Abstract
Background & Aims
Constipation is a common clinical problem that negatively impacts quality of life and is associated with significant health care costs. Activation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is the primary pathway that drives fluid secretion in the intestine, which maintains lubrication of luminal contents. We hypothesized that direct activation of CFTR would cause fluid secretion and reverse the excessive dehydration of stool found in constipation.
Methods
A cell-based, high-throughput screen was performed for 120,000 drug-like, synthetic small molecules. Active compounds were characterized for mechanism of action and one lead compound was tested in a loperamide-induced constipation model in mice.
Results
Several classes of novel CFTR activators were identified, one of which, the phenylquinoxalinone CFTRact-J027, fully activated CFTR chloride conductance with an half-maximal effective concentration (EC50) of approximately 200 nmol/L, without causing an increase of cytoplasmic cyclic adenosine monophosphate. Orally administered CFTRact-J027 normalized stool output and water content in a loperamide-induced mouse model of constipation with a 50% effective dose of approximately 0.5 mg/kg; CFTRact-J027 was without effect in cystic fibrosis mice lacking functional CFTR. Short-circuit current, fluid secretion, and motility measurements in mouse intestine indicated a prosecretory action of CFTRact-J027 without direct stimulation of intestinal motility. Oral administration of 10 mg/kg CFTRact-J027 showed minimal bioavailability, rapid hepatic metabolism, and blood levels less than 200 nmol/L, and without apparent toxicity after chronic administration.
Conclusions
CFTRact-J027 or alternative small-molecule CFTR-targeted activators may be efficacious for the treatment of constipation.
Keywords: CFTR, Constipation, High-Throughput Screening, Loperamide
Abbreviations used in this paper: cAMP, cyclic adenosine monophosphate; CFTR, cystic fibrosis transmembrane conductance regulator; CSBM, complete spontaneous bowel movement; DMSO, dimethyl sulfoxide; EC50, half-maximal effective concentration; FRT, Fischer rat thyroid; IP, intraperitoneal; PBS, phosphate-buffered saline; YFP, yellow fluorescent protein
Summary.
Activation of the cystic fibrosis transmembrane conductance regulator chloride channel drives fluid secretion in the intestine. High-throughput screening identified a small-molecule cystic fibrosis transmembrane conductance regulator activator that increases intestinal fluid secretion. Oral administration of the activator corrected constipation in a mouse model.
Constipation is a common clinical complaint in adults and children that negatively impacts quality of life. The prevalence of chronic constipation has been estimated to be 15% in the US population, with annual health care costs estimated at approximately 7 billion dollars, with more than 800 million dollars spent on laxatives.1, 2 The mainstay of constipation therapy includes laxatives that increase stool bulk, such as soluble fiber; creation of an osmotic load, such as polyethylene glycol; or stimulation of intestinal contraction, such as the diphenylmethanes. There also are surface laxatives that soften stool such as docusate sodium and probiotics such as Lactobacillus paracasei.3 The Food and Drug Administration–approved drug linaclotide, a peptide agonist of the guanylate cyclase C receptor, acts by inhibiting visceral pain, stimulating intestinal motility, and increasing intestinal secretion.4, 5 A second approved drug, lubiprostone, a prostaglandin E analog, is thought to activate a putative enterocyte ClC-2 channel,6 although the mechanistic data are less clear. Despite the wide range of therapeutic options, there is a continued need for safe and effective drugs to treat constipation.
Intestinal fluid secretion involves active Cl- secretion across the enterocyte epithelium through the basolateral membrane Na+/ K+/ 2Cl- cotransporter, the luminal membrane cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel, and the Ca2+-activated Cl- channel. The electrochemical and osmotic forces created by Cl- secretion drive Na+ and water secretion.7 In cholera and traveler’s diarrhea, CFTR is activated strongly by bacterial enterotoxins through increase of intracellular cyclic nucleotides.8, 9 CFTR is an attractive target to increase intestinal fluid secretion in constipation because it is expressed robustly throughout the intestine and its activation can strongly increase intestinal fluid secretion. An activator targeting CFTR directly is unlikely to produce the massive, uncontrolled intestinal fluid secretion seen in cholera because the enterotoxins in cholera act irreversibly to produce a sustained increase of cytoplasmic cyclic adenosine monophosphate (cAMP), which not only activates CFTR but also basolateral K+ channels, which increase the electrochemical driving force for Cl- secretion; cholera enterotoxins also inhibit the luminal NHE3 Na+/H+ exchanger involved in intestinal fluid absorption.10, 11
Motivated by these considerations and the continuing need for safe and effective drug therapy of constipation, we report the identification and characterization of a nanomolar potency, CFTR-targeted, small-molecule activator, and provide proof of concept for its prosecretory action in the intestine and efficacy in constipation.
Materials and Methods
Materials
High-throughput screening was performed using a diverse collection of 120,000 drug-like synthetic compounds obtained from ChemDiv, Inc (San Diego, CA) and Asinex (Winston-Salem, NC). For structure-activity analysis, 600 commercially available analogs (ChemDiv, Inc) of active compounds identified in the primary screen were tested. Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise.
CFTRact-J027 Synthesis
Potassium carbonate (2.5 g, 18.4 mmol) and benzyl bromide (0.73 mL, 6.2 mmol) were added to a solution of o-phenylenediamine (1 g, 9.24 mmol) in dimethylformamide (DMF) (30 mL), and then stirred overnight at ambient temperature. The reaction mixture was diluted with CH2Cl2, washed with water, dried over MgSO4, and concentrated under reduced pressure. The residue was purified by flash chromatography to yield the intermediate N1-benzylbenzene-1,2-diamine as a brown liquid. 1H NMR (300 MHz, CDCl3): δ 7.45–7.31 (m, 5H), 6.86–6.69 (m, 4H), 4.35 (s, 2H), 3.50 (br, 3H); MS: m/z 199 (M+H)+. Then, a solution of the intermediate (400 mg, 2 mmol) and 5-nitroisatin (380 mg, 2 mmol) in acetic acid (5 mL) was refluxed for 2 hours. The reaction mixture was cooled to room temperature and solvent was removed under reduced pressure. The residue was dissolved with methanol to crystallize 3-(2-amino-5-nitrophenyl)-1-benzylquinoxalin-2(1H)-one (CFTRact-J027) as a yellow solid with more than 99% purity. 1H NMR (300 MHz, [DMSO] d6): δ 9.15 (d, 1H, J = 2.8 Hz), 8.07 (dd, 1H, J = 2.7, 9.2 Hz), 7.97 (dd, 1H, J = 1.2, 7.9 Hz), 7.82 (br, 2H), 7.60-7.27 (m, 7H), 6.92 (d, 1H, J = 9.2 Hz), 5.59 (br, 2H); 13C NMR (75 MHz, DMSO-d6): δ 155.0, 154.6, 153.3, 136.3, 135.3, 132.8, 132.2, 131.0, 130.0, 129.5, 129.1, 127.7, 127.3, 126.8, 124.1, 116.1, 115.9, 115.4, 45.9; MS: m/z 373 (M+H)+.
Cell Culture
Fischer rat thyroid (FRT) cells stably co-expressing human wild-type CFTR and the halide-sensitive yellow fluorescent protein (YFP)-H148Q were generated as previously described.12 Cells were cultured on plastic in Coon’s-modified Ham’s F12 medium supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. For high-throughput screening, cells were plated in black 96-well microplates (Corning-Costar Corp, Corning, NY) at a density of 20,000 cells per well. Screening was performed 24–48 hours after plating.
High-Throughput Screening
Screening was performed using a Beckman Coulter (Brea, CA) integrated system equipped with a liquid handling system and 2 FLUOstar fluorescence plate readers (BMG Labtechnologies, Durham, NC), each equipped with dual syringe pumps and 500 ± 10 nm excitation and 535 ± 15 nm emission filters (see Galietta et al12 for more detail). CFTR- and YFP-expressing FRT cells were grown at 37°C/5% CO2 for 24–48 hours after plating. At the time of assay, cells were washed 3 times with phosphate-buffered saline (PBS) and then incubated for 10 minutes with 60 μL of PBS containing test compounds (at 10 μmol/L) and a low concentration of forskolin (125 nmol/L). Each well was assayed individually for I- influx in a plate reader by recording fluorescence continuously (200 ms/point) for 2 seconds (baseline) and then for 12 seconds after rapid (<1 s) addition of 165 μL of PBS in which 137 mmol/L Cl- was replaced by I-. The initial rate of I- influx was computed by determining exponential regression. All compound plates contained negative controls (DMSO vehicle) and positive controls (20 μmol/L forskolin).
Short-Circuit Current Measurement
Short-circuit current was measured in FRT cells stably expressing wild-type human CFTR cultured on porous filters as described.12 The basolateral solution contained (in mmol/L): 130 NaCl, 2.7 KCl, 1.5 KH2PO4, 1 CaCl2, 0.5 MgCl2, 10 glucose, and 5 Na-HEPES (pH 7.3, 37°C). In the apical solution, 65 mmol/L NaCl was replaced by Na gluconate, and CaCl2 was increased to 2 mmol/L, and the basolateral membrane was permeabilized with 250 μg/mL amphotericin B. Short-circuit current was measured in freshly harvested adult mouse colon at 37°C using symmetric Krebs-bicarbonate buffer (pH 7.4, in mmol/L: 117 NaCl, 4.7 KCl, 1.2 MgCl2, 2.5 CaCl2, 11.1 D-glucose, 1.2 KH2PO4, and 24.8 NaHCO3).
cAMP Assay
Intracellular cAMP activity was measured using a GloSensor luminescence assay (Promega Corp, Madison, WI). FRT null cells were stably transfected with the pGloSensor cAMP plasmid and plated on white 96-well microplates and grown to confluence. Cells were washed 3 times with PBS and incubated with 5 μmol/L CFTRact-J027 for 10 minutes in the absence and presence of 100 nmol/L forskolin. cAMP was assayed according to the manufacturer’s instructions.
Animals
Animal experiments were approved by the University of California San Francisco Institutional Animal Care and Use Committee. Animals were housed in communal cages with standard rodent chow and water available ad libitum. Wild-type CD1 mice and CFTR ΔF508 homozygous mice were bred in the University of California San Francisco Laboratory Animal Resource Center.
Pharmacokinetics
Female CD1 mice were treated with 10 mg/kg CFTRact-J027 (saline containing 5% DMSO and 10% Kolliphor HS 15) either intraperitoneally (IP) or orally. Blood was collected at 15, 30, 60, 150, 240, and 360 minutes after treatment by orbital puncture and centrifuged at 5000 rpm for 15 minutes to separate plasma. Plasma samples (60 μL) were mixed with 300 μL acetonitrile and centrifuged at 13,000 rpm for 20 minutes, and 90 μL of the supernatant was used for liquid chromatography–mass spectrometry. The solvent system consisted of a linear gradient from 5% to 95% acetonitrile over 16 minutes (0.2 mL/min flow). Mass spectra was acquired on a mass spectrometer (Waters 2695 and Micromass ZQ, Milford, MA) using electrospray (+) ionization, mass ranged from 100 to 1500 daltons, cone voltage was 40 V. Calibration standards were prepared in plasma from untreated mice, to which known amounts of CFTRact-J027 were added.
In Vitro Metabolic Stability
CFTRact-J027 (5 μmol/L) was incubated for specified times at 37°C with mouse liver microsomes (1 mg protein/mL; Sigma-Aldrich) in potassium phosphate buffer (100 mmol/L) containing 1 mmol/L reduced nicotinamide adenine dinucleotide phosphate, as described.13 The mixture then was chilled on ice, and 0.5 mL of ice-cold ethyl acetate was added. Samples were centrifuged for 15 minutes at 3000 rpm, the supernatant evaporated to dryness, and the residue was dissolved in 100 μL mobile phase (acetonitrile:water, 3:1) for liquid chromatography–mass spectrometry and assayed as described earlier.
Murine Model of Constipation
Female CD1 mice (age, 8–10 wk) were administered loperamide (0.3 mg/kg, IP; Sigma-Aldrich) to produce constipation. Various amounts of CFTRact-J027 (0.1, 0.3, 1, 3, and 10 mg/kg) were given at the same time (for IP administration) or 1 hour before (for oral administration) loperamide. Control mice were treated with vehicle only. Some mice were treated orally with lubiprostone (0.5 mg/kg; Sigma-Aldrich) or linaclotide (0.5 mg/kg; Toronto Research Chemicals, Inc, Toronto, Ontario, Canada). After loperamide injection, mice were placed individually in metabolic cages with food and water provided ad libitum. Stool samples were collected for 3 hours, and total stool weight and number of fecal pellets were quantified. To measure stool water content the stool samples were dried at 80°C for 24 hours and water content was calculated as follows: (wet weight - dry weight)/wet weight. Similar studies were performed in cystic fibrosis mice (ΔF508 homozygous) lacking functional CFTR. Some studies were performed using the chemically similar but inactive analog of CFTRact-J027: 3-(2-amino-5-nitrophenyl)-1-(methyl)-2(1H)-quinoxalinone.
In Vivo Intestinal Transit and Ex Vivo Intestinal Contractility
Whole-gut transit time was determined using an orally administered marker (200 μL, 5% Evans Blue, 5% gum Arabic) and measuring the time of its appearance in stool. Mice were administered loperamide and CFTRact-J027 (10 mg/kg) or vehicle intraperitoneally at zero time. For ex vivo contractility measurements, mice were euthanized by avertin overdose (200 mg/kg, 2,2,2-tribromethanol; Sigma-Aldrich) and ileum and colon segments of approximately 2-cm length were isolated and washed with Krebs–Henseleit buffer (pH 7.4, in mmol/L: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, and 11 D-glucose). The ends of the intestinal segments were tied, connected to a force transducer (Biopac Systems, Goleta, CA), and tissues were transferred to an organ chamber (Biopac Systems) containing Krebs–Henseleit buffer at 37°C aerated with 95% O2, 5% CO2. Ileum and colon were stabilized for 60 minutes with resting tensions of 0.5 and 0.2 g, respectively, and solutions were changed every 15 minutes. Effects of CFTRact-J027 on baseline and loperamide-suppressed isometric intestinal contractions were recorded.
In Vivo Intestinal Secretion and Absorption
Mice (wild-type or CF) were given access to 5% dextrose water but not solid food for 24 hours before experiments. Mice were anesthetized with isoflurane, and body temperature was maintained during surgery at 36°C –38°C using a heating pad. A small abdominal incision was made to expose the small intestine, and closed midjejunal loops (length 2–3 cm) were isolated by sutures. Loops were injected with 100 μL vehicle alone or 100 μg CFTRact-J027 in vehicle. The abdominal incision was closed with sutures, and mice were allowed to recover from anesthesia. Intestinal loops were removed at 90 minutes and loop length and weight were measured to quantify fluid secretion. Intestinal absorption was measured in CF mice (to prevent secretion) as described earlier, except that the loops were removed at 0 or 30 minutes. Absorption was calculated as follows: 1 - loop weight at 30 minutes)/loop weight at 0 minutes.
Chronic Administration and Toxicity Studies
Mice were administered 10 mg/kg CFTRact-J027 or vehicle orally once a day for 7 days. One hour after the final dose mice were treated with loperamide (0.3 mg/kg, IP) and stool was collected for 3 hours. In vivo toxicity was assessed in these mice by measuring the lung wet/dry weight ratio, complete blood count (Hemavet 950FS; Drew Scientific, Inc, Miami Lakes, FL) and serum chemistry (Idexx Laboratories, Inc, Sacramento, CA) 4 hours after the last CFTRact-J027 dose. In vitro cytotoxicity was measured in FRT cells incubated with 25 μmol/L CFTRact-J027 for 8 and 24 hours. Cytotoxicity was measured by Alamar Blue assay according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
Statistical Analysis
Experiments with 2 groups were analyzed with the Student's t test, when there were 3 groups or more analysis was performed with 1-way analysis of variance and the post hoc Newman–Keuls multiple comparisons test. A P value less than .05 was taken as statistically significant.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Identification and In Vitro Characterization of Small-Molecule CFTR Activators
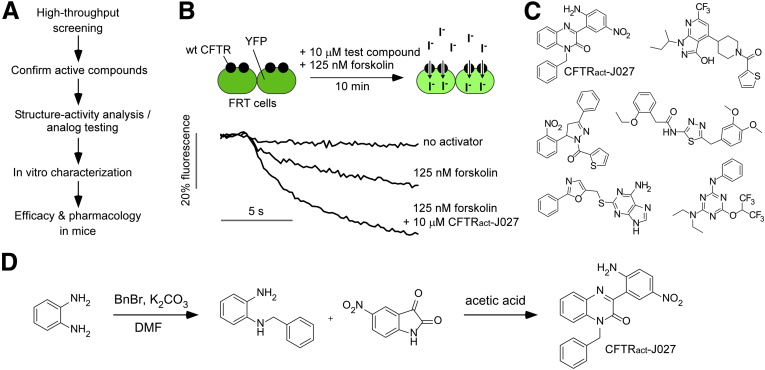
The initial goal was to identify a potent, CFTR-targeted activator with prosecretory activity in the intestine to test its efficacy in a mouse model of constipation. Figure 1A summarizes the project strategy. The compounds evaluated here included small molecules identified in prior CFTR activator/potentiator screens14 and from a new screen of synthetic small molecules not tested previously. The most active compounds emerging from the screen, along with commercially available chemical analogs, were prioritized based on an initial mechanism-of-action study (assay of cAMP increase), in vitro toxicity, prosecretory action in mouse intestine, and efficacy in a mouse model of constipation. Figure 1B shows the cell-based plate reader screening method in which the initial rate of iodide influx was measured in FRT cells stably expressing human wild-type CFTR and a YFP fluorescent halide sensor after extracellular addition of iodide. CFTR activation increased the initial slope of the fluorescence quenching curve. A low concentration of forskolin (125 nmol/L) was included in the screen to recapitulate the basal level of CFTR phosphorylation in vivo because CFTR is not phosphorylated at baseline in FRT cells.15
Figure 1.
Identification of small-molecule CFTR activators. (A) Project overview. (B) CFTR activator screen using FRT cells co-expressing human wild-type CFTR and YFP iodide–sensing protein. Test compounds at 10 μmol/L were added for 10 minutes at room temperature in the presence of forskolin (125 nmol/L) before iodide addition. Examples of data from single wells of a 96-well plate showing CFTR activation by CFTRact-J027 are shown. (C) Structures of CFTR activators emerging from the screen. (D) Synthesis of CFTRact-J027. DMF, dimethylformamide.
Figure 1C shows the chemical structures of 6 classes of candidate CFTR activators identified from the screens. Based on the criteria listed earlier, we focused further studies on CFTRact-J027, a 3-phenyl-quinoxalinone with drug-like properties. CFTRact-J027 was synthesized in pure crystalline form in 2 steps (Figure 1D).
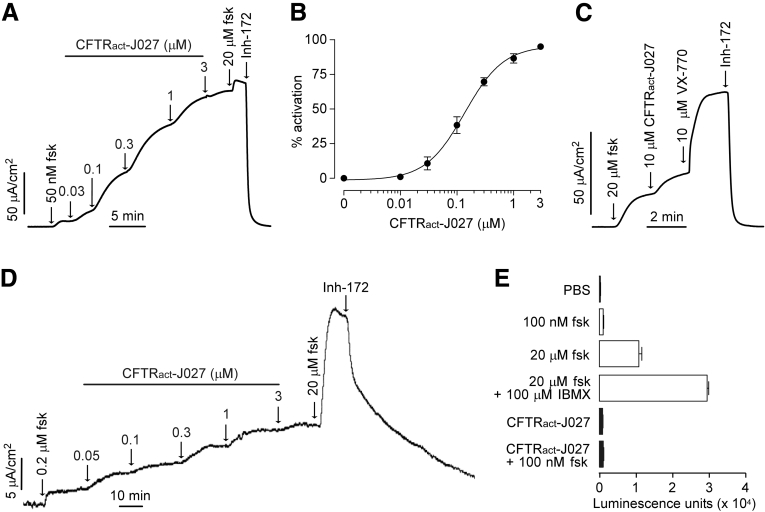
Short-circuit current measurements in CFTR-expressing FRT cells showed that CFTRact-J027 fully activated CFTR (Figure 2A) because the cAMP agonist forskolin produced a minimal further increase in current, with an EC50 of approximately 200 nmol/L (Figure 2B). Little CFTR activation by CFTRact-J027 was seen in the absence of forskolin (data not shown), suggesting the need for some level of basal phosphorylation as expected in vivo. Interestingly, CFTRact-J027 was only a weak potentiator of ΔF508-CFTR, as studied in FRT cells expressing ΔF508-CFTR after overnight incubation with the corrector VX-809, which promotes plasma membrane targeting of the mutant channel (Figure 2C). Cl- secretion in freshly isolated mouse colon showed a concentration-dependent increase in short-circuit current, with an EC50 of approximately 300 nmol/L (Figure 2D). The increase in current at high CFTRact-J027 levels was increased further by forskolin, which may be a consequence of activation of a basolateral membrane cAMP-sensitive K+ channel that increases the driving force for apical membrane Cl- secretion. The increase in current was fully reversed by a CFTR-selective inhibitor. The reduced effect of CFTRact-J027 on intact intestine could also result from limited drug access to the crypts or intestinal tissue metabolism of the drug. Figure 2E shows that CFTRact-J027 does not increase cellular cAMP when added alone, and does not increase cAMP further when added together with forskolin, suggesting that CFTR activation by CFTRact-J027 involves a direct interaction mechanism rather than indirect action through cAMP increase. Direct activation of protein kinase A by CFTRact-J027 is unlikely to occur because little or no activation of CFTR by CFTRact-J027 is seen in the absence of a cAMP agonist.
Figure 2.
Characterization of CFTR activation by CFTRact-J027. Short-circuit current measured in FRT cells expressing (A) human wild-type CFTR and (C) ΔF508-CFTR showing responses to indicated concentrations of forskolin (fsk), CFTRact-J027, and VX-770. The ΔF508-CFTR–expressing FRT cells were corrected with 3 μmol/L VX-809 at 37°C for 24 hours before measurement. CFTRinh-172 (Inh-172, 10 μmol/L) was added where indicated (representative of 3 experiments for each). (B) CFTRact-J027 concentration-dependent activation of wild-type CFTR Cl- current (means ± SEM; n = 3 cultures). (D) Short-circuit current in mouse colon showing responses to indicated concentrations of forskolin (fsk), CFTRact-J027, and CFTRinh-172 (representative of 3 experiments). (E) Assay of cAMP concentration in FRT cells measured after a 10-minute incubation with indicated concentrations of forskolin and 5 μmol/L CFTRact-J027. Positive controls included forskolin (100 nmol/L and 20 μmol/L), and forskolin plus 3-isobutyl-1-methylxanthine (IBMX, 100 μmol/L) (means ± SEM, n = 4–8).
CFTRact-J027 Normalizes Stool Output in a Mouse Model of Constipation
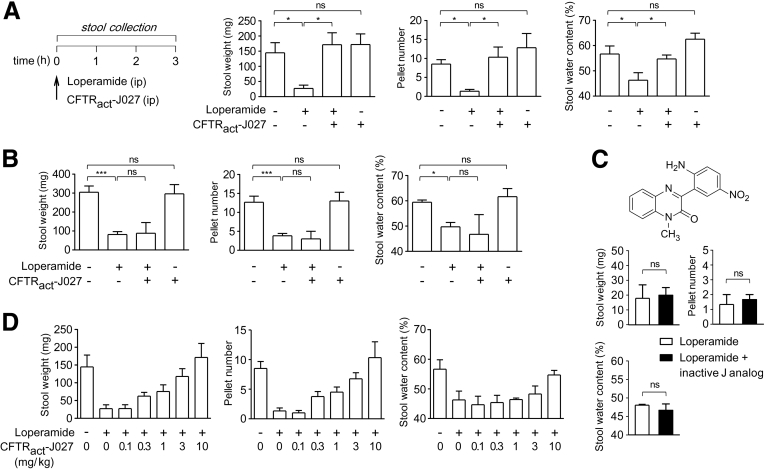
CFTRact-J027 was studied in a loperamide-induced model of constipation in mice in which stool weight, pellet number, and water content were measured for 3 hours after intraperitoneal loperamide administration (Figure 3A). Intraperitoneal administration of CFTRact-J027 at 10 mg/kg normalized each of the stool parameters. CFTRact-J027 did not affect stool output or water content in control (non–loperamide-treated) mice. Importantly, CFTRact-J027 was without effect in cystic fibrosis mice lacking functional CFTR (Figure 3B). Also an inactive chemical analog of CFTRact-J027 was not effective in wild-type mice (Figure 3C). These results support a CFTR-selective action of CFTRact-J027. Also, the lack of efficacy of CFTRact-J027 in cystic fibrosis mice shows that the action of CFTRact-J027 is not caused by opioid-receptor antagonism. Dose-response studies in mice showed a 50% effective dose of 2 mg/kg in the loperamide model by IP administration of CFTRact-J027 (Figure 3D).
Figure 3.
CFTRact-J027 normalizes stool output and water content in loperamide-treated mice. (A) Mouse model of constipation with loperamide (left). Three-hour stool weight, number of pellets, and stool water content in mice (means ± SEM, 6 mice per group). (B) Same study as shown in panel A, but with cystic fibrosis mice lacking functional CFTR (3–6 mice per group). (C) Same study as shown in panel A, but with an inactive chemical analog of CFTRact-J027 (structure shown, 3 mice per group). (D) Dose-response for intraperitoneal administration of CFTRact-J027 in loperamide-treated mice (4–6 mice per group). One-way analysis of variance was used for panels A and B, the Student's t test was used for panel C. *P < .05, ***P < .001.
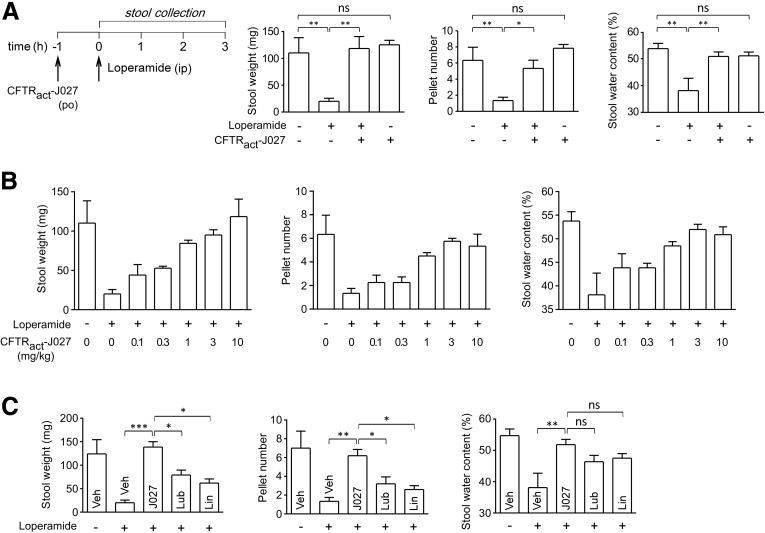
Oral administration of 10 mg/kg CFTRact-J027 1 hour before loperamide administration also was effective in normalizing stool output and water content in loperamide-treated mice, with no effect in control mice (Figure 4A). The median effective dose for oral administration was 0.5 mg/kg, which was substantially lower than that for IP administration (Figure 4B). In parallel studies, oral administration of the approved drugs lubiprostone or linaclotide at 250- to 500-fold greater mg/kg doses than given to human beings for treatment of constipation were less effective in normalizing stool output, producing 50% and 35%, respectively, of the maximal CFTRact-J027 response (Figure 4C).
Figure 4.
Orally administered CFTRact-J027 normalizes stool output and water content in loperamide-treated mice. (A) Study protocol (left) and stool output, pellet number, and water content as performed in Figure 3 (means ± SEM, 6 mice per group). (B) Dose-response study of CFTRact-J027 administered orally in loperamide-treated mice (4–6 mice per group). (C) Same study as shown in panel A, but with oral lubiprostone (Lub) (0.5 mg/kg) or linaclotide (Lin) (0.5 mg/kg) (5–6 mice per group). One-way analysis of variance, *P < .05, **P < .01, ***P < .001. Veh, vehicle.
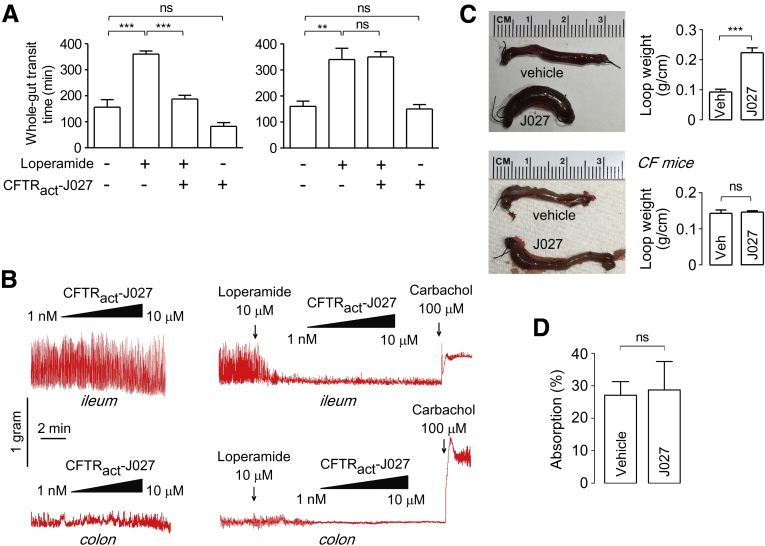
CFTRact-J027 Actions on Intestinal Transit, Motility, and Fluid Transport
CFTRact-J027 action on intestinal transit and motility was measured in vivo and in isolated intestinal strips. Whole-gut transit time, as measured by the appearance of a marker in the stool after bolus oral gavage at the time of IP loperamide and CFTRact-J027 administration, was normalized by CFTRact-J027 (Figure 5A, left panel). CFTRact-J027 had no effect on whole-gut transit time in cystic fibrosis mice (right panel). In vitro measurements of intestinal contraction showed no effect of CFTRact-J027 added alone or in the presence of 10 μmol/L loperamide in isolated mouse ileum and colon strips (Figure 5B). CFTRact-J027 thus may increase intestinal transit in vivo by stimulating motility by secretion-induced stretch of the gut wall or other indirect mechanisms, without direct effect on intestinal smooth muscle. The lack of compound effect on loperamide-induced suppression of intestinal contractions shows that CFTRact-J027 does not work by antagonizing the opioid effect.
Figure 5.
CFTRact-J027 actions on intestinal fluid secretion, absorption, and motility. (A) Whole-gut transit time in control and loperamide-treated wild-type (left) and cystic fibrosis (right) mice (means ± SEM, 3–5 mice per group). Where indicated, loperamide (0.3 mg/kg) and CFTRact-J027 (10 mg/kg) were administered IP at 0 time (means ± SEM, 6 mice per group). (B) Contraction of isolated intestinal strips. Ileum and colon strips (∼2 cm) were suspended in Krebs–Henseleit buffer with 0.5 g and 0.2 g tension, respectively. Where indicated, CFTRact-J027, loperamide, and carbachol were added to the organ chamber. (C) Intestinal fluid secretion was measured in closed midjejunal loops in wild-type mice (upper panel). Loops were injected with 100 μL vehicle or 100 μg CFTRact-J027. Loop weight/length was measured at 90 minutes (means ± SEM, 4 loops per group). Similar experiments were performed in cystic fibrosis mice (lower panel, 4 loops per group). (D) Intestinal fluid absorption was measured in midjejunal loops in cystic fibrosis mice. Loops were injected with 100 μL vehicle or 100 microgram CFTRact-J027. Loop weight/length was measured at 30 minutes. Summary of fluid absorption (means ± SEM, 4 loops per group). One-way analysis of variance was used for panel A , Student's t test was used for panel C and D. ∗∗P < .01, ∗∗∗P < .001.
To directly investigate the effects of CFTRact-J027 on intestinal fluid secretion and absorption, an in vivo closed-intestinal loop model was used. CFTRact-J027 was injected into closed midjejunal loops and fluid accumulation was measured at 90 minutes. CFTRact-J027 produced a 140% increase in loop weight/length ratio, indicating fluid secretion into the intestinal lumen in wild-type mice (Figure 5C, upper panel), but was without effect in cystic fibrosis mice (lower panel), supporting a CFTR-selective mechanism of action. A closed-loop model also was used to study CFTRact-J027 action on intestinal fluid absorption. Fluid without or with CFTRact-J027 was injected into closed midjejunal loops of cystic fibrosis mice (to avoid confounding fluid secretion) and fluid absorption was measured at 30 minutes. CFTRact-J027 did not affect intestinal fluid absorption (Figure 5D).
CFTRact-J027 Pharmacology and Toxicity in Mice
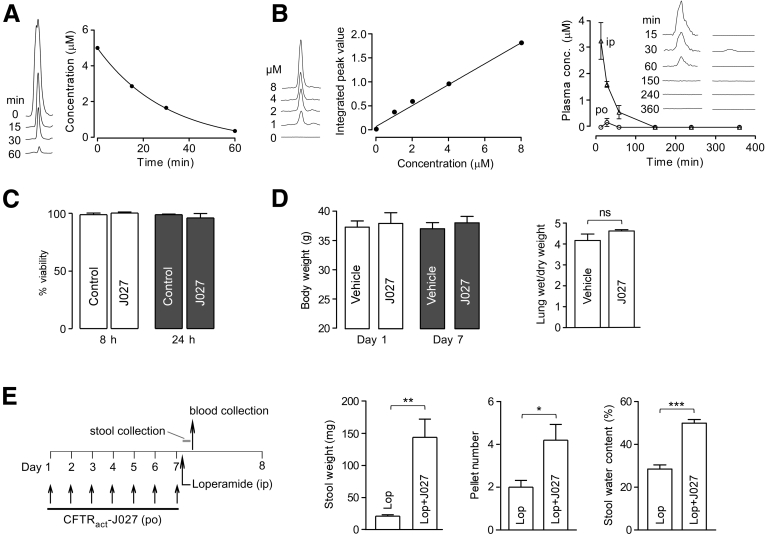
The in vitro metabolic stability of CFTRact-J027 was measured by incubation with mouse liver microsomes in the presence of reduced nicotinamide adenine dinucleotide phosphate. CFTRact-J027 was metabolized rapidly, with an approximately 21-minute elimination half-life, with only 7% of the original compound remaining at 60 minutes (Figure 6A).
Figure 6.
CFTRact-J027 pharmacokinetics, tissue distribution, and toxicity. (A) In vitro metabolic stability of CFTRact-J027 assayed in mouse liver microsomes after incubation for specified times. (B) Standard plasma concentration curve for liquid chromatography–mass spectrometry (left) and kinetics of CFTRact-J027 concentration in plasma determined by liquid chromatography–mass spectrometry after bolus IP or oral administration of 10 mg/kg CFTRact-J027 at zero time (right, means ± SEM, 3 mice per group). (C) In vitro toxicity measured by Alamar Blue assay in FRT cells (means ± SEM; n = 3 cultures). (D) Body weight and lung wet/dry weight ratio in mice receiving 10 mg/kg CFTRact-J027 orally for 7 days (means ± SEM, 5 mice per group). (E) Chronic administration protocol (left) and efficacy of oral CFTRact-J027 after 7-day administration (means ± SEM, 5 mice per group). Student t test, *P < .05, **P < .01, ***P < .001. conc, concentration; Lop, loperamide.
Pharmacokinetics was measured in mice after bolus intraperitoneal or oral administration of 10 mg/kg CFTRact-J027. After IP administration, the serum CFTRact-J027 concentration decreased with an elimination half-life of approximately 16 minutes, and was undetectable at 150 minutes (Figure 6B). After oral administration, the serum CFTRact-J027 concentration reached 180 nmol/L at 30 minutes and was undetectable at other time points (Figure 6B). Together, these results suggest rapid compound metabolism by liver, resulting in minimal systemic exposure of orally administered CFTRact-J027.
Preliminary toxicologic studies of CFTRact-J027 were performed in cell cultures and mice. CFTRact-J027, at a concentration of 25 μmol/L, which is near its solubility limit, did not show cytotoxicity as measured by the Alamar Blue assay (Figure 6C). In the 7-day–treated mice, CFTRact-J027 did not affect the major serum chemistry and blood parameters (Table 1), and it did not change body weight or produce airway/lung fluid accumulation (Figure 6D).
Table 1.
Complete Blood Count and Serum Chemistries of Mice Treated for 7 Days With 10 mg/kg CFTRact-J027 or Vehicle Orally Once per Day
| Vehicle | CFTRact-J027 | P value | |
|---|---|---|---|
| Hemoglobin level, g/dL | 13.3 ± 0.2 | 12.8 ± 0.3 | >.05 |
| Leukocyte level, 103/μL | 1.9 ± 0.3 | 1.9 ± 0.5 | >.05 |
| Thrombocyte level, 103/μL | 790 ± 109 | 900 ± 48 | >.05 |
| Total protein level, g/dL | 4.7 ± 0.2 | 5.2 ± 0.1 | >.05 |
| Albumin level, g/dL | 2.6 ± 0.1 | 2.9 ± 0.03 | >.05 |
| Globulin level, g/dL | 2.1 ± 0.1 | 2.2 ± 0.1 | >.05 |
| ALT level, U/L | 52 ± 16 | 44 ± 6 | >.05 |
| AST level, U/L | 131 ± 17 | 105 ± 11 | >.05 |
| ALP level, U/L | 47 ± 8.5 | 53 ± 2.5 | >.05 |
| Total bilirubin level, mg/dL | 0.1 ± 0 | 0.1 ± 0 | >.05 |
| Glucose level, mg/dL | 156 ± 22 | 164 ± 6 | >.05 |
| Cholesterol level, mg/dL | 121 ± 14 | 121 ± 6 | >.05 |
| CK level, U/L | 344 ± 85 | 312 ± 62 | >.05 |
| Sodium level, mmol/L | 149 ± 2.3 | 151 ± 0.7 | >.05 |
| Potassium level, mmol/L | 5.0 ± 0.1 | 4.4 ± 0.1 | >.05 |
| Chloride level, mmol/L | 113 ± 1 | 115 ± 1 | >.05 |
| Calcium level, mg/dL | 8.5 ± 0.2 | 8.5 ± 0.04 | >.05 |
| Phosphorus level, mg/dL | 6.6 ± 0.9 | 6.8 ± 0.3 | >.05 |
| BUN level, mg/dL | 15.3 ± 3 | 18.4 ± 1.2 | >.05 |
| Creatinine level, mg/dL | 0.2 ± 0 | 0.2 ± 0 | >.05 |
| Bicarbonate level, mmol/L | 15.3 ± 1.6 | 16 ± 1.7 | >.05 |
NOTE. Mean ± SEM are shown, 5 mice per group. Student's t test was used.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatinine kinase.
Finally, to determine whether chronically administered CFTRact-J027 retained efficacy, mice were treated orally for 7 days with 10 mg/kg CFTRact-J027 or vehicle, and loperamide was given 1 hour after the final dose. Figure 6E shows that chronically administered CFTRact-J027 remained effective in normalizing stool output and water content after loperamide.
Discussion
We identified by high-throughput screening a nanomolar affinity, small-molecule CFTR activator, CFTRact-J027, and showed its prosecretory action in mouse intestine and its efficacy in normalizing stool output in a loperamide-induced mouse model of constipation. Constipation remains a significant clinical problem in outpatient and hospitalized settings. Opioid-induced constipation is a common adverse effect in patients after surgery, undergoing chemotherapy, and with chronic pain.
CFTR-targeted activation adds to the various mechanisms of action of anticonstipation therapeutics. It is notable that pure CFTR activation is able to produce a robust Cl- current and fluid secretion response in the intestine, without causing global increase of cyclic nucleotide concentration, direct stimulation of intestinal contractility, or alteration of intestinal fluid absorption. Linaclotide, a peptide agonist of the guanylate cyclase C receptor that increases intestinal cell cGMP concentration, has the closest mechanism of action to a pure CFTR activator. Linaclotide inhibits activation of colonic sensory neurons and activates motor neurons, which reduces pain and increases intestinal smooth muscle contraction; in addition, an increase in cGMP concentration in enterocytes may activate CFTR and have a prosecretory action.4, 5 The uroguanylin analog plecanatide has a similar mechanism of action and preliminary data showed comparable efficacy with linaclotide for treatment of chronic constipation.16 A second approved drug, the prostaglandin E analog lubiprostone, is thought to activate a putative enterocyte ClC-2 channel6 and it has been reported that lubiprostone also activates CFTR via prostanoid-receptor signaling.17 Compared with these drugs, a pure CFTR activator has a single, well-validated mechanism of action and does not produce a global cyclic nucleotide response in multiple cell types. Of note, linaclotide and lubiprostone showed limited efficacy in clinical trials. In terms of the primary efficacy end point (weekly frequency of ≥3 complete spontaneous bowel movements [CSBM] and increase of ≥1 CSBM from baseline for at least 9 weeks of the 12-week treatment period), linaclotide was effective in approximately 20% of chronic constipation patients, of whom approximately 5% also responded to placebo, and linaclotide significantly increased CSBM per week (∼2.5) compared with placebo (0.9).18 The overall response rate to lubiprostone was approximately 13% in constipation-predominant irritable bowel syndrome patients, of whom approximately 7% responded to placebo.19 Based on our mouse data showing substantially greater efficacy of CFTRact-J027 compared with supramaximal doses of linaclotide or lubiprostone, we speculate that CFTR activators may have greater efficacy in clinical trials.
CFTRact-J027 is substantially more potent for activation of wild-type CFTR than VX-770 (ivacaftor), the Food and Drug Administration–approved drug for treatment of CF caused by certain CFTR gating mutations. In FRT cells expressing wild-type CFTR, short-circuit current measurement showed nearly full activation of CFTR by CFTRact-J027 at 3 μmol/L whereas VX-770 maximally activated CFTR by only 15% (data not shown). However, CFTRact-J027 was substantially less potent than ivacaftor as a potentiator of defective chloride channel gating of the most common CF-causing mutation, ΔF508, which is not unexpected because potentiator efficacy in CF is mutation-specific. In addition to its potential therapeutic utility for constipation, a small-molecule activator of wild-type CFTR may be useful for treatment of chronic obstructive pulmonary disease and bronchitis, asthma, cigarette smoke–induced lung dysfunction, dry eye, and cholestatic liver disease.20, 21, 22
There have been several prior reports on the biological properties of the core quinoxalinone scaffold found in CFTRact-J027. Substituted quinoxalinones were reported as selective antagonists of the membrane efflux transporter multiple-drug-resistance protein 1.23 Quinoxalinones also have been reported to show antidiabetic activity by stimulating insulin secretion in pancreatic INS-1 cells,24 and inhibitory activity against serine proteases for potential therapy of thrombotic disorders.25 Recently, quinoxalinones have been reported to inhibit aldose reductase.26 These reports suggest that the quinoxalinone scaffold has drug-like properties. Synthetically, quinoxalinone can be prepared in 1–4 steps from commercially available starting materials,27 which allows facile synthesis of targeted analogs.
In addition to compound-specific, off-target actions, the potential side-effect profile of a CFTR activator could include prosecretory activity in the airway/lungs and various glandular and other epithelia. Off-target effects for constipation therapy could be limited by oral administration of a CFTR activator with limited intestinal absorption and/or rapid systemic clearance to minimize systemic exposure. CFTRact-J027, when administered orally at a high dose (10 mg/kg), showed very low bioavailability with blood levels well below the EC50 for CFTR activation, which may be owing to first-pass effect as evidenced by its rapid in vitro metabolism in liver microsomes. CFTRact-J027 did not show significant in vitro cytotoxicity at a concentration of 25 μmol/L, more than 100-fold greater than its EC50 for CFTR activation, or in vivo toxicity in mice in a 7-day study at a maximal efficacious dose. The potentially most significant off-target action, stimulation of lung/airway fluid secretion, was not seen as evidenced by normal lung water content in the 7-day–treated mice. These limited toxicity studies offer proof of concept for application of a CFTR activator in constipation, but will require a more extensive data set for further preclinical development.
In summary, our data provide evidence for the prosecretory action of a CFTR activator in mouse intestine and proof of concept for its use in the treatment of various types of constipation, which could include opioid-induced constipation, chronic idiopathic constipation, and irritable bowel syndrome with constipation predominance.
Acknowledgments
The authors thank Drs Mark Donowitz (Johns Hopkins University) and Jerry Turner (Univ Chicago) for helpful advice and suggestions.
Footnotes
Conflicts of interest These authors disclose the following: Onur Cil, Marc Levin, and Alan Verkman are named inventors on a provisional patent filing, with rights owned by the University of California, San Francisco, California. The remaining authors disclose no conflicts.
Funding Supported by grants DK099803, DK72517, DK101373, DK35124, EB00415, and EY13574 from the National Institutes of Health, a Research Development Program grant from the Cystic Fibrosis Foundation, and a grant from the Guthy-Jackson Charitable Foundation (A.S.V.); National Institutes of Health/National Center for Advancing Translational Sciences University of California San Francisco-Clinical and Translational Science Institute UL1 TR000004 (M.H.L. and A.S.V.); a Research to Prevent Blindness Career Development Award and National Institutes of Health K08 grant EY023981 (M.H.L.); grants 31471099 and 81173109 from the National Natural Science Foundation of China (T.M.); and National Institutes of Health K12 grant HD000850 (J.R.T.).
Contributor Information
Tonghui Ma, Email: tonghuima@dmu.edu.cn.
Alan S. Verkman, Email: Alan.Verkman@ucsf.edu.
References
- 1.Pinto Sanchez M.I., Bercik P. Epidemiology and burden of chronic constipation. Can J Gastroenterol. 2011;25(Suppl B):11B–15B. doi: 10.1155/2011/974573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mugie S.M., Di Lorenzo C., Benninga M.A. Constipation in childhood. Nat Rev Gastroenterol Hepatol. 2011;8:502–511. doi: 10.1038/nrgastro.2011.130. [DOI] [PubMed] [Google Scholar]
- 3.Menees S., Saad R., Chey W.D. Agents that act luminally to treat diarrhoea and constipation. Nat Rev Gastroenterol Hepatol. 2012;9:661–674. doi: 10.1038/nrgastro.2012.162. [DOI] [PubMed] [Google Scholar]
- 4.Castro J., Harrington A.M., Hughes P.A. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3',5'-monophosphate. Gastroenterology. 2013;145:1334–1346. doi: 10.1053/j.gastro.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Busby R.W., Bryant A.P., Bartolini W.P. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649:328–335. doi: 10.1016/j.ejphar.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Fei G., Raehal K., Liu S. Lubiprostone reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouse. J Pharmacol Exp Ther. 2010;334:333–340. doi: 10.1124/jpet.110.166116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiagarajah J.R., Donowitz M., Verkman A.S. Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol. 2015;12:446–457. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field M., Fromm D., Al-Awqati Q. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972;51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao M.C., Guandalini S., Smith P.L. Mode of action of heat-stable Escherichia coli enterotoxin tissue and subcellular specificities and role of cyclic GMP. Biochim Biophys Acta. 1980;632:35–46. doi: 10.1016/0304-4165(80)90247-0. [DOI] [PubMed] [Google Scholar]
- 10.Subramanya S.B., Rajendran V.M., Srinivasan P. Differential regulation of cholera toxin-inhibited Na-H exchange isoforms by butyrate in rat ileum. Am J Physiol. 2007;293:G857–G863. doi: 10.1152/ajpgi.00462.2006. [DOI] [PubMed] [Google Scholar]
- 11.Hecht G., Hodges K., Gill R.K. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G370–G378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 12.Galietta L.J.V., Springsteel M.F., Eda M. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 13.Esteva-Font C., Cil O., Phuan P.W. Diuresis and reduced urinary osmolality in rats produced by small-molecule UT-A-selective urea transport inhibitors. FASEB J. 2014;28:3878–3890. doi: 10.1096/fj.14-253872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma T., Vetrivel L., Yang H. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 15.Namkung W., Park J., Seo Y. Novel amino-carbonitrile-pyrazole identified in a small molecule screen activates wild-type and ΔF508 cystic fibrosis transmembrane conductance regulator in the absence of a cAMP agonist. Mol Pharmacol. 2013;84:384–392. doi: 10.1124/mol.113.086348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri M. Guanylate cyclase C agonists: emerging gastrointestinal therapies and actions. Gastroenterology. 2015;148:483–487. doi: 10.1053/j.gastro.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Bijvelds M.J., Bot A.G., Escher J.C. Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–985. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Lembo A.J., Schneier H.A., Shiff S.J. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–536. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- 19.https://www.amitizahcp.com/efficacy-indication-1/. Accessed December 2015.
- 20.Gras D., Chanez P., Vachier I. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013;140:290–305. doi: 10.1016/j.pharmthera.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25–36. doi: 10.1016/j.jceh.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin M.H., Verkman A.S. CFTR-regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest Ophthalmol Vis Sci. 2005;46:1428–1434. doi: 10.1167/iovs.04-1314. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence D.S., Copper J.E., Smith C.D. Structure-activity studies of substituted quinoxalinones as multiple-drug-resistance antagonists. J Med Chem. 2001;44:594–601. doi: 10.1021/jm000282d. [DOI] [PubMed] [Google Scholar]
- 24.Botton G, Valeur E, Kergoat M. Preparation of quinoxalinone derivatives as insulin secretion stimulators useful for the treatment of diabetes. PCT Int Appl 2009, WO 2009109258 approval date: Sep 11, 2009 (patent).
- 25.Dudley DA, Edmunds JJ. Preparation of quinoxalinones as serine protease inhibitors for treatment of thrombotic disorders. PCT Int Appl 1999, WO 9950254 approval date: Oct 07, 1999 (patent).
- 26.Qin X., Hao X., Han H. Design and synthesis of potent and multifunctional aldose reductase inhibitors based on quinoxalinones. J Med Chem. 2015;58:1254–1267. doi: 10.1021/jm501484b. [DOI] [PubMed] [Google Scholar]
- 27.Shaw A.D., Denning C.R., Hulme C. One-pot two-step synthesis of quinoxalinones and diazepinones via a tandem oxidative amidation-deprotection-cyclization sequence. Synthesis. 2013;45:459–462. [Google Scholar]