Abstract
Objective:
We sought to determine via a cross-sectional study the contribution of (1) the right hemisphere's speech-relevant white matter regions and (2) interhemispheric connectivity to speech fluency in the chronic phase of left hemisphere stroke with aphasia.
Methods:
Fractional anisotropy (FA) of white matter regions underlying the right middle temporal gyrus (MTG), precentral gyrus (PreCG), pars opercularis (IFGop) and triangularis (IFGtri) of the inferior frontal gyrus, and the corpus callosum (CC) was correlated with speech fluency measures. A region within the superior parietal lobule (SPL) was examined as a control. FA values of regions that significantly predicted speech measures were compared with FA values from healthy age- and sex-matched controls.
Results:
FA values for the right MTG, PreCG, and IFGop significantly predicted speech fluency, but FA values of the IFGtri and SPL did not. A multiple regression showed that combining FA of the significant right hemisphere regions with the lesion load of the left arcuate fasciculus—a previously identified biomarker of poststroke speech fluency—provided the best model for predicting speech fluency. FA of CC fibers connecting left and right supplementary motor areas (SMA) was also correlated with speech fluency. FA of the right IFGop and PreCG was significantly higher in patients than controls, while FA of a whole CC region of interest (ROI) and the CC-SMA ROI was significantly lower in patients.
Conclusions:
Right hemisphere white matter integrity is related to speech fluency measures in patients with chronic aphasia. This may indicate premorbid anatomical variability beneficial for recovery or be the result of poststroke remodeling.
Aphasia is a common and devastating symptom of left hemisphere stroke. Although many patients recover to some degree, this recovery is often incomplete even with intensive speech therapy.1–4 While recent studies have succeeded in predicting recovery potential by quantifying the injury to relevant gray and white matter structures in the lesional left hemisphere,5,6 the right hemisphere's role in recovery of speech-language functions remains both unclear and controversial.7–10 Patients with small left hemisphere lesions may recruit perilesional areas with some involvement of right hemisphere homotops,7,8,11–13 while recovery for those with larger left lesions may occur entirely via the right hemisphere.14–21 However, some studies have suggested right hemisphere remodeling as a maladaptive mechanism or epiphenomenon.22,23 In the current study, we examine the contribution of the right hemisphere to fluency recovery by relating white matter integrity of right speech-motor homotops and the corpus callosum (CC) to a patient's arcuate fasciculus (AF) lesion load (LL) and chronic speech fluency measures.
Diffusion tensor imaging (DTI) is a useful means of studying white matter in vivo. One DTI-based measurement, fractional anisotropy (FA), has demonstrated effectiveness in correlating alterations in white matter integrity with both speech and motor outcomes after stroke or experimental interventions.24–26 We correlated FA values of (1) right hemisphere speech homotops, (2) the entire CC, and (3) selected CC fiber tracts with speech fluency measures, then compared FA values of patients vs healthy controls to detect patterns of callosal or right hemisphere white matter differences.
METHODS
Patient group.
Patients were recruited from the population of stroke patients seen at Beth Israel Deaconess Medical Center in Boston and via print and online advertisements from the greater Boston area between 2008 and 2012. Eligibility criteria included a history of left hemisphere stroke at least 6 months prior to assessment, age 12–80 years, right-handedness, and expressive aphasia despite acute rehabilitation. Exclusion criteria included bihemispheric or brainstem infarcts, history of stroke prior to the one causing aphasia, other concomitant neurologic diseases/disorders, and significant cognitive impairments (defined as <50% correct on the Raven Colored Progressive Matrices27).
Control group.
To examine potential differences in diffusion measures of right hemisphere and CC regions of interest (ROIs) between patients and controls, a group of 13 age- and sex-matched healthy controls underwent DTI (2 female; age 63 ± 14.7 years). All controls were native English speakers, right-handed, and had normal cognitive ability as evidenced by a score in the normal range on the Shipley/Hartford scale (an equivalent of IQ)28 or attainment of postsecondary degrees.
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Board of Beth Israel Deaconess Medical Center approved this study and all participants gave written informed consent.
Behavioral data.
All patients performed a battery of language tests assessing spontaneous speech production, naming, repetition, and comprehension (table e-1 on the Neurology® Web site at Neurology.org). Patients were evaluated for speech fluency and accuracy of content elicited during conversational interviews (comprised of questions about biographical data, medical history, daily activities, and a description of a routine procedure) and complex picture descriptions (e.g., the Cookie Theft picture from the Boston Diagnostic Aphasia Examination and the picnic picture from the Western Aphasia Battery, as well as other similar pictures). Patients' responses were videotaped and transcribed by a rater blinded to patients' other behavioral/cognitive and imaging measures. Our study focused on 2 speech fluency/efficiency measures: words per minute (words/min, a measure of speech fluency) and correct information units per minute (CIUs/min, a measure of speech “efficiency”).29 Words/minute takes into account all words spoken in response to a prompt including both correct and informative words as well as repeated, incorrect, or otherwise irrelevant words. In contrast, CIUs/min takes into account only words/phrases that are relevant, accurate, intelligible, and informative in the context of a conversation or complex picture, and excludes exclamations, perseverations, and meaningless utterances. More extensive details of the speech assessments can be found in previous publications.5
Our group of healthy age- and sex-matched controls did not undergo any fluency measures or naming tests. However, published data of a healthy control group suggests that the range of CIUs/min can be from 92 to 175, and words/min can be from 105 to 198.29
Structural and diffusion MRI.
All stroke patients were scanned with a 3T General Electric (Cleveland, OH) MRI scanner using a standard radiofrequency head coil. T1-weighted MRI (voxel resolution 0.93 × 0.93 × 1.5 mm) were spatially normalized to the SPM T1 template (isotropic 2-mm voxel size) in SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK) implemented in MATLAB (The Mathworks Inc., Natick, MA). Patients' T1 image normalizations were supported by excluding the chronic ischemic lesion from the registration algorithm before normalization.30
Additionally, all patients underwent DTI. The diffusion images were acquired using a diffusion-weighted, single-shot, spin-echo, echoplanar imaging sequence (echo time 86.9 milliseconds, repetition time 10,000 milliseconds, field of view 240 mm, slice thickness 2.5 mm resulting in a voxel size of 2.5 mm3, no skip, number of excitations 1, axial acquisition, 30 noncollinear directions with b value of 1,000 seconds/mm2). There were 5 volumes acquired with b value of 0 s/mm2 for 24 patients, and 6 volumes acquired with b value of 0 s/mm2 for the remainder. All DTI processing and analysis was done in FSL (www.fmrib.ox.ac.uk) and has been described in detail elsewhere.26
ROI generation and FA extraction.
All individual ROIs were extracted from the Harvard-Oxford Atlas in FSL (www.cma.mgh.harvard.edu/fsl_atlas.html) and multiplied with FSL's FMRIB58 1 mm FA template at an FA threshold of 0.3 to ensure inclusion of white matter only. Our right hemisphere ROIs included the white matter underlying the pars opercularis and pars triangularis of the inferior frontal gyrus (IFGop, IFGtri), the middle temporal gyrus (MTG), and the precentral gyrus (PreCG) including the primary motor and premotor cortices, all of which have been shown to be consistently involved in speech-motor and language tasks in both normal healthy controls and in patients with aphasia.9,31 In addition, these regions are served by and connected via the AF, of which the segment connecting Broca and Wernicke right hemisphere homotops has been found to be larger in aphasic patients with better recovery of fluency after left hemisphere stroke.32 For a control region, we chose the white matter underlying the posterior superior parietal cortex (figure 1) within the superior parietal lobule (SPL). We chose this region due to a lack of a priori information that this region was involved in speech motor functions. In addition, we generated left hemisphere ROIs of white matter underlying the IFGop, MTG, and PreCG (homotop to the right hemisphere ROIs found to significantly predict speech measures in correlation analysis; see Results) in order to calculate lesion/ROI overlap ratios. Combined right and combined left hemisphere ROIs were generated by summing these same 3 individual ROIs (IFGop, MTG, and PreCG) from each hemisphere.
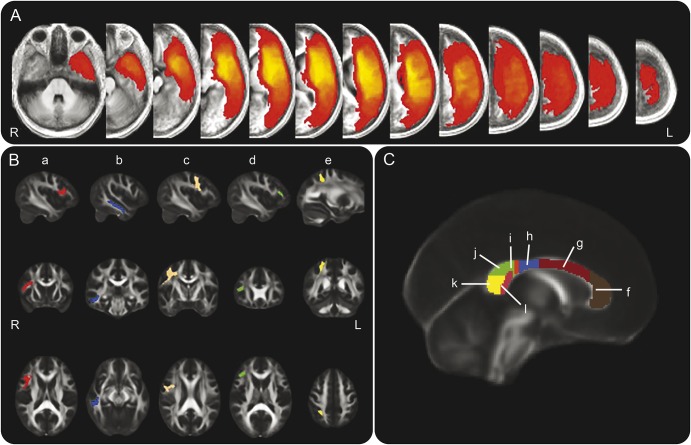
Figure 1. Lesion density map and regions of interest (ROI).
(A) Lesion density map created by summation of all patients' lesion maps. Intensities convey number of patients whose lesions include a given voxel (red = 1; bright yellow = 33). (B) Right hemisphere white matter ROIs used for extracting diffusivity values: (a) right pars opercularis of the inferior frontal gyrus (IFGop) ROI (Brodmann area 44, ROI spans X = 50 to 28, Y = 27 to −4, Z = 27 to 7), (b) right middle temporal gyrus (MTG) ROI (ROI spans X = 59 to 39, Y = −5 to −44, and Z = 4 to −27), (c) right precentral gyrus (PreCG) ROI (ROI spans X = 55 to 6, Y = 10 to −38, and Z = 72 to 10), (d) right pars triangularis of the inferior frontal gyrus ROI (ROI spans X = 46 to 35, Y = 33 to 23, and Z = 21 to 67), (e) right superior parietal lobule ROI (ROI spans Z = 32 to 15, Y = −57 to −38, Z = 62 to 38). All hemispheric ROIs were extracted from the Harvard-Oxford atlas in FSL and thresholded at a fractional anisotropy (FA) value of 0.3 so as to include only white matter. Left hemisphere ROIs were generated using the same Harvard-Oxford regions in the left hemisphere; right and left combined hemispheric ROIs are the summation of 3 individual ROIs in the specified hemisphere: IFGop, MTG, and PreCG (the ROIs which, in the right hemisphere, were significant predictors of speech fluency). (C) Corpus callosum (CC) ROIs corresponding to locations of fibers crossing between the left and right frontal (f), supplementary motor (g), motor (h), somatosensory (i), parietal (j), occipital (k), and temporal (l) areas. The ROI referred to as the entire CC ROI is the sum of these 7 individual ROIs. All CC ROIs were drawn by hand according to a previously published schematic.33 ROIs are displayed overlaid onto FSL's FMRIB-58 FA template. All coordinates are in Montreal Neurological Institute 152 space.
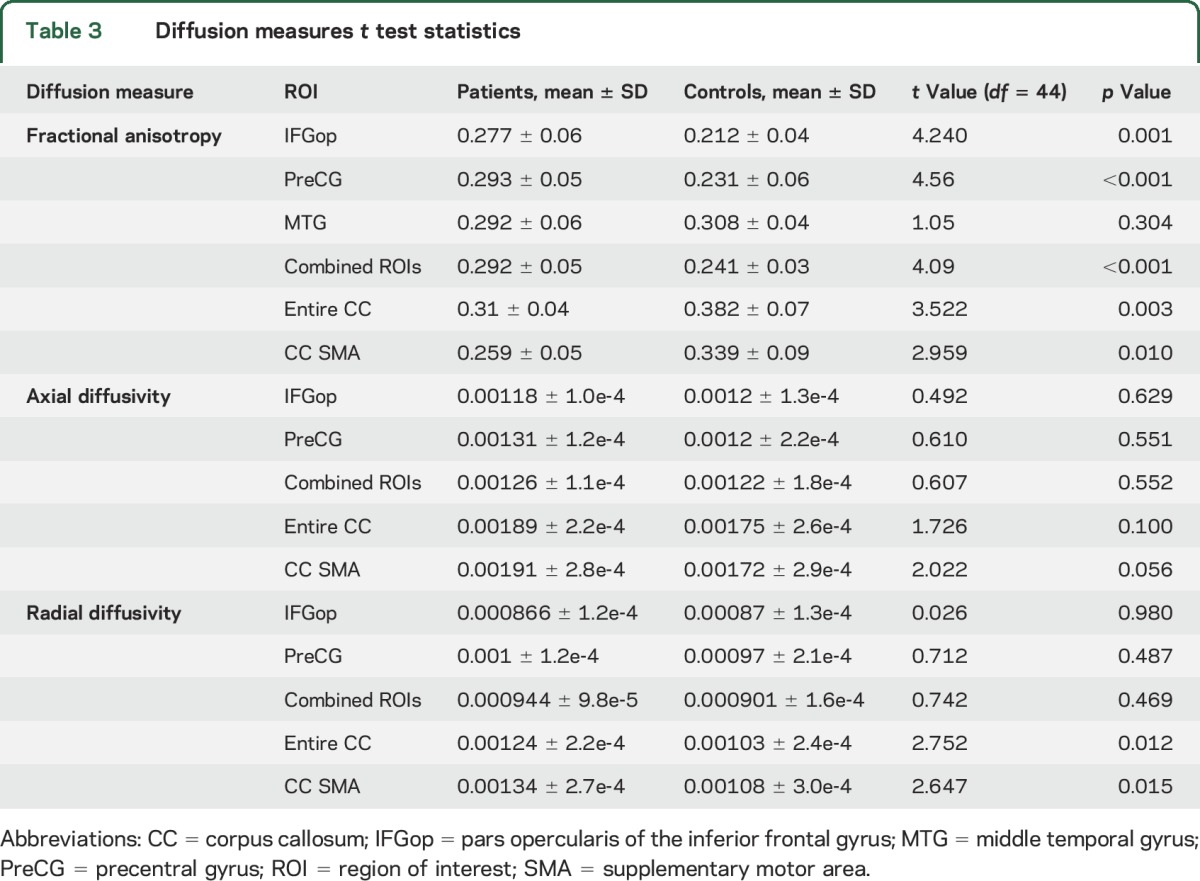
Seven CC ROIs were drawn by hand on 9 sagittal slices in a classification scheme similar to a well-established schematic based on DTI fiber tracking.33 These ROIs (figure 1) correspond to areas of the CC thought to be used by fibers crossing between left and right cortical regions including the supplementary motor area (SMA) region. CC ROIs were also summed to create a whole CC ROI. Mean FA, axial diffusivity (AD), and radial diffusivity (RD) values from all ROIs are shown in table 3.
Table 3.
Diffusion measures t test statistics
LL and lesion/ROI overlap calculation.
To assess the extent of damage to the white matter serving speech-motor function, a LL of the left AF was calculated for each patient. LL seeks to measure the overlap of a patient's lesion with a fiber map of a particular white matter tract.34 In this case, we chose to use a probabilistically derived canonical left AF tract for which LL had been previously shown to be an effective predictor of speech-fluency recovery.5
All patients had lesion maps drawn by a single rater on normalized T1-weighted MRI in MRIcro (http://www.mccauslandcenter.sc.edu/mricro) using the coregistered T2 image to guide lesion mapping. A second rater (blinded to the first rater's results) drew additional lesion maps for 20 patients, achieving an interrater reliability >0.9 for lesion map volume. LL was calculated by overlaying each patient's lesion map (using the maps drawn by the first rater) with the aforementioned probabilistically derived canonical left AF tract. For a given patient, each voxel included in both the lesion map and the canonical tract was multiplied by its probability of inclusion in the tract and the volume of the voxel, with the sum of all such calculations giving the LL for that patient. Previous studies have shown that LL is a better predictor of behavioral outcomes after stroke than is lesion volume.6,34
To determine the extent to which each left hemisphere ROI was affected by a patient's lesion, we calculated a second LL variable between the lesion and left hemisphere ROIs, which we termed lesion/ROI overlap ratio for each patient, according to the following equation:
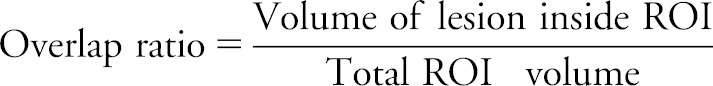 |
This lesion/ROI overlap ratio provides a decimal representation for the proportion of each left hemisphere ROI affected by the lesion in each patient. To carry out this calculation, we normalized our left hemisphere ROIs to the SPM5 T1 template brain using FSL's FLIRT (linear transform) function. This transformation was chosen for optimal alignment with lesion maps drawn previously on brains normalized in SPM5. We then masked each patient's lesion map with each left hemisphere ROI and calculated an overlap ratio.
Statistical analyses.
All statistical analyses were done in Predictive Analytics Software, SPSS (17.0.2) (Chicago, IL). Linear and multiple regressions were run to assess the ability of each right hemisphere and CC ROI as well as combinations of right hemisphere and CC ROIs to predict speech measures. LL was included in the multiple regressions to determine whether a combination of AF-LL and right hemisphere FA values would improve upon predictions made using AF-LL alone. Since the output of the LL equation is a volumetric measurement, the cube root of left AF-LL was used for all correlations and regressions.35,36 In addition, we correlated lesion/ROI overlap ratios for left hemisphere ROIs with CC ROI FA values to determine whether the degree of left hemisphere lesion/ROI overlap could predict CC white matter integrity.
Two-tailed independent t tests assuming unequal variance were run between patient and control groups comparing FA values for each right hemisphere and CC ROI that significantly predicted both speech fluency measures.
RESULTS
Patient statistics.
Our study group consisted of 33 patients with left hemisphere lesions (5 female; age 57.8 ± 8.7 years) who comprise a subgroup of the 50 patients reported previously5 for whom we had isotropic DTI scans. All patients were native English speakers and at least 6 months poststroke (mean onset-to-assessment time 30.6 ± 29.6 months), but had varying degrees of speech recovery. Thirty-two of 33 patients were right handed, with one being mixed handed. All patients had some degree of speech fluency impairment in the acute phase, which progressed to various forms of aphasia in the chronic stage (see table e-1, patients' speech assessment details).
Correlation and regression analyses.
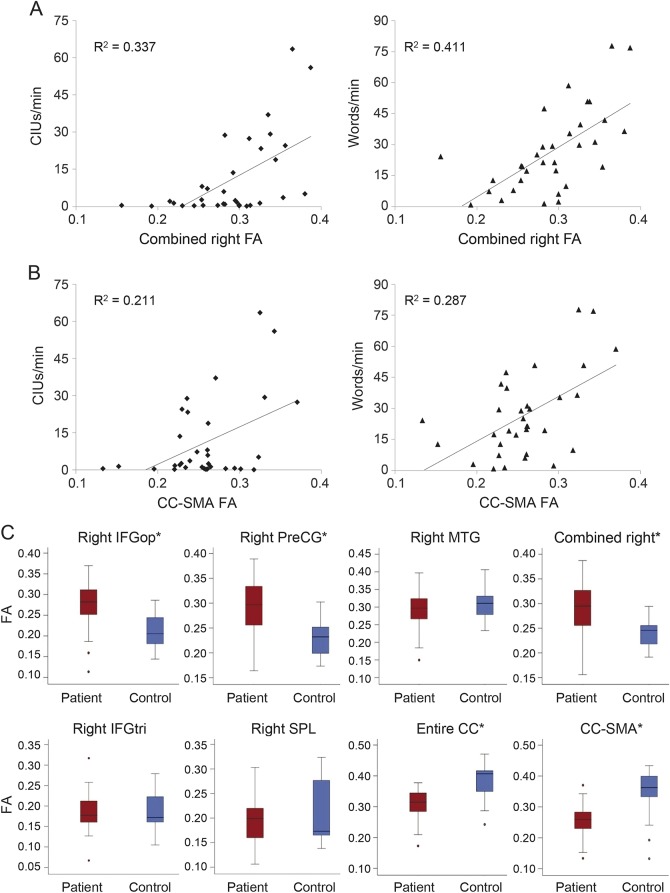
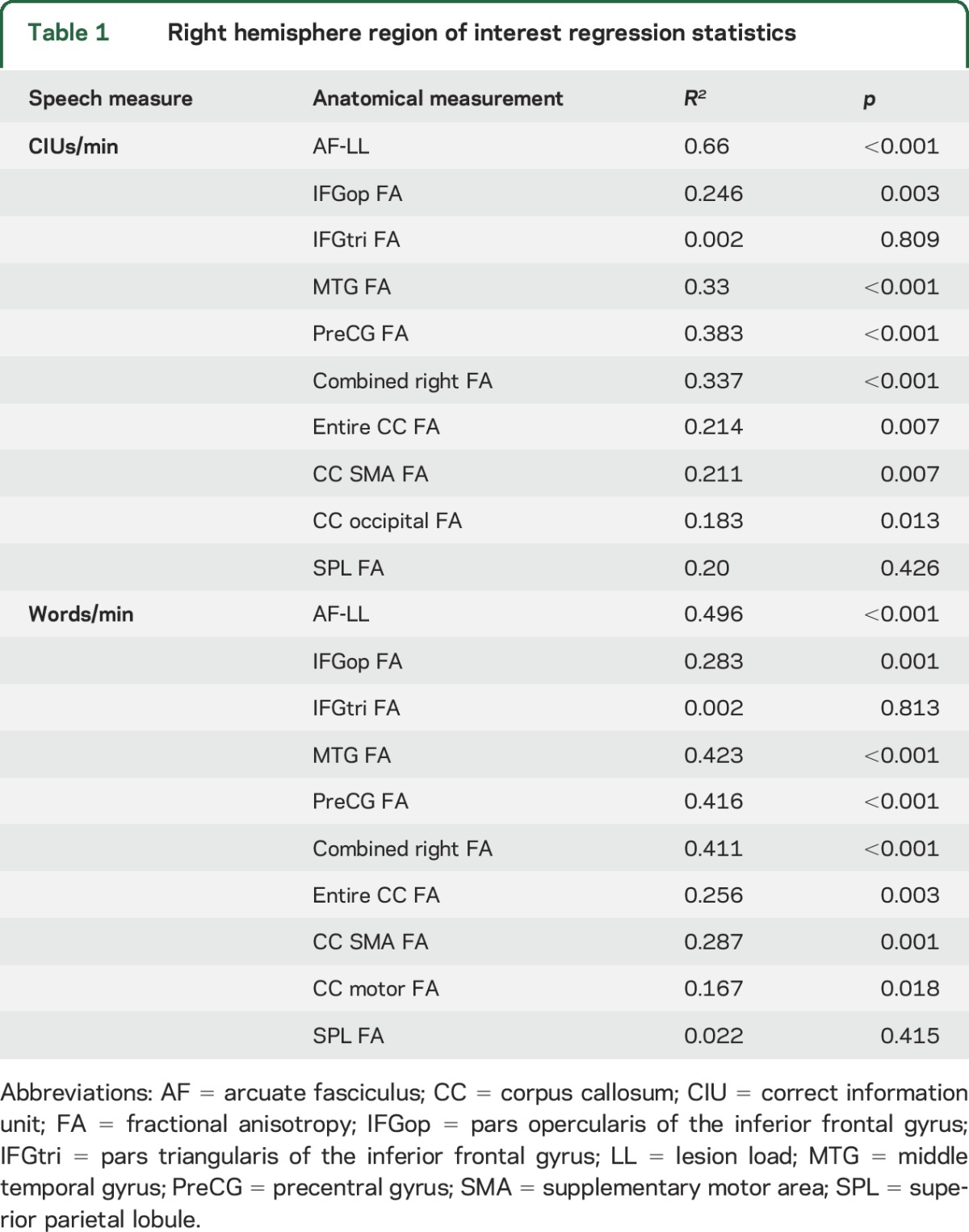
FA values of the white matter underlying the right MTG, IFGop, PreCG, and combined regions of those ROIs all significantly predicted both speech fluency measures (see figure 2 for regression graphs and mean FA values, and table 1 for p and R2 values), with the MTG and PreCG generally having the highest correlations. FA values of the IFGtri and SPL (control) regions did not significantly predict speech fluency (p > 0.05); therefore, these regions were not included in further analyses. FA values of the CC-SMA ROI significantly predicted both speech measures, while FA values of the CC-occipital ROI predicted only CIUs/min and FA values from the CC-motor ROI predicted only words/min. FA values for the entire CC predicted both CIUs/min and words/min, though this significance was driven primarily by the CC-SMA ROI.
Figure 2. Fractional anisotropy (FA) and speech fluency measures regression and FA boxplots.
(A) Scatterplots of correct information units (CIUs)/min and words/min vs FA of the combined right hemisphere region of interest (ROI). FA of this combined ROI predicts outcomes on both speech measures (R2CIUs = 0.337, pCIUs < 0.001, R2words = 0.411, pwords < 0.001). (B) Scatterplots of CIUs/min and words/min vs FA of the corpus callosum (CC) area consisting of fibers crossing between the left and right supplementary motor areas (SMA). FA of this region also predicts outcomes on both speech measures (R2CIUs = 0.211, pCIUs = 0.007, R2words = 0.287, pwords = 0.001). (C) Boxplots of FA in patients and controls from each right hemisphere ROI as well as from the CC ROIs found to significantly predict both CIUs/min and words/min. *Significant difference between groups, although only precentral gyrus (PreCG), combined right hemisphere (RHem) ROI, and the entire CC ROI survived Bonferroni correction (threshold p value = 0.008). See table 3 for t statistics and p values. IFGop = pars opercularis of the inferior frontal gyrus; IFGtri = pars triangularis of the inferior frontal gyrus; MTG = middle temporal gyrus.
Table 1.
Right hemisphere region of interest regression statistics
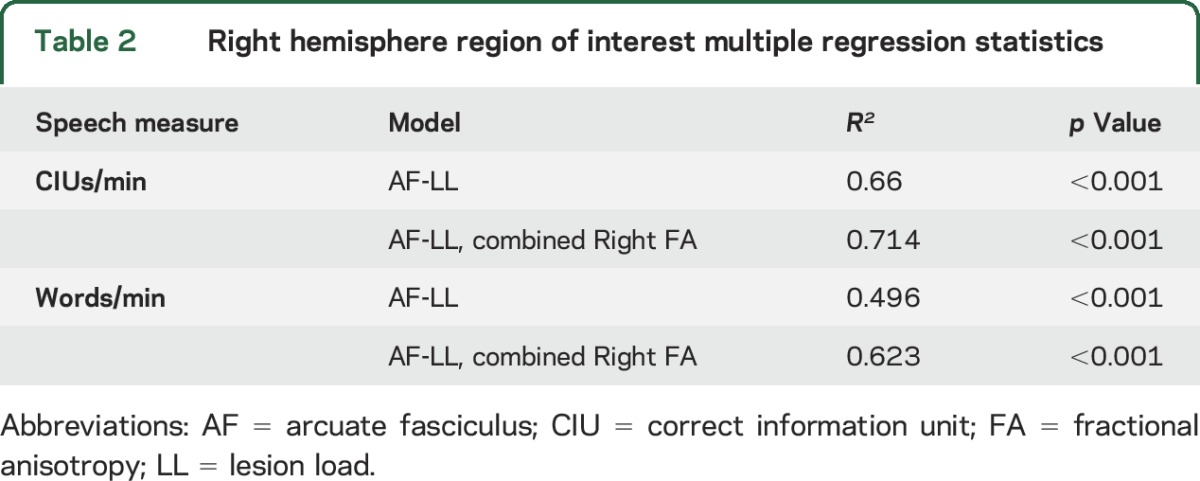
Stepwise multiple regressions using left AF-LL, FA values of the combined significant right hemisphere ROIs (RHem-FA), and CC-SMA ROI FA values as independent variables found that the most predictive model for both CIUs/min and words/min included AF-LL and RHem-FA but not CC-SMA ROI FA (table 2). A partial correlation showed that Rhem-FA was still predictive of both speech fluency measures when AF-LL was held constant (partial r-CIUs = 0.398, partial pCIUs = 0.024, partial rwords = 0.502, partial pwords = 0.003). Since R2 values do not convey directionality, it should be noted that higher behavioral measures were associated with both higher FA values in right hemisphere regions and with lower AF-LL.
Table 2.
Right hemisphere region of interest multiple regression statistics
Diffusion measures analysis.
FA values were significantly higher in patients than controls in the right IFGop, PreCG, and combined RHem-ROI (see table 3 for t and p values). In contrast, FA values were significantly lower in patients than controls for the whole CC ROI and the CC-SMA ROI (see figure 2C). Only the right IFGop, PreCG, combined RHem-ROI, and whole CC ROI survived Bonferroni correction (threshold p value for α = 0.05 with 6 comparisons = 0.008). Somewhat surprisingly, we found no significant difference in FA values between groups for the MTG despite the predictive value of MTG FA with regard to speech fluency.
To further examine and better interpret these differences in FA values, we compared AD and RD between groups in regions found to have significant between-group FA differences. RD was significantly higher in patients in the whole CC ROI and CC-SMA ROI, while AD was higher in patients in these areas but not significantly so (0.05 < p ≤ 0.1, see also table 3).
Left hemisphere lesion/ROI overlap and CC fiber FA.
To test whether the degree of damage to left hemisphere homotops of our right hemisphere ROIs could predict CC FA values, we correlated lesion/ROI overlap ratios in the combined left hemisphere ROI (LHem-ROI) with FA values of the CC ROIs that significantly predicted both speech measures. We found a significant correlation between lesion/ROI overlap ratio of the combined LHem-ROIs and FA values in the entire CC ROI and CC-SMA ROI, indicating lower FA in these regions in patients with more significant left hemisphere damage (RWhole CC = −0.372, pWhole CC = 0.033, RCC-SMA = −0.371, pCC-SMA = 0.034). To determine whether the significance of the correlation was being driven by any one left hemisphere ROI, we correlated lesion/ROI overlap ratio of each left hemisphere ROI with FA values of the whole CC ROI and CC-SMA ROI. Only the PreCG lesion/ROI overlap ratio was significant with both regions (RWhole CC = −0.401, pwhole CC = 0.021, RCC-SMA = −0.423, pCC-SMA = 0.014). In addition, IFGop lesion/ROI overlap ratio showed a trend toward significance for the whole CC ROI (R = −0.311, p = 0.079).
DISCUSSION
As shown previously,5,6 LL of the left AF is a strong predictor of speech fluency after stroke. Our new model, which combines left AF-LL and FA values of right hemisphere speech-relevant white matter regions, explains between 62% (words/min) and 71% (CIUs/min) of the variance in speech fluency outcomes.
The right hemisphere regions we examined are all connected via the AF, which has been shown previously to have higher volume in the right hemispheres of better-recovered patients with left hemisphere stroke-induced aphasia, possibly indicating an increase in signal efficiency.32 Using a more direct measurement (FA), our results show that white matter integrity in 3 right hemisphere speech homotops served by this tract is indeed higher in better-recovered patients. Interestingly, FA values of our right MTG ROI were predictive of speech fluency but showed no significant difference between groups. This may suggest that premorbid differences in WM integrity of the right MTG or differential language lateralization contributed to recovery of speech fluency.
In addition, we observed decreased FA in our whole CC ROI as well as the CC-SMA ROI, though the latter finding did not survive Bonferroni correction. Given previous imaging studies showing that axonal loss and demyelination associated with Wallerian degeneration (WD) can be detected in DTI as decreased FA and increased RD in white matter distant from the injury,37,38 we hypothesize that our CC findings are due to WD caused by the left hemisphere lesion.39 Our finding that increased lesion/ROI overlap ratio in the left hemisphere predicted lower FA values in the whole CC ROI and CC-SMA ROI lends additional support to this hypothesis.
To our knowledge, no previous study has shown an association between higher FA values of right hemisphere speech-motor homotops and higher speech fluency measures in aphasic patients after left hemisphere stroke. Our findings indicate that right hemisphere white matter regions must play a role in speech-motor and language recovery, whether due to prestroke anatomical variability providing a favorable condition for recovery from left hemisphere lesions or due to poststroke remodeling. We hypothesize that the observed between-group differences in FA values of the right PreCG and IFGop, combined with the lack of observed difference in FA values between groups in the right MTG, IFGtri, or SPL control region, indicate that poststroke reorganization of right hemisphere white matter in the PreCG and IFGop region is likely. This interpretation would suggest right hemisphere remodeling as a beneficial mechanism rather than a maladaptive one or an epiphenomenon, which is in contrast with interpretations of some previous studies.13,22,23,40
A principal limitation of our study is that it does not allow us to identify any time points at which these right hemisphere or CC changes may occur or to conclusively determine whether or not these differences are preexisting. In addition, the larger representation of men in our study groups (85% for both patients and controls) may limit the cross-gender generalizability of our findings. However, our results provide strong evidence that WM integrity of right hemisphere speech-motor homotops is (1) an important factor in recovery of speech fluency and (2) related to the degree of damage to left hemisphere speech-motor regions. In addition, our finding that FA values of right hemisphere speech-motor homotops used in conjunction with AF-LL form a strong predictor of speech fluency in chronic stroke patients may be relevant for future clinical use. Longitudinal studies will be necessary to establish a detailed timeline of right hemisphere and CC FA changes during both natural and experimentally facilitated speech recovery after left hemisphere stroke.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Sarah Marchina for contributions to data collection and technical assistance.
GLOSSARY
- AD
axial diffusivity
- AF
arcuate fasciculus
- CC
corpus callosum
- CIU
correct information unit
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- IFGop
pars opercularis of the inferior frontal gyrus
- IFGtri
pars triangularis of the inferior frontal gyrus
- LHem-ROI
left hemisphere region of interest
- LL
lesion load
- MTG
middle temporal gyrus
- PreCG
precentral gyrus
- RD
radial diffusivity
- RHem-FA
fractional anisotropy values of the combined significant right hemisphere regions of interest
- ROI
region of interest
- SMA
supplementary motor area
- SPL
superior parietal lobule
- WD
Wallerian degeneration
Footnotes
Supplemental data at Neurology.org
Editorial, page 1566
AUTHOR CONTRIBUTIONS
Ethan Pani: drafting and revising manuscript for content, analysis and interpretation of data, statistical analysis. Xin Zheng: revising the manuscript for content, analysis and interpretation of data. Jasmine Wang: revising the manuscript for content, analysis and interpretation of data, acquisition of data. Andrea Norton: revising the manuscript for content, acquisition of data. Gottfried Schlaug: drafting and revising the manuscript for content, study concept and design, analysis and interpretation of data, study supervision and coordination, obtaining funding.
STUDY FUNDING
Supported by NIH (1RO1 DC008796, 3R01DC008796-02S1, R01 DC009823-01), the Richard and Rosalyn Slifka Family Fund, the Tom and Suzanne McManmon Family Fund, and the Mary Crown and William Ellis Fund.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain 1977;1:1–18. [DOI] [PubMed] [Google Scholar]
- 2.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry 1986;49:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol 1995;38:659–666. [DOI] [PubMed] [Google Scholar]
- 4.Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37:1379–1384. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Marchina S, Norton AC, Wan CY, Schlaug G. Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci 2013;7:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 2011;42:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang 2006;98:118–123. [DOI] [PubMed] [Google Scholar]
- 8.Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain 2006;129:1371–1384. [DOI] [PubMed] [Google Scholar]
- 9.Turkeltaub PE, Messing S, Norise C, Hamilton RH. Are networks for residual language function and recovery consistent across aphasic patients? Neurology 2011;76:1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaug G, Marchina S, Wan CY. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychol Rev 2011;21:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 1999;45:430–438. [DOI] [PubMed] [Google Scholar]
- 12.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology 2007;69:200–213. [DOI] [PubMed] [Google Scholar]
- 13.Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology 2000;55:1883–1894. [DOI] [PubMed] [Google Scholar]
- 14.Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia. Two case studies. Cortex 1989;25:555–566. [DOI] [PubMed] [Google Scholar]
- 15.Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron 2002;36:159–170. [DOI] [PubMed] [Google Scholar]
- 16.Cappa SF, Vallar G. The role of the left and right hemispheres in recovery from aphasia. Aphasiology 1992;6:359–372. [Google Scholar]
- 17.Cappa SF, Perani D, Grassi F, et al. A PET follow-up study of recovery after stroke in acute aphasics. Brain Lang 1997;56:55–67. [DOI] [PubMed] [Google Scholar]
- 18.Kinsbourne M. The right hemisphere and recovery from aphasia. In: Stemmer B, Whitaker HA, ed. Handbook of Neurolinguistics. New York: Academic Press; 1998:386–393. [Google Scholar]
- 19.Selnes OA. Recovery from aphasia: activating the “right” hemisphere. Ann Neurol 1999;45:419–420. [PubMed] [Google Scholar]
- 20.Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke's aphasia: a positron emission tomographic study. Ann Neurol 1995;37:723–732. [DOI] [PubMed] [Google Scholar]
- 21.Zipse L, Norton A, Marchina S, Schlaug G. When right is all that is left: plasticity of right hemisphere tracts in a young aphasic patient. Ann NY Acad Sci 2012;1252:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin PI, Naeser MA, Theoret H, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang 2004;25:181–191. [DOI] [PubMed] [Google Scholar]
- 23.Postman-Caucheteux WA, Birn RM, Pursley RH, et al. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci 2010;22:1299–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puig J, Blasco G, Daunis IEJ, et al. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke 2013;44:2016–2018. [DOI] [PubMed] [Google Scholar]
- 25.Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 2011;33:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca's aphasia. Brain Lang 2014;136:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raven JC. Coloured Progressive Matrices. Oxford: Oxford Psychologists Press; 1995. [Google Scholar]
- 28.Paulson MJ, Lin TT. Predicting WAIS IQ from Shipley-Hartford scores. J Clin Psychol 1970;26:453–461. [DOI] [PubMed] [Google Scholar]
- 29.Nicholas LE, Brookshire RH. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech Hear Res 1993;36:338–350. [DOI] [PubMed] [Google Scholar]
- 30.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage 2001;14:486–500. [DOI] [PubMed] [Google Scholar]
- 31.Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. NeuroImage 2006;33:628–635. [DOI] [PubMed] [Google Scholar]
- 32.Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain 2014;137:2027–2039. [DOI] [PubMed] [Google Scholar]
- 33.Hofer S, Frahm J. Topography of the human corpus callosum revisited: comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage 2006;32:989–994. [DOI] [PubMed] [Google Scholar]
- 34.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA stroke study group. Stroke 1999;30:2355–2359. [DOI] [PubMed] [Google Scholar]
- 36.van den Elskamp IJ, Knol DL, Uitdehaag BM, Barkhof F. Modeling MR imaging enhancing-lesion volumes in multiple sclerosis: application in clinical trials. AJNR Am J Neuroradiol 2011;32:2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 2000;69:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 2005;26:132–140. [DOI] [PubMed] [Google Scholar]
- 39.Iizuka H, Sakatani K, Young W. Selective cortical neuronal damage after middle cerebral artery occlusion in rats. Stroke 1989;20:1516–1523. [DOI] [PubMed] [Google Scholar]
- 40.Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol 2001;50:620–629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.