Abstract
Purpose
To evaluate if the preoperative administration of levosimendan in patients with right ventricular (RV) dysfunction, pulmonary hypertension, and high perioperative risk would improve cardiac function and would also have a protective effect on renal and neurological functions, assessed using two biomarkers neutrophil gelatinase-associated lipocalin (N-GAL) and neuronal enolase.
Methods
This is an observational study. Twenty-seven high-risk cardiac patients with RV dysfunction and pulmonary hypertension, scheduled for cardiac valve surgery, were prospectively followed after preoperative administration of levosimendan. Levosimendan was administered preoperatively on the day before surgery. All patients were considered high risk of cardiac and perioperative renal complications. Cardiac function was assessed by echocardiography, renal function by urinary N-GAL levels, and the acute kidney injury scale. Neuronal damage was assessed by neuron-specific enolase levels.
Results
After surgery, no significant variations were found in mean and SE levels of N-GAL (14.31 [28.34] ng/mL vs 13.41 [38.24] ng/mL), neuron-specific enolase (5.40 [0.41] ng/mL vs 4.32 [0.61] ng/mL), or mean ± SD creatinine (1.06±0.24 mg/dL vs 1.25±0.37 mg/dL at 48 hours). RV dilatation decreased from 4.23±0.7 mm to 3.45±0.6 mm and pulmonary artery pressure from 58±18 mmHg to 42±19 mmHg at 48 hours.
Conclusion
Preoperative administration of levosimendan has shown a protective role against cardiac, renal, and neurological damage in patients with a high risk of multiple organ dysfunctions undergoing cardiac surgery.
Keywords: levosimendan, preoperative, kidney, brain, acute renal failure
Introduction
Levosimendan is an inotropic agent with vasodilatory and protective effects, acting via the calcium channels in cardiac myofilaments and the opening of adenosine triphosphate (ATP)-sensitive potassium channels in vascular smooth muscle cells and ATP-sensitive potassium channels in cardiac mitochondria.1
Through this mechanism, levosimendan increases the contractile force of cardiac muscle fibers, causes peripheral vasodilatation in both arteries and veins, and protects the heart against ischemic events; the KATP channels in the mitochondria have a predominant role in the protection of the myocardium from ischemia and reperfusion injury.2 Several studies have demonstrated the benefit of preoperative levosimendan administration in patients with ventricular dysfunction.3–5
Right ventricular (RV) failure is associated with higher mortality rates than left ventricle failure, reaching up to 48% in patients admitted to intensive care units (ICUs).6 Mortality in patients with RV dysfunction and pulmonary hypertension is further increased with concomitant renal dysfunction; preoperative treatment with levosimendan in these patients appears to improve RV function and reduces postoperative pulmonary hypertension.6–8
Neurological and renal complications after cardiac surgery are frequent and have a significant impact on morbidity and mortality. Recently, cognitive impairment has been identified in 83% of patients undergoing cardiac surgery, and so the incidence of neurological damage in this type of surgery is high.9 For its part, acute renal failure prolongs hospital stay and increases the mortality risk by between three and nine times depending on severity; small increases in creatinine worsen the prognosis of these patients, especially those with RV dysfunction.10,11
Several studies have assessed the possible beneficial effects of levosimendan on renal function after cardiac surgery. Bragadottir et al12 reported an improvement in glomerular filtration rate, renal flow, and oxygenation, which was independent of the improvement in cardiac function.12 The possible neuroprotective effects of levosimendan have also been studied by several authors.13,14
We hypothesized that the preoperative administration of levosimendan in patients with RV dysfunction, pulmonary hypertension, and high perioperative risk would improve cardiac function and would also have a protective effect on renal and neurological function.
Methods
This prospective study was approved by the Institutional Review Board of University Hospital Virgen de la Victoria, Málaga, Spain. Written informed consent was obtained from all subjects. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Twenty-seven high cardiac risk patients (defined as having RV dysfunction with pulmonary hypertension and EUROSCORE II >5%), scheduled for cardiac valve surgery, were prospectively followed after preoperative administration, once they gave informed consent. Inclusion criteria were RV dysfunction: tricuspid annulus diameter >35 (mm) and pulmonary hypertension (systolic pulmonary pressure >30 mmHg), scheduled valve surgery (replacement or repair), a Cleveland Clinic Acute Kidney Injury (AKI) score (>7= high risk), and EuroSCORE II values >5%.15,16 Criteria for exclusion included the history of adverse reaction to levosimendan, combined surgery (not cardiac surgery, eg, carotid surgery), preoperative renal dysfunction (creatinine clearance <50 mL/min), hypersensitivity to levosimendan or any of the excipients, hemodynamic instability (systolic blood pressure <90 mmHg and tachycardia, >100 beats/min), mechanical ventricular obstruction, history of Torsade de Pointes, or previous neurological dysfunction. The study aimed to evaluate the neurological and renal benefits of the administration of levosimendan in patients considered at high risk of cardiac and renal dysfunction due to RV dysfunction and pulmonary hypertension, all prior to a cardiac valvular surgery, a surgical procedure with known higher risks of both systems complications.
Levosimendan was administered via continuous infusion at a rate of 0.1 μg/kg/min until a dose of 12.5 μg was reached on the day prior to surgery. During administration, patients were monitored with continuous electrocardiography (ECG) and pulse oximetry. Blood pressure was also recorded every 30 minutes.
Anesthesia and surgical procedure
All patients were monitored upon arrival in the operating room with continuous ECG, invasive blood pressure monitoring through the right radial artery, pulse oximetry, capnography, monitoring of hypnosis through the bispectral index (BIS; BIS XP®, Aspect Medical Systems, Newton, MA, USA), as well as temperature of the pharynx, bladder, and blood. Cardiac function was assessed with transesophageal echocardiography (Philips Sonos 7500) and pulmonary artery catheter (PAC-Edwards Lifesciences, Irvine, CA, USA). Induction and intraoperative maintenance of anesthesia were performed in a similar way in all cases, with sevoflurane (Sevorane®, Abbott, Louvain-la-Neuve, Belgium) as the anesthetic drug before and after extracorporeal circulation and with propofol during the cardiopulmonary bypass (CPB). The various hypnotics were adjusted to maintain a BIS value of between 45 and 60, ensuring hypnosis and avoiding overdose. Neuromuscular relaxation was maintained with cisatra-curium (Nimbex®, GlaxoSmithKline, London, UK) infusion (1–2 μg/kg/min). Analgesia was performed with intravenous remifentanil infusion at a rate of 0.1–0.2 μg/kg/min (Ultiva®, GlaxoSmithKline, Genval, Belgium).
At the end of the surgery, all patients were transferred to the ICU and were sedated with propofol and remifentanil until extubation.
Study protocol
Postoperative hemodynamic management was standardized as follows: adrenaline, dobutamine, and/or noradrenaline were administered by continuous infusion if cardiac index was low (<2.4 L/min/m2 and/or central venous oxygen saturation was <65%), if vascular resistance was normal or low, and if pulmonary capillary wedge pressure was higher than 15 mmHg (to avoid situations of low cardiac output and hypoperfusion; Table 1). If urine output was <1 mL/kg/h 1 hour after hemodynamic optimization, 500 mL of Ringer’s lactate was administered for 10 minutes and repeated if the result was not satisfactory, always provided that pulmonary wedge pressures did not exceed 15 mmHg; and if there was no response, 10 mg of furosemide was administered every 30 minutes until a favorable response was achieved. If urine output was >1 mL/kg/h, no diuretic drug was administered.
Table 1.
Inotropic management (protocol)
| Cardiac index | Cardiac index <2.4 L/min/m2 and/or central venous oxygen saturation was <65% and wedge pressure >15 mmHg | Cardiac index <2.4 L/min/m2 and/or central venous oxygen saturation was <65% and wedge pressure <15 mmHg |
|---|---|---|
| Systolic blood pressure >70 mmHg | Dobutamine (initial dose of 5 μg/kg/min) | Volume bolus (500 mL of Ringer’s solution) |
| Systolic blood pressure <70 mmHg | Adrenaline ± noradrenaline | Volume bolus (500 mL of Ringer’s solution) |
Urine output was monitored for the first 48 hours and also if postoperative renal replacement therapy was necessary. Epidemiological data on age, sex, previous treatment, EuroSCORE II, Cleveland score, CPB time, ischemia time, and type of surgery were recorded in all patients.
Analytical measurements
Hemoglobin, troponin I, CK-MB, creatinine, urea, lactate, and central venous saturation were recorded before administration of levosimendan, on arrival in the operating room, on arrival in the ICU, and 24 and 48 hours after surgery. NT-proBNP value was recorded before administration of levosimendan and 24 hours after surgery. Urine samples for neutrophil gelatinase-associated lipocalin (N-GAL) and enolase testing were collected after administration of the drug and 2 hours after arrival in the ICU (after surgery).
Hemodynamic measurements
Echocardiographic measurements were performed to assess RV dilatation (tricuspid annulus diameter) and pulmonary hypertension (if present) at baseline and 48 hours postoperatively. If a low cardiac output syndrome was diagnosed during admission (cardiac index <2.4 L/min/m2, pulmonary wedge pressure >15 mmHg, and/or central venous saturation <65%), the number and dose of inotropic and/or vasoactive drugs administered during the first 48 postoperative hours was recorded.
Renal function
Postoperative renal dysfunction was assessed with the AKI classification. Small changes in creatinine may be associated with impaired renal function.17 Creatinine values and urine output were recorded before administration of levosimendan and at 24–48 hours after administration.
Sample size
The primary endpoint was N-GAL in urine. A difference of 500 ng/dL between baseline and postoperative values was considered as significant. The incidence of postoperative renal dysfunction of 20%. Twenty-six patients were required to obtain a β statistical power of 80% and an α error of 0.05. For enolase, a sample of 16 patients was needed to detect an increase of 50% from baseline, a beta statistical power of 80%, and an α error of 0.05.
Statistical analyses
Descriptives
Continuous variables are presented as total number of cases (n), the mean ± standard deviation, median and standard error (SE) values, and interquartile range p25–p75 (IQRp25–p75). Qualitative variables were described using the number of cases (n).
Univariate analysis
For the statistical analysis, the Shapiro–Wilk test was initially performed to check the normal distribution of the variables. Continuous variables were compared using Student’s t-test, ANOVA, or Kruskal–Wallis or Mann–Whitney nonparametric tests, as appropriate. Qualitative variables were analyzed using the χ2 test or Fisher’s exact test when necessary (if N <20, or if any value in the table of expected values was <5). When the χ2 test was used, the Yates correction was applied in all cases.
Results
Patients’ epidemiological and surgical data are listed in Table 2.
Table 2.
Patients’ preoperative epidemiological characteristics and surgical data
| Sex (male/female) | 14/13 |
| Age (years) | 67±8 |
| Height (cm) | 164±5 |
| EuroScore | 8±1 |
| ASA class | III±1 |
| Cleveland score | 9±2 |
| Type of surgery | |
| Mitral | 4/27 |
| Aortic mitral | 5/27 |
| Mitral-tricuspid | 13/27 |
| Tricuspid | 2/27 |
| Aortic-mitral-tricuspid | 3/27 |
| Cardiopulmonary bypass time (min) | 110±21 |
| Time of ischemia (min) | 74±12 |
| Preoperative treatment | |
| β-Blockers | 15 |
| ACE inhibitors/ARA II | 12 |
| Nitrates | 5 |
| Calcium antagonist blockers | 4 |
| Diuretics | 21 |
| Bronchodilating agents | 14 |
| Acetyl salicylic acid | 6 |
| Sildenafil/bosentan | 18/27 |
Note: Data are given as absolute numbers or median ± SD.
Abbreviations: ASA, American Society of Anesthesiologists (physical status); min, minutes; ACE, angiotensin-converting enzyme; ARA, angiotensin receptor blockers; SD, standard deviation.
Pulmonary arterial pressure showed a significantly lower value after surgery, with values decreasing from 58±18 (31.17–48.89) mmHg to 42±19 (40–64.8) mmHg post operatively (P=0.001); this finding was also observed regarding dilatation of the RV that decreased from 4.23±0.7 (3.5–5.8) mm to 3.45±0.6 (2.5–4.8) mm (P=0.044; Table 3). Total volume infused during levosimendan administration was as usual (1,000 mL of Ringer’s solution for 24 hours).
Table 3.
Hemodynamic parameters
| Hemodynamic parameters | Baseline
|
48 hours
|
||
|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | |
| Tricuspid annulus diameter (mm) | 4.23±0.7 | 3.5–5.8 | 3.05±0.6* | 2.5–4.8 |
| Heart rate (bpm) | 70±9 | 65–78 | 71±7 | 70–80 |
| Mean blood pressure (mmHg) | 70±9 | 72–81 | 71±7 | 72–79 |
| Central venous pressure (mmHg) | 12±4 | 11–14 | 10±4 | 9–12 |
| Pulmonary artery systolic pressure (mmHg) | 58±19 | 31.17–48.89 | 42±18* | 40–64.77 |
Note:
Significant variations P<0.05.
Abbreviations: SD, standard deviation; CI, confidence interval.
Baseline central venous saturation was 75%±5% (68%–79%), and at 48 hours, it was 70%±4% (67%–78%), with no significant variations. Baseline NT-proBNP values rose from 2,014 (79) (1,883–5,106) pg/mL to 3,456 (211) (3,062–9,091) pg/mL, the differences being significant (P=0.006). Baseline lactate values were 0.91±0.3 (0.78–1.07) mmol/L and 1.42±0.62 (1.37–1.73) mmol/L after 48 hours (Table 4).
Table 4.
Biochemical and analytical parameters assessed during the study
| Biochemical parameters | Baseline
|
2 hours after surgery
|
48 hours
|
|||
|---|---|---|---|---|---|---|
| Mean ± SD or mean (SEM) | 95% CI | Mean ± SD or mean (SEM) | 95% CI | Mean ± SD or mean (SEM) | 95% CI | |
| Central venous saturation (%) | 75±5 | 68–79 | – | – | 70±4 | 67–78 |
| Lactate (mmol/L) | 0.91±0.3 | 0.78–1.07 | – | – | 1.42±0.62 | 1.37–1.73 |
| NT-ProBNP (pg/mL) | 2,014 (79) | 1,883–5,106* | – | – | 3,456 (211)** | 3,062–9,091 |
| Creatinine (mg/dL) | 1.06±0.24 | 1.04–1.44 | – | – | 1.25±0.37 | 1.22–1.68 |
| N-GAL (ng/mL) | 14.31 (28.34) | 0.87–278.5 | 13.41 (38.24) | 0.34–360.2 | – | – |
| Neuron-specific enolase (ng/mL) | 5.40 (0.41) | 0.11–9.12 | 4.32 (0.61) | 0.11–17.15 | – | – |
Notes:
Significant variations (P<0.05).
Value reported at 24 hours.
Abbreviations: N-GAL, neutrophil gelatinase-associated lipocalin; CI, confidence interval; SD, standard deviation; SEM, standard error of the mean.
N-GAL value at baseline was 14.31±28. ng/mL (0.87–278.5), and value after 24 hours was 13.41 (38.24) (0.34–360.2) ng/mL, and enolase value at baseline was 5.40 (0.41) (0.11–9.12) ng/mL, and value after 24 hours was 4.32 (0.61) (0.11–17.15) ng/mL; the differences were not significant in either case. In both sets of measurements, the Mann–Whitney test was performed, since the distribution was not normal (as in the case of NT-proBNP, but not in the case of the rest of the parameters; Table 4 and Figures 1 and 2).
Figure 1.
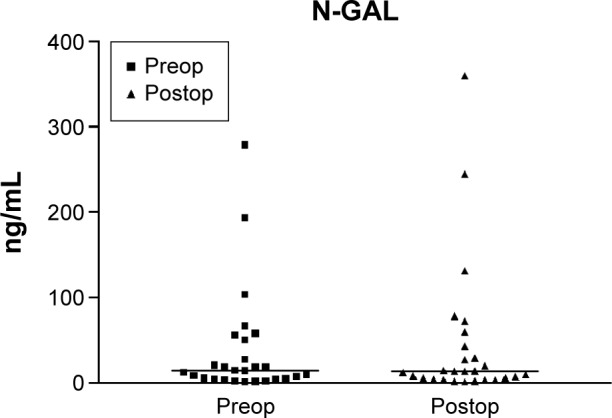
Preoperative and postoperative N-GAL values.
Notes: The scatter graph shows N-GAL values before and after surgery in 27 patients preoperatively administered levosimendan and the mean of the N-GAL concentration in urine at these two time points. Preop was the preoperative values (before levosimendan) and postop was postoperative values (2 hours after ICU admission postsurgery). Mean values were 14.31 ng/mL prior to levosimendan infusion and 13.41 ng/mL 2 hours after ICU admission. The variations were not statistically significant (P>0.05).
Abbreviations: N-GAL, neutrophil gelatinase-associated lipocalin; ICU, intensive care unit.
Figure 2.
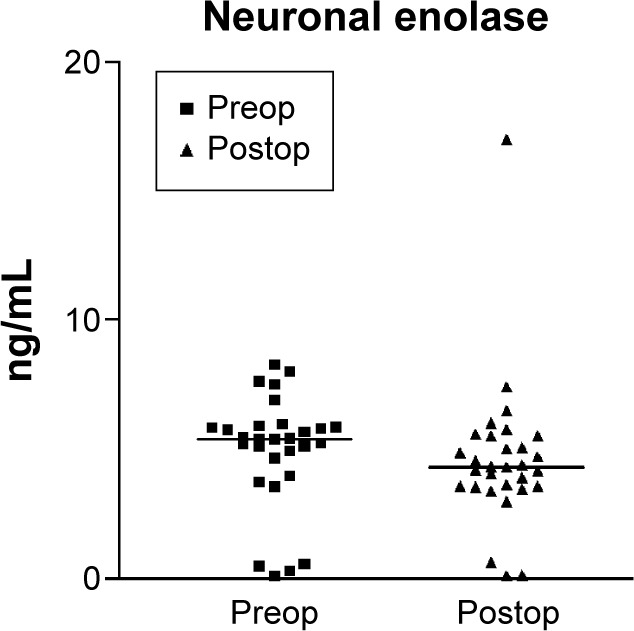
Preoperative and postoperative neuron-specific enolase values.
Notes: The scatter graph shows neuron-specific enolase values before and after surgery in 27 patients preoperatively administered levosimendan and the medians of neuron-specific enolase concentrations in urine at these two time points. Preop was the preoperative values (before levosimendan) and postop was postoperative values (2 hours after ICU admission postsurgery). The median values were 5.4 ng/mL prior to levosimendan infusion and 4.32 ng/mL 2 hours after ICU admission. The variations were not statistically significant (P>0.05).
Abbreviation: ICU, intensive care unit.
With regard to renal function parameters, baseline creatinine was 1.06±0.24 (1.04–1.44) mg/dL, rising to 1.25±0.37 (1.22–1.68) mg/dL at 48 hours, with no significant variation (P>0.05). Urine output in the first 24 hours was 1.37±0.3 (1.11–1.71) mL/kg/h, decreasing to 1.02±0.23 (0.96–1.45) mL/kg/h at 48 hours. The AKI stage remained unchanged at 48 hours. No patients required renal replacement therapy (Table 4).
Of the 27 patients, six needed support with adrenaline for the first 48 hours (0.01±0.02 [0–0.02] μg/kg/min). Noradrenaline vasoactive support was necessary in five patients at 48 hours (0.018±0.03 [0–0.03] μg/kg/min). No patient received support with dobutamine.
Troponin values were not significant at baseline but reached a peak of 8.63±7.23 (5.87–11.40) ng/mL at 24 hours, decreasing at 48 hours to values of 5.22±3.4 (2.9–5.5) ng/mL. Similarly, baseline CK-MB were not significant but reached a peak of 55.5±46 (36.10–71.71) ng/dL at 24 hours, decreasing to 18.50±12.48 (13.16–22.87) ng/dL after 48 hours.
Discussion
In previous works, levosimendan has been shown to protect the heart and may also preserve the kidneys and the brain.18,19 Levosimendan is an inotropic agent with vasodilatory effects, thanks to a triple mechanism of action: through calcium channels in cardiac myofilaments and opening of the ATP-sensitive potassium channels in smooth muscle cells and the ATP-sensitive potassium channels of the mitochondria of cardiac cells. Through this triple mechanism, levosimendan increases the contractile force of the cardiac muscle fibers, causes both peripheral arterial and venous vasodilatation, and exerts a cardioprotective effect against ischemia. The opening of KATP channels in the mitochondria has a predominant role in the protection of the myocardium from ischemia and reperfusion injury. The drug exerts its cardioprotective effect through several mechanisms: it reduces preload and afterload, thus improving cardiac work, and its action on the ATP potassium-sensitive channels in the mitochondria of cardiac cells protects the heart against myocardial ischemia by achieving cell stabilization and preserving homeostasis, thanks to the correction of the mitochondrial function.1,2
Besides cardioprotection against ischemic events and the antistunning effect, levosimendan may also reduce ischemia-related damage in other organs.1 In this study, we evaluated its effect on the heart, kidney, and central nervous system.
Perioperative renal dysfunction is a serious acute complication after cardiac surgery, being associated with prolonged hospitalization and mortality rates that vary from 30% to 70% according to previous studies.20,21 Although the renal function subsequently recovers, long-term mortality rates in this group of patients are increased.10 In patients with RV dysfunction and pulmonary hypertension, small increases in creatinine represent an independent predictor of mortality; instead in these patients, perioperative kidney preservation is important.7 Several scales are available for the diagnosis of acute renal failure. In our case, we used the current reference scale for the diagnosis of renal failure (AKI) derived from the RIFLE scale.17 Research using this scale has shown that even small increases in creatinine or decreased urine output worsen prognosis in these patients.10,11 In our study, we evaluated postoperative changes in renal function in patients with high perioperative cardiac risk assessed by the Euroscore II scale and a high baseline value of NT-proBNP, which is an independent predictor of poor prognosis and morbidity in these patients.22
Patients with a high risk of postoperative renal dysfunction were assessed with the Cleveland scale. No significant changes in renal function were found at 48 hours postsurgery. Baseline and postoperative values of urinary N-GAL showed no significant differences and were below the established cutoff points for the diagnosis of renal dysfunction after cardiac surgery.23,24
The Portland study reported a decrease in creatinine values in patients with heart failure treated with levosimendan.25 Levin et al26 found fewer cases of renal dysfunction in patients with low cardiac output treated with levosimendan compared with patients who received dobutamine. In our view, these results are related not only to the cardiac benefit of the drug but also to its effects on the kidney, which have been described previously by Bragadottir et al:12 renal vasodilation, increased flow, and glomerular filtration rate, without increasing renal oxygen demand.12 The preconditioning mediated by the action on ATP-dependent K channels may also be involved.27–29
Our study was conducted in patients with RV dysfunction and pulmonary hypertension. In line with previous research, we found a decrease in the diameter of the tricuspid annulus and pulmonary pressures after administration of levosimendan and the surgical procedure.30–32 Conceivably, then, the reduction in renal venous pressure due to the improved ventricular function may also have contributed to the renal protection.
The study also evaluated the central nervous system by assessing the values of neuron-specific enolase, a marker of neurological damage that rises in the immediate postoperative period and returns to baseline values after 24 hours.33,34 In our study, enolase values did not change significantly vis-à-vis baseline, a finding that suggests the possibility that levosimendan has a neuroprotective effect, preserving neuronal tissue in these patients who are undergoing surgical procedures with a high risk of neurological dysfunction. Rubio-Regidor et al9 concluded that neurological complications immediately after cardiac surgery are a major cause of morbidity and mortality, increasing the use of health resources and leading to functional limitations in the patients who survive, since as many as 83% of patients undergoing cardiac surgery may present cognitive alterations.9
Several studies have demonstrated the neuroprotective properties of levosimendan and its positive impact on nerve function after transient ischemic episodes.35–38 The neuroprotective effect may be mediated through mitochondrial KATP channels, due to its action during ischemia reperfusion processes.39 In our opinion, our findings may be due to the improvement in the cardiac situation and the possible neuroprotective effects.
The main limitations of our study are the absence of a control group to check the variations in the group of untreated patients. We could just hypothesize that the pre- and poste-valuation within patients previously identified as high risk of postoperative organ dysfunction is sufficient to assess the properties of the drug at multiorgan level, as has been done by other authors, but we need to confirm it by other randomized studies.5,8,32,40,41 Larger studies in cardiac surgery are warranted to clarify the renal and neuronal effects of levosimendan and adverse effects.42
Conclusion
In addition to its previously described cardioprotective effects, levosimendan also preserves renal and neurological function. The drug appears to achieve this preservation through its action on KATP-dependent channels at the mitochondrial level and through the improved organ perfusion it leads to, thanks to its inodilator properties.
Acknowledgments
The authors would like to thank the Servicio de Anestesia Hospital Universitario Virgen de la Victoria and Instituto de Salud Carlos III CIBERobn, Spain.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors do not have a financial relationship with the organization that sponsored the research and report no further conflicts of interest.
References
- 1.Orriach Guerrero JL, Ramirez Fernandez A, Iglesias P, et al. Preoperative levosimendan. A new way for organoprotection. Curr Pharm Des. 2014;20(34):5476–5483. doi: 10.2174/1381612820666140325121452. [DOI] [PubMed] [Google Scholar]
- 2.du Toit EF, Genis A, Opie LH, Pollesello P, Lochner A. A role for the RISK pathway and K(ATP) channels in pre- and post-conditioning induced by levosimendan in the isolated guinea pig heart. Br J Pharmacol. 2008;154(1):41–50. doi: 10.1038/bjp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tritapepe L, De Santis V, Vitale D, et al. Levosimendan pretreatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth. 2009;102(2):198–204. doi: 10.1093/bja/aen367. [DOI] [PubMed] [Google Scholar]
- 4.Levin R, Degrange M, Del Mazo C, Tanus E, Porcile R. Preoperative levosimendan decreases mortality and the development of low cardiac output in high-risk patients with severe left ventricular dysfunction undergoing coronary artery bypass grafting with cardiopulmonary bypass. Exp Clin Cardiol. 2012;17(3):125–130. [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrero-Oriach JL, Navarro-Arce I, Iglesias P, Galán-Ortega M, Rubio-Navarro M, Cruz Mañas J. Preoperative levosimendan for right ventricular dysfunction before heart valve replacement surgery. Rev Esp Cardiol. 2013;66(12):999–1000. doi: 10.1016/j.rec.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Campo A, Mathai SC, Le Pavec J, et al. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38(2):359–367. doi: 10.1183/09031936.00148310. [DOI] [PubMed] [Google Scholar]
- 7.Sztrymf B, Souza R, Bertoletti L, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010;35(6):1286–1293. doi: 10.1183/09031936.00070209. [DOI] [PubMed] [Google Scholar]
- 8.Ersoy O, Boysan E, Unal EU, et al. Effectiveness of prophylactic levosimendan in high-risk valve surgery patients. Cardiovasc J Afr. 2013;24(7):260–264. doi: 10.5830/CVJA-2013-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio-Regidor M, Pérez-Vela JL, Escribá-Bárcena A, et al. Neurological complications in cardiac surgery post-operative period. Med Intensiva. 2007;31(5):241–250. doi: 10.1016/s0210-5691(07)74817-9. [DOI] [PubMed] [Google Scholar]
- 10.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119(4):495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 11.Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009;88(1):124–130. doi: 10.1016/j.athoracsur.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Bragadottir G, Redfors B, Ricksten SE. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: a randomized placebo-controlled study. Crit Care Med. 2013;41(10):2328–2335. doi: 10.1097/CCM.0b013e31828e946a. [DOI] [PubMed] [Google Scholar]
- 13.Varvarousi G, Stefaniotou A, Varvaroussis D, Aroni F, Xanthos T. The role of Levosimendan in cardiopulmonary resuscitation. Eur J Pharmacol. 2014;740:596–602. doi: 10.1016/j.ejphar.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Kelm RF, Wagenführer J, Bauer H, Schmidtmann I, Engelhard K, Noppens RR. Effects of levosimendan on hemodynamics, local cerebral blood flow, neuronal injury, and neuroinflammation after asphyctic cardiac arrest in rats. Crit Care Med. 2014;42(6):e410–e419. doi: 10.1097/CCM.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 15.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16(1):162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 16.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic Euro-SCORE. Eur Heart J. 2003;24:882–883. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV. Acute kidney injury network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parissis JT, Adamopoulos S, Antoniades C, et al. Effects of levosimendan on circulating pro-inflammatory cytokynes and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am J Cardiol. 2004;93(10):1309–1312. doi: 10.1016/j.amjcard.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 19.Papp Z, Édes I, Fruhwald S, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 2012;159(2):82–87. doi: 10.1016/j.ijcard.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129(5):1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 21.Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 22.Sear JW. Kidney dysfunction in the postoperative period. Br J Anaesth. 2005;95(1):20–32. doi: 10.1093/bja/aei018. [DOI] [PubMed] [Google Scholar]
- 23.Tuladhar SM, Püntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardio-vasc Pharmacol. 2009;53(3):261–266. doi: 10.1097/FJC.0b013e31819d6139. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu A, Sujatha DI, Ninan B, Vijayalakshmi MA. Neutrophil gelatinase associated lipocalin as a biomarker for acute kidney injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg. 2010;24(4):525–531. doi: 10.1016/j.avsg.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso JS, Ferreira J, de Sá EP, et al. Levosimendan in daily intensive care practice – the experience of 15 centers. The PORTLAND study. J Cardiac Fail. 2004;10:131. [PubMed] [Google Scholar]
- 26.Levin RL, Degrange MA, Porcile R, et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome. Rev Esp Cardiol. 2008;61(5):471–479. [PubMed] [Google Scholar]
- 27.McCully D, Levitsky S. Mitochondrial ATP-sensitive potassium channels in surgical cardioprotection. Arch Biochem Biophys. 2003;420:237–245. doi: 10.1016/j.abb.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Papp JG, Pollesello P, Varró AF, Végh AS. Effect of levosimendan and-milrinone on regional myocardial ischemia/reperfusion-induced arrhythmias in dogs. J Cardiovasc Pharmacol Ther. 2006;11(2):129–135. doi: 10.1177/1074248406289286. [DOI] [PubMed] [Google Scholar]
- 29.Metzsch C, Liao Q, Steen S, Algotsson L. Levosimendan cardioprotection reduces the metabolic response during temporary regional coronary occlusion in an open chest pig model. Acta Anaesthesiol Scand. 2007;51(1):86–93. doi: 10.1111/j.1399-6576.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 30.Parissis JT, Paraskevaidis I, Bistola V, et al. Effects of levosimendan on right ventricular function in patients with advanced heart failure. Am J Cardiol. 2006;98(11):1489–1492. doi: 10.1016/j.amjcard.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 31.Poelzl G, Zwick RH, Grander W, et al. Safety and effectiveness of levosimendan in patients with predominant right heart failure. Herz. 2008;33(5):368–373. doi: 10.1007/s00059-008-3051-2. [DOI] [PubMed] [Google Scholar]
- 32.Russ MA, Prondzinsky R, Carter JM, et al. Right ventricular function in myocardial infarction complicated by cardiogenic shock: improvement with levosimendan. Crit Care Med. 2009;37(12):3017–3023. doi: 10.1097/CCM.0b013e3181b0314a. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann M, Ebert AD, Galazky I, Wunderlich MT, Kunz WS, Huth C. Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke. 2000;31(3):645–650. doi: 10.1161/01.str.31.3.645. [DOI] [PubMed] [Google Scholar]
- 34.Gao F, Harris DN, Sapsed-Byrne S. Time course of neuronespecific enolase and S-100 protein release during and after coronary artery bypass grafting. Br J Anaesth. 1999;82(2):266–267. doi: 10.1093/bja/82.2.266. [DOI] [PubMed] [Google Scholar]
- 35.Katircioglu SF, Seren M, Parlar AI, et al. Levosimendan effect on spinal cord ischemia-reperfusion injury following aortic clamping. J Card Surg. 2008;23(1):44–48. doi: 10.1111/j.1540-8191.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 36.Lafci B, Yasa H, Ilhan G, et al. Protection of the spinal cord from ischemia: comparative effects of levosimendan and iloprost. Eur Surg Res. 2008;41(1):1–7. doi: 10.1159/000121394. [DOI] [PubMed] [Google Scholar]
- 37.Roehl AB, Hein M, Loetscher PD, et al. Neuroprotective properties of levosimendan in an in vitro model of traumatic brain injury. BMC Neurol. 2010;10:97. doi: 10.1186/1471-2377-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayanagi K, Gáspár T, Katakam PV, Busija DW. Systemic administration of diazoxide induces delayed preconditioning against transient focal cerebral ischemia in rats. Brain Res. 2007;1168:106–111. doi: 10.1016/j.brainres.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 39.Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110(95):986–995. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sponga S, Ivanitskaia E, Potapov E, Krabatsch T, Hetzer R, Lehmkuhl H. Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J. 2012;58(1):6–11. doi: 10.1097/MAT.0b013e318239f401. [DOI] [PubMed] [Google Scholar]
- 41.Guerrero Orriach JL, Galán Ortega M, Ramírez Fernandez A, et al. Assessing the effect of preoperative levosimendan on renal function in patients with right ventricular dysfunction. J Clin Monit Comput. 2016 Jan 13; doi: 10.1007/s10877-016-9827-7. Epub. [DOI] [PubMed] [Google Scholar]
- 42.Yilmaz MB, Grossini E, Silva Cardoso JC, et al. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther. 2013;27(6):581–590. doi: 10.1007/s10557-013-6485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


