Abstract
Background
Few studies have evaluated associations between low to moderate arsenic levels and chronic kidney disease (CKD). The objective was to evaluate the associations of inorganic arsenic exposure with prevalent and incident CKD in American Indian adults.
Methods
We evaluated the associations of inorganic arsenic exposure with CKD in American Indians who participated in the Strong Heart Study (SHS) in 3,851 adults aged 45–74 years in a cross-sectional analysis, and 3,119 adults with follow-up data in a prospective analysis. Inorganic arsenic, monomethylarsonate, and dimethylarsinate were measured in urine at baseline. CKD was defined as eGFR≤60 mL/min/1.73m2, kidney transplant or dialysis.
Results
CKD prevalence was 10.3%. The median (IQR) concentration of inorganic plus methylated arsenic species (total arsenic) in urine was 9.7 (5.8, 15.7) μg/L. The adjusted OR (95% CI) of prevalent CKD for an interquartile range in total arsenic was 0.7 (0.6, 0.8), mostly due to an inverse association with inorganic arsenic (OR 0.4 (0.3, 0.4)). Monomethylarsonate and dimethylarsinate were positively associated with prevalent CKD after adjustment for inorganic arsenic (OR 3.8 and 1.8). The adjusted HR of incident CKD for an IQR in ΣAs was 1.2 (1.03, 1.41). The corresponding HR for inorganic arsenic, monomethylarsonate and dimethylarsinate were 1.0 (0.9, 1.2), 1.2 (1.00, 1.3) and 1.2 (1.0, 1.4).
Conclusions
The inverse association of urine inorganic arsenic with prevalent CKD suggests that kidney disease affects excretion of inorganic arsenic. Arsenic species were positively associated with incident CKD. Studies with repeated measures are needed to further characterize the relationship between arsenic and kidney disease development.
INTRODUCTION
Strong evidence suggests that chronic exposure to arsenic from drinking water1 and food2 is involved in the development of cancer3 and cardiovascular disease.4–7 Other health outcomes that have been associated with arsenic exposure include type 2 diabetes,8–10 respiratory outcomes,11 and neurodevelopmental and reproductive abnormalities.12, 13 Fewer epidemiologic studies have evaluated associations between arsenic and chronic kidney disease (CKD).14 Most studies on arsenic and renal outcomes have focused on proteinuria, showing that high arsenic exposure levels15 and low arsenic exposure levels16 are associated with increased albuminuria or proteinuria. In two small studies from Taiwan (Taipei and Central Taiwan), moderate arsenic exposure levels were associated with prevalent CKD, defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2.17, 18 In Southeastern Michigan, an ecologic study found a positive association between moderate arsenic concentrations in drinking water (mean 11 μg/L) and kidney disease mortality.19 Little is known, however, about the association between arsenic and CKD at low-moderate levels of exposure.
The objective of this study was to investigate the association between inorganic arsenic exposure, by measuring inorganic (arsenite and arsenate) and methylated (monomethylarsonate and dimethylarsinate)) arsenic species in urine, and CKD, defined as eGFR <60 mL/min/1.73 m2, kidney transplant, or dialysis. The sum of inorganic and methylated arsenic species in urine is an established biomarker of recent and ongoing arsenic exposure.20 Because kidney excretion is the primary route for arsenic elimination,20 it is possible that kidney function affects excretion and concentrations of arsenic species in urine.21, 22 Little is known, however, about how the inorganic and methylated species are excreted in the kidney or if they are affected by GFR. In this study, we evaluated the cross-sectional association of baseline urine arsenic concentrations with prevalent CKD and the prospective association of baseline urine arsenic concentrations with incident CKD among participants free of CKD at baseline. The study was conducted using data from the Strong Heart Study, a population-based study in American Indian communities from Arizona, Oklahoma and North and South Dakota.23 The study population is characterized by low to moderate levels of arsenic exposure through drinking water and food.24 Water arsenic levels in the study areas also include some levels that are higher than the national average as well as the above the current arsenic standard of 10 μg/L. Additionally, due to the high prevalence of diabetes in the Strong Heart Study, the participants are at increased risk for developing CKD. In a previous study, we confirmed that the sum of inorganic and methylated arsenic concentrations measured in urine samples collected as baseline was an appropriate biomarker of arsenic exposure over a 10-year period in our study population.24 We have also previously reported that urine arsenic was associated with prevalent albuminuria, with no difference for inorganic or methylated arsenic species.16
METHODS
Study population
The Strong Heart Study was originally funded by the National Heart Lung and Blood Institute to evaluate risk factors for cardiovascular disease in American Indian communities.23, 24 Men and women 45–74 years of age from 13 tribes and communities in Arizona, Oklahoma, and North and South Dakota were invited to participate.23, 25 A total of 4,549 participants were recruited (62% response rate). The baseline visits took place between 1989 and 1991 and two follow-up visits took place in 1993–1995 and 1997–1999. The response rates among participants who were alive at the time of the visits were 88% and 89% at visits 2 and 3, respectively.
We used data from 3,974 Strong Health Study participants in whom we have measured urine arsenic at the baseline visit. We excluded 78 participants missing serum creatinine, 7 participants missing fasting glucose, and 38 participants missing other variables of interest, leaving 3,851 participants for the cross-sectional analysis. For the prospective analyses, we further excluded the 395 participants who had CKD (eGFR≤60 ml/min/1.73m2, dialysis or kidney transplant) at baseline as well as 331 participants who missed both follow-up visits, leaving 3,119 participants for the prospective analyses. The Strong Health Study protocol and consent form were approved by local institutional review boards, participating tribes and the Indian Health Service. All participants provided informed consent.
Urine arsenic
Urine collected at baseline was frozen and shipped to the MedStar Health Research Institute, Washington DC where it was stored at −80°C. In 2009, up to 1 mL urine sample aliquots were shipped to the Trace Element Laboratory at Graz University, Austria for arsenic analyses. The analytical methods and quality control criteria for the measurement of urine arsenic in the Strong Heart Study have been described in detail.26 In summary, inorganic arsenic species (arsenite and arsenate, measured together under oxidized conditions), methylated arsenic species (monomethylarsonate and dimethylarsinate) and arsenobetaine plus other arsenic cations were determined by high performance liquid chromatography (HPLC; Agilent 1100, Agilent Technologies, www.agilent.com) coupled to inductively coupled plasma - mass spectrometry (ICP-MS) (Agilent 7700x ICPMS). The inter-assay coefficient of variation was below 5% for all arsenic species. The limit of detection was 0.1 μg/L for inorganic arsenic, monomethylarsonate, dimethylarsinate, and arsenobetaine and other arsenic cations. The percentage of samples below the limit of detection was 5.2% for inorganic arsenic, 0.8% for monomethylarsonate, 0.03% for dimethylarsinate, and 2.1% for arsenobetaine and other arsenic cations. Samples below the limit of detection were replaced by the limit of detection divided by the square root of two.
We used the sum of inorganic (arsenite, arsenate) and methylated (monomethylarsonate, dimethylarsinate) arsenic species as our biomarker of inorganic arsenic exposure. In addition, we evaluated inorganic arsenic, monomethylarsonate, and dimethylarsinate separately, as well as arsenobetaine, an arsenical found in seafood that is characterized by very low toxicity. Urine arsenic concehntrations (μg/L) were divided by urine creatinine concentrations (g/L) to account for urine dilution and expressed in μg/g creatinine. The Spearman’s correlation coefficient of inorganic arsenic (arsenate, arsenite) with monomethylarsonate, dimethylarsinate and arsenobetaine were 0.84, 0.73, and 0.01, respectively.
Plasma and urine creatinine measures
Serum creatinine was measured in fasting venous blood samples by an automated alkaline-picrate rate method (Roche Diagnostics, www.rocheusa.com/portal/usa) using Hitachi 717 platform (Hitachi Ltd, www.hitachi.com) at all visits.27 eGFR was calculated from creatinine, age, and sex using the Modification of Diet in Renal Disease equation without the ethnicity factor.28 Prevalent CKD was defined as eGFR ≤60 ml/min/1.73 m2 or presence of kidney transplant or dialysis at baseline.29 Incident CKD was defined as eGFR ≤60 ml/min/1.73 m2 or presence of kidney transplant or dialysis at either follow-up visits.
Urine creatinine was measured in spot urine samples collected during the baseline visit at the Laboratory of the National Institute of Diabetes and Digestive and Kidney Diseases Epidemiology and Clinical Research Branch, Phoenix, Arizona by an automated alkaline picrate methodology.23
Other variables
Information on age, gender, education, smoking status, hypertension medication use, dialysis, and kidney transplant was collected by trained and certified interviewers using standardized questionnaires.23 Physical exam measures (height, weight, systolic, and diastolic blood pressure) were performed by trained nurses and medical assistants following a standardized protocol. Methods to measure blood pressure, body mass index, fasting glucose, 75-g oral glucose tolerance test, and hemoglobin A1c (HbA1c) have been described.23 Diabetes was defined as a fasting glucose ≥126 mg/dL, a 2-h post-load plasma glucose ≥200 mg/dL, an HbA1c ≥6.5%, or the use of insulin or an oral hypoglycemic agent.30
Statistical analyses
Statistical analyses were performed in Stata 11.2 (Stata Corporation, www.stata.com) and R 2.15.2 (R Project, www.r-project.org). Urine arsenic concentrations were right skewed and natural log transformed. Quartiles were generated based on the distribution of urine arsenic concentrations in the overall study sample.
For the cross-sectional analysis, we used logistic regression models to estimate adjusted odds ratios for prevalent CKD by urine arsenic concentrations at baseline. For the prospective analysis, we used Cox proportional hazards models stratified by study location with age as time scale and age at baseline treated as staggered entries. Follow up time was calculated in years from the date of the baseline visit to the date at the first visit with CKD for participants with incident CKD, and to the date at the last visit for those without incident CKD. This follow up time was added to the participant’s age at the baseline visit. In both logistic and Cox proportional hazards regression models, arsenic concentrations were entered as quartiles (comparing quartiles 2–4 to the lowest quartile), as log-transformed continuous variables (comparing an interquartile range in log-transformed arsenic levels), and as restricted quadratic splines in separate models. P-values for linear trend were obtained by including a continuous variable with the medians corresponding to each quartile of the arsenic distribution in the regression models.31
Models were progressively adjusted. Initially, we adjusted for age (only for logistic models as Cox models already accounted for age), gender, study location (only for logistic models as Cox models were stratified by location), education, body mass index, smoking status, systolic blood pressure, and hypertension medication (Model 2 in prospective models only). Because arsenic has been associated with diabetes prevalence and poor diabetes control in our population,30 we ran additional models adjusting for diabetes and fasting glucose levels (Model 3). Results were similar adjusting for HbA1c instead of fasting glucose levels (data not shown). For the prospective association between baseline urine arsenic and incident CKD, because glomerular filtration rate could interfere with arsenic excretion in urine,32 a possibility further supported by our cross-sectional findings especially for inorganic arsenic species, in Model 2 we also adjusted for baseline eGFR. This is a common approach to look at CKD progression. In this model, we are evaluating the association between urine arsenic concentrations in urine with incident CKD that are independent of baseline eGFR.
In sensitivity analyses, we accounted for urine dilution adjusting for urine creatinine instead of dividing arsenic by creatinine,33 and adjusting for specific gravity.34 In analyses adjusted for specific gravity, we also excluded participants with prevalent diabetes and albuminuria. The results of the sensitivity analyses were similar (Supplemental Table 1). To account for high mortality rates in the study population,35 we fitted Cox proportional hazards models using the Fine and Gray method of handling competing risks36 with death as the competing event with similar findings (data not shown). Because the exact date of CKD development was unknown and our ascertainment of the study outcome was only available at three visits, we used Poisson regression as an alternative modeling strategy with consistent findings. Using Poisson regression we also estimated absolute incidence rates across arsenic quartile categories given mean values of covariates (model 3 and model 4).
RESULTS
Cross-sectional findings
The prevalence of CKD at baseline was 10.3% (395/3,851). Participants with prevalent CKD were more likely to be older, female, from Arizona, less educated and former smokers (Table 1). Participants with prevalent CKD were also more likely to have diabetes and higher fasting glucose levels, to use anti-hypertensive medication, and to have higher blood pressure levels. Median (IQR) urine inorganic plus methylated arsenic concentrations were lower in participants with CKD (8.9 (5.2, 14.6) μg/g) compared to participants without CKD (9.8 (5.9, 15.7) μg/g). Inorganic arsenic, monomethylarsonate, and dimethylarsinate concentrations were also lower in participants with CKD (Table 1). Arsenobetaine concentrations were similar in participants with and without CKD(Table 2).
Table 1.
CKD status by participant characteristics at baseline. Data are median (interquartile range) or percentages
| Participant Characteristics | Prevalent CKD | Incident CKD | ||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| Overall No. | 3456 | 395 | 2617 | 502 |
| Age, years | 54.3 (49, 61.1) | 63 (55.8, 68.1) | 53.4 (48.5, 60.1) | 58.3 (52.5, 64.4) |
| Gender (%) | ||||
| Male | 43 | 23 | 43 | 32 |
| Female | 57 | 77 | 57 | 68 |
| Study Region (%) | ||||
| Arizona | 34 | 35 | 33 | 39 |
| Oklahoma | 33 | 37 | 33 | 33 |
| South Dakota | 33 | 29 | 34 | 28 |
| Education, years | 12 (9, 13) | 11 (9, 12) | 12 (9, 13) | 11 (8, 12) |
| Smoking Status (%) | ||||
| Never | 34 | 36 | 32 | 36 |
| Former | 32 | 40 | 33 | 36 |
| Current | 35 | 24 | 35 | 28 |
| BMI, kg/m2 | 30.1 (26.6, 34.3) | 29.1 (26.1, 33.3) | 30.1 (26.6, 34.5) | 30.5 (27, 34.3) |
| Diabetes Status (%) | ||||
| No | 52 | 36 | 56 | 31 |
| Yes | 48 | 64 | 44 | 69 |
| Fasting Glucose, mg/dL | 115 (110, 174.5) | 125 (102, 191) | 111 (99, 154) | 160.5 (109. 260) |
| Hypertension Medication (%) | ||||
| No | 79 | 52 | 81 | 71 |
| Yes | 21 | 48 | 19 | 29 |
| Systolic BP, mmHg | 124 (113, 136) | 134 (119, 153) | 123 (113, 135) | 130.5 (118, 144) |
| As concentrations | ||||
| iAs+MMA+DMA, μg/gb | 9.8 (5.9, 15.7) | 8.9 (5.2, 14.6) | 9.5 (5.7, 15.4) | 11.2 (6.2, 18.2) |
| iAs (inorganic arsenic) | 0.8 (0.4, 1.5) | 0.4 (0.2, 0.9) | 0.8 (0.4, 1.5) | 0.8 (0.4, 1.5) |
| MMA (monomethylarsonate) | 1.3 (0.8, 2.2) | 1.2 (0.7, 2.2) | 1.3 (0.8, 2.2) | 1.4 (0.8, 2.5) |
| DMA (dimethylarsinate) | 7.4 (4.5, 12.0) | 7.3 (4.0, 11.7) | 7.1 (4.4, 11.7) | 8.5 (4.8, 13.8) |
| Arsenobetaine, μg/g | 0.7 (0.4, 1.5) | 0.7 (0.4, 2.1) | 0.7 (0.4, 1.6) | 0.7 (0.4, 1.4) |
BMI: body mass index, BP: blood pressure, MMA: monomethylarsonate, DMA: dimethylarsinate
Sum of inorganic and methylated arsenic species
BMI indicates body mass index; BP, blood pressure; DMA, dimethylarsinate; iAs, inorganic arsenic; MMA, monomethylarsonate.
Table 2.
Baseline participant characteristics by urine arsenic concentrations for participants without CKD at baseline and with at least one follow-up visit (N=3119). Data are median (interquartile range) or percentages
| Population | Sum of inorganic and methylated (μg/g creatinine) | ||||
|---|---|---|---|---|---|
| ≤5.8 | 5.8 – 9.7 | 9.7 – 15.6 | ≥15.6 | ||
| Number | 3119 | 772 | 781 | 784 | 782 |
| Sex | |||||
| Male | 41 | 51 | 40 | 41 | 34 |
| Female | 59 | 49 | 60 | 59 | 66 |
| Age, years | 54.3 (49.61) | 54.4 (48.6 61.1) | 54 (48.9, 59.9) | 54.7 (49.3, 61.4) | 54.2 (49.3) |
| Study Location | |||||
| Arizona | 34. | 7 | 26 | 43 | 60 |
| Oklahoma | 33 | 70 | 38 | 17 | 6 |
| South Dakota | 33 | 23 | 36 | 40 | 34 |
| Education, years | 12 (9, 13) | 12 (11, 14) | 12, (10. 14) | 11 (8, 12) | 10 (8, 12) |
| BMI, kg/m2 | 30.2 (26.6, 34.5) | 30 (27, 34) | 30.5 (27.1, 34.9) | 30.4 (26.5, 34.6) | 29.8 (26.2, 34.3) |
| Smoking status | |||||
| Never | 32 | 31 | 31 | 32 | 35 |
| Former | 34 | 35 | 36 | 33 | 32. |
| Current | 34 | 34 | 34 | 35 | 33 |
| Diabetes | |||||
| No | 53 | 64 | 55 | 53 | 39 |
| Yes | 47 | 36 | 45 | 47 | 61 |
| Fasting Glucose, mg/dL | 115 (100, 176) | 108 (11, 131) | 111 (98, 152) | 113.5 (101, 166) | 135 (104, 243) |
| Hypertension Medication | |||||
| No | 80 | 78 | 81 | 80 | 80 |
| Yes | 20 | 22 | 19 | 20 | 20 |
| Systolic BP, mmHg | 124 (113, 136) | 124.5 (115, 135) | 123 (112, 134) | 124 (113, 138) | 125 (114, 138) |
| eGFR, ml/min/1.73m2 | 82.4 (75.5, 94.5) | 80.4 (71.5, 90.5) | 81.8 (74.3, 94.0) | 82.4 (76.4, 94.8) | 90.2 (78.5, 105.1) |
| As concentrations | |||||
| iAs+MMA+DMA | 9.7 (5.8, 15.7) | 4.2 (3.4, 5.0) | 7.5 (6.6, 8.6) | 12.3 (10.9, 13.8) | 21.8 (18.3, 28.7) |
| iAs (inorganic arsenic) | 0.8 (0.4, 1.5) | 0.3 (0.2, 0.4) | 0.6 (0.4, 0.8) | 1.1 (0.7, 1.4) | 2.1 (1.4, 3.3) |
| MMA | 1.3 (0.8, 2.2) | 0.6 (0.4, 0.8) | 1.0 (0.8, 1.3) | 1.7 (1.3, 2.1) | 2.9 (2.2, 4.3) |
| DMA | 7.3 (4.4, 12.1) | 3.2 (2.6, 3.8) | 5.8 (5.1, 6.6) | 9.3 (8.2, 10.6) | 16.8 (14.0, 21.9) |
| Urine arsenobetaine | 0.7 (0.4, 1.5) | 0.6 (0.4, 1.1) | 0.7 (0.4, 1.9) | 0.7 (0.4, 1.6) | 0.8 (0.5, 1.6) |
The OR for CKD comparing the 75th to the 25th percentile of inorganic plus methylated arsenic was 0.7 (0.6, 0.8) after adjusting for sociodemographic and CKD risk factors (Table 3). In separate analyses for each arsenic species, a markedly strong inverse association was observed for inorganic arsenic (OR for CKD comparing the 75th to the 25th percentile was 0.4 (0.3, 0.4). For monomethylarsonate and dimethylarsinate, the inverse associations were weaker and they became positive after adjustment for inorganic arsenic (OR (95% CI) 3.8 (2.8, 5.1) for monomethylarsonate, and 1.8 (1.4, 2.3) for dimethylarsinate). Urine arsenobetaine was not associated with prevalent CKD. In restricted quadratic spline models, we observed an inverse association between the sum of inorganic and methylated arsenic concentrations and prevalent CKD, which was mostly driven by a strong inverse association with inorganic arsenic (Figure 1). For monomethylarsonate and dimethylarsinate the inverse association was weaker and it could be related to the correlation between inorganic arsenic and monomethylarsonate and dimethylarsinate species. After adjustment for inorganic arsenic levels, the associations between monomethylarsonate and prevalent CKD, and dimethylarsinate and prevalent CKD became strongly positive (Figure 1). The inverse association for the sum of inorganic and methylated arsenic species was observed in all participant subgroups evaluated, except in participants younger than 55 years of age (Figure 2).
Table 3.
Odds ratios (95% confidence interval) for prevalent CKD by quartile of urine arsenic concentrations
| Sum of inorganic and methylated, μg/g | Cases/noncases | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Quartiles | ||||
| ≤6.9 | 117/849 | 1.0 (ref) | 1.0 (ref) | – |
| 6.9 – 11.3 | 95/865 | 0.8 (0.6, 1.1) | 0.7 (0.5, 1.00) | – |
| 11.3 – 18.6 | 94/869 | 0.8 (0.6, 1.1) | 0.6 (0.4, 0.9) | – |
| ≥18.6 | 89/873 | 0.7 (0.6, 1.00) | 0.5 (0.3, 0.7) | – |
| p-trend | 0.08 | <0.01 | – | |
| IQR (15.6 vs 5.8) | 395/3456 | 0.8 (0.7, 1.00) | 0.7 (0.6, 0.8) | – |
| Inorganic arsenic, μg/g | ||||
| IQR (1.4 vs 0.3) | 395/3456 | 0.5 (0.4, 0.5) | 0.4 (0.3, 0.4) | – |
| MMA, μg/g | ||||
| IQR (2.2 vs 0.7) | 395/3456 | 0.9 (0.8, 1.0) | 0.8 (0.7, 1.0) | 3.8 (2.8, 5.1) |
| DMA, μg/g | ||||
| IQR (12.0 vs 4.4) | 395/3456 | 0.9 (0.8, 1.0) | 0.7 (0.6, 0.9) | 1.8 (1.4, 2.3) |
| Arsenobetaine, μg/g | ||||
| IQR (1.6 vs 0.4) | 395/3456 | 1.0 (0.9, 1.1) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) |
BMI: body mass index, BP: blood pressure, MMA: monomethylarsonate, DMA: dimethylarsinate, IQR: interquartile range
Model 1 is unadjusted.
Model 2 is adjusted for age and gender, location, education, smoking status, BMI, hypertension medication, SBP, diabetes status, and fasting glucose
Model 3 is additionally adjusted for inorganic arsenic (arsenite, arsenate)
Figure 1.
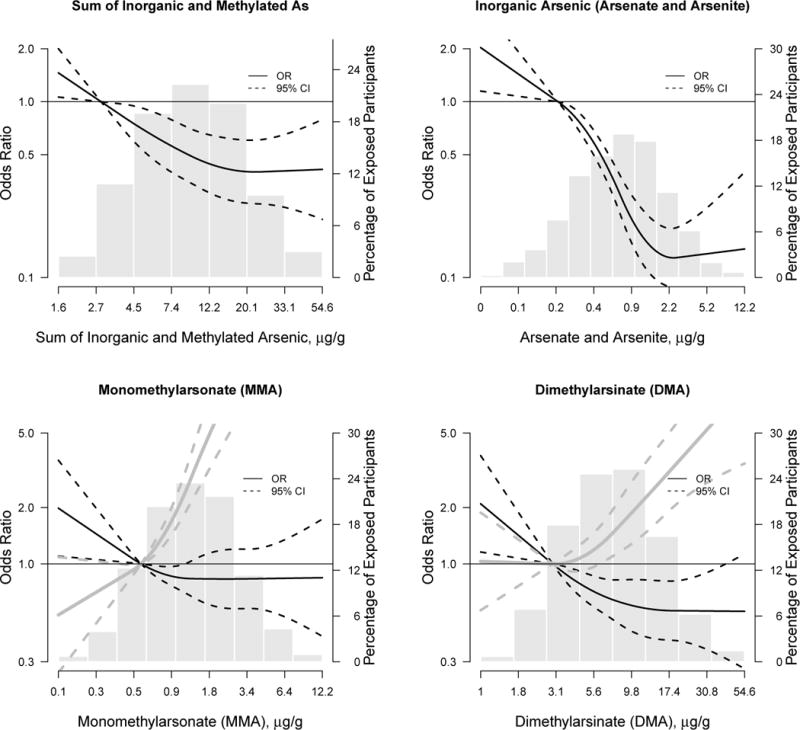
Odds ratios for prevalent chronic kidney disease by total arsenic (the sum of inorganic and methylated arsenic species), inorganic arsenic (arsenate and arsenite), monomethylarsonate arsenic, and dimethylarsinate.
Black lines (solid lines) represent odds ratios and 95% confidence intervals (dashed lines) based on restricted quadratic spline models for log transformed arsenic with 3 knots and adjusted as in Table 3, model 3. Gray lines (solid lines) are the odds ratios and 95% CI (dashed lines) for monomethylarsonate and dimethylarsinate after adjustment for urine inorganic arsenic levels. The reference was set at the 10th percentile of the urine arsenic biomarker distribution. Odds ratios were adjusted for age (continuous), sex, study region, body mass index (continuous), education, smoking status, diabetes status, hypertensive medication, systolic blood pressure (continuous), and fasting glucose (continuous). For inorganic plus methylated arsenic species the p-value for a linear and non-linear dose-response relationships were 0.02 and 0.13, respectively. For inorganic arsenic, the p-values for linear and non-linear dose-response were 0.01 and 0.005. For monomethylarsonate, p-values for linear and non-linear dose-response were, respectively, 0.02 and 0.19 before and 0.10 and 0.003 after adjustment for inorganic arsenic. For dimethylarsinate, p-values for linear and non-linear dose-response were 0.01 and 0.14 before and 0.92 and 0.02, after adjustment for inorganic arsenic.
Figure 2.
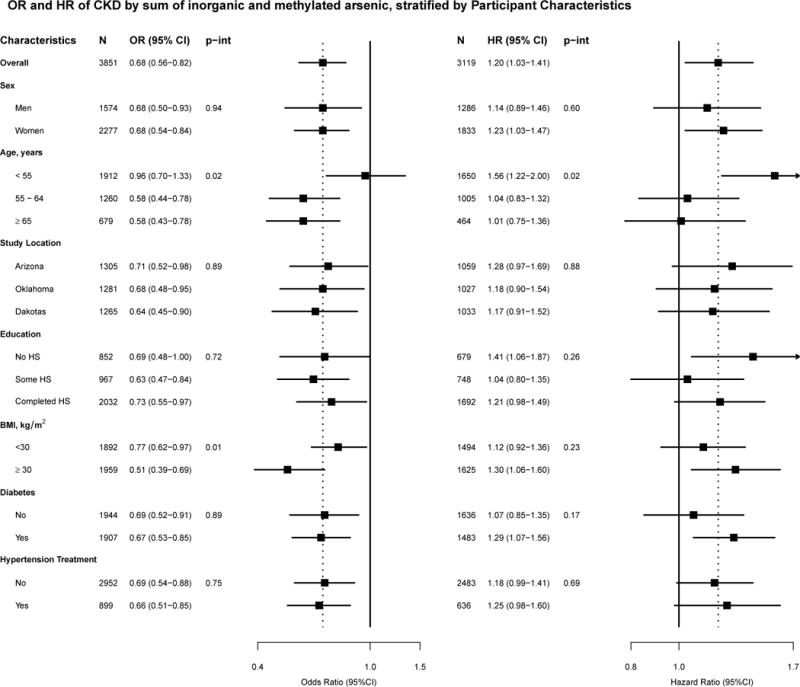
Odds ratios (OR) and hazard ratios (HR) for chronic kidney disease comparing the 75th to 25th percentile of the sum of inorganic and methylated arsenic species, stratified by participant characteristics
Odds ratios are adjusted for age (years), sex, study region, body mass index (continuous), education, smoking status, diabetes status, hypertensive medication, systolic blood pressure (continuous), and fasting glucose (continuous)
Hazard ratios were adjusted for age (continuous), sex, body mass index (continuous), education (continuous), smoking status, diabetes status, hypertensive medication, systolic blood pressure (continuous), baseline eGFR (continuous), and fasting glucose (continuous). Baseline hazard was stratified by study region.
Modeling eGFR as a continuous variable, higher urine arsenic was associated with higher eGFR levels in all models. After adjustment for all variables in model 3 (Supplemental Table 2), an interquartile range in urine arsenic levels was associated with a mean difference of 5.2 (95% CI 4.1, 6.3) ml/min/1.73 m2 for the sum of inorganic and methylated arsenic, 7.9 (6.9, 9.0) ml/min/1.73 m2 for inorganic arsenic, 3.0 (1.9, 4.0) ml/min/1.73 m2 for monomethylarsonate and 4.8 (3.7, 5.8) ml/min/1.73 m2 for dimethylarsinate. After adjusting for inorganic arsenic concentrations, the corresponding mean difference in eGFR was −0.1 (−1.4, 1.1) for monomethylarsonate and 2.5(1.2, 3.7) ml/min/1.73 m2 for dimethylarsinate. In analyses restricted to 3,456 participants without CKD and in 1,356 participants with eGFR ≥90 ml/min/1.73m2, the mean difference in eGFR for an interquartile range in inorganic plus methylated arsenic concentrations were 4.4 (3.4, 5.5) ml/min/1.73 m2 and 3.4 (1.5, 5.5) ml/min/1.73 m2, respectively.
Prospective findings
The incidence rate of CKD over the study period was 23.5 per 1,000 person-years. Mean follow-up time was 6.86 years. Similar to the cross-sectional analysis, participants who developed CKD were more likely to be older, female, from Arizona, less educated, former smokers, and more likely to have diabetes, use anti-hypertension medication, and have higher blood pressure and higher fasting glucose levels (Table 1). Participants with incident CKD had higher inorganic plus methylated arsenic concentrations in baseline urine (median 11.2 (interquartile range 6.2, 18.2) μg/g creatinine) compared to those without incident CKD (9.5 (5.7, 15.4) μg/g creatinine). Looking at the individual species, baseline monomethylarsonate and dimethylarsinate concentrations were higher in participants with than without incident CKD. Baseline inorganic arsenic species and arsenobetaine concentrations were similar in participants with and without incident CKD.
The hazard ratio (95%CI) for incident CKD comparing the 75th to the 25th percentile of inorganic plus methylated arsenic species was 1.2 (1.0, 1.4) after adjusting for all sociodemographic and kidney disease risk factors evaluated (Table 4, model 3). In analyses of the individual species separately, the corresponding hazard ratios were 1.0 (0.9, 1.2) for inorganic arsenic, 1.2 (1.00, 1.3) for monomethylarsonate and 1.2 (1.0, 1.4) for dimethylarsinate. In flexible dose-response models, a positive prospective association with CKD was observed for the sum of inorganic and methylated arsenic species, as well as for monomethylarsonate and especially for dimethylarsinate (Figure 3); by contrast, there was no association for inorganic arsenic. The association between the sum of inorganic and methylated arsenic species and incident CKD was similar across participant characteristics (Figure 2).
Table 4.
Hazard ratios (95% confidence interval) for incident CKD by urine arsenic concentrations
| Sum of inorganic and methylated, μg/g | Cases/noncases | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Quartiles | ||||
| ≤5.7 | 109/663 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 5.8 – 9.7 | 110/671 | 1.0 (0.8, 1.4) | 1.1 (0.8, 1.4) | 1.1 (0.8, 1.4) |
| 9.7 – 15.6 | 128/656 | 1.2 (0.9, 1.5) | 1.2 (0.9, 1.7) | 1.2 (0.9, 1.6) |
| ≥15.6 | 155/627 | 1.4 (1.0, 1.9) | 1.6 (1.2, 2.2) | 1.3 (0.9, 1.8) |
| p-trend | 0.01 | <0.01 | 0.12 | |
| IQR (15.6 vs 5.8) | 502/2617 | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.5) | 1.2 (1.0, 1.4) |
| Inorganic arsenic, μg/g | ||||
| IQR (1.5 vs 0.4) | 502/2617 | 0.9 (0.8, 1.1) | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.2) |
| MMA, μg/g | ||||
| IQR (2.2 vs 0.8) | 502/2617 | 1.1 (0.9, 1.2) | 1.2 (1.0, 1.4) | 1.2 (1.00, 1.3) |
| DMA, μg/g | ||||
| IQR (12.1 vs 4.4) | 502/2617 | 1.3 (1.1, 1.5) | 1.3 (1.2, 1.6) | 1.2 (1.0, 1.4) |
| Arsenobetaine, μg/g | ||||
| IQR (1.5 vs 0.4) | 502/2617 | 1.1 (0.9, 1.2) | 1.0 (0.9, 1.1) | 1.1 (1.00, 1.2) |
BMI: body mass index, BP: blood pressure, MMA: monomethylarsonate, DMA: dimethylarsinate, IQR: interquartile range
Model 1 is unadjusted.
Model 2 is adjusted for age and gender, location, education, smoking status, BMI, hypertension medication, SBP, and baseline eGFR
Model 3 is additionally adjusted for diabetes status and fasting glucose
Figure 3. Hazard ratio for incident CKD by the sum of inorganic and methylated arsenic species.
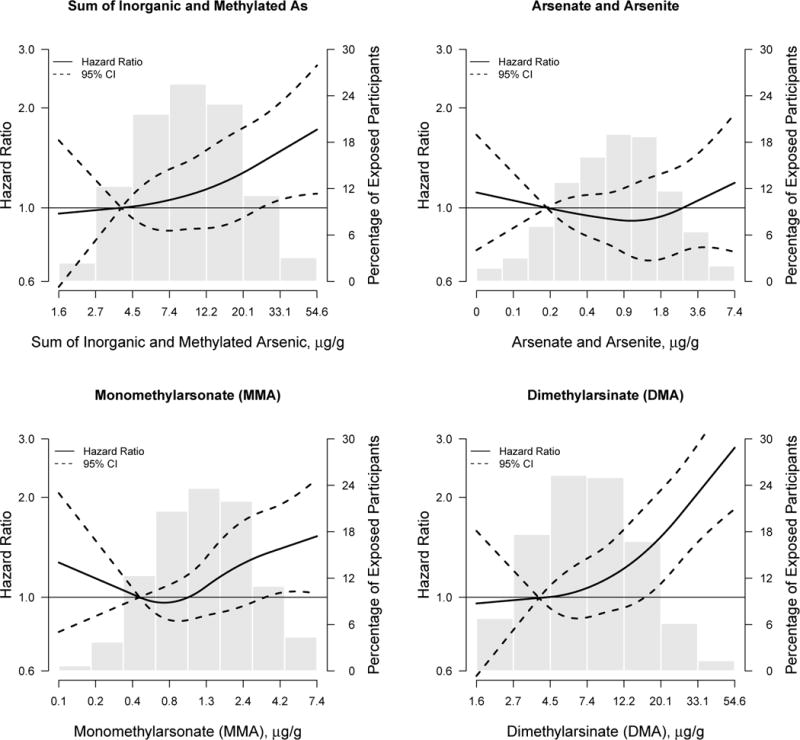
Lines represent hazard ratios (solid lines) and 95% confidence intervals (dashed lines) based on restricted quadratic spline models for log transformed arsenic with 3 knots. The reference was set at the 10th percentile of the urine arsenic biomarker distribution. Hazard ratios were adjusted for age (continuous), sex, body mass index (continuous), education (continuous), smoking status, diabetes status, hypertensive medication, systolic blood pressure (continuous), eGFR (continuous) and fasting glucose (continuous). Baseline hazard was stratified by study region. The p-value for linear dose-response relationship for the sum of inorganic and methylated species was 0.80 and the p-value for a non-linear dose-response was 0.69. The p-value for a linear dose-response relationship for inorganic arsenic was 0.31 and the p-value for a non-linear dose-response was 0.47. For monomethylarsonate, the p-value for a linear dose-response relationship was 0.32 and 0.23 for a non-linear dose-response relationship. For dimethylarsinate, the p-value for a linear dose-response relationship was 0.91 and 0.61 for a non-linear dose-response relationship.
DISCUSSION
In this population, primarily from rural communities in the western United States, participants with prevalent CKD had lower concentrations of inorganic plus methylated arsenic species measured in baseline urine, which was mostly explained by a strong inverse association with inorganic arsenic. This finding suggests that glomerular filtration contributes importantly to inorganic arsenic excretion. For urinary monomethylarsonate and dimethylarsinate, we found a positive association with prevalent CKD after adjustment for inorganic urinary arsenic concentrations. Prospectively, baseline urine concentrations of inorganic plus methylated arsenic species were associated with incident CKD. The prospective association between arsenic and CKD remained after adjustment for sociodemographic and CKD risk factors, including baseline eGFR, as well as in most participant subgroups. In analyses of the individual species, the prospective associations remained present for monomethylarsonate and dimethylarsinate but no association was observed with inorganic arsenic concentrations.
The major route of arsenic excretion is through the kidneys.20 Little is known, however, about the precise pathways of excretion for the different arsenic species. In an experiment in dogs, arsenate and arsenite were filtered through the glomeruli and partly reabsorbed by the tubules.37 Arsenic excretion was decreased in several studies of animals with impaired kidney function. In rabbits, arsenic excretion was reduced in a dose-responsive manner following partial and subtotal nephrectomy.38 None of those animal studies have evaluated the impact of kidney function on the methylated arsenic species.
Few human studies have evaluated arsenic excretion in the presence of impaired kidney function. Dialysis was needed to remove sodium arsenite from blood in 2 patients with acute sodium arsenite intoxication and acute kidney failure.39 When kidney function recovered, urine total arsenic excretion increased.39 A pharmacologic study of arsenic trioxide treatment in 20 cancer patients with varying kidney function levels found that total arsenic excretion was reduced in patients with impaired kidney function.32 Impaired kidney function also resulted in decreased percentage of total arsenic excreted as arsenite as well as with an increase in plasma monomethylarsonate and dimethylarsinate concentrations.32 However, only in cases of severe renal impairment did the internal dose of arsenite increase.32 In a cancer patient on hemodialysis treated with arsenic trioxide, plasma total arsenic concentration increased in the presence of arsenic trioxide treatment and hemodialysis was not sufficient to reduce plasma arsenic concentrations.40
Our cross-sectional findings suggest that chronically reduced glomerular function reduces the excretion of inorganic arsenic but not of monomethylarsonate or dimethylarsinate, or of arsenobetaine. These findings are thus consistent with reverse causation, in which CKD and decreased GFR impair urine arsenic excretion, specifically inorganic arsenic exposure. Decreased inorganic arsenic elimination through the kidneys could result in increased arsenic internal dose and higher risk of arsenic related health effects among individuals with CKD. In blood, the longer retention of inorganic arsenic species would allow them to be further methylated. In our study, however, we have no measurements of arsenic species in blood. Several small studies in patients with CKD, including participants on dialysis, have shown increased arsenic concentrations in serum compared to healthy controls, although levels of urine arsenic were not reported (and urine assessment can be difficult to do in patients on dialysis).21, 22, 41, 42 The impact could occur across the range of kidney filtration, rather than just with CKD, since the association between arsenic, especially inorganic arsenic, and eGFR was present even at eGFR levels > 90 mL/min/1.73 m2. The marked change in the direction of the association between monomethylarsonate and dimethylarsinate after adjustment for inorganic arsenic needs to be interpreted cautiously. Although our analysis can support that the evidence that methylated species are a risk factor for CKD and the result is consistent with the prospective analyses, we cannot rule out that the moderate to strong correlation between the inorganic and methylated arsenic species results in an artifact.
A second possible explanation for the observed inverse association between arsenic and prevalent CKD that becomes positive with incident CKD is hyperfiltration. Hyperfiltration is the presence of an elevated GFR in the early stages of kidney disease before a decline in GFR in later stages of kidney disease. Hyperfiltration is well established in the early stages of kidney diseases associated with diabetes and obesity,43 and with sickle cell disease.44 It has also been proposed for environmental nephrotoxicants such as lead 45, 46. A caveat to this hypothesis is that little is know about whether arsenic could induce hyperfiltration; as no human or animal studies, to our knowledge, have evaluated the possible role of arsenic exposure in hyperfiltration.
Few experimental studies have evaluated the role of arsenic in kidney disease development. In vitro and animal studies support the role of high arsenic exposure levels in kidney damage,47, 48 although the relevance of those studies is unclear as arsenic concentrations were very high. For instance, subcytotoxic but still high arsenite (10 μmol/L) and arsenate (25 μmol/L) concentrations inhibited mitochondrial metabolism in proximal tubule cells.49 Arsenic can also influence inflammatory processes in in vitro models, as measured by increased IL-6 and IL-8 expression,50 and reactive oxygen species pathways.51 In rodents exposed to 5 mg/kg arsenic trioxide, markers of kidney function (serum urea nitrogen and serum creatinine), markers of kidney injury (urine N-acetyl- β -D-glucosaminidase), and markers of reactive oxygen species increased in arsenic trioxide treated kidney tissue compared to controls.52 Through these mechanisms, arsenic could also play a role in arseni- related kidney damage.53
In human populations, ecologic studies estimating standardized mortality ratios (SMRs) have found associations between high arsenic levels in drinking water and kidney disease mortality including evidence from Antofagasta, Chile54 and Southwestern Taiwan.55 At lower levels of arsenic exposure, an ecological study in Southeastern Michigan (mean water arsenic 11 μg/L) found elevated kidney disease mortality compared to the rest of Michigan in both men (SMR 1.28, 95% CI 1.15–1.42) and women (SMR 1.38, 95% CI 1.25–1.52).19 In another ecological study of residents of Millard County in Utah (median arsenic concentrations ranging from 14 to 166 μg/L in selected towns), the SMR for mortality due to nephritis and nephrosis was increased among men (SMR 1.72, 95% CI 1.13 – 2.50), but non-increased among women (SMR 1.21, 95% CI 0.66– 2.03).56 In a systematic review, the overall pooled SMR was 1.29 (95% CI 1.10 – 1.51).14 Our prospective results are consistent with those ecologic findings.
The major strengths of our study were the availability of arsenic speciation and the ability to compare cross-sectional and prospective findings. The laboratory techniques for assessing urine arsenic are state-of-the art and highly sensitive, resulting in few participants below the limit of detection (less than 6% for inorganic arsenic).26 The speciation of arsenic in urine allowed us to distinguish exposure to inorganic arsenic, confirm that organic arsenic from seafood was low, and evaluate different associations with different species. The protocols for recruitment, interviews, examinations, and collection and storage of biological samples were highly standardized. The losses to follow-up were low and we had little missing data.
Our study has several limitations. First, eGFR levels were only measured at baseline and two follow-up visits. Therefore, the exact date of CKD development was unknown. Second, as the direct determination of GFR is not feasible in large population studies, we estimated GFR using the CKD-EPI equation for baseline and cross-sectional analyses and the MDRD equation for follow-up assessment of incident CKD. The MDRD equation was developed in individuals with kidney disease, and as a result, the equation has greater imprecision and underestimates GFR in individuals with GFR> 60 ml/min/1.73m2.57 In the Strong Heart Study, the isotope dilution mass spectrometry (IDMS)-calibrated serum creatinine measures required for the CKD-EPI were only available at baseline, so that it could not be used for outcome assessment. Third, we used spot urine samples to measure arsenic exposure, which must be adjusted for urine dilution. Our main analysis divided by urine creatinine to account for urine dilution. In sensitivity analyses, we adjusted for specific gravity, with consistent findings (Table S1). Also, we could not evaluate arsenite and arsenate separately, as both species were measured simultaneously under oxidized conditions.26 Fourth, our study population has a high burden of obesity, diabetes and CKD. Generalization to a population with a different disease profile may be limited. However, the prospective associations remained after adjustment for CKD risk factors, including diabetes and fasting glucose levels, which have been associated with arsenic in the Strong Heart Study population30 and other studies in American Indians8 and Northern Mexicans,58 and could be potential mediators of the association between arsenic and CKD. Finally, competing risks such as censoring by death could be a problem in this study population, characterized by a high burden of disease.35 Our sensitivity analyses using the Fine and Gray method to handle competing risks resulted in similar findings.
CONCLUSIONS
Prevalent CKD was inversely associated with the sum of inorganic and methylated arsenic concentrations in urine, and particularly with inorganic arsenic concentrations. These cross-sectional findings suggest that kidney disease is associated with the excretion of inorganic arsenic species. Prospectively, inorganic plus methylated urine arsenic concentrations, especially monomethylarsonate and dimethylarsinate concentrations, were positively associated with incident CKD. These findings could support the hypothesis that arsenic is a kidney disease risk factor. Studies with repeated measures of arsenic species in urine and blood as well as renal dysfunction endpoints are needed to further characterize the association between arsenic exposure, excretion of the arsenic species, and CKD development.
Supplementary Material
References
- 1.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123(2):305–32. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Administration USFaD, editor. FDA. Arsenic in Rice. U.S Food and Drug Administration; 2012. [Google Scholar]
- 3.International Agency for Research on C, editor. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2004. [Google Scholar]
- 4.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease:an updated systematic review. Current atherosclerosis reports. 2012;14(6):542–55. doi: 10.1007/s11883-012-0280-x. Epub 2012/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Annals of internal medicine. 2013;159(10):649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. Epub 2013/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakharyan RA, Wildfang E, Aposhian HV. Enzymatic Methylation of Arsenic Compounds. Toxicol Appl Pharmacol. 1996;140(1):77–84. Epub 1996/09/01. [PubMed] [Google Scholar]
- 8.Kim NH, Mason CC, Nelson RG, Afton SE, Essader AS, Medlin JE, et al. Arsenic exposure and incidence of type 2 diabetes in Southwestern American Indians. American journal of epidemiology. 2013;177(9):962–9. doi: 10.1093/aje/kws329. Epub 2013/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Current diabetes reports. 2013;13(6):831–49. doi: 10.1007/s11892-013-0432-6. Epub 2013/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environmental health perspectives. 2012;120(12):1658–70. doi: 10.1289/ehp.1104579. Epub 2012/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental health perspectives. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. Epub 2013/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohel N, Vahter M, Ali M, Rahman M, Rahman A, Streatfield PK, et al. Spatial patterns of fetal loss and infant death in an arsenic-affected area in Bangladesh. Int J Health Geogr. 2010;9:53. doi: 10.1186/1476-072X-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong AP, Xiao K, Choi KC, Wang G, Chan MH, Ho CS, et al. Associations between microRNA (miR-21, 126, 155 and 221), albuminuria and heavy metals in Hong Kong Chinese adolescents. Clinica chimica acta; international journal of clinical chemistry. 2012;413(13–14):1053–7. doi: 10.1016/j.cca.2012.02.014. Epub 2012/03/13. [DOI] [PubMed] [Google Scholar]
- 14.Hong F, Jin TY, Lu GD, Yin ZY. Renal dysfunction in workers exposed to arsenic and cadmium. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi = Chinese journal of industrial hygiene and occupational diseases. 2003;21(6):432–6. Epub 2004/02/06. [PubMed] [Google Scholar]
- 15.Chen Y, Parvez F, Liu M, Pesola GR, Gamble MV, Slavkovich V, et al. Association between arsenic exposure from drinking water and proteinuria: results from the Health Effects of Arsenic Longitudinal Study. Int J Epidemiol. 2011:828–35. doi: 10.1093/ije/dyr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W, et al. Urine arsenic and prevalent albuminuria: evidence from a population-based study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013;61(3):385–94. doi: 10.1053/j.ajkd.2012.09.011. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JW, Chen HY, Li WF, Liou SH, Chen CJ, Wu JH, et al. The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere. 2011;84(1):17–24. doi: 10.1016/j.chemosphere.2011.02.091. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh YM, Chung CJ, Shiue HS, Chen JB, Chiang SS, Yang MH, et al. Urinary arsenic species and CKD in a Taiwanese population: a case-control study. Am J Kidney Dis. 2009;54(5):859–70. doi: 10.1053/j.ajkd.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler BA, Chou SJ, Jones RL, Chen CJ. Arsenic. In: Nordberg GF, Fowler BA, Nordberg M, Freiberg LT, et al., editors. Handbook on the Toxicology of Metals. Amsterdam: Elsevier; 2007. pp. 367–443. [Google Scholar]
- 21.Zhang X, Cornelis R, De Kimpe J, Mees L, Vanderbiesen V, De Cubber A, et al. Accumulation of arsenic species in serum of patients with chronic renal disease. Clinical chemistry. 1996;42(8 Pt 1):1231–7. Epub 1996/08/01. [PubMed] [Google Scholar]
- 22.Zhang X, Cornelis R, Mees L, Vanholder R, Lameire N. Chemical speciation of arsenic in serum of uraemic patients. The Analyst. 1998;123(1):13–7. doi: 10.1039/a704841f. Epub 1998/05/15. [DOI] [PubMed] [Google Scholar]
- 23.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 24.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. EnvironHealth Perspect. 2009;117(9):1428–33. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee ET, Howard BV, Wang W, Welty TK, Galloway JM, Best LG, et al. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation. 2006;113(25):2897–905. doi: 10.1161/CIRCULATIONAHA.105.593178. [DOI] [PubMed] [Google Scholar]
- 26.Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Analytical Methods. 2012;4:406–13. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shara NM, Wang H, Mete M, Al-Balha YR, Azalddin N, Lee ET, et al. Estimated GFR and Incident Cardiovascular Disease Events in American Indians: The Strong Heart Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;60(5):795–803. doi: 10.1053/j.ajkd.2012.06.015. Epub 2012/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. Epub 1999/03/13. [DOI] [PubMed] [Google Scholar]
- 29.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 30.Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W, et al. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. American journal of epidemiology. 2012;176(10):865–74. doi: 10.1093/aje/kws153. Epub 2012/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agresti A. Categorical Data Analysis. Wiley-Interscience; 2002. [Google Scholar]
- 32.Sweeney CJ, Takimoto C, Wood L, Porter JM, Tracewell WG, Darwish M, et al. A pharmacokinetic and safety study of intravenous arsenic trioxide in adult cancer patients with renal impairment. Cancer chemotherapy and pharmacology. 2010;66(2):345–56. doi: 10.1007/s00280-009-1169-4. Epub 2009/11/17. [DOI] [PubMed] [Google Scholar]
- 33.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vij HS, Howell S. Improving the specific gravity adjustment method for assessing urinary concentrations of toxic substances. American Industrial Hygiene Association journal. 1998;59(6):375–80. doi: 10.1080/15428119891010622. Epub 1998/07/22. [DOI] [PubMed] [Google Scholar]
- 35.Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988. The Strong Heart Study. Am J Epidemiol. 1998;147(11):995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 36.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 37.Ginsburg JM. RENAL MECHANISM FOR EXCRETION AND TRANSFORMATION OF ARSENIC IN THE DOG. Am J Physiol. 1965;208:832–40. doi: 10.1152/ajplegacy.1965.208.5.832. [DOI] [PubMed] [Google Scholar]
- 38.De Kimpe J, Cornelis R, Mees L, Vanholder R, Verhoeven G. 74As-arsenate metabolism in Flemish Giant rabbits with renal insufficiency. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. 1999;13(1–2):7–14. doi: 10.1016/S0946-672X(99)80017-0. Epub 1999/08/13. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri ND, Upham T, Barton CH. Hemodialysis clearance of arsenic. Clinical toxicology. 1980;17(3):451–6. doi: 10.3109/15563658008989995. Epub 1980/10/01. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Sasaki M, Oshimi K, Sugimoto K. Arsenic trioxide in a hemodialytic patient with acute promyelocytic leukemia. Acta haematologica. 2009;122(1):52–3. doi: 10.1159/000243724. Epub 2009/10/10. [DOI] [PubMed] [Google Scholar]
- 41.Palaneeswari MS, Rajan PM, Silambanan S, Jothimalar Blood Arsenic and Cadmium Concentrations in End-Stage Renal Disease Patients who were on Maintenance Haemodialysis. Journal of clinical and diagnostic research: JCDR. 2013;7(5):809–13. doi: 10.7860/JCDR/2013/5351.2945. Epub 2013/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Cornelis R, De Kimpe J, Mees L, Lameire N. Speciation of arsenic in serum, urine, and dialysate of patients on continuous ambulatory peritoneal dialysis. Clinical chemistry. 1997;43(2):406–8. Epub 1997/02/01. [PubMed] [Google Scholar]
- 43.Amann K, Benz K. Structural renal changes in obesity and diabetes. Seminars in nephrology. 2013;33(1):23–33. doi: 10.1016/j.semnephrol.2012.12.003. Epub 2013/02/05. [DOI] [PubMed] [Google Scholar]
- 44.Becker AM. Sickle cell nephropathy: challenging the conventional wisdom. Pediatric nephrology. 2011;26(12):2099–109. doi: 10.1007/s00467-010-1736-2. Epub 2011/01/05. [DOI] [PubMed] [Google Scholar]
- 45.de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, et al. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environmental health perspectives. 2006;114(4):584–90. doi: 10.1289/ehp.8202. Epub 2006/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver VM, Lee BK, Ahn KD, Lee GS, Todd AC, Stewart WF, et al. Associations of lead biomarkers with renal function in Korean lead workers. Occupational and environmental medicine. 2003;60(8):551–62. doi: 10.1136/oem.60.8.551. Epub 2003/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Molecular biology reports. 2012;39(12):11201–16. doi: 10.1007/s11033-012-2029-6. Epub 2012/10/17. [DOI] [PubMed] [Google Scholar]
- 48.Tsukamoto H, Parker HR, Gribble DH, Mariassy A, Peoples SA. Nephrotoxicity of sodium arsenate in dogs. Am J Vet Res. 1983;44(12):2324–30. [PubMed] [Google Scholar]
- 49.Peraza MA, Carter DE, Gandolfi AJ. Toxicity and metabolism of subcytotoxic inorganic arsenic in human renal proximal tubule epithelial cells (HK-2) Cell Biol Toxicol. 2003;19(4):253–64. doi: 10.1023/b:cbto.0000003970.60896.49. [DOI] [PubMed] [Google Scholar]
- 50.Escudero-Lourdes C, Medeiros MK, Cardenas-Gonzalez MC, Wnek SM, Gandolfi JA. Low level exposure to monomethyl arsonous acid-induced the over-production of inflammation-related cytokines and the activation of cell signals associated with tumor progression in a urothelial cell model. Toxicol Appl Pharmacol. 2010;244(2):162–73. doi: 10.1016/j.taap.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al GI, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, et al. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51(7):1271–88. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Zhao H, Shao Y, Wang P, Wei Y, Zhang W, et al. Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats. Nutrition research and practice. 2014;8(1):46–53. doi: 10.4162/nrp.2014.8.1.46. Epub 2014/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malyszko J, Malyszko JS, Pawlak K, Mysliwiec M. Visfatin and apelin, new adipocytokines, and their relation to endothelial function in patients with chronic renal failure. Adv Med Sci. 2008;53(1):32–6. doi: 10.2478/v10039-008-0024-x. [DOI] [PubMed] [Google Scholar]
- 54.Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in Young Adults following in Utero and Childhood Exposure to Arsenic in Drinking Water. Environmental health perspectives. 2012;120(11):1527–31. doi: 10.1289/ehp.1104867. Epub 2012/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu HF, Yang CY. Decreasing trend in renal disease mortality after cessation from arsenic exposure in a previous arseniasis-endemic area in southwestern Taiwan. Journal of toxicology and environmental health Part A. 2005;68(5):319–27. doi: 10.1080/15287390590900804. Epub 2005/04/01. [DOI] [PubMed] [Google Scholar]
- 56.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. EnvironHealth Perspect. 1999;107(5):359–65. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. Journal of the American Society of Nephrology: JASN. 2007;18(10):2749–57. doi: 10.1681/ASN.2007020199. Epub 2007/09/15. [DOI] [PubMed] [Google Scholar]
- 58.Coronado-Gonzalez JA, Del Razo LM, Garcia-Vargas G, Sanmiguel-Salazar F, Escobedo-de la PJ. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104(3):383–9. doi: 10.1016/j.envres.2007.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


