Abstract
Background
The identification and use of novel compounds alone or in combination hold promise for the fight against NRAS mutant melanoma.
Material/Methods
We screened a kinase-specific inhibitor library through combining it with α-Mangostin in NRAS mutant melanoma cell line, and verified the enhancing effect of α-Mangostin through inhibition of the tumorigenesis pathway.
Results
Within the kinase inhibitors, retinoic acid showed a significant synergistic effect with α-Mangostin. α-Mangostin also can reverse the drug resistance of retinoic acid in RARa siRNA-transduced sk-mel-2 cells. Colony assay, TUNEL staining, and the expressions of several apoptosis-related genes revealed that α-Mangostin enhanced the effect of retinoic acid-induced apoptosis. The combination treatment resulted in marked induction of ROS generation and inhibition of the AKT/S6 pathway.
Conclusions
These results indicate that the combination of these novel natural agents with retinoid acid may be clinically effective in NRAS mutant melanoma.
MeSH Keywords: Antineoplastic Agents, Melanoma, Tretinoin
Background
Melanoma is a potentially fatal cutaneous cancer, with a significant increase in the number of cases diagnosed in the world in the past few years [1]. Due to its poor response to standard chemotherapy, the primary treatment for melanoma is surgical excision [2]. NRAS is an important oncogene mutated in 15% to 20% of melanoma cases compared to BRAF mutants in approximately 50% of cases. NRAS mutants are correlated with a higher rate of mitosis and no targeted therapies are currently available [3,4]. The progress of research on treatments for melanoma harboring NRAS mutation has been slow [5], and effective single-agent chemotherapies are currently limited [6]. Thus, for long-term response rates and minimizing drug toxicity and adverse effects, combining inhibition and multiple treatments are already underway [7].
There are extensive studies showing that retinoic acid can inhibit the cell proliferation and induce cell differentiation in a variety of human cancers [8]. The anti-proliferation effect of retinoic acid in the B16 murine melanoma cell model has been reported [9], however, the in vitro response of melanoma cell lines to retinoic acid is quite variable [10]. Resistance to retinoid treatment and the adverse effects limit the use of retinoic acid as a single-agent chemotherapy [11,12]; therefore, it is mainly used in combination with other antitumor agents for the treatment of cancer [13,14].
α-Mangostin is a natural product, been widely studied as a candidate treatment of various cancers [15,16]. The cytotoxic activity of α-Mangostin also has been demonstrated in melanoma cell lines [17]. The use of these novel compounds alone or in combination therapies may greatly help in the fight against NRAS mutant melanoma.
Material and Methods
Human melanoma cell lines culture
Sk-mel-2 cell (human NRAS mutant melanoma cell line) was kept in our laboratory. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; fetal calf serum, 10%; penicillin, 100 000 U/l; streptomycin sulphate, 100 mg/l; Life Technologies, Grand Island, NY). Cells were incubated at 37°C in 5% CO2, 5% humidity, and passaged at 2×104 cells/ml when near-confluent monolayers were achieved. Cells were free from Mycoplasma contamination.
Drugs and treatment
α-Mangostin was purchased from Sigma, and retinoic acid was purchased from LC Laboratories (MA). All the drugs were dissolved in dimethyl sulfoxide (DMSO) and were added directly to the culture medium of melanoma cells at the concentrations to be tested. Melanoma cells incubated with culture medium with DMSO served as controls. The stock solutions of the drugs were prepared according to the manufacturer’s specification and stored at −20°C.
Screening assay
The kinase inhibitor library was screened in sk-mel-2 cell line alone or in combination with α-Mangostin to determine the synergistic effect. The screening was performed in sk-mel-2 cell line plating in 384-well clear bottom plates (Corning, Tewksbury, MA) with 20 000 cell/ml density. Kinase inhibitors were pin-transferred into each well at the original concentration of 10 mM with or without α-Mangostin (5 μM). The cell proliferation and cytotoxicity were measured using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI) at 48 h using automated high-throughput analysis.
Cell proliferation assay
Drug cytotoxicity was determined using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI) as previously described. Briefly, cells were plated in a 96-well clear bottom plate (Corning, Tewksbury, MA) at the density of 20 000 cells/ml in culture medium (DMEM supplemented with 10% FBS and penicillin/streptomycin), drugs were added to the first well of each row at the concentration of 10 μM alone or in combination with α-Mangostin (5 μM), then double-diluted by more than 10 times. After 48 h of culture, 8 ul of CellTiter-Glo (10μM, in PBS) was added into each well and incubated for 10 min at room temperature. The absorbance at a wavelength of 490 nm was read using a EnVision® Multilabel Plate Reader (Envision, Perkin Elmer) using ultrasensitive luminescence. The experiments were done in triplicate.
Colony formation assay
Sk-mel-2 cells were seeded in 6-well plates at the density of 100 cells/well, and drugs were added into each well at the concentration of 5 uM alone or with α-Mangostin (2 μM) for 6 days. Cells were fixed with 4% paraformaldehyde, and colonies were stained with 0.1% crystal violet.
Immunofluorescence microscopy
Sk-mel-2 cells mounted on glass slides were fixed with 4% paraformaldehyde for 20 min, and permeabilized with PBS containing 0.1% Triton X-100 and 0.1% glycine for 2 min on ice. The TUNEL staining was performed using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Germany). Cells were co-stained with 4′6′-diamidino-2-phenylindole (DAPI) to visualize nuclei. The images were taken using a FSX100 all-in-one microscope (Olympus Corporation, Japan).
Western blotting
Cells were lysed in 1× radioimmunoprecipitation assay (RIPA) lysis buffer (Life Technologies, Grand Island, NY), and the protein concentration was determined by BCA protein Assay Kit (Thermo Scientific). Equal amounts of the proteins were electrophoresed on 4–15% Bis-Tris Gels (BioRad, USA) and transferred onto polyvinylidene difluoride membranes. Membranes were blocked for 1 h at room temperature with 5% BSA in Tris-Buffered Saline and Tween 20, incubated with various primary antibodies diluted in blocking buffer at 4°C overnight, and detected with either anti-rabbit (1:5000) or anti-mouse (1:5000) secondary antibodies for 1 h at room temperature. The final immunoreactive products were detected by enhanced chemiluminescence (ECL) system (Promega, Madison, WI). The human anti-S6, anti-phospho-S6, anti-AKT, anti-phospho-AKT, anti-Mcl-1, anti-P21, anti-cleaved PARP, anti-PARP, and anti-beta-actin antibodies were purchased from Cell Signaling Technologies (Dedham, MA).
Determination of ROS
The CellROX Oxidative Stress Reagents (Life Technology, Grand Island, NY, USA) provides a cell-based assay for measuring primarily hydrogen peroxide, along with hydroxyl, peroxyl, and other ROS levels within a cell. Cells were incubated with 5 μM CellROX Oxidative Stress Reagents for 30 min, after which they were washed with PBS, cells were co-stained with 4′6′-diamidino-2-phenylindole (DAPI) to visualize nuclei, and the amount of fluorescence was determined using an FSX100 all-in-one microscope (Olympus Corporation, Japan).
RNA interference studies
Transfection of SK-mel-2 cells was performed with Attractgene Transfection Reagent according to the manufacturer’s protocol (Qiagen, Germany) with siRNAs against RARα and control siRNA (Santa Cruz, CA). Transfected cells were treated with α-Mangostin (2μM), retinoic acid (2 μM), or their combination for 48 h for further studies.
Statistical analysis
The differences and variances were analyzed using the t test for unpaired observations. The results are presented as mean ±SEM and P<0.05 was considered statistically significant.
Results
Screening of the kinase inhibitors and primary hit selection
A total of 2000 compounds in the kinase-specific inhibitor library were screened with or without the combination of α-Mangostin, and 10 compounds were identified to be significantly inhibiting cell proliferation and inducing cell death after combined with α-Mangostin in sk-mel-2 cells (Figure 1). Several kinase inhibitors, such as Rapamycin, retinoic acid, and Torin1, were previously reported to have the ability to inhibit tumor growth in various cancers [18–20]. Some of them were being studied in treating melanoma alone or with other combinations. These compounds include inhibitors targeting mTOR (Rapamycin, Torin1), GSK3 (CHIR99021), and VEGFR (Sorafenib) which are closely connected with cancer formation, growth, and metastasis [21–23].
Figure 1.
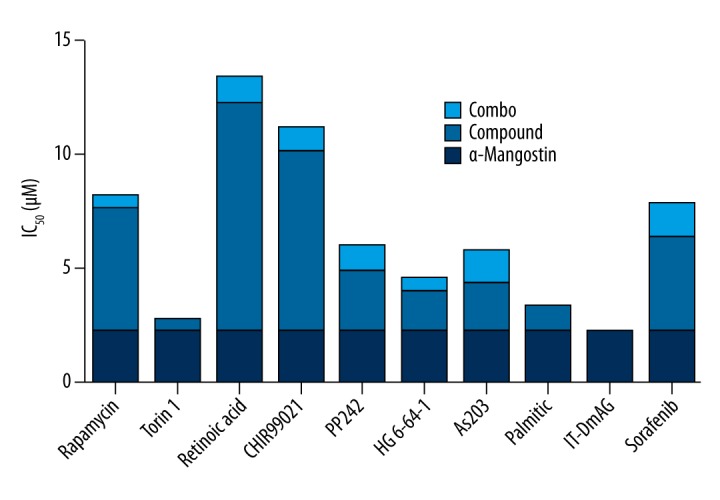
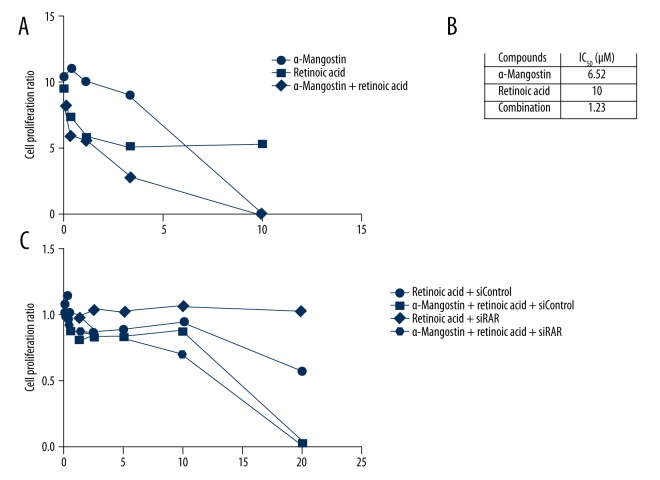
Screening of kinase-specific inhibitors with α-Mangostin. The dosage-dependent cell proliferation ratio assay of drugs and drug combination in sk-mel-2 cells was performed with or without the presence of α-Mangostin (5 μM). The synergistic effect of drug combination was shown in the form of half-lethal dosage IC50. Ten compounds were selected, and strong synergistic effects of those compounds combined with α-Mangostin respectively were found. A marked decrease is seen in the half-lethal dosage IC50 in combination groups compared to the single-agent groups.
Secondary hit selection and verification of the combination effect
We further verified the efficacy of 5 lead compounds with α-Mangostin by CellTiter-Glo Luminescent Cell Viability Assay. Improved IC50 was shown in combination groups. Within all these compounds, we found that retinoic acid showed a significant inhibition effect after combining with α-Mangostin. The IC50 of retinoic acid was decreased nearly by 8-fold in sk-mel-2 cells in combination with α-Mangostin (Figure 2A, 2B). To evaluate the correlation of RAR (retinoic acid receptor) level with retinoic acid resistance in NRAS mutant melanoma, we inhibited the RARα expression in sk-mel-2 cells using RARα siRNA, and determined the sensitivities of retinoic acid, α-Mangostin, and their combination by CellTiter-Glo Luminescent Cell Viability Assay in RARα siRNA-transduced cells and control cells. The results showed that RARα siRNA-transduced sk-mel-2 cells were more sensitive to retinoic acid combined with α-Mangostin, which means that α-Mangostin can significantly reverse the retinoic acid resistance in RARα siRNA-transduced sk-mel-2 cells (Figure 2C).
Figure 2.
The combination effect of α-Mangostin and retinoic acid was verified. (A, B) Within all these compounds, we found the effect of retinoic acid (10 μM) and verified its synergistic effect with α-Mangostin (5μM) in sk-mel-2 cells. The half-lethal dosage IC50 was decreased to 1.23 μM, nearly 8 times higher than the 10 μM in the single-agent group. (C) We determined the correlation of the synergistic effect with RAR level in RARα siRNA-transduced sk-mel-2 cells, showing that cells without RAR expression were not sensitive to retinoic acid, but the synergistic effect of retinoic acid and α-Mangostin was significantly improved.
α-Mangostin activates retinoic acid-induced apoptosis
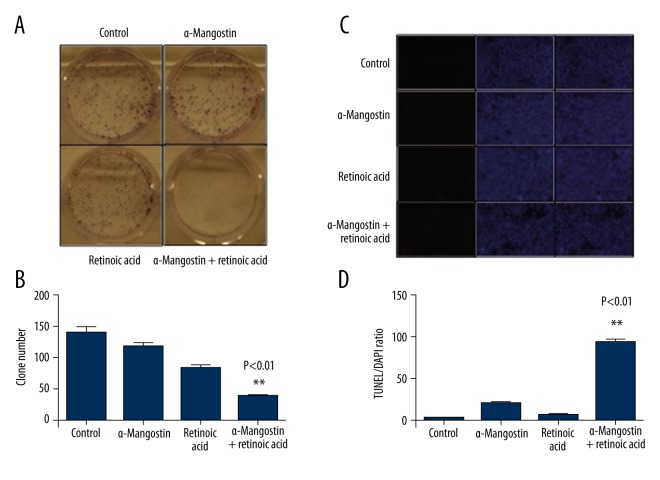
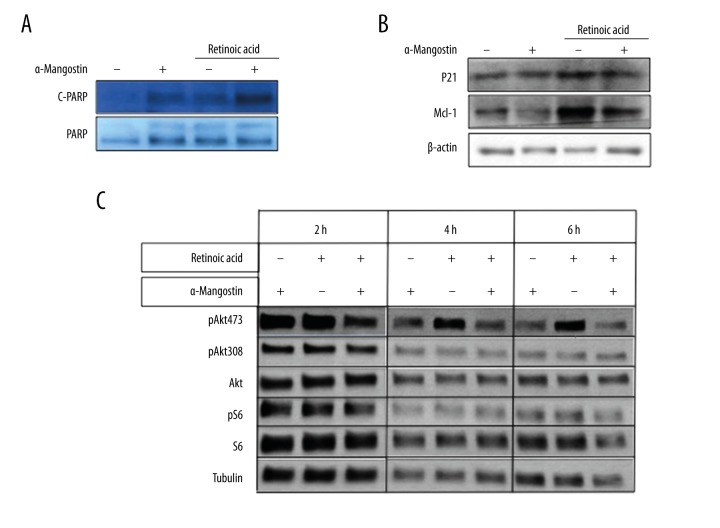
We examined the effect of retinoic acid, α-Mangostin, and their combination on the survival of sk-mel-2 cells by colony survival assay. Colony formation was completely inhibited in cells treated with retinoic acid combined with α-Mangostin for 5 days. In contrast, retinoic acid or α-Mangostin alone showed little effect under the same condition (Figure 3A, 3B). To study whether retinoic acid combined with α-Mangostin could result in increased apoptosis compared to being used alone, we further examined the growth-inhibitory effects in sk-mel-2 cells via TUNEL (Terminal deoxynucleotidyl transferase dUTP nick-end labeling) staining assay. As shown in Figure 3C and 3D, apoptotic bodies, as an indication of DNA fragmentation and activated apoptotic cascades, were markedly increased in the retinoic acid combined with α-Mangostin treatment group. Thus, the enhancing effect of α-Mangostin on retinoic acid-induced cell apoptosis was determined. We also noticed that the expression of cleaved PARP, which is an apoptotic marker [24], was increased with the treatment of retinoic acid and α-Mangostin (Figure 4A). P21 and Mcl-1, which are anti-apoptotic proteins [25, 26], were significantly down-regulated with 2 μM of retinoic acid and α-Mangostin treatment for 8 h in sk-mel-2 cells (Figure 4B).
Figure 3.
α-Mangostin activates retinoic acid-induced apoptosis. (A) Clonogenic survival assay was performed to examine the synergistic effect of the combination of retinoic acid and α-Mangostin on the survival of sk-mel-2 cells. Cell colonies were stained blue. (B) Colony numbers were counted. The paired t test was used to check the statistical significance (P<0.001). (C) The growth-inhibitory effects were examined via TUNEL staining. Slices were cultured in medium containing retinoic acid (2 μM), α-Mangostin (2 μM), or their combination for 3 h and stained for TUNEL (red) or 4′,6-diamidino-2-phenylindole (DAPI; blue) to label all cells. Representative images of 3 independent experiments are shown. D. The numbers of positive cell were counted. The paired t test was used to assess the statistical significance (P<0.001).
Figure 4.
α-Mangostin is synergistic with retinoic acid on inhibiting apoptosis-related genes and AKT signaling pathway. (A) The expression of cleaved PARP and PARP were analyzed by Western blot in sk-mel-2 cells exposed to retinoic acid (2 μM) in either the presence or absence of α-Mangostin (5 μM) for 8 h. The expression of cleaved PARP was increased with the combination of retinoic acid and α-Mangostin. (B) As anti-apoptotic proteins, MCL-1 and P21 expression levels were suppressed with retinoic acid (2 μM) and α-Mangostin (5 μM) treatment for 8 h in sk-mel-2 cells. (C) Down-regulation of phosphorylation of AKT and S6 is much more significant within 2 h after the treatment with the combination of retinoic acid (2 μM) and α-Mangostin (5 μM) than by single agents.
α-Mangostin is synergistic with retinoic acid on pAKT down-regulation in SK-mel-2 cell line
To further explore the underlying mechanisms of the effect, we next analyzed the expression of a variety of proteins involved in the regulation of cell growth and vitality in sk-mel-2 cells after single-agent and combination therapy with retinoic acid and α-Mangostin. The AKT survival pathway has been shown as an important pathway involved in cancer growth [27]. The effects of retinoic acid with or without α-Mangostin on the expression of pAKT, AKT, pS6, and S6 in sk-mel-2 cells were analyzed by Western blot. It revealed that their combination could inhibit the phosphorylation of AKT and S6, with no effect on the total expression of AKT and S6. Time course analysis showed that the inhibition of phospho-AKT by α-Mangostin was observed within 2 h after the treatment (Figure 4C). Thus, α-Mangostin may sensitize NRAS mutant melanoma to the apoptotic effect of retinoic acid through its inhibition of the AKT pathway.
The combined effect of α-Mangostin and retinoic acid on ROS level
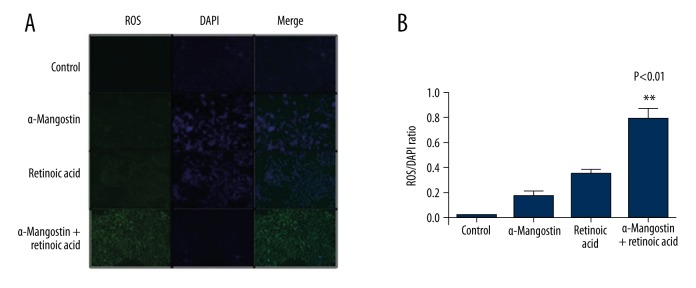
Since the generation of ROS is known to induce apoptosis in melanoma cells [28], we investigated whether the combination of α-Mangostin and retinoic acid-induced apoptosis is related to ROS generation. For this, we treated sk-mel-2 cells with α-Mangostin, retinoic acid, or their combination. The combination treatment resulted in marked induction of ROS generation at 8 h, signifying that the combination-induced apoptosis is mediated through ROS generation (Figure 5). Therefore, α-Mangostin might sensitize melanoma to the apoptotic effect of retinoic acid through its induction of ROS level.
Figure 5.
α-Mangostin enhanced the apoptotic effect of retinoic acid through its induction of ROS level. (A) SK-MEL-2 cells were treated with retinoic acid (5 μM), α-Mangostin (5 μM), or their combination for 8 h. High levels of ROS were detected in the combination group. Reactive oxygen species (ROS) production was detected by staining with dichlorofluorescein diacetate (DCFDA), which turns into a fluorescent compound (green) upon oxidation and all the cells were stained for the nucleus (blue, DAPI). Representative images of 3 independent experiments are shown. (B) The numbers of positive cell were counted. The paired t test was used to assess statistical significance (P=0.005).
Discussion
The incidence of melanoma is rapidly increasing around the world [29]. BRAF mutations contribute to ~50% of cutaneous melanomas, which is the most common type [30]. In contrast, NRAS mutations are detected in ~20% of cutaneous melanomas, with advanced high-risk tumor features and low survival rate in clinical melanoma patients. Rapid and successful advances have been made in targeted therapies of BRAF mutations; however, there are still no effective therapeutics against NRAS mutant melanomas [31]. NRAS is an important oncogene in the biology of melanomas that contain NRAS mutations [32]; therefore, it is necessary to understand the related mechanisms and the benefits of multiple treatments to guide therapeutics against different types of melanoma. Some studies have lighted the possibility that both the RAS/RAF/MEK/ERK and the PI3K/AKT pathways are necessary for inducing and maintaining the malignant phenotype in NRAS mutant melanomas [33,34]. To date, there is no targeted therapy that directly targets NRAS. Many approaches have been addressed and studied, including various kinds of combined inhibitors, such as the combined use of MEK inhibitors and RAF inhibitors, and the combination of PI3K pathway inhibitors with ERK pathway inhibitors, and some of them are currently being evaluated in clinical trials [7,35]. There is evidence that combined targeting of the PI3K and ERK pathways may enhance the responses in NRAS mutant melanoma [36], that the activation of AKT is required for melanoma initiation, and that the binding of RAS to PI3K p110 plays a critical role in the tumorigenesis of RAS mutant melanoma [37]. The above evidence suggests that combined therapeutics are necessary for the treatment of melanoma, especially that with high incidence of drug resistance.
Many studies have shown that retinoic acid can inhibit cell proliferation and induce cell differentiation in a variety of human cancers, and its use in cancer therapy has been extensively studied in recent decades [38,39]. Most retinoids show limited clinical efficacy in treatment of solid malignancies; they have adverse effects and are associated with frequent development of resistance [40]. Much effort has been spent on developing use of retinoic acid alone or in combination with other related chemotherapeutic agents to keep its antitumor activity, as well as to minimize the toxicity in the prevention and treatment of cancers [14]. There is evidence that retinoic acid can induce growth arrest and differentiation in a B16 murine melanoma cell model, and its derivatives have been used for treatment of primary melanoma; however, the response of different melanoma cell lines against retinoic acid is quite variable [41]. There is a growing need for novel therapeutics, such as enhancing antitumor activity with combined chemotherapeutic agents.
Our studies addressed an innovative combination with α-Mangostin, a natural product and the active component of some commercial nutraceuticals. Although it has been extensively studied for its wide range of biological activities, including antitumor, antioxidant, anti-inflammatory activities, the details of the mechanisms related in its activities are currently unknown [42]. Previous research has shown that α-Mangostin can inhibit the proliferation of several melanoma cell lines in vitro [17], probably through the inhibition of the PI3K/mTOR pathway [43]. Our lab has studied melanoma for many years; we have identified α-Mangostin as a candidate pigmentation inhibitor from a large numbers of compounds using a high-throughput, high-content screening approach, showing the possibility of α-Mangostin as an anti-melanoma agent [44]. We therefore screened a kinase-specific inhibitor compound library in human melanoma cell lines, including BRAF mutant and NRAS mutant melanoma, to explore the effect of α-Mangostin on enhancing inhibition and reversing chemo-resistance to the kinase-specific inhibitors. We found α-Mangostin can significantly enhance the inhibition of retinoic acid in an NRAS mutant melanoma cell line and further verified this effect in terms of the decreasing phosphorylation levels of related proteins in the PI3K pathway, as well as through its induction of ROS level [45]. The specific mechanism is unclear, but there is evidence that CF31 can inhibit RXR (retinoid X receptor) signaling through its unique RXR-α binding mode [46]. We hypothesize that α-Mangostin, as a kind of xanthone, may act synergistically with retinoic acid through targeting the binding or activity of retinoid-related receptors [47].
Conclusions
The need for novel, natural, and effective chemotherapies for NRAS mutant melanoma is growing. Our studies on the details of the mechanism are underway, and a number of approaches are being developed. Our novel combination therapy with natural products holds promise.
Footnotes
Source of support: Departmental sources
References
- 1.Frangos JE, Duncan LM, Piris A, et al. Increased diagnosis of thin superficial spreading melanomas: A 20-year study. J Am Acad Dermatol. 2012;67:387–94. doi: 10.1016/j.jaad.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Gigliofiorito P, Segreto F, Piombino L, et al. Managing malignant melanoma. Plast Reconstr Surg. 2014;133:437–38e. doi: 10.1097/01.prs.0000438448.78400.40. [DOI] [PubMed] [Google Scholar]
- 3.Lee le M, Feun L, Tan Y. A case of intracranial hemorrhage caused by combined dabrafenib and trametinib therapy for metastatic melanoma. Am J Case Rep. 2014;15:441–43. doi: 10.12659/AJCR.890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedorenko IV, Gibney GT, Smalley KS. NRAS mutant melanoma: Biological behavior and future strategies for therapeutic management. Oncogene. 2013;32:3009–18. doi: 10.1038/onc.2012.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DB, Puzanov I. Treatment of NRAS-mutant melanoma. Curr Treat Options Oncol. 2015;16:15. doi: 10.1007/s11864-015-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soon CW, Algazi AP, Cha EN, et al. NRAS-mutant melanoma: Response to chemotherapy. Arch Dermatol. 2011;147:626–27. doi: 10.1001/archdermatol.2011.85. [DOI] [PubMed] [Google Scholar]
- 7.Drug combo shows promise in NRAS-mutant melanoma. Cancer Discov. 2014;4:OF2. doi: 10.1158/2159-8290.CD-NB2014-098. [DOI] [PubMed] [Google Scholar]
- 8.Schenk T, Stengel S, Zelent A. Unlocking the potential of retinoic acid in anticancer therapy. Br J Cancer. 2014;111:2039–45. doi: 10.1038/bjc.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi N, Imai M, Komori Y. Inhibitory effects of p-alkylaminophenol on melanogenesis. Bioorg Med Chem. 2014;22:4677–83. doi: 10.1016/j.bmc.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Hoal E, Wilson EL, Dowdle EB. Variable effects of retinoids on two pigmenting human melanoma cell lines. Cancer Res. 1982;42:5191–95. [PubMed] [Google Scholar]
- 11.Liu CY, Francis JH, Brodie SE, et al. Retinal toxicities of cancer therapy drugs: Biologics, small molecule inhibitors, and chemotherapies. Retina. 2014;34:1261–80. doi: 10.1097/IAE.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 12.Demary K, Wong L, Liou JS, et al. Redox control of retinoic acid receptor activity: a novel mechanism for retinoic acid resistance in melanoma cells. Endocrinology. 2001;142:2600–5. doi: 10.1210/endo.142.6.8201. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro MP, Silva FS, Paixao J, et al. The combination of the antiestrogen endoxifen with all-trans-retinoic acid has anti-proliferative and anti-migration effects on melanoma cells without inducing significant toxicity in non-neoplasic cells. Eur J Pharmacol. 2013;715:354–62. doi: 10.1016/j.ejphar.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Chan SY, Ho PC. Comparison of the in vitro and in vivo effects of retinoids either alone or in combination with cisplatin and 5-fluorouracil on tumor development and metastasis of melanoma. Cancer Chemother Pharmacol. 2008;63:167–74. doi: 10.1007/s00280-008-0763-1. [DOI] [PubMed] [Google Scholar]
- 15.Mizushina Y, Kuriyama I, Nakahara T, et al. Inhibitory effects of alpha-Mangostin on mammalian DNA polymerase, topoisomerase, and human cancer cell proliferation. Food Chem Toxicol. 2013;59:793–800. doi: 10.1016/j.fct.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Shan T, Cui XJ, Li W, et al. alpha-Mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of Stat3 signaling pathway. Acta Pharmacol Sin. 2014;35:1065–73. doi: 10.1038/aps.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beninati S, Oliverio S, Cordella M, et al. Inhibition of cell proliferation, migration and invasion of B16-F10 melanoma cells by alpha-Mangostin. Biochem Biophys Res Commun. 2014;450:1512–17. doi: 10.1016/j.bbrc.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–35. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Song M, DiPaola RS, Cracchiolo BM, et al. Phase 2 trial of paclitaxel, 13-cis retinoic acid, and interferon alfa-2b in the treatment of advanced stage or recurrent cervical cancer. Int J Gynecol Cancer. 2014;24:1636–41. doi: 10.1097/IGC.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkin J, Halova L, Ferguson J, et al. Torin1-mediated TOR kinase inhibition reduces Wee1 levels and advances mitotic commitment in fission yeast and HeLa cells. J Cell Sci. 2014;127:1346–56. doi: 10.1242/jcs.146373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu K, Liu P, Wei W. mTOR signaling in tumorigenesis. Biochim Biophys Acta. 2014;1846:638–54. doi: 10.1016/j.bbcan.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCubrey JA, Steelman LS, Bertrand FE, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881–911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Adjei AA. Targeting Angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist. 2015;20:660–73. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qurishi Y, Hamid A, Sharma PR, et al. NF-kappaB down-regulation and PARP cleavage by novel 3-alpha-butyryloxy-beta-boswellic acid results in cancer cell specific apoptosis and in vivo tumor regression. Anticancer Agents Med Chem. 2013;13:777–90. doi: 10.2174/1871520611313050012. [DOI] [PubMed] [Google Scholar]
- 25.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–49. [PubMed] [Google Scholar]
- 26.Fujise K, Zhang D, Liu J, Yeh ET. Regulation of apoptosis and cell cycle progression by MCL1. Differential role of proliferating cell nuclear antigen. J Biol Chem. 2000;275:39458–65. doi: 10.1074/jbc.M006626200. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Rajendran V, Sethumadhavan R, Purohit R. AKT kinase pathway: A leading target in cancer research. ScientificWorldJournal. 2013;2013:756134. doi: 10.1155/2013/756134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai J, Lei Y, An GL, He L. Down-regulation of deacetylase HDAC6 inhibits the melanoma cell line A375.S2 growth through ROS-dependent mitochondrial pathway. PLoS One. 2015;10:e0121247. doi: 10.1371/journal.pone.0121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo JA, Fisher DE. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science. 2014;346:945–49. doi: 10.1126/science.1253735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529–45. ix. doi: 10.1016/j.hoc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Fedorenko IV, Gibney GT, Sondak VK, Smalley KS. Beyond BRAF: Where next for melanoma therapy? Br J Cancer. 2015;112:217–26. doi: 10.1038/bjc.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelleher FC, McArthur GA. Targeting NRAS in melanoma. Cancer J. 2012;18:132–36. doi: 10.1097/PPO.0b013e31824ba4df. [DOI] [PubMed] [Google Scholar]
- 33.Bello DM, Ariyan CE, Carvajal RD. Melanoma mutagenesis and aberrant cell signaling. Cancer Control. 2013;20:261–81. doi: 10.1177/107327481302000404. [DOI] [PubMed] [Google Scholar]
- 34.Yajima I, Kumasaka MY, Thang ND, et al. RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant melanoma progression and therapy. Dermatol Res Pract. 2012;2012:354191. doi: 10.1155/2012/354191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atefi M, Titz B, Avramis E, et al. Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol Cancer. 2015;14:27. doi: 10.1186/s12943-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkpatrick DS, Bustos DJ, Dogan T, et al. Phosphoproteomic characterization of DNA damage response in melanoma cells following MEK/PI3K dual inhibition. Proc Natl Acad Sci USA. 2013;110:19426–31. doi: 10.1073/pnas.1309473110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Hernandez I, Baquero P, et al. Dual inhibition of (V600E)BRAF and the PI3K/AKT/mTOR pathway cooperates to induce apoptosis in melanoma cells through a MEK-independent mechanism. Cancer Lett. 2012;314:244–55. doi: 10.1016/j.canlet.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 38.Evans TR, Kaye SB. Retinoids: present role and future potential. Br J Cancer. 1999;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doldo E, Costanza G, Agostinelli S, et al. Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. Biomed Res Int. 2015;2015:624627. doi: 10.1155/2015/624627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos B, Weisang S, Osswald F, et al. Retinoid resistance and multifaceted impairment of retinoic acid synthesis in glioblastoma. Glia. 2015;63(10):1850–59. doi: 10.1002/glia.22849. [DOI] [PubMed] [Google Scholar]
- 41.Niles RM. Vitamin A (retinoids) regulation of mouse melanoma growth and differentiation. J Nutr. 2003;133:282S–86S. doi: 10.1093/jn/133.1.282S. [DOI] [PubMed] [Google Scholar]
- 42.Jindarat S. Xanthones from mangosteen (Garcinia mangostana): Multi-targeting pharmacological properties. J Med Assoc Thai. 2014;97(Suppl 2):S196–201. [PubMed] [Google Scholar]
- 43.Xu Q, Ma J, Lei J, et al. alpha-Mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. Biomed Res Int. 2014;2014:546353. doi: 10.1155/2014/546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Chen H, Kolev V, et al. High-throughput, high-content screening for novel pigmentation regulators using a keratinocyte/melanocyte co-culture system. Exp Dermatol. 2014;23:125–29. doi: 10.1111/exd.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng HB, Bo Y, Shen WX, et al. Longikaurin E induces apoptosis of pancreatic cancer cells via modulation of the p38 and PI3K/AKT pathways by ROS. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:623–34. doi: 10.1007/s00210-015-1107-4. [DOI] [PubMed] [Google Scholar]
- 46.Wang GH, Jiang FQ, Duan YH, et al. Targeting truncated retinoid X receptor-alpha by CF31 induces TNF-alpha-dependent apoptosis. Cancer Res. 2013;73:307–18. doi: 10.1158/0008-5472.CAN-12-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.di Masi A, Leboffe L, De Marinis E, et al. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]