Abstract
Recent studies underscore that prostaglandin-E2 (PGE2) exerts mostly proinflammatory effects in chronic CNS and peripheral disease models, mainly through a specific prostanoid receptor EP2. However, very few highly characterized EP2 receptor antagonists have been reported until recently, when Pfizer and Emory University published two distinct classes of EP2 antagonists with good potency, selectivity and pharmacokinetics. The purpose of this article is to evaluate recently published patents WO 2012177618 A1 and US-2014/0179750 A1 from Emory, which describe a number of cinnamic amide- and amide-derivatives as a potent antagonists of EP2 receptor, and their neuroprotective effects in in vitro and in an in vivo model. A selected compound from this patent(s) also attenuates prostate cancer cell growth and invasion in vitro, suggesting these compounds should be developed for therapeutic use.
Keywords: Neuroinflammation, status epilepticus, anti-cancer, neuroprotection, cinnamic amides, amides, competitive antagonism
1. Introduction
Cyclooxygenase-2 (COX-2) evolved as one of the major contributors to the inflammation following a brain or peripheral injury. Thus COX-2 inhibitors were developed and used in animal models and humans in the past1, 2. However, COX-2 catalyzes the synthesis of five prostanoids (PGD2, PGE2, PGF2, PGI2 and TXA2), which activate a total of 11 prostanoid receptors (DP1 and DP2 activated by PGD2; EP1, EP2 EP3 and EP4 by PGE2; FPα and FPβ by PGF2; IP by PGI2; TPα and TP by TXA2)3–5. Some of these receptors share common downstream signaling pathways. For example, EP2, EP4, DP1 and IP stimulate adenylate cyclase to elevate cAMP (via Gs coupled-protein), which promotes protein kinase A (PKA), and the exchange protein activated by cAMP (Epac) mediated signaling6, 7. Others such as DP2 and EP3 (via Gi coupled-protein) impede cAMP signaling; EP1, FP and TP receptors promote (via Gq coupled-protein) calcium mediated signaling. Individually these receptors display ‘Jekyll and Hyde nature’, similar to COX-2, depending on the disease condition3, 4. Although, COX-2 inhibitors proved to be efficacious in ameliorating inflammation and pain in humans with osteoarthritis and rheumatoid arthritis8, 9, they have not provided a clear benefit to the rodent models of inflammatory neurodegenerative disease epilepsy10, and to humans with Alzheimer’s diseases11, 12 and ALS13. Instead, they resulted in adverse cardiovascular effects upon chronic use14. One of the important reasons for these adverse effects was due to inhibition of IP receptor15, 16. As a result, two COX-2 drugs rofecoxib (Vioxx) and valdecoxib (Bextra) were withdrawn from the USA market. Induction of COX-2 following a brain injury or excessive neuronal activity in the brain is often associated with induction of a membrane bound prostaglandin E synthase-1 (mPGES-1), which produces PGE2 from COX-2 derived intermediate PGH2. Thus, it appears a future anti-inflammatory therapy should be targeted through a specific prostanoid receptor or a prostanoid synthase enzyme downstream of COX-2, rather than generic block of entire COX-2 cascade3, 4, 17–19.
PGE2 is the major product of COX-2, but, it activates four receptors EP1-EP4. Studies show that each of these four receptors display (‘yin-yang’ nature) either protective or deleterious role depending on disease model20. EP2 receptor is widely distributed in the brain and periphery21. In the brain, EP2 is expressed on both neurons and microglia cells19, 22. It has been demonstrated that acute activation of EP2 was beneficial in stroke and glaucoma models22, 23, whereas chronic activation was deleterious in models of Alzheimer’s, Parkinson’s and ALS diseases18, 19, 24. Furthermore, studies indicate that EP2 mediates tumorigenesis, and promotes tumor angiogenesis by attenuating apoptosis25–27. EP2 inhibition has been shown to impair several cell survival pathways and activates apoptotic pathways in a model of endometriosis28 suggesting ‘Jekyll and Hyde’ nature either pro-apoptotic, or anti-apoptotic signaling leading to a beneficial outcome in two different disease conditions. However, a vast majority of the studies are conducted with EP2 gene knockout models and use of poorly selective or in vivo unstable EP2 agonists (e.g. PGE2 and butaprost (Figure 1)). Pharmacological inhibition studies were limited until recently when a Emory University group published key results demonstrating proof of concept that a short term exposure of EP2 antagonist is anti-inflammatory in a pilocarpine induced acute brain injury model of status epilepticus29, and, is anti-proliferative in vitro cultures30, subsequent to the filing of the patents WO 2012/177618 A1 and US-2014/0179750 A131, 32, which are the subjects of current discussion below.
Figure 1.
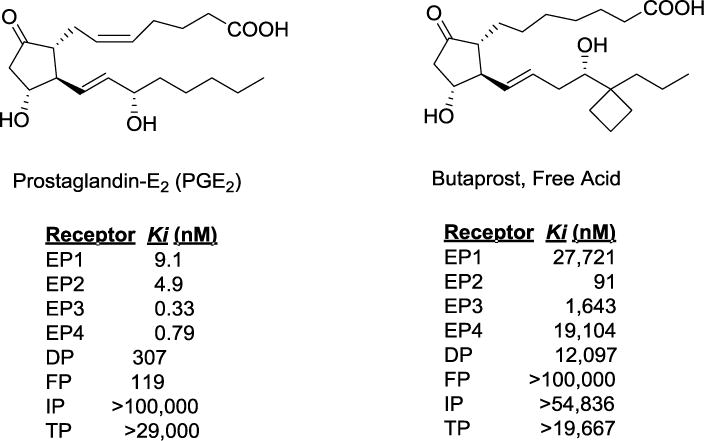
Structures and bioactivity of EP2 receptor agonist PGE2 (endogenous) and synthetic derivative butaprost free acid. Values are obtained from33.
2 Chemistry
Initial hits 3 and 4 (Scheme 1) were identified though a high-throughput screening campaign by using a TR-FRET assay on human EP2 receptors expressed on C6-glioma cell line. These two compounds belong to a cinnamic amide chemical class, where one of amide (CONH2) proton is substituted by a two carbon linker with an indole ring at the end. Medicinal chemistry on these hits generated number of compounds with modification on phenyl ring replacing one or two methoxyl groups of 3 and 4 with one or two fluorines, or a chlorine, bromine, or methyl group. Indole ring was also decorated with one fluorine atom, a methyl or trifluoromethyl group. Interestingly, these modifications retained the EP2 potency at nanomolar level (Schild KB ≤ 50 nM).
Scheme 1.
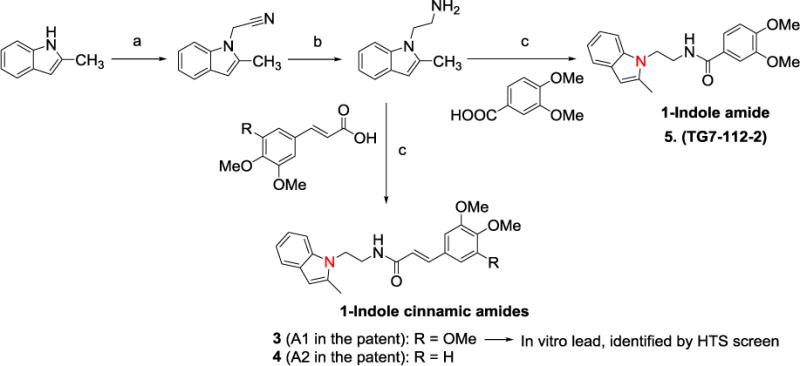
Synthesis of 1-indole cinnamic amide and amide EP2 antagonists. Reagents and conditions: a. NaH, bromoacetonitrile, DMF, 75% b. Lithium aluminum hydride (LAH), tetrahydrofuran (THF), c. a substituted cinnamic acid or a benzoic acid or a heterocyclic acid, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDCI), dimethylaminopyridine (DMAP), CH2Cl2, 70–80%.
Cinnamic amides may act as Michael acceptors and pose a potential threat for drug development, thus investigators also developed several amide analogs as EP2 antagonists. About 150 analogs have been synthesized that fall in either one of the Markush structures M1–M6 (Figure 2). A majority of the structural modifications are envisioned, for example substitution on the indole ring and replacements for indole ring (M1 and M4), right side phenyl ring, and also at the linker region (M4 and M5). Compound(s) belongs to each claim (M1–M6) are synthesized and characterized by 1H-NMR, LCMS, HRMS, and elemental analysis.
Figure 2.
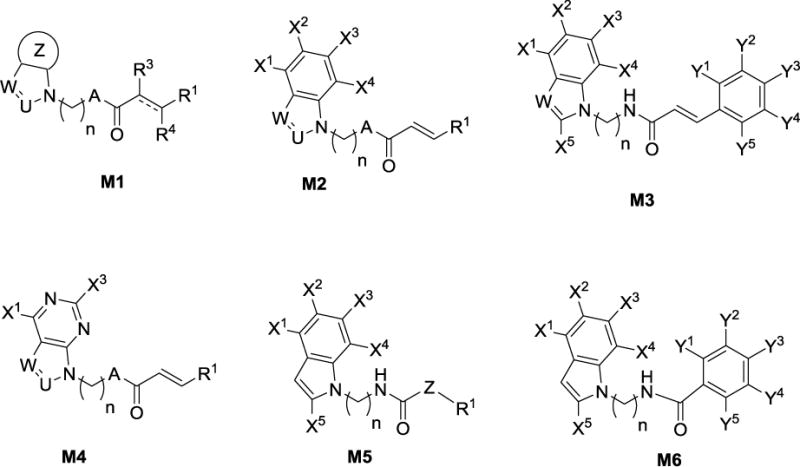
Key Markush structures illustrated in the patent application31, 32 to cover the structural modifications possible on this scaffold to protect the intellectual property
The synthesis was straight forward and completed in 3 steps for 3, 4 and many other derivatives. Briefly, 2-methyl indole was alkylated using sodium hydride as a base, and the resulting cyanide product was reduced with lithium aluminum hydride to synthesize amine precursor which was used to couple to either substituted cinnamic acids or benzoic acids, benzamidazole- and quinoline-carboxylic acids to furnish final cinnamic amides or amides (Scheme 1). Moreover, isomeric 3-indole derivatives and synthetically challenging benzofuran replacements (belongs to M1–M3) were also synthesized. Furthermore, derivatives with one methylene and three methylenes (linker) between indole and cinnamic amide were also synthesized analogously.
It is noteworthy to draw few comparisons between structures from the Emory patents31, 32 to the structures previously published in patents from Bayer Schering Pharma34. As shown in Figure 3, Emory group also created several isomeric 3-indole derivatives as illustrated by 6 and 7 (along with novel 1–indoles 3 and 4 derivatives), which partially overlap with previously published dimainopyrimidines (e.g. 8) with tryptamine moiety34. However, the cinnamic amide moiety makes 6, 7 and their derivatives structurally novel. Likewise, the only amide compound 5 partially overlaps with previously published structure 9 in an abandoned patent35 (personal communication from Emory University OTT). Nonetheless, structures such as 10 and 11, which emerged from the 5 by the SAR study, are completely novel and may serve as the leads for further development.
Figure 3.
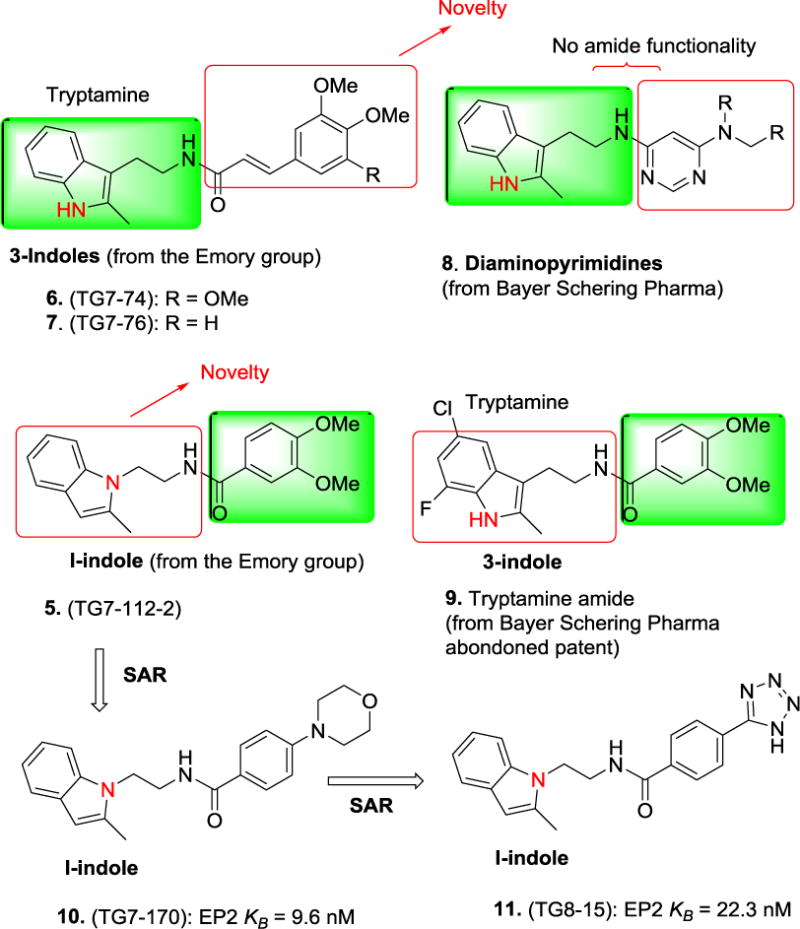
Structural comparison between compounds from the Emory patents31, 32 to the one published in patents from Bayer Schering Pharma34, 35. SAR = Structure activity relationship study. Filled green boxes are used to indicate similarity, whereas the unfilled red boxes are used to indicate the differences.
3. Biology
Unlike several other previous patents, particularly on the EP2 antagonists34–39, which reported relatively little or no biology, the Emory group31, 32 presented a significant biological data to suggest EP2 antagonists are therapeutically useful. First, a cAMP-mediated TR-FRET assay was used to determine the potency. Schild KB which was defined as 2-fold rightward shift of PGE2 EC50 on human EP2 and EP4 receptors; or a rightward shift of BW25C (DP1 agonist) EC50 on DP1 receptors, expressed on C6-glioma cells was used to characterize the potency and selectivity of the compounds. The data indicate that several compounds including 3 are >10-fold selective to EP2 against DP1 receptor and >1000-fold selective against EP4 receptor. But recent reports from Emory laboratory suggest that the selectivity against DP1 receptor has been improved to 1000-fold40 and a vast majority of the compounds display high selectivity against EP4 and IP receptors and insignificant cytotoxicity on parent C6-glioma cells40, 41. However, only the compound 3 and its trifluoromethylindole derivative 12 (Figure 4) were used to demonstrate a proof of concept either in vitro or in vivo models.
Figure 4.
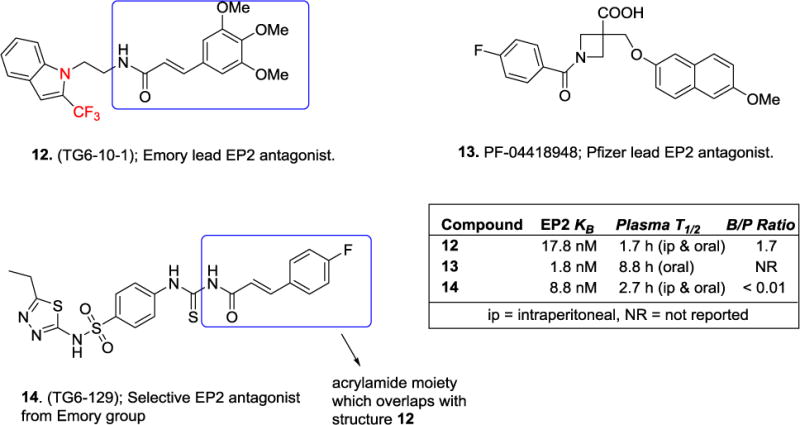
Lead EP2 antagonists currently available for in vivo use. EP2 Schild KB (derived from a cAMP-mediated-TR-FRET assay) and pharmacokinetics are shown in inset box. Blue boxes are used to indicate the similarity.
Status epilepticus (SE), a continuous seizure activity for more than 30 min, causes a significant inflammatory injury to the brain and is associated with significant delayed mortality (30 day mortality is 35%) in humans and laboratory rodent models (30–40%). Mouse and rat models of SE exhibit higher levels of COX-2 (and hence PGE2) along with several inflammatory mediators, starting from 2h until 4 days. These agents play a deleterious role in over activation of brain resident macrophage microglia and astrocytes, subsequently leading to a robust neuronal death, memory, and behavioral deficits. Thus, SE model presents an opportunity to test whether EP2 receptor plays a proinflammatory role and whether an EP2 antagonist blunts EP2 mediated deleterious pathology after SE. The data presented in the patents31, 32 suggest that a brief exposure of EP2 antagonist an hour after mice entered into SE by pilocarpine (a muscarinic receptor agonist), suppresses the delayed mortality by 30%, accelerates the recovery of mice by day 4 (determined by weight regain and nest building), suppresses the upregulation of several proinflammatory mediators including IL-1β, IL-6 and TNF-α, breakdown of blood brain barrier and neurodegeneration (by 50–66%) in hippocampus sub-regions (CA1, CA3 and hilus), in comparison to a vehicle treatment suggesting EP2 antagonist is useful to mitigate the inflammatory response after brain injury. The details of the study were published recently29, 42.
A significant body of evidence suggests that upregulation of COX-2 correlates well with high levels of PGE2 in tumor tissues43–45, and administration of PGE2 enhances the colon cancer in an azoxymethane-induced colon tumor model46, suggesting antagonism of PGE2 receptors could be a novel strategy for anti-cancer drug development. EP2 gene knockout models also established that EP2 receptor plays pivotal role in tumorigenesis and metastasis25–27. Thus, EP2 can be targeted by small molecule antagonist. While in vivo biological studies are still to be conducted, the early results presented in this patent and subsequently in a recent publication30 demonstrate that an EP2 antagonist 3 attenuates EP2 agonist butaprost (Figure 1) mediated PC3 prostate cancer cell growth and invasion in vitro.
4. Expert opinion
The patents31, 32 from the Emory laboratory underscore the importance of EP2 antagonists for therapeutic discovery, but they also raise a number of issues to be resolved before an EP2 antagonist is taken forward for human use. First, it should be determined whether a pharmacological inhibition of basal EP2 receptor activity in normal and healthy mice will have any detrimental effects, because EP2 global knockout mice displayed several adverse effects such as reduced fertility and pups with reduced litter size47, 48; systolic hypertension when fed high salt-diet48; cognitive, social memory deficits; impaired spatial learning49, 50, suggesting EP2 plays a physiological role. Thus, it is important to demonstrate whether chronic, study state exposure of EP2 antagonist in rodents will have any adverse effects on all these fronts, particularly on maintenance of arterial blood pressure, ovulation, and fertility. However, studies from the Dingledine laboratory51 established that EP2 inhibition recapitulates many features of COX-2 inhibition restricted to forebrain neurons in the SE model51; several other studies from the laboratories of Andreasson18, 19, 52, 53 and Montine54–56 demonstrated that EP2 exacerbates the inflammatory signaling and oxidative stress, drives brain micro environment for neurotoxicity and suppress the beneficial functions of microglia. Thus, EP2 antagonism should be explored as a novel therapeutic strategy, not only for anti-inflammation and neuroprotection, but also for anti-cancer and endometriosis3, 4.
Until now, very few selective EP2 antagonists have been discovered, including a brain permeable compound 12 with brain-to-plasma ratio (1.7) and plasma half-life 1.7 h. In the SE model study, three injections of 12 at 4, 21 and 30 hrs (5 mg/kg, ip) after SE seem beneficial, but it is difficult to ascertain whether a steady state inhibition of EP2 receptor was achieved by this regimen. It is unclear whether the efficacy can be repeated in this model if and when a novel compound with a prolonged plasma half-life and brain pharmacokinetics is used. Moreover, from the bioactivity, compound 12 is only 10-fold selective to EP2 over DP1, thus it is also possible that 12 may be working through DP1 receptors. Furthermore, the data is insufficient to ascertain the biological effect by 12 in the SE model is only through its interaction with EP2 receptor. A related compound 14, which shares structural similarity with 12 (Figure 4) is also available for use in peripheral disease models. Compound 14 has a prolonged plasma half-life (2.7 h) and about 500-fold higher concentrations in plasma than its EP2 Schild KB (8.8 nM) until 8 h after ip injection57.
While Bayer Schering Pharma has not advanced any of their products, Pfizer seems to be a real competitor in the development of EP2 antagonists. Pfizer created a compound 13, an azetidine carboxylic acid derivative with high selectivity to EP2 against all other prostanoid receptors and showed that it attenuates a butaprost induced cutaneous blood flow in rats58. However, its brain penetration properties were not reported to suggest whether it will be a useful compound for in vivo use in brain injury models and it is unknown whether Pfizer is exploring EP2 antagonists in any CNS models.
Therapeutic applications of a clinically useful EP2 antagonist would be enormous. Although the lead antagonist 13 may potentially result in idiosyncratic drug reactions due to the acrylamide and the trimethoxyphenyl functional groups59, 60, the compounds 10 and 11 will not. Overall, as it was claimed in the patents31, 32, 34, an optimized EP2 antagonist may be useful for treating a variety of inflammatory diseases such as arthritis and IBD; neurodegenerative diseases such as SE, epilepsy, AD, PD, and ALS; women’s diseases such as menorrhagia, dysmenorrhea, and endometriosis; and other diseases such as cancer and COPD.
Acknowledgments
Financial disclosure
The authors were supported by NIH (VOINSO58158) and ADDF (grant 20131001).
Abbreviations
- cAMP
cyclic adenosine monophosphate
- ALS
amyotrophic lateral sclerosis
- TR-FRET
time-resolved fluorescence resonance energy transfer
- OTT
office of technology transfer
- POC
proof of concept
- SE
status epilepticus
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- COPD
chronic obstructive pulmonary disease
- IBD
inflammatory bowel disease
Footnotes
competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.DeWitt DL. Cox-2-selective inhibitors: the new super aspirins. Mol Pharmacol. 1999 Apr;55(4):625–31. [PubMed] [Google Scholar]
- 2.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 3.Ganesh T. Prostanoid Receptor EP2 as a Therapeutic Target. J Med Chem. 2014 Jun 12;57(11):4454–65. doi: 10.1021/jm401431x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013 Jul;34(7):413–23. doi: 10.1016/j.tips.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta. 2014 Jul 17; doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Hirata T, Narumiya S. Prostanoid receptors. Chem Rev. 2011 Oct 12;111(10):6209–30. doi: 10.1021/cr200010h. [DOI] [PubMed] [Google Scholar]
- 7.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999 Oct;79(4):1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 8.Cannon GW, Caldwell JR, Holt P, McLean B, Seidenberg B, Bolognese J, et al. Rofecoxib, a specific inhibitor of cyclooxygenase 2, with clinical efficacy comparable with that of diclofenac sodium: results of a one-year, randomized, clinical trial in patients with osteoarthritis of the knee and hip. Rofecoxib Phase III Protocol 035 Study Group. Arthritis Rheum. 2000 May;43(5):978–87. doi: 10.1002/1529-0131(200005)43:5<978::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Clemett D, Goa KL. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs. 2000 Apr;59(4):957–80. doi: 10.2165/00003495-200059040-00017. [DOI] [PubMed] [Google Scholar]
- 10.Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, et al. Cyclooxygenase-2 in epilepsy. Epilepsia. 2014 Jan;55(1):17–25. doi: 10.1111/epi.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003 Jun 4;289(21):2819–26. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 12.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011 Jul;7(4):402–11. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006 Jul;60(1):22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Cannon PJ. COX-2 Inhibitors and Cardiovascular Risk. Science. 2012 Jun 15;336(6087):1386–87. doi: 10.1126/science.1224398. [DOI] [PubMed] [Google Scholar]
- 15.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004 Dec 10;306(5703):1954–57. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 16.Grosser T, Yu Y, Fitzgerald GA. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33. doi: 10.1146/annurev-med-011209-153129. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006 May;116(5):1391–9. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005 Nov 2;25(44):10180–7. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang X, Wang Q, Shi J, Lokteva L, Breyer RM, Montine TJ, et al. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008 Sep;64(3):304–14. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010 Apr;91(3–4):104–12. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastien L, Sawyer N, Grygorczyk R, Metters KM, Adam M. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem. 1994 Apr 22;269(16):11873–7. [PubMed] [Google Scholar]
- 22.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004 Jan 7;24(1):257–68. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasanna G, Carreiro S, Anderson S, Gukasyan H, Sartnurak S, Younis H, et al. Effect of PF-04217329 a prodrug of a selective prostaglandin EP(2) agonist on intraocular pressure in preclinical models of glaucoma. Exp Eye Res. 2011 Sep;93(3):256–64. doi: 10.1016/j.exer.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Jin J, Shie FS, Liu J, Wang Y, Davis J, Schantz AM, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnini S, Finetti F, Solito R, Terzuoli E, Sacchetti A, Morbidelli L, et al. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J. 2007 Aug;21(10):2418–30. doi: 10.1096/fj.06-7581com. [DOI] [PubMed] [Google Scholar]
- 26.Kabashima K, Nagamachi M, Honda T, Nishigori C, Miyachi Y, Tokura Y, et al. Prostaglandin E2 is required for ultraviolet B-induced skin inflammation via EP2 and EP4 receptors. Lab Invest. 2007 Jan;87(1):49–55. doi: 10.1038/labinvest.3700491. [DOI] [PubMed] [Google Scholar]
- 27.Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006 Nov 9;25(53):7019–28. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- 28.Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009 Aug;23(8):1291–305. doi: 10.1210/me.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3591–6. doi: 10.1073/pnas.1218498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther. 2013 Feb;344(2):360–7. doi: 10.1124/jpet.112.200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang J, Ganesh T, Du Y, Thepchatri P, Quan Y, Dingledine R. US 2014/0179750 A1. Prostaglandin receptor EP2 antagonists, derivatives, compositions, and uses related thereto. 2014 Jun 26;
- 32.Jiang J, Ganesh T, Du Y, Thepchatri P, Quan Y, Dingledine RJ. WO 2012177618 A1. Preparation of indole and benzimidazole derivatives as prostaglandin receptor EP2 antagonists. 2012 Dec 27;
- 33.Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000 Jan 17;1483(2):285–93. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 34.Buchmann B, Brauer N, Koppititz M, Peters O, Ter Laak A, Lindenthal B, et al. WO 2008/152093 A3. Diaminopyrimidines as modulators of the EP2 receptor. 2008 Decemeber;
- 35.Buchmann, et al. BSP EP2 149 552 A1 5,6-Substituted benzamide-derivative as modulators of EP2 receptor European patent 2008.
- 36.Braeuer N, Buchmann B, Koppitz M, Langer G, Lindenthal B, Peters O, et al. WO2008028689 A1. N-(1-phthalazin-1-ylpiperidin-4-yl)amides as EP2 receptor modulators. 2008 Mar 13;
- 37.Buchmann B, Braeuer N, Koppitz M, Peters O, Ter Laak A, Lindenthal B, et al. WO 2009007422 A1. Thienopyrimidylamines as modulators of the EP2 receptor. 2009 Jan 15;
- 38.Dack KN, Skerratt SE, Yeap SK. WO 2008/139287 A1. Azetidine derivatives and their use as prostaglandin E2 antagonists. 2008 Novemebr;
- 39.Koppitz M, Lindenthal B, Antonious Ter L, Wintermantel T, Peters O, Langer G, et al. WO2010012397 A1. Dérivés de n-(indol-3-ylalkyl)-(hétéro)arylamide comme modulateurs du récepteur. 2010 Feb 4;
- 40.Ganesh T, Jianxiong J, Dingledine R. Development of second generation EP2 antagonists with high selectivity. Eur J Med Chem. 2014;82:521–535. doi: 10.1016/j.ejmech.2014.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganesh T, Jiang J, Yang MS, Dingledine R. Lead Optimization Studies of Cinnamic Amide EP2 Antagonists. J Med Chem. 2014 May 22;57(10):4173–84. doi: 10.1021/jm5000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, Qui M, et al. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):3149–54. doi: 10.1073/pnas.1120195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001 Oct;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 44.Loh JK, Hwang SL, Lieu AS, Huang TY, Howng SL. The alteration of prostaglandin E2 levels in patients with brain tumors before and after tumor removal. J Neurooncol. 2002 Apr;57(2):147–50. doi: 10.1023/a:1015782809966. [DOI] [PubMed] [Google Scholar]
- 45.Murakami M, Kudo I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des. 2006;12(8):943–54. doi: 10.2174/138161206776055912. [DOI] [PubMed] [Google Scholar]
- 46.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003 May;24(5):985–90. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 47.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10501–6. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999 Feb;5(2):217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 49.Savonenko A, Munoz P, Melnikova T, Wang Q, Liang X, Breyer RM, et al. Impaired cognition, sensorimotor gating, and hippocampal long-term depression in mice lacking the prostaglandin E2 EP2 receptor. Exp Neurol. 2009 May;217(1):63–73. doi: 10.1016/j.expneurol.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H, Zhang J, Breyer RM, Chen C. Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J Neurochem. 2009 Jan;108(1):295–304. doi: 10.1111/j.1471-4159.2008.05766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011 Oct 19;31(42):14850–60. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansson JU, Pradhan S, Lokteva LA, Woodling NS, Ko N, Brown HD, et al. Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J Neurosci. 2013 Oct 2;33(40):16016–32. doi: 10.1523/JNEUROSCI.2203-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, et al. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Invest. 2014 Dec 8; doi: 10.1172/JCI77487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shie FS, Breyer RM, Montine TJ. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005 Apr;166(4):1163–72. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005 Oct;52(1):70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- 56.Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathology. 2005 Apr;15(2):134–38. doi: 10.1111/j.1750-3639.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganesh T, Jiang J, Shashidharamurthy R, Dingledine R. Discovery and characterization of carbamothioylacrylamides as EP selective antagonists. ACS Med Chem Lett. 2013 Jul 11;4(7):616–21. doi: 10.1021/ml400112h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.af Forselles KJ, Root J, Clarke T, Davey D, Aughton K, Dack K, et al. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. Br J Pharmacol. 2011 Dec;164(7):1847–56. doi: 10.1111/j.1476-5381.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA. Drug-protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem Res Toxicol. 2004 Jan;17(1):3–16. doi: 10.1021/tx034170b. [DOI] [PubMed] [Google Scholar]
- 60.Stepan AF, Walker DP, Bauman J, Price DA, Baillie TA, Kalgutkar AS, et al. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem Res Toxicol. 2011 Sep 19;24(9):1345–410. doi: 10.1021/tx200168d. [DOI] [PubMed] [Google Scholar]


