Abstract
The manipulation of seed phosphorus is important for seedling growth and environmental P sustainability in agriculture. The mechanism of regulating P content in seed, however, is poorly understood. To study regulation of total P, we focused on phytic acid (inositol hexakisphosphate; InsP6) biosynthesis-related genes, as InsP6 is a major storage form of P in seeds. The rice (Oryza sativa L.) low phytic acid mutant lpa1-1 has been identified as a homolog of archael 2-phosphoglycerate kinase. The homolog might act as an inositol monophosphate kinase, which catalyzes a key step in InsP6 biosynthesis. Overexpression of the homolog in transgenic rice resulted in a significant increase in total P content in seed, due to increases in InsP6 and inorganic phosphates. On the other hand, overexpression of genes that catalyze the first and last steps of InsP6 biosynthesis could not increase total P levels. From the experiments using developing seeds, it is suggested that the activation of InsP6 biosynthesis in both very early and very late periods of seed development increases the influx of P from vegetative organs into seeds. This is the first report from a study attempting to elevate the P levels of seed through a transgenic approach.
Keywords: ectopic expression, mineral element, molecular breeding, Oryza sativa L., phosphorus, phytic acid, seed, translocation
1. Introduction
Early development of seedlings is completely dependent on seed nutrient reserves. Seeds accumulate a large amount of phosphorus (P) to sustain seedling growth. Seeds store P mainly in the form of phytic acid (inositol hexakisphosphate; InsP6), with approximately 70% to 80% of total P stored in the form of InsP6 [1]. After imbibition, phytase hydrolyzes InsP6 in seeds, and the resulting available P is remobilized into shoots and roots.
Initial seedling growth is supported by available P in the seeds. As the plant develops, it proceeds from P-heterotrophy (P supply from seed) to P-autotrophy (uptake of external P via roots). In maize (Zea mays L.), the P-heterotrophic growth phase continues for 4 d after sowing, and the P-autotrophic phase starts after 16 d after sowing [2]. From 5 to 15 d after sowing, seedling growth is supported by both seed P and external P. The supply of nutrients from seed reserves to support early seedling development is, therefore, substantial. Ros et al. (1997) investigated the effect of seed P levels on early growth of rice and confirmed the beneficial effects from an increase in seed P content on plant growth and, in particular, the growth of roots [3].
In Arabidopsis thaliana L., reduced total P content was observed in seeds of the atpap26 mutant, in which acid phosphatase activity was markedly reduced in the leaves. There was also a significant decrease in remobilization of P from old, senesced leaves to new leaves and to seeds [4]. Seed germination of the atpap26 mutant was delayed. These results suggest that a reduction in total P content has a negative effect on seed performance. On the contrary, higher seed P content has led to more rapid seedling emergence and larger biomass in several species [5,6].
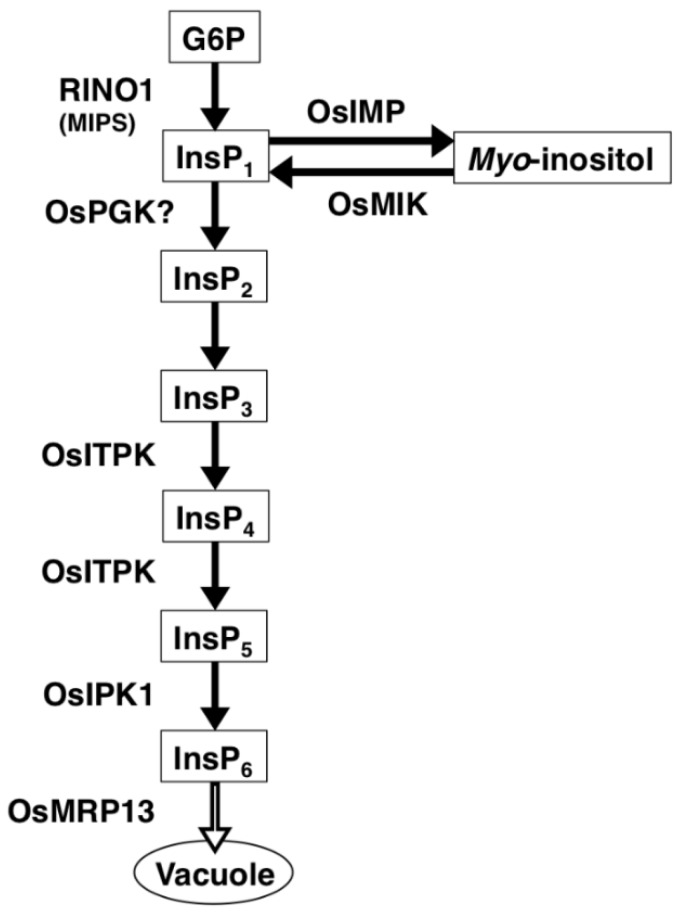
Control of seed total P content is important not only for seed performance but also for environmental sustainability of P in agriculture [1]. However, the control mechanism of total P content in seed is poorly understood. The majority of seed P is stored in the form of InsP6, so it is plausible that total P content in seed might increase if the InsP6 content is increased. In fact, InsP6 and total P contents are closely correlated in beans [7,8]. To elevate the InsP6 level in a seed, it is important to activate the InsP6 biosynthetic pathway by increasing the expression level of the rate-limiting enzymes in that pathway. Myo-inositol 3-phosphate synthase (MIPS), which catalyzes the first step of InsP6 biosynthesis and inositol metabolism, has been considered as a key enzyme in these pathways [9] (Figure 1). There are two MIPS genes in the rice genome, RINO1 (Os03g0192700) and RINO2 (Os10g0369900). RINO1 is responsible for InsP6 biosynthesis in rice seeds, because RINO1 transcript levels are extremely high in developing seeds and RINO2 mRNA is scarcely detected [10]. Another important step is the last step of InsP6 biosynthesis, from inositol pentaphosphate (InsP5) to InsP6, which is catalyzed by inositol 1,3,4,5,6-pentakisphosphate 2-kinase (IPK1) (Figure 1). The rice IPK1 gene, OsIPK1 (Os04g0661200), is highly expressed in developing seeds [10]. Activation of the first or last step of InsP6 biosynthesis might lead to activation of InsP6 biosynthesis and an increase in total P content in seeds.
Figure 1.
Scheme for biosynthesis of phytic acid (Ins P6) and inositol in rice.
The step from InsP1 to inositol diphosphate (InsP2) is another key step in the InsP6 biosynthetic pathway, as InsP1 lies at an important branch point between InsP6 biosynthesis and inositol metabolism (Figure 1). Kim et al. (2008) identified a rice low phytic acid mutant (lpa1) as a homolog of 2-phosphoglycerate kinase (2-PGK), which catalyzes the step from 2-phosphoglycerate to 2,3-bisphosphoglycerate in archaea [11]. From structural analogy of substrates and products, the protein encoded by rice lpa1 might function as an InsP1 kinase, which catalyzes the step from InsP1 to InsP2 [1,11]. If the protein catalyzes the key branch point step of InsP6 biosynthesis, overexpression of the protein might lead to an increase in InsP6 accumulation in seeds.
In the rice genome, there are two putative 2-PGK homologs, which were identified by a BLAST search. We designated the homologs as OsPGK1 (Lpa1; Os02g0819400) and OsPGK2 (Os09g057220). Alignment of the deduced amino acid sequence of OsPGK1 indicated an approximately 60% similarity to OsPGK2. OsPGK1 might play a major role in phytic acid biosynthesis in developing seeds, because the mutation of this gene resulted in a severe lpa phenotype [11,12]. The expression pattern of OsPGK1 is immature seed-specific, which is apparent from the microarray data in RiceXPro (http://ricexpro.dna.affrc.go.jp).
In this study, to increase InsP6 content in rice seeds, we generated transgenic rice plants that overexpressed the rice genes RINO1, OsIPK1, and OsPGK1 under the control of an actin promoter. We designated the RINO1, OsIPK1, and OsPGK1 overexpressors as RINO1ox, OsIPK1ox, and OsPGK1ox, respectively. We investigated InsP6 and total P content in seeds from these transgenic plants and discovered that only OsPGK1ox significantly increased InsP6 and total P contents in the seed, compared to the non-transformant (NT) seed. We discuss the causal mechanisms of these increases.
2. Results
2.1. The Effect of RINO1 or OsIPK1 Overexpression on InsP6 Accumulation in Seeds
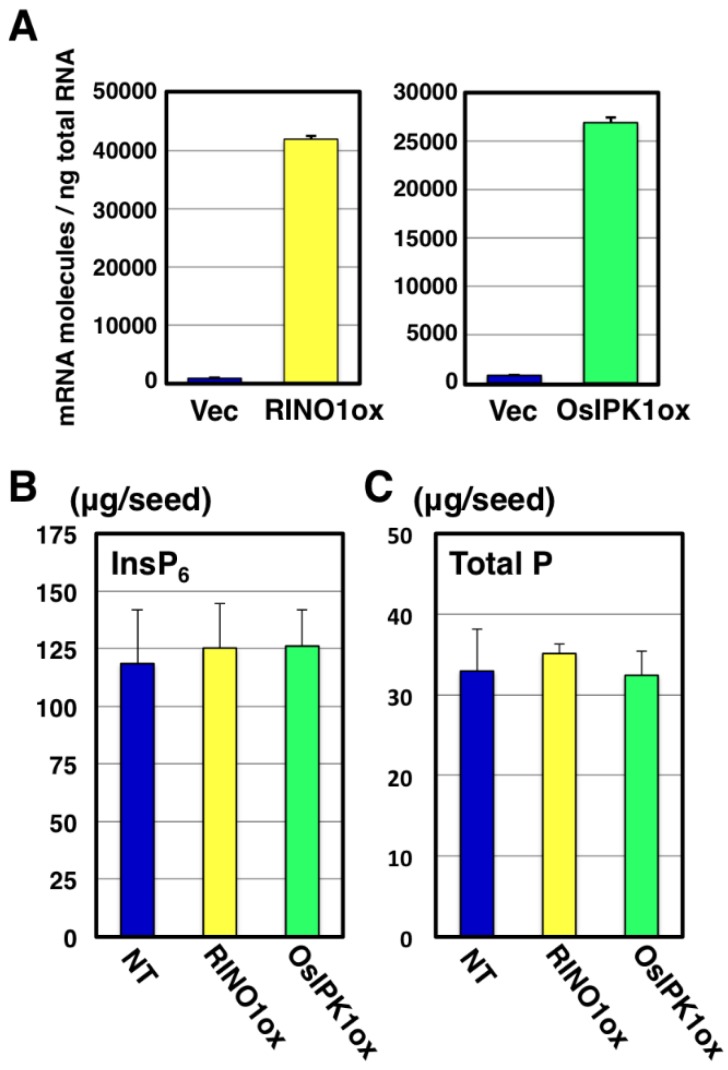
We first observed the seed phenotypes of RINO1ox and OsIPK1ox, as these enzymes have been well characterized in InsP6 biosynthesis [9,10]. From the three fixed progeny lines of each transformant, we selected a RINO1ox line and an OsIPK1ox line, based on their high expression in T3 plants (Figure 2A). InsP6 content in T4 seeds was measured by ion chromatography. InsP6 content in the RINO1ox and OsIPK1ox seeds was slightly higher than in NT seed, albeit not significantly so. (Figure 2B). Seed total P content in both transgenic plants was identical to that of NT seed (Figure 2C).
Figure 2.
Gene expression and seed phenotype of RINO1ox and OsIPK1ox transgenic plants. (A) Quantitative RT-PCR analysis of RINO1 (left) and OsIPK1 (right) genes with cDNA templates from leaves of vector control (Vec) and RINO1ox or OsIPK1ox plants 7 d after germination (n = 3); (B) InsP6 content in mature seeds obtained from non-transformant (NT) and two transgenic plants was determined by ion chromatography (n ≥ 7); (C) Total P content in NT and two transgenic seeds was measured by colorimetric assay (n ≥ 5). Each value (A to C) represents the mean ± SD.
2.2. Seed Phenotype of the OsPGK1 Overexpressing Transformants
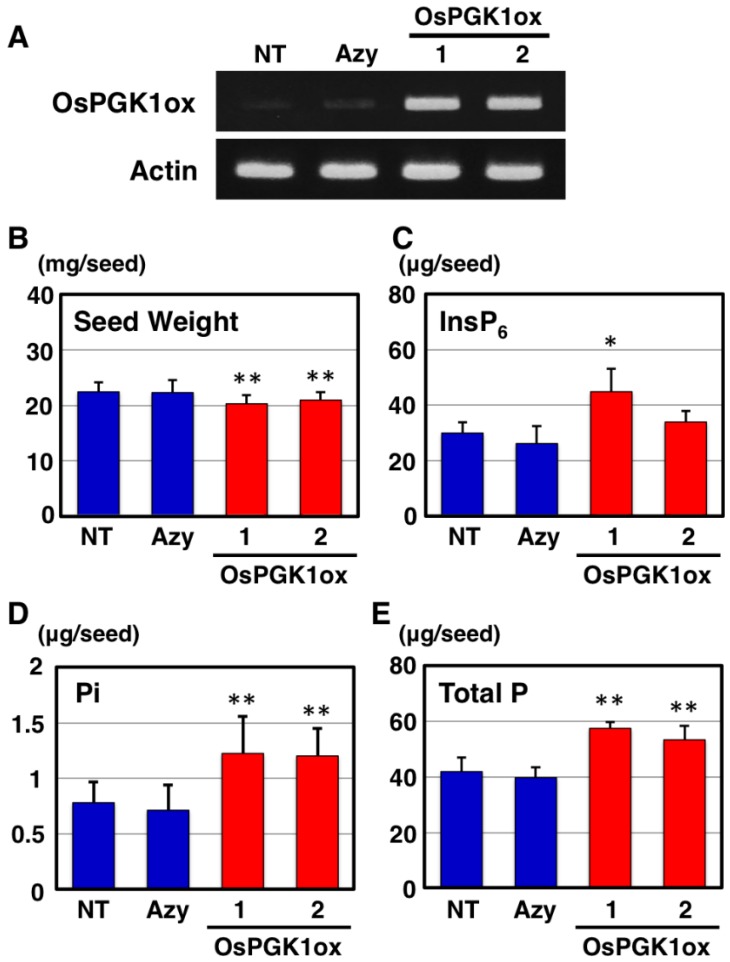
As a next step, we generated transgenic rice plants carrying a rice 2-PGK homolog gene (OsPGK1), driven by a rice actin promoter. Five primary transformants (OsPGK1ox) were obtained after regeneration from antibiotic-resistant calli. All plants produced viable T1 seeds. After measuring InsP6 content in T1 seeds, we selected two transgenic lines (OsPGK1ox-1 and OsPGK1ox-2), which exhibited increased seed InsP6 contents, and the fixed progeny lines were used in subsequent experiments. We confirmed overexpression of the OsPGK1 gene in these transgenic lines (Figure 3A). We selected an azygous line (AZ), which was the transformed line without the transgene, derived from an OsPGK1ox-2 line.
Figure 3.
Gene expression and seed phenotype of OsPGK1ox transgenic plants. (A) Semi-quantitative RT-PCR analysis of the expression of OsPGK1. Total RNA was extracted from flowers of non-transformants (NT), azygous plants (Azy), and two independent OsPGK1ox lines (1 and 2) just before flowering. Actin was used as a reference; (B) Seed weights (n = 20); (C) InsP6 content was determined by ion chromatography (n = 4); (D) Inorganic phosphate content was measured by colorimetric assay using molybdate staining (n = 10); (E) Total P content was measured by ICP-OES analysis (n = 6). Each value (B to E) represents the mean ± SD. * and ** indicate p < 0.05 and p < 0.01, respectively.
We examined the phenotype of T4 seeds. Although the dried mature seed weights of the OsPGK1ox lines were significantly less than weights of NT and AZ seeds (Figure 3B), there were no significant differences between the NT and OsPGK1ox lines in terms of germination rate, early seedling growth, and plant height. There was a significant increase in total InsP6 content in the OsPGK1ox-1 seeds and a slight increase in total InsP6 content in the OsPGK1ox-2 seeds compared with NT and AZ seeds (Figure 3C). Contrary to expectations, Pi content also increased in the seeds of OsPGK1ox lines (Figure 3D). Seed total P content was significantly higher in both transgenic lines than in NT and AZ (Figure 3E). Total P content increased by 1.29-fold for OsPGK1ox-2 seeds and 1.37-fold for OsPGK1ox-1 seeds, compared with NT seeds.
2.3. Accumulation of InsP6 and Total Phosphorus in Developing Seeds of OsPGK1ox
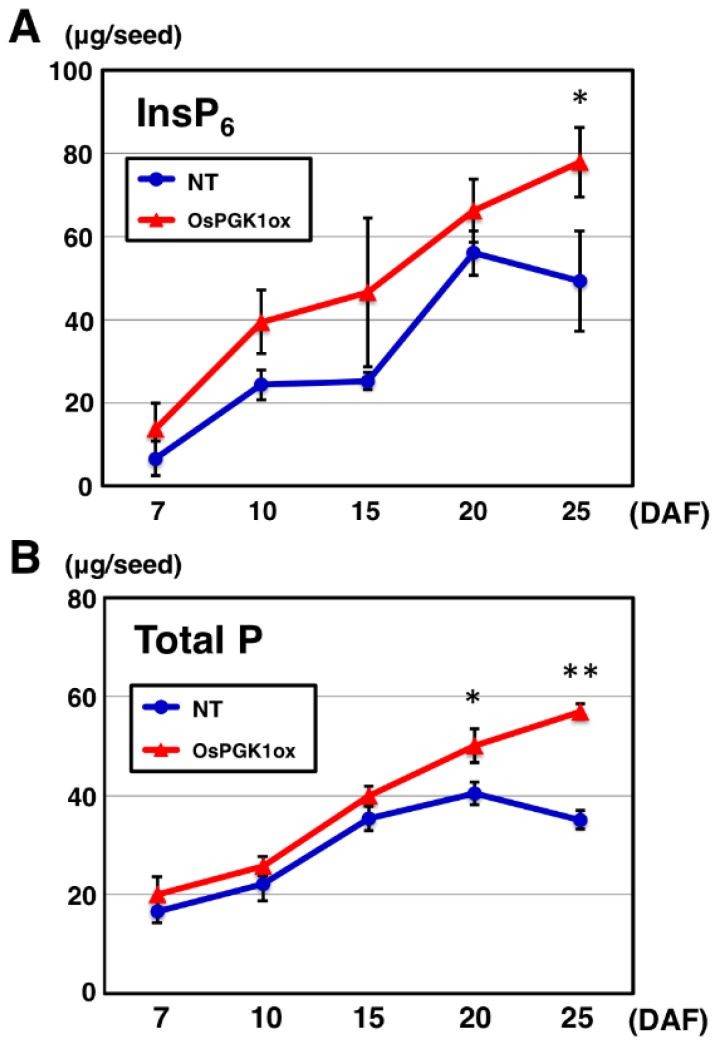
To determine changes in InsP6 content during seed development, seeds of OsPGK1ox-1 and NT were analyzed by ion chromatography at 7, 10, 15, 20, and 25 d after flowering (DAF; Figure 4A). InsP6 content in NT seeds increased from 7 to 20 DAF and stopped at 20 DAF. In contrast, the increase in InsP6 content in the OsPGK1ox-1 seeds continued from 7 to 25 DAF. InsP6 content in the OsPGK1ox-1 seeds at 25 DAF was significantly higher than InsP6 content in the NT seeds. Although InsP6 content in the OsPGK1ox-1 seeds was slightly higher than InsP6 content in the NT seeds from 7 to 20 DAF, the rates of InsP6 content increase were similar for the OsPGK1ox-1 and NT seeds during this period (Figure 4A).
Figure 4.
Changes in InsP6 (A) and total P (B) content in immature seeds of OsPGK1ox-1 and NT during seed development from 7 to 25 d after flowering (DAF). InsP6 and total P contents were determined by ion chromatography and ICP-OES analyses, respectively. Each value represents the mean ± SD of three replicates. * and ** indicate p < 0.05 and p < 0.01, respectively.
Similar results were obtained for total P content in developing seeds analyzed by inductively coupled plasma optical-emission spectrometry (ICP-OES; Figure 4B). Total P content in NT seeds rapidly increased until 15 DAF, and then gradually increased between 15 and 20 DAF. The increase in total P content stopped at 20 DAF in the NT seeds. In contrast, the increase in total P content in the OsPGK1ox-1 seeds continued from 7 to 25 DAF. Total P content in the OsPGK1ox-1 seeds at 20 and 25 DAF was significantly higher than total P content in the NT seeds. These results indicate that an influx of P from vegetative organs to seeds continued after 20 DAF only in the OsPGK1ox-1 plants. Although total P content in the OsPGK1ox-1 line, from 7 to 20 DAF, was slightly higher than total P content in the NT line, the rates of increase in total P were similar for OsPGK1ox-1 and NT seeds during this period (Figure 4B).
2.4. Mineral Contents in the Seeds of OsPGK1ox
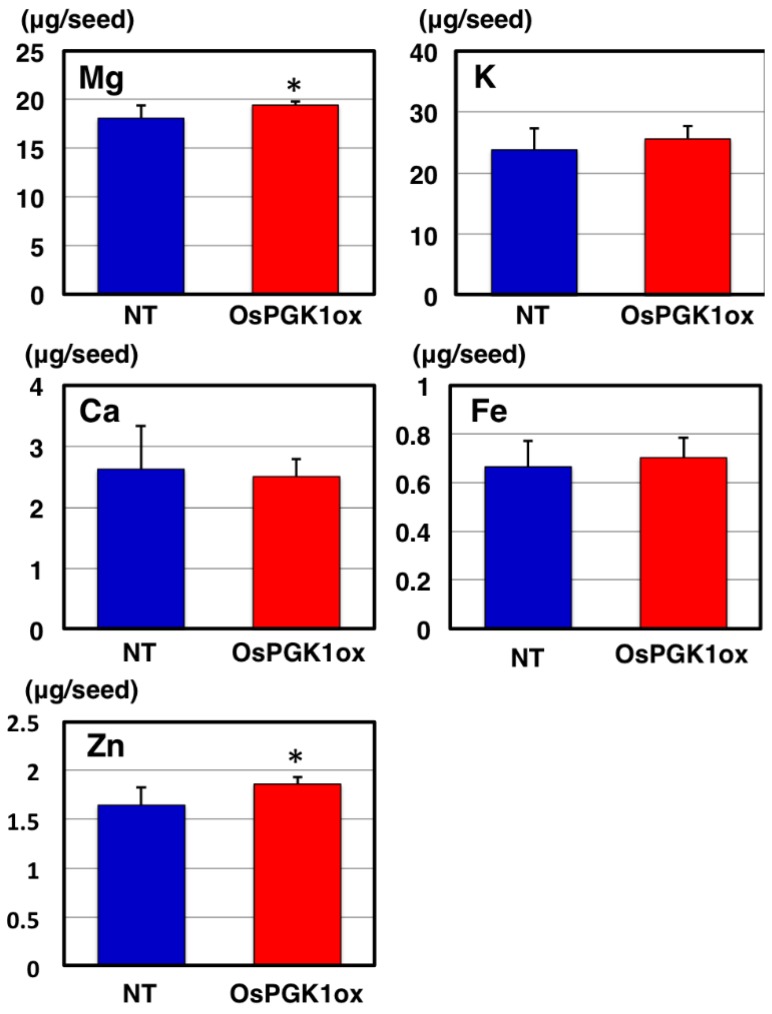
In OsPGK1ox plants, a reduced number of grains per panicle was observed compared to NT panicles. The reductions were 40.4% for OsPGK1ox-1 and 27.1% for OsPGK1ox-2. We measured the contents of several mineral elements (Mg, K, Ca, Fe, and Zn) in OsPGK1ox-1 seeds, using ICP-OES analysis, to determine if the decrease in grains per panicle affected mineral influx from vegetative organs into seeds (Figure 5). Calcium was the negative control, because the influx of Ca into a seed via the phloem is limited during seed development [13]. The OsPGK1ox seeds tended to exhibit increased contents of mineral elements, except for Ca, as compared with NT seeds (Figure 5). Contents of mineral elements in the OsPGK1ox seeds increased by 1.07-fold for Mg and K, 1.05-fold for Fe, and 1.13-fold for Zn, compared to the NT seeds. However, total P content in the same OsPGK1ox-1 seeds was 1.37-fold higher than total P content in the NT seeds (Figure 3E).
Figure 5.
Contents of Mg, K, Ca, Fe, and Zn in mature seeds of OsPGK1ox and NT. The mineral contents were measured by ICP-OES analysis. The analysis in Figure 3E and Figure 5 was performed simultaneously using the same seed samples. Each value represents the mean ± SD of six replicates. * indicates p < 0.05.
2.5. Expression of Phytic Acid Biosynthesis-related Genes in OsPGK1ox
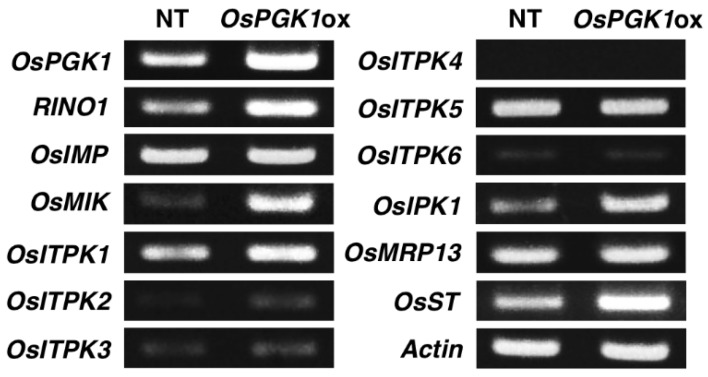
To determine whether the overexpression of OsPGK1 affects expression levels of other phytic acid-biosynthesis related genes, we analyzed the expression of several genes (RINO1, OsIMP, OsMIK, OsITPK, OsIPK1, OsMRP13, and OsST; Figure 1) by RT-PCR (Figure 6). OsIMP is a myo-inositol monophosphatase, OsMIK is a myo-inositol kinase, and OsITPK is an inositol 1,3,4-triskisphosphate 5/6-kinase. OsMRP13/OsABCC13 is an ortholog of AtMRP5, which is a putative transporter of InsP6 [14]. OsST is an ortholog of barley (Hordeum vulgare L.) HvST, which is a member of the sulfate transporter gene family and is the causal gene of a barley lpa mutant [15].
Figure 6.
Semi-quantitative RT-PCR analysis of the expression of 13 phytic acid biosynthesis-related genes in the OsPGK1ox and NT plants. Total RNA was extracted from the roots of non-transformant (NT) and OsPGK1ox-1 3 d after germination. Actin was used as a reference.
In our previous studies, real-time RT-PCR analysis of mRNA levels revealed strong up-regulation of the phytic acid-biosynthesis related genes (e.g., RINO1, OsIPK1, and OsITPK) in immature rice seeds. The expression levels of these genes were more than tenfold greater in seeds than in vegetative tissues [10]. It is difficult to detect changes in the expression levels of the genes using analysis of total RNA extracted from immature seeds. To detect transcript level changes induced by overexpression of OsPGK1, we used total RNA extracted from roots as RT-PCR templates. InsP6 in roots could be detected by ion chromatography even if the InsP6 content was 1% of that of seeds (data not shown). Therefore, we concluded that the InsP6 synthetic pathway is also active in roots, even though activity was lower in roots than in seeds.
To determine the effect of overexpression of OsPGK1 on expression of the other genes, we used total RNA extracted from roots for RT-PCR analysis. Among phytic acid biosynthesis-related genes, expression of the OsMIK, OsITPK2, OsITPK3, OsITPK4, and OsITPK6 genes was barely detected in the NT roots (Figure 6). However, expression of OsMIK gene was markedly induced, and expression of OsITPK2 and OsITPK3 genes was slightly induced in OsPGK1ox roots. Additionally, the expression levels of RINO1, OsITPK1, and OsIPK1 in OsPGK1ox were higher than in the NT (Figure 6). The expression of OsST was also higher in OsPGK1ox than in NT, although we do not know its function in InsP6 biosynthesis. The expression levels of transcripts of OsIMP and a putative phytic acid transporter, OsMRP13, displayed similar differences between OsPGK1ox and NT.
3. Discussion
There have been many reports on lpa mutants and on lpa transgenic plants [1,16]. In lpa seeds, the reduction in InsP6 content is often accompanied by a molar-equivalent increase in Pi content, so total P content is almost unchanged. There are, however, two lpa mutants that have a reduction in total P content. Arabidopsis atmrp5, a putative transporter for InsP6, reduced total P content by 35% [14], and barley lpa1-1/hvst, a homolog of a sulfate transporter, reduced total P content by 15% [15,17]. There have been no reports on mutants or transgenic plants with increases in InsP6 or total P content. To our knowledge, this is the first report of significant increases in both InsP6 and total P contents in seeds.
Overexpression of RINO1 or OsIPK1 was not effective in activating InsP6 biosynthesis (Figure 2). In the RINO1ox plants, accumulation of InsP1 and its dephosphorylated product, inositol, has been observed [18]. This suggests that InsP1 is rapidly dephosphorylated into inositol in RINO1ox plants (Figure 1), which might explain the limited increase in InsP6 in RINO1ox seeds (Figure 2). For OsIPK1ox, it is possible that there is a rate-limiting step before the last step of InsP6 biosynthesis, which is catalyzed by OsIPK1, and, therefore, overexpression of OsIPK1 has a very slight effect on activation of InsP6 biosynthesis. We do not know if expression of InsP6 biosynthesis-related genes is induced in both RINO1ox and OsIPK1ox lines. In any case, the fact that only the overexpression of OsPGK1 led to activation of InsP6 biosynthesis suggests that OsPGK1 is a key gene for InsP6 synthesis and that OsPGK1 is involved in the branch and, probably, the rate-limiting step from InsP1 to InsP2.
From the RT-PCR analysis results, we revealed that the expression of many phytic acid biosynthesis-related genes increased in OsPGK1ox plants (Figure 6). Among these genes, the expression of OsMIK, myo-inositol kinase, which catalyzes the step from myo-inositol to InsP1, was markedly induced in OsPGK1ox plants. If OsPGK1 is involved in the branch step from InsP1 to InsP2, overexpression of OsPGK1 might lead to a deficiency in InsP1. It is plausible that the expression of OsMIK and RINO1 might be raised in OsPGK1ox to compensate for the InsP1 deficiency.
The rates of InsP6 content increase, from 7 to 20 DAF, were similar for the OsPGK1ox and NT seeds (Figure 4A). This indicated that the effect of overexpression of OsPGK1 was barely observed from 7 to 20 DAF. In contrast, after 20 DAF, synthesis of InsP6 continued in the OsPGK1ox seed, but not in the NT seed (Figure 4A). Also, InsP6 content in the OsPGK1ox seed was higher than that in NT seed at 7 DAF, although the difference was not significant.
Expression levels of the InsP6 biosynthesis-related genes are usually highest between 7 and 21 DAF [10]. Microarray analysis revealed that the level of transcripts in OsPGK1 seed is also high during that period, but not during the other periods (RiceXPro). It is plausible that the overexpression of OsPGK1 was very effective in activating InsP6 biosynthesis before 7 DAF and after 20 DAF, which explained the increase in InsP6 content in OsPGK1ox seeds at very early and very late periods (Figure 4A). We plan to compare the gene expression levels in OsPGK1ox and NT seeds before 7 DAF and after 20 DAF in future studies.
In this study, the increase in InsP6 content was accompanied by an increase in total P content. In the OsPGK1ox line, the increase in total P content continued after 20 DAF and total P content at 7 DAF was higher than total P content in the NT line, as was the case for InsP6. This indicates that influx of P from vegetative organs into seeds is more active in OsPGK1ox, and the activity of InsP6 biosynthesis affects the influx of P. The signal for determining the amount of P influx and involvement of OsPGK1 in P influx has not been elucidated. In Arabidopsis, AtPAP26 encoding purple acid phosphatase is up-regulated during leaf senescence and the atpap26 mutant displayed delayed leaf senescence and reduced seed total P content [4]. This indicates that AtPAP26 plays a key role in remobilization of P from old leaves to seeds. Waters and Grusak (2008) investigated quantitative trait loci (QTL) that control seed P concentration in two Arabidopsis recombinant inbred line populations [19]. Some phosphate transporters belonging to the phosphate transporter 1 (Pht1) family were listed as candidate genes in the QTL regions [19]. The Pht1 Pi transporters are active in the uptake of Pi from the soil and its translocation within the plant, and some transporters might affect P remobilization from vegetative organs into seeds. Attention should be paid to both the phosphatases and the Pi transporters that are specific for P remobilization.
In this study, the number of grains per panicle was greatly reduced in the OsPGK1ox plant. The level of myo-inositol in the OsPGK1ox cells might be lower than NT cells, because the overexpression of OsPGK1 induced the high expression of OsMIK gene (Figure 1 and Figure 6). myo-Inositol is a central compound in diverse biochemical processes, including signal transduction, stress protection, cell wall biogenesis, growth regulation, and membrane trafficking [20]. Therefore, it is possible that a decrease of myo-inositol level in shoot apical meristems caused by ectopic expression of OsPGK1 leads to an alteration in the development of inflorescences and floral organs in the OsPGK1ox plant. This might result in the reduced number of grains per panicle.
The reduced number of grains per panicle might influence the translocation of mineral elements. However, contents of mineral elements other than P increased only slightly in the OsPGK1ox line (Figure 5). Therefore, the large increase in P content in the seeds of OsPGK1ox was mainly due to the specific response to overexpression of OsPGK1, not to the reduced number of grains per panicle. We plan to attempt to generate transgenic plants that overexpress OsPGK1 under the control of a seed specific promoter, such as the 18-kDa oleosin promoter, which promotes expression in the embryo and aleurone layer, where phytic acid is synthesized [16].
Many reports have suggested that seed P content has a beneficial effect on seed performance, in terms of germination rate and early seedling growth [3,4,5,6]. However, OsPGK1ox seed performance was similar to that of NT seed, although seed total P content in OsPGK1ox increased by 1.3 to 1.4-fold. Further study is needed to determine the reason for identical seed performance of the OsPKG1ox and NT plants.
Control of the total P content of seeds is important to enhance P sustainability and decrease the environmental impacts of agriculture [1]. We hope that this report provides the first step toward manipulating seed total P.
4. Experimental Section
4.1. Transformation of Rice Plants
OsIPK1 or OsPGK1 cDNA were amplified by RT-PCR using total RNA prepared from immature seeds (cv. Nipponbare) at 10 DAF. The gene-specific primer pairs were 5'-CTGATTCTGTGTGGGGATGG-3' and 5'-AAATTCGGCCTACTGCTGAG-3' for OsIPK1, and 5'-GGGAGGCCTCTTCTTGATTC-3' and 5'-TTGACACCGGAGGCACTATG-3' for OsPGK1. Amplified cDNA with a length of 1578 bp for OsIPK1 or 2523 bp for OsPGK1 was cloned into the binary vector pBIAct/nos containing the rice Actin1 promoter [21] and nos terminator. The method of plasmid construction was similar to that described previously [18]. Transgenic rice (Oryza sativa L., cv. Kitaake) was produced by the Agrobacterium-mediated method and grown in a glasshouse. The method to produce RINO1-overexpressing rice was described previously [18].
The presence of introduced genes was confirmed by PCR using specific primer sets for the Act1 promoter (5'-TCCCTCAGCATTGTTCATCG-3') and the RINO1 (5'-ACCAGCTCCGTCGTGTCGTA-3'), OsIPK1 (5'-GGTGCCGGTTGTCCCTTGTC-3'), and OsPGK1 (5'-GCCTTGCATCCCATGAGTTG-3') genes.
4.2. Measurements of Seed Components
InsP6 content in T4 seeds was measured by ion-chromatography and the Pi levels of T4 seeds were measured by molybdate-staining assay. The detailed method of Pi and InsP6 measurements was described previously [22]. Total P content was determined by either colorimetric assay or by inductively coupled plasma optical-emission spectrometry (ICP-OES) analysis. The detailed methods using the colorimetric assay were described in Kuwano et al. (2009) [16]. The methods of ICP-OES analysis of total P and other minerals (Mg, K, Ca, Fe, and Zn) were described previously [23]. Before analysis, the developing T4 seeds at 7, 10, 15, 20, 25 DAF were vacuum-freeze dried for 2 d and mature seeds were dried for 2 d at 60 °C.
4.3. Expression Analysis
Quantitative RT-PCR analysis of RINO1 and OsIPK1 genes was performed with cDNA templates from leaves of vector control plants carrying the empty vector, RINO1ox (T3), or OsIPK1ox (T3) plants at 7 d after germination. The detailed methods were described in Suzuki et al. (2007) [10].
For RT-PCR analysis, total RNA was prepared from flowers just before flowering or roots at 3 d after germination of NT (cv. Kitaake) and OsPGK1ox plants. The detailed methods of RT-PCR analysis were described previously [23]. The Actin gene was used as the control. The gene-specific PCR primer sets are listed in Table S1. The experiment was repeated three times with three biologically independent RNA samples.
5. Conclusions
To regulate total P in seeds, we focused on InsP6 biosynthesis-related genes because InsP6 is a major storage form of P in seeds. We generated transgenic rice plants that overexpressed InsP6-biosynthesis key genes, RINO1, OsIPK1, and OsPGK1. Only the overexpression of OsPGK1 resulted in a significant increase in seed total P, due to the increases in InsP6 and Pi. It is strongly suggested that the overexpression of OsPGK1 may lead the increase in influx of P from vegetative organs into seeds and may activate InsP6 biosynthesis. This is a first report to elevate the seed P levels through a transgenic approach.
Acknowledgments
The ICP-OES analysis was supported by Japan Advanced Plant Science Network. The authors are grateful to the members of Laboratory of Plant Molecular Genetics at the University of Tokyo for comments, and participation in discussions.
Supplementary Files
Author Contributions
Kaoru T. Yoshida and Yusuke Tagashira conceived and designed the experiments; Tomoe Shimizu, Masanobu Miyamoto and Kaoru T. Yoshida generated the transgenic rice and selected the progeny lines; Yusuke Tagashira performed the other experiments; Sho Nishida contributed the analysis of ICP-OES; Kaoru T. Yoshida and Yusuke Tagashira wrote the paper; Kaoru T. Yoshida, Yusuke Tagashira and Sho Nishida read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Raboy V. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 2009;177:281–296. doi: 10.1016/j.plantsci.2009.06.012. [DOI] [Google Scholar]
- 2.Nadeem M., Mollier A., Morel C., Prud’homme L., Vives A., Pellerin S. Remobilization of seed phosphorus reserves and their role in attaining phosphorus autotrophy in maize (Zea mays L.) seedlings. Seed Sci. Res. 2014;24:187–194. doi: 10.1017/S0960258514000105. [DOI] [Google Scholar]
- 3.Ros C., Bell R.W., White P.F. Effect of seed phosphorus and soil phosphorus applications on early growth of rice (Oryza sativa L.) cv. IR66. Soil Sci. Plant Nutr. 1997;43:499–509. doi: 10.1080/00380768.1997.10414777. [DOI] [Google Scholar]
- 4.Robinson W.D., Carson I., Ying S., Ellis K., Plaxton W.C. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphate remobilization. New Phytol. 2012;196:1024–1029. doi: 10.1111/nph.12006. [DOI] [PubMed] [Google Scholar]
- 5.De Marco D.G. Effect of seed weight, and seed phosphorus and nitrogen concentration on the early growth of wheat seedlings. Aust. J. Exp. Agric. 1990;30:545–549. [Google Scholar]
- 6.Thompson B.D., Bell R.W., Bolland M.D.A. Low seed phosphorus concentration depresses early growth and nodulation of narrow-leafed lupin. J. Plant Nutr. 1992;15:1193–1214. doi: 10.1080/01904169209364390. [DOI] [Google Scholar]
- 7.Lolas G.M., Markakis P. Phytic acid and other phosphorus compounds of beans (Phaseolus vulgaris L.) J. Agric. Food Chem. 1975;23:13–15. doi: 10.1021/jf60197a016. [DOI] [Google Scholar]
- 8.Cichy K.A., Caldas G.V., Snapp S.S., Blair M.W. QTL analysis of seed iron, zinc, and phosphorus levels in an Andean bean population. Crop Sci. 2009;49:1742–1750. doi: 10.2135/cropsci2008.10.0605. [DOI] [Google Scholar]
- 9.Yoshida K.T., Wada T., Koyama H., Mizobuchi-Fukuoka R., Naito S. Temporal and spatial patterns of accumulation of the transcript of myo-inositol-1-phosphate synthase and phytin-containing particles during seed development in rice. Plant Physiol. 1999;119:65–72. doi: 10.1104/pp.119.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki M., Tanaka K., Kuwano M., Yoshida K.T. Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): Implications for the phytic acid biosynthetic pathway. Gene. 2007;405:55–64. doi: 10.1016/j.gene.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.I., Andaya C.B., Goyal S.S., Tai T.H. The rice OsLpa1 gene encodes a novel protein involved in phytic acid metabolism. Theor. Appl. Genet. 2008;117:769–779. doi: 10.1007/s00122-008-0818-z. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H.-J., Liu Q.-L., Ren X.-L., Wu D.-X., Shu Q.-Y. Gene identification and allele-specific marker development for two low phytic acid mutations in rice (Oryza sativa L.) Mol. Breed. 2008;22:603–612. doi: 10.1007/s11032-008-9202-6. [DOI] [Google Scholar]
- 13.Epstein E., Bloom A.J. Mineral Nutrition of Plants: Principles and Perspectives. 2nd ed. Sinauer Associates, Inc.; Sunderland, UK: 2004. p. 400. [Google Scholar]
- 14.Nagy R., Grob H., Weder B., Green P., Klein M., Frelet-Barrand A., Schjoerring J.K., Brearley C., Martinoia E. The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage. J. Biol. Chem. 2009;284:33614–33622. doi: 10.1074/jbc.M109.030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye H., Zhang X.Q., Broughton S., Westcott S., Wu D., Lance R., Li C. A nonsense mutation in a putative sulphate transporter gene results in low phytic acid in barley. Funct. Integr. Genomics. 2011;11:103–110. doi: 10.1007/s10142-011-0209-4. [DOI] [PubMed] [Google Scholar]
- 16.Kuwano M., Mimura T., Takaiwa F., Yoshida K.T. Generation of stable “low phytic acid” transgenic rice through antisense repression of the 1D-myo-inositol 3-phosphate synthase gene (RINO1) using the 18-kDa oleosin promoter. Plant Bioltechnol. J. 2009;7:96–105. doi: 10.1111/j.1467-7652.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- 17.Ockenden I., Dorsch J.A., Reid M.M., Lin L., Grant L.K., Raboy V., Lott J.N.A. Characterization of the storage of phosphorus, inositol phosphate and cations in grain tissues of four barley (Hordeum vulgare L.) low phytic acid genotypes. Plant Sci. 2004;167:1131–1142. [Google Scholar]
- 18.Kusuda H., Koga W., Kusano M., Oikawa A., Saito K., Hirai M.Y., Yoshida K.T. Ectopic expression of myo-inositol 3-phosphate synthase induces a wide range of metabolic changes and confers salt tolerance in rice. Plant Sci. 2015;232:49–56. doi: 10.1016/j.plantsci.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Waters B.M., Grusak M.A. Quantitative trait locus mapping for seed mineral concentrations in two Arabidopsis thaliana recombinant inbred populations. New Phytol. 2008;179:1033–1047. doi: 10.1111/j.1469-8137.2008.02544.x. [DOI] [PubMed] [Google Scholar]
- 20.Irvine R.F., Schell M.J. Back in the water: The return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W., McElroy D., Wu R. Analysis of rice Act1 5’region activity in transgenic rice plants. Plant Cell. 1991;3:1155–1165. doi: 10.1105/tpc.3.11.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwano M., Ohyama A., Tanaka Y., Mimura T., Takaiwa F., Yoshida K.T. Molecular breeding for transgenic rice with low-phytic-acid phenotype through manipulating myo-inositol 3-phosphate synthase gene. Mol. Breed. 2006;18:263–272. doi: 10.1007/s11032-006-9038-x. [DOI] [Google Scholar]
- 23.Iwai T., Takahashi M., Oda K., Terada Y., Yoshida K.T. Dynamic changes in the distribution of minerals in relation to phytic acid accumulation during rice seed development. Plant Physiol. 2012;160:2007–2014. doi: 10.1104/pp.112.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.