by reprogrammed cells in the presence of autologous DCs. PBMCs alone and DCs alone were used as negative controls for the production of IFN-γ. PBMCs from a total of six patients had HER-2/neu-reactive immune responses when cells were reprogrammed, regardless of variation in HER-2/neu expression amongst individual tumors (Figure 3A). There was not a significant difference between reprogrammed cells and IL-2 PBMC in the background levels of IFN-γ production. Two of these patients also displayed a HER-2/neu-reactive immune response when PBMCs were cultured in IL-2 alone (IL-2 PBMC); however the differences were not significant over basal IFN-γ production (Figure 3B). These data, together with data presented in Supplemental Figure 3, suggest that reprogrammed PBMCs are composed of an enriched frequency of specific tumor antigen-sensitized T cell clones, resulting in enhanced anti-HER-2/neu reactivity. This is further supported by our observation that antigen-specific responses were undetectable when IL-2 PBMCs, i.e. non-reprogrammed PBMCs, from the same six patients were cultured with HER-2/neu pulsed DCs.
Figure 3. Reprogrammed PBMCs produce enhanced anti-HER-2/neu responses.
A) Reprogrammed PBMC (n=6) or B) IL-2 PBMC (n=2) were cultured with autologous monocyte-derived DCs pulsed with HER-2/neu ICD for 20hrs, followed by detection of IFN-γ in the culture supernatant. Data represent Least Squares Means ± SEM.
Rescue of reprogrammed PBMCs from MDSC immunosuppression is associated with an increased frequency of activated NKT cells
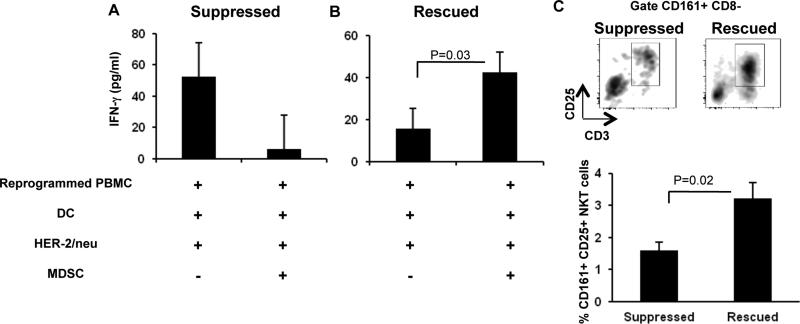
We previously reported that murine splenocytes treated with B/I and γ-c cytokines rescued T cells from MDSC immunosuppression; this phenomenon was dependent on the presence of activated NK and/or NKT cells [30]. Therefore, we tested the ability of breast cancer patients’ PBMCs to overcome MDSC immunosuppression after ex vivo reprogramming. To determine this, reprogrammed PBMCs were stimulated with HER-2/neu-pulsed DCs, with or without addition of autologous MDSCs. PBMCs, DCs, and MDSCs alone were used as negative controls for the production of IFN-γ. Surprisingly, reprogrammed PBMCs showed variable results in response to MDSC suppression. Some patients’ reprogrammed cells with a detectable IFN-γ response appeared to be suppressed, as determined by reduced secretion of IFN-γ in the presence of MDSC. Although t-test analysis showed that this suppression was not statistically significant (Figure 4A, n=4), we observed appreciable decreases in HER-2/neu reactive IFN-γ release in the presence of MDSC for these 4 patients. Conversely, we found some patients’ reprogrammed PBMCs with a weak IFN-γ response (n=6) were not only resistant to MDSC-mediated suppression, but actually exhibited increased secretion of IFN-γ in the presence of MDSCs (Figure 4B), hereafter referred to as a “rescued” response. We hypothesized that such variations in response to MDSCs may be related to the frequency of activated NK or NKT cells in reprogrammed PBMCs. To test this, we calculated the Pearson correlation coefficient (PCC) to determine if the presence of activated NK and/or NKT cells was associated with rescuing anti-HER-2/neu-specific immune responses from MDSC-mediated suppression. Indeed, we found that the rescue of reprogrammed PBMCs from suppression had a high positive correlation with the presence of activated CD161+ NKT cells (PCC= 0.82, p=0.048, n=6) and it is likely that it also had positive correlation with activated CD56+ NKT cells (PCC= 0.72, p= 0.077, n=7). Since only activated CD161+ NKT cells showed a statistically significant positive correlation (p=0.048), we asked whether an optimal frequency of CD161+ NKT cells was required to rescue the immune response from MDSC suppression. We examined the frequency of these cells among the “suppressed” and “rescued” reprogrammed PBMCs. We found that a frequency of 3% activated CD161+ NKT cells amongst all lymphocytes was sufficient to rescue HER-2/neu-specific IFN-γ production in the presence of MDSCs (Figure 4C); a frequency of 1.5% of these cells, 2-fold less, was observed in samples which exhibited suppression of IFN-γ secretion in the presence of MDSCs (Figure 4C). We observed no significant differences when comparing activated CD56+ NK, CD56+ NKT or activated CD161+ NK cells between the rescued and suppressed responses (data not shown). Therefore, our data suggest that reprogrammed PBMCs with a sufficient frequency of activated CD161+ NKT cells (3%) are resistant to MDSC-mediated suppression of anti-HER-2/neu immune responses.
Figure 4. Reprogrammed PBMCs overcome MDSC-mediated immune suppression.
A) Reprogrammed PBMC were cultured with monocyte-derived DCs pulsed with HER-2/neu ICD and sorted MDSCs for 20 hrs, followed by detection of IFN-γ in the culture supernatant. Data represent Least Squares Means ± SEM in reprogrammed PBMCs that were either suppressed (A, n=4) or showed resistance to MDSC (B, n=6). C) Reprogrammed PBMCs were analyzed by flow cytometry to determine the frequency of activated CD161+ NKT cells amongst all lymphocytes in suppressed (n=2) and rescued (n=6) samples. Data represent Mean ± SEM. P value calculated using unpaired one-tailed t-test.
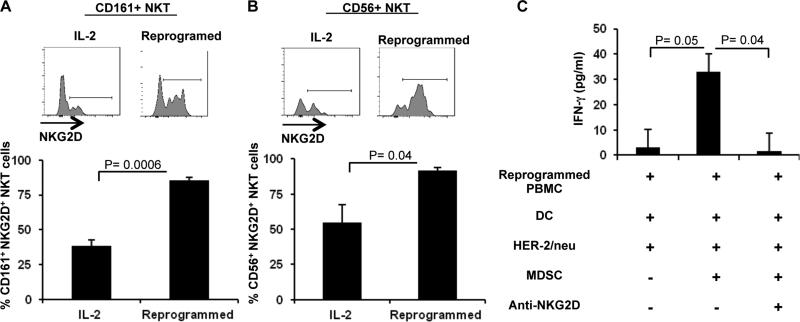
In order to determine the potential mechanism(s) through which activated NKT cells overcome MDSC suppression, we examined the status of the NKG2D activating receptor among PBMCs after cellular reprogramming. We observed upregulation of NKG2D expression on CD161+NKT cells (Figure 5A) and CD56+NKT cells among reprogrammed PBMCs (Figure 5B) when compared to PBMCs cultured in IL-2 alone. We did not observe significant NKG2D upregulation in CD56+ or CD161+ NK cells or CD8+ T cells (data not shown). We then sought to determine if the upregulation of this activating receptor following cellular reprogramming may be involved in rescuing HER-2/neu-specific immune responses from MDSC suppression. As a preliminary assessment, we blocked the NKG2D receptor by adding anti-human NKG2D blocking antibody to the culture containing reprogrammed PBMCs and MDSCs. As shown in Figure 6C, blockade of the NKG2D receptor abrogated the ability of reprogrammed PBMCs to secrete IFN-γ in the presence of HER-2/neu-pulsed DCs and MDSCs in 2/2 patients examined.
Figure 5. NKT cells may rescue reprogrammed PBMCs from MDSC suppression via an NKG2D-dependent pathway.
PBMCs were either maintained in IL-2 for six days (IL-2) or were reprogrammed with B/I and γ-c cytokines (Reprogrammed) and subsequently analyzed for NKG2D expression in CD161+ NKT cells (A) or CD56+ NKT cells (B) using flow cytometry (n=5). Data represent Mean ± SEM. C) Reprogrammed PBMCs were cultured in the presence of HER-2/neu ICD pulsed DCs with or without sorted MDSCs and anti-NKG2D blocking antibody (n=3). Data represent Least Squares Means ± SEM.
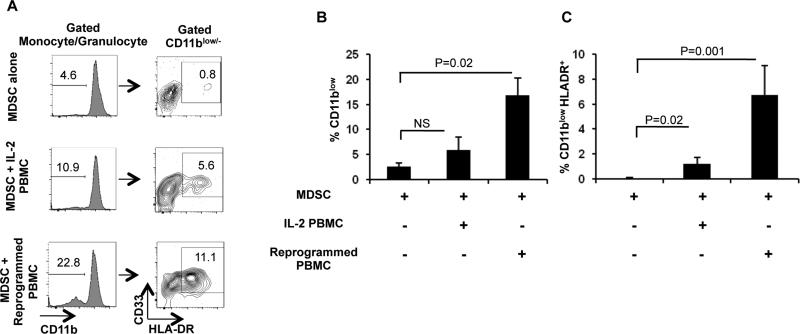
Figure 6. MDSCs upregulate expression of HLA-DR upon co-culture with reprogrammed PBMCs.
A) Sorted MDSCs were cultured alone (MDSC alone), in the presence of IL-2 (MDSC+ IL-2 PBMC) or reprogrammed cells (MDSC+ Reprogrammed PBMC) for 20hrs. Flow cytometry was then used to determine frequency of CD11b expression and percentage of HLADR+ cells. B) Frequency of CD11b loss after 20hr culture (n=3). C) Frequency of HLA-DR expression gated on CD11blo/− cells (n=3). Data represent Least Squares Means ± SEM.
MDSCs mature into functional dendritic cells upon culture with reprogrammed and activated NKT cells
Our observations that anti-HER-2/neu-specific immune responses were boosted in the presence of reprogrammed PBMCs and MDSCs led us to question if MDSCs were undergoing maturation into functional DCs, as has been demonstrated in animal models [35, 36]. To determine this, we performed a 20-hr co-culture of sorted MDSCs with reprogrammed PBMCs; MDSCs were then harvested and subjected to multi-color flow cytometry to analyze potential phenotypic modulations. As shown in Figure 6A-B, significant downregulation of CD11b expression was observed on MDSCs cultured with reprogrammed PBMCs compared with MDSCs cultured alone; significant loss of CD11b expression was not observed on MDSCs cultured with IL-2 PBMCs. CD11b downregulation was associated with significant upregulation of HLA-DR as observed upon culture of reprogrammed PBMCs and MDSCs compared with MDSCs alone and MDSCs cultured with IL-2 PBMCs (Figure 6C), which is indicative of maturation of MDSCs into DCs [37, 38]. We did not observe a significant upregulation of HLA-DR in the CD11bhigh population after the co-culture (data not shown). It is likely that there may exist a positive correlation of both activated CD161+ NKT cells (PCC=0.703) and CD56+ NKT cells (PCC=0.977) within reprogrammed PBMCs in inducing CD11b downregulation and HLA-DR upregulation compared with lymphocytes cultured only in IL-2.
Discussion
The main objective of this study was to determine the clinical applicability of a cellular reprogramming protocol that we developed in the FVBN202 transgenic mouse model of spontaneous mammary carcinoma for adoptive cellular therapy of breast cancer [30]. We have shown that the proposed protocol is effective for the reprogramming of human PBMCs. The differentiated and expanded T cells were composed of a predominantly CD62L+ Tm phenotype. B/I activation was required for the reprogramming of activated NKT cells and NK cells as well as CD8+ T cells, whereas the combination of three γ-c cytokines alone contributed to the phenotypic differentiation of Tm cells. Furthermore, high throughput sequencing of TcR Vβ showed that B/I activation was critical for the clonal expansion of specific TcR Vβ clones. Reprogrammed PBMCs were also found to be resistant to MDSC suppression, provided that the frequency of activated CD161+ NKT cells approached 3% or more of total lymphocytes. Additionally, activated NKT cells showed a positive correlation in the rescue of HER-2/neu-reactive T cells from MDSC suppression. Our preliminary observations suggest that NKT cells may protect T cells from MDSC suppression via an NKG2D-dependent signaling pathway, resulting in CD11bhigh HLA-DR− MDSCs maturing into CD11blow HLA-DR+ DCs. Because the primary objective of this study was to determine the clinical applicability of our reprogramming protocol, we were not able to expand our studies on the NKG2D pathway. However, our preliminary observation suggests NKG2D plays an important role in the crosstalk between NKT cells, MDSCs and T cells. This pathway requires further investigation. Furthermore, one of the main logistical issues with ACT is the retention of tumor-reactive immune cells after the freeze/thaw cycle. We determined that freeze/thaw conditions negatively affected the frequency of the Tm subset of previously reprogrammed PBMCs. Importantly, however, activated NKT cells, which we found to be the cellular mediators responsible for rescuing T cells from MDSC suppression, were unaffected by cryopreservation. These findings suggest that the storage of PBMC with subsequent reprogramming for infusion is likely to be efficacious, thus providing a feasible strategy for clinical adaptation. Although, HER-2/neu-specific IFN-γ production by the reprogrammed PBMCs was measured at picogram concentrations, we expect a higher frequency of tumor-reactive immune cells when lymphocytes from tumor-draining lymph nodes or tumor-infiltrating lymphocytes are used for reprogramming as a source of ACT in future studies [8, 30, 39, 40].
Other groups have reported that T central memory (Tcm) phenotypes are more effective than T effector (Te) phenotypes in generating long-lasting protection against tumor cells [41, 42]. We have also made similar observations in animal models in which protection from tumor re-challenge was associated with the enrichment of Tm cells within reprogrammed lymphocytes [30]. Here, we also observed an enrichment of Tm following ex vivo reprogramming of PBMC derived from breast cancer patients, suggesting the potential to establish a long-lived immune response against tumor antigens upon reinfusion during ACT, which may protect breast cancer patients from future distant relapse and metastasis.
Tumor-induced immune suppression mediated by MDSCs poses a challenge to immunotherapy of breast cancer [43]. As such, devising strategies to overcome MDSCs may be critical for improved efficacy of ACT [5, 44]. Similar to previous studies in animal models which suggest that NKT cells have a role in the conversion of MDSCs into DCs [30, 35, 36]; we now report a similar phenomenon in breast cancer patients. The physiological role of NKT cells in tumor immunity is yet to be fully defined, but it appears a primary function of these cells is to activate DCs to promote antigen-specific T cell responses [45-47]. Furthermore, the ability of NKT cells to direct myeloid lineage cellular differentiation is becoming apparent. Hedge and colleagues [48] have demonstrated the ability of NKT cells to induce the differentiation of monocytes into DCs as a result of secretion of GM-CSF and IL-13 and, similarly, others have reported on the ability of innate immune cells to induce the maturation of monocytes into DCs [49-51]. In animal models, NKT cells exhibit a regulatory function towards MDSCs through a CD1d-dependent mechanism [35]. In patients with breast carcinoma, however, NKG2D-dependent signaling may be involved in the crosstalk between activated NKT cells and MDSC, resulting in the maturation of MDSCs into DCs, thereby rescuing immune responses from MDSC suppression. We observed downregulation of CD11b and upregulation of HLA-DR upon culture of MDSCs with reprogrammed PBMC containing activated NKT cells, a phenotypic modulation which has been previously associated with the maturation of precursor cells into DCs [35, 37, 38]. Interestingly, it has been shown that NKG2D may function as a costimulatory molecule in the context of weak CD1d-restricted TcR signaling [52].
Recently, it was reported that reduced levels of the NKG2D receptor on immune cells can predict poor outcome in breast cancer patients [53]. NKG2D-ligands, known for their NK/NKT cell activating function, were found to be widely expressed across all breast cancer subtypes [53, 54], though expression of these ligands on human MDSCs is yet to be demonstrated. Recent studies using adoptively transferred T cells engineered to express a chimeric NKG2D receptor were found to alter myeloid cells of the local tumor microenvironment to promote anti-tumor immune cell activity in murine models [55, 56]. Zhou and colleagues [57] have described the ability of macrophages to stimulate NK cells via NKG2D signaling while being protected from direct NK cell cytolysis by means of inhibitory ligand-receptor signaling. As macrophages and MDSCs share a common lineage, this study suggests that MDSCs may also have the capacity to interact with activated NKT cells via NKG2D without inducing direct NKT cell cytolysis of MDSCs. Collectively with our work described here, these studies suggest a more comprehensive investigation about the potential role of NKG2D-dependent signaling in shifting the immunosuppressive tumor microenvironment into immunostimulatory.
The results of our study offer strategies to improve adoptive cellular therapy of breast cancer. These include: i) inclusion of activated NKT cells in adoptive T cell therapy in order to induce MDSC maturation into DCs to overcome immune suppression and rescue anti-tumor T cell responses, and ii) the activation of PBMCs using B/I followed by γ-c cytokines to enrich the frequency of Tm cells and to selectively expand T cells with specificity for endogenously expressed tumor-antigens. Our preclinical studies showed that reprogrammed Tm cells persisted in animals after complete rejection of the tumor such that animals were able to reject recall tumor challenge few months after the rejection of primary tumor [30]. These results also suggest conducting pilot studies in order to determine: i) if lymphocytes harvested from lymph nodes of breast cancer patients may be better than PBMC for the expansion and reprogramming of tumor-sensitized immune cells. Our preclinical studies show that secondary lymphoid tissues including lymph nodes and spleen are enriched with tumor-sensitized T cells compared with peripheral T cells, thus they serve as potent sources for reprogramming of the immune cells for ACT [30, 58]; and ii) if MDSC accumulation is associated with tumor burden. Our results raised a number of questions for future studies. These include: Do all the clonally expanded T cells react with tumor-antigens? Do NKT cell-mediated conversion of MDSC to DCs involves NKG2D pathway? Are MIC-A and MIC-B receptors involved in the crosstalk between NKT cells and MDSC?
Supplementary Material
Acknowledgements
We thank Julie Farnsworth for her expertise in cell sorting and immense dedication to furthering the research at our institution. We also thank Laura Graham for assisting with the ex vivo expansion of PBMCs. We gratefully acknowledge the support of VCU Massey Cancer Center and the Commonwealth Foundation for Cancer Research.
This research was supported by grant funding from Virginia's Commonwealth Health Research Board (M. H. Manjili) and Adaptive Biotechnologies Young Investigator Award (K. K. Payne). Flow cytometry supported in part by NIH grant P30 CA016059.
Abbreviations
- ACT
adoptive cellular therapy
- B/I
bryostatin and ionomycin
- DC
dendritic cell
- ICD
intracellular domain
- MDSC
myeloid derived suppressor cell
- PBMC
peripheral blood mononuclear cells
- PCC
Pearson correlation coefficient
- Tcm
T central memory cells
- TcR
T cell receptor
- Te
T effector cells
- Tm
T memory cells
- Vβ
variable beta
- γ-c
gamma chain
Footnotes
Conflict of interest:
M.H. Manjili and H.D. Bear hold a pending patent assigned to Virginia Commonwealth University entitled: Composition and method for immunologic treatment of cancer, prevention of cancer recurrence and metastasis, and overcoming immune suppressor cells (US Patent application 13/877,536).
All other authors declare no conflict of interest.
References
- 1.Sotomayor EM, Borrello I, Levitsky HI. Tolerance and cancer: a critical issue in tumor immunology. Crit Rev Oncog. 1996;7:433–456. doi: 10.1615/critrevoncog.v7.i5-6.30. [DOI] [PubMed] [Google Scholar]
- 2.Guckel B, Rentzsch C, Nastke MD, et al. Pre-existing T-cell immunity against mucin-1 in breast cancer patients and healthy volunteers. J Cancer Res Clin Oncol. 2006;132:265–274. doi: 10.1007/s00432-005-0064-6. [DOI] [PubMed] [Google Scholar]
- 3.Goodell V, Waisman J, Salazar LG, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–454. doi: 10.1158/1535-7163.MCT-07-0386. [DOI] [PubMed] [Google Scholar]
- 4.Rentzsch C, Kayser S, Stumm S, et al. Evaluation of pre-existent immunity in patients with primary breast cancer: molecular and cellular assays to quantify antigen-specific T lymphocytes in peripheral blood mononuclear cells. Clin Cancer Res. 2003;9:4376–4386. [PubMed] [Google Scholar]
- 5.Morales JK, Kmieciak M, Knutson KL, et al. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastula A, Marcinkiewicz J. Myeloid-derived suppressor cells: a double-edged sword? Int J Exp Pathol. 2011;92:73–78. doi: 10.1111/j.1365-2613.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales JK, Kmieciak M, Graham L, et al. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:941–953. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 10.Tuve S, Chen BM, Liu Y, et al. Combination of tumor site-located CTL-associated antigen-4 blockade and systemic regulatory T-cell depletion induces tumor-destructive immune responses. Cancer Res. 2007;67:5929–5939. doi: 10.1158/0008-5472.CAN-06-4296. [DOI] [PubMed] [Google Scholar]
- 11.Bernhard H, Neudorfer J, Gebhard K, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16:4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Dudley ME, Restifo NP. Cancer immunotherapy. N Engl J Med. 2008;359:1072. doi: 10.1056/NEJMc081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Lorenzo G, Buonerba C, Kantoff PW. Immunotherapy for the treatment of prostate cancer. Nat Rev Clin Oncol. 2011;8:551–561. doi: 10.1038/nrclinonc.2011.72. [DOI] [PubMed] [Google Scholar]
- 17.Cantrell DA. T cell antigen receptor signal transduction pathways. Cancer Surv. 1996;27:165–175. [PubMed] [Google Scholar]
- 18.Chatila T, Silverman L, Miller R, et al. Mechanisms of T cell activation by the calcium ionophore ionomycin. J Immunol. 1989;143:1283–1289. [PubMed] [Google Scholar]
- 19.Kazanietz MG, Lewin NE, Gao F, et al. Binding of [26-3H]bryostatin 1 and analogs to calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1994;46:374–379. [PubMed] [Google Scholar]
- 20.Chin CS, Miller CH, Graham L, et al. Bryostatin 1/ionomycin (B/I) ex vivo stimulation preferentially activates L-selectinlow tumor-sensitized lymphocytes. Int Immunol. 2004;16:1283–1294. doi: 10.1093/intimm/dxh130. [DOI] [PubMed] [Google Scholar]
- 21.Tuttle TM, Inge TH, Wirt CP, et al. Bryostatin 1 activates T cells that have antitumor activity. J Immunother (1991) 1992;12:75–81. doi: 10.1097/00002371-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan JT, Ernst B, Kieper WC, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillet AH, Bugault F, Theze J, et al. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. J Immunol. 2009;182:6267–6277. doi: 10.4049/jimmunol.0801933. [DOI] [PubMed] [Google Scholar]
- 25.Gordy LE, Bezbradica JS, Flyak AI, et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187:6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Z, Konieczny BT, Lakkis FG. The dual role of IL-2 in the generation and maintenance of CD8+ memory T cells. J Immunol. 2000;165:3031–3036. doi: 10.4049/jimmunol.165.6.3031. [DOI] [PubMed] [Google Scholar]
- 27.Bessoles S, Fouret F, Dudal S, et al. IL-2 triggers specific signaling pathways in human NKT cells leading to the production of pro- and anti-inflammatory cytokines. J Leukoc Biol. 2008;84:224–233. doi: 10.1189/jlb.1007669. [DOI] [PubMed] [Google Scholar]
- 28.de Lalla C, Festuccia N, Albrecht I, et al. Innate-like effector differentiation of human invariant NKT cells driven by IL-7. J Immunol. 2008;180:4415–4424. doi: 10.4049/jimmunol.180.7.4415. [DOI] [PubMed] [Google Scholar]
- 29.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kmieciak M, Basu D, Payne KK, et al. Activated NKT cells and NK cells render T cells resistant to myeloid-derived suppressor cells and result in an effective adoptive cellular therapy against breast cancer in the FVBN202 transgenic mouse. J Immunol. 2011;187:708–717. doi: 10.4049/jimmunol.1100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kmieciak M, Toor A, Graham L, et al. Ex vivo expansion of tumor-reactive T cells by means of bryostatin 1/ionomycin and the common gamma chain cytokines formulation. J Vis Exp. 2011;(47):2381. doi: 10.3791/2381. doi:10.3791/2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kmieciak M, Payne KK, Idowu MO, et al. Tumor escape and progression of HER-2/neu negative breast cancer under immune pressure. J Transl Med. 2011;9:35–5876-9-35. doi: 10.1186/1479-5876-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kmieciak M, Gowda M, Graham L, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89–5876-7-89. doi: 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier J, Roberts C, Avent K, et al. Fractal organization of the human T cell repertoire in health and after stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:366–377. doi: 10.1016/j.bbmt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Ko HJ, Lee JM, Kim YJ, et al. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 36.Lee JM, Seo JH, Kim YJ, et al. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int J Cancer. 2012;131:741–751. doi: 10.1002/ijc.26411. [DOI] [PubMed] [Google Scholar]
- 37.Luft T, Pang KC, Thomas E, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 38.Zhang Y, Harada A, Wang JB, et al. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood. 1998;92:118–128. [PubMed] [Google Scholar]
- 39.Dudley ME, Gross CA, Somerville RP, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152–2159. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knutson KL, Schiffman K, Cheever MA, et al. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369-377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 41.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perret R, Ronchese F. Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours? Tissue Antigens. 2008;72:187–194. doi: 10.1111/j.1399-0039.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 43.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One. 2011;6(11):e27690. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mundy-Bosse BL, Thornton LM, Yang HC, et al. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–87. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno M, Molling JW, von Mensdorff-Pouilly S, et al. IFN-gamma-producing human invariant NKT cells promote tumor-associated antigen-specific cytotoxic T cell responses. J Immunol. 2008;181:2446–2454. doi: 10.4049/jimmunol.181.4.2446. [DOI] [PubMed] [Google Scholar]
- 46.Stober D, Jomantaite I, Schirmbeck R, et al. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170:2540–2548. doi: 10.4049/jimmunol.170.5.2540. [DOI] [PubMed] [Google Scholar]
- 47.Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 48.Hegde S, Jankowska-Gan E, Roenneburg DA, et al. Human NKT cells promote monocyte differentiation into suppressive myeloid antigen-presenting cells. J Leukoc Biol. 2009;86:757–768. doi: 10.1189/jlb.0209059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang AL, Colmenero P, Purath U, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–2493. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldszmid RS, Caspar P, Rivollier A, et al. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerosa F, Baldani-Guerra B, Nisii C, et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuylenstierna C, Bjorkstrom NK, Andersson SK, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol. 2011;41:1913–1923. doi: 10.1002/eji.200940278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamessier E, Sylvain A, Thibult ML, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madjd Z, Spendlove I, Moss R, et al. Upregulation of MICA on high-grade invasive operable breast carcinoma. Cancer Immun. 2007;7:17. [PMC free article] [PubMed] [Google Scholar]
- 55.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–6947. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spear P, Barber A, Rynda-Apple A, et al. Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-gamma and GM-CSF. J Immunol. 2012;188:6389–6398. doi: 10.4049/jimmunol.1103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z, Zhang C, Zhang J, et al. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS One. 2012;7:e36928. doi: 10.1371/journal.pone.0036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parviz M, Chin CS, Graham LJ, et al. Successful adoptive immunotherapy with vaccine-sensitized T cells, despite no effect with vaccination alone in a weakly immunogenic tumor model. Cancer Immunol Immunother. 2003;52(12):739–750. doi: 10.1007/s00262-003-0405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.