Abstract
Objective
Post-transplant cyclophosphamide is increasingly used as graft-versus-host disease (GvHD) prophylaxis in the setting of bone marrow transplantation. No data have been published on the use of single-agent GvHD prophylaxis with post-transplant cyclophosphamide in the setting of peripheral blood stem cell transplantation (PBSCT).
Methods
In a phase II trial, 11 patients with myeloma or lymphoma underwent conditioning with fludarabine and busulfan followed by T-replete PBSCT and application of 50 mg/kg/d of cyclophosphamide on day+3 and +4 without other concurrent immunosuppression (IS).
Results
Median time to leukocyte, neutrophil, and platelet engraftment was 18, 21, and 18 d. The incidence of grade II–IV and grade III–IV GvHD was 45% and 27%, with a non-relapse mortality (NRM) of 36% at one and 2 yr. After median follow-up of 927 d, overall and relapse-free survival was 64% and 34%. Three patients did not require any further systemic IS until day+100 and thereafter. Analysis of immune reconstitution demonstrated rapid T- and NK-cell recovery. B- and CD3+/CD161+NK/T-cell recovery was superior in patients not receiving additional IS.
Conclusion
Post-transplant cyclophosphamide as sole IS in PBSCT is feasible and allows rapid immune recovery. Increased rates of severe acute GvHD explain the observed NRM and may advise a temporary combination partner such as mTor-inhibitors in the PBSCT setting.
Keywords: allogeneic hematopoietic stem cell transplantation, post-transplant cyclophosphamide, graft-versus-host disease
Acute graft-versus-host disease (GvHD) and chronic GvHD are major barriers to successful allogeneic stem cell transplantation (1). Standard prophylaxis regimens for GvHD comprise combinations of the calcineurin inhibitors cyclosporine or tacrolimus together with either methotrexate or mycophenolate mofetil (2–6). These combination strategies largely contribute to the acute toxicity of the procedure. Despite this prophylaxis, a significant proportion of patients still experiences GvHD (1). Furthermore, continuous immunosuppression (IS) delays immune reconstitution and may diminish the desired graft-versus-tumor effect as well as predispose to infections (7). Based on animal models on tolerance induction by cyclophosphamide (8–10), Luznik et al. (11) pioneered an approach using high-dose cyclophosphamide applied after transplantation in a murine model. Since then, post-transplant cyclophosphamide has been used as component of GvHD prophylaxis in the haploidentical setting (12–19). In the setting of matched donor transplantation, post-transplant cyclophosphamide has been used as single-agent GvHD prophylaxis in bone marrow transplantation after myeloablative conditioning with acceptable rates of acute and chronic GvHD (20–22). In these clinical trials, bone marrow was used as stem cell source, as it contains significantly lower T-cell numbers. Vice versa, based on higher T-cell numbers, the incidence of acute and chronic GvHD could be higher in peripheral blood transplants (23–25). No data have been published so far on post-transplant cyclophosphamide as single-agent GvHD prophylaxis in peripheral blood stem cell transplantation (PBSCT) in the non-haploidentical setting. For haploidentical transplantation, Castagna et al. (18) demonstrated similar results for peripheral blood and bone marrow transplants (acute and chronic GvHD 33% vs. 25% and 13% vs. 13%, respectively). In a matched-control analysis presented at ASH 2012, Alousi et al. (26) reported a significantly higher incidence of grade II–IV and grade III–IV acute GvHD as well as chronic GvHD in matched related or unrelated transplants and post-transplant cyclophosphamide as only GvHD prophylaxis when compared to patients receiving conventional GvHD prophylaxis. Thirty percent of these patients received peripheral blood transplants. A single center study recently demonstrated feasibility of peripheral blood transplants with post-transplant cyclophosphamide followed by short-term sirolimus as GvHD prophylaxis with cumulative incidences of grade II–IV acute GVHD, grade III–IV acute GVHD, all chronic GVHD, and severe chronic GVHD of 41%, 15%, 32%, and 12%, respectively (27).
Post-transplant cyclophosphamide on day+3 and +4 after transplant is considered to act mainly on alloreactive T cells rapidly and early dividing upon encounter with patient cells (28–31). In the haploidentical setting, early and favorable immune recovery has been reported (17, 30, 32, 33). In this phase II pilot trial, we assessed the efficacy and safety of post-transplant cyclophosphamide as sole GvHD-prophylaxis as well as its impact on immune recovery in related and unrelated PBSCT following reduced-intensity conditioning in patients with myeloma or lymphoma.
Material and methods
Study design and patients
The study (ClinicalTrials.gov NCT01283776, EudraCT number: 2010-022058-18) included adult patients undergoing allogeneic PBSCT for multiple myeloma, non-Hodgkin’s lymphoma or Hodgkin’s disease. The proportion of patients not requiring additional systemic immunosuppressive treatment within 100 d after transplant was chosen as the primary endpoint. The study was designed as an early phase II clinical study according to Simon’s two-stage phase II procedure (34). Eleven evaluable patients out of up to 13 patients could be included. In a first step, five patients were enrolled on the trial. Eight more patients could be recruited for the second stage of the trial if one or more patients did not require any systemic IS until day+100. The primary endpoint would be met if at least three of 11 evaluable patients would not require systemic IS. A total of 12 patients received treatment on this trial approved by the local institutional review board after informed consent was granted in accordance with the Declaration of Helsinki. Eligibility criteria included the following: patients with multiple myeloma or non-Hodgkin’s lymphoma or Hodgkin’s disease who have received an allogeneic PBSCT following reduced-intensity conditioning; age of at least 18 yr; written informed consent according to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use/Good Clinical Practice (ICH/GCP) and national/local regulations; transplantation of stem cells from one of the following donors: Human Leucocyte Antigen (HLA)-identical sibling donor, HLA-matched unrelated donor (MUD), HLA-mismatched related donor or unrelated donor (mMUD), if not mismatched in more than one single HLA antigen as defined by high-resolution HLA-typing for HLA-A, B, C, DRB1, and DQB1; Karnofsky-Index ≥80%. Exclusion criteria included known intolerance to cyclophosphamide, severe organ dysfunction, pregnancy or breastfeeding, presence of hemorrhagic cystitis or urinary tract obstruction, and presence of uncontrolled infections. High-resolution HLA-typing was performed for HLA-A, B, C, DRB1, and DQB1.
Regimen and supportive care
For all patients, conditioning consisted of fludarabine 30 mg/m2 per day over 5 consecutive days at 10 am accompanied on the first 3 d by busulfan at 3.2 mg/kg/d intravenously given in four daily divided doses at 8 am, 2 pm, 8 pm, and 2 am. Cyclophosphamide was administered intravenously on days 3 and 4 after transplant at 50 mg/kg per day. Mesna (20% of the cyclophosphamide dose as bolus prior to cyclophosphamide, 80% of the cyclophosphamide dose over 20 h from the time of cyclophosphamide infusion) was administered on all days of cyclophosphamide administration. Colony-stimulating factors were not given. Supportive care measures were administered according to institutional protocols and included prophylaxis against Pneumocystis jirovecii, fungal infection, and herpes zoster/simplex infections. All blood products except for the allograft were irradiated before transfusion. Supportive care measures were identical for recipients of related and unrelated allografts. Patients had weekly measurements of CMV copy numbers by polymerase chain reaction until discharge for the time of the study (day+100). Pre-emptive therapy was initiated when more than 1000 copies CMV/mL were detected.
Regimen-related toxicity
Toxic effects were categorized using the NCI Common Terminology Criteria for Adverse Events, Version 4.0.
Engraftment and donor chimerism
Leukocyte recovery was defined as the first of 3 consecutive days with an absolute leukocyte count greater than 1 × 109/L. Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count greater than 0.5 × 109/L. Platelet recovery was defined as platelet counts greater than 20 × 109/L without platelet transfusion in the preceding 5 d. Donor chimerism was assessed on days+30 and +100 after transplantation within the study observation and thereafter every 3 months up to the end of year 2.
Diagnosis and treatment of GvHD
Acute cutaneous and acute liver GvHD were diagnosed clinically and, if ambiguous, confirmed pathologically. A clinical diagnosis of intestinal GVHD was confirmed histologically. Acute GVHD was graded according to the Keystone criteria (35). First-line therapy of clinically significant acute cutaneous GVHD consisted of methylprednisolone (MP) 1–2.5 mg/ kg/d orally or intravenously, while patients with acute GVHD of liver and intestine received cyclosporine or tacrolimus and MP. Chronic GVHD was graded according to the National Institutes of Health criteria (36).
Flow cytometry
T-cell (CD3/CD4), NK-cell, and B-cell absolute numbers were analyzed by our routine central laboratory using flow cytometry and the TruCount system (Becton & Dickinson, www.bd.com). For analysis of T-regulatory cells and CD3+ CD161+ NK/T cells, whole blood (30 mL) was collected in EDTA tubes at day+30, +90, and +150 after allo-SCT. Peripheral blood mononuclear cells were isolated by density-gradient centrifugation (Pancol®, www.pan-biotech.com). Cells were stained with the following fluorescence-labeled antibodies: CD20 Pacific Blue and CD161 PerCP-Cy5.5 (www.biolegend.com), CD4 Pacific Blue, CD25 PE-Cy7, CD127 AlexaF700, CD3 APC-H7 (www.bd.com), CD19 Alexa780 APC (www.eBioscience.com), CD45 Pacific Orange (www.lifetechnologies.com).
Flow cytometry was performed using a Gallios® cytometer (Beckman Coulter, www.beckmancoulter.com). Data were analyzed by Kaluza® flow cytometry software (Beckman Coulter).
Statistical analysis
Statistical analysis was performed using GraphPad Prism® 5 (www.graphpad.com) and IBM SPSS22 (www.ibm.com). Event time distributions for overall survival (OS) and relapse-free survival (EFS) were estimated with the method of Kaplan and Meier. Probabilities of non-relapse mortality (NRM) and relapse were calculated using cumulative incidence estimates. Patients were considered to have died of NRM if there was no evidence of disease persistence or progression before death. NRM and relapse were treated as competing risk events.
Results
Patient, donor, and graft characteristics
Eleven patients suffering from lymphoma or myeloma were included in the study. The median number of previous therapies was four including high-dose therapy followed by autologous transplantation in ten patients. Median time from auto to allotransplant was 375 d. Six patients were transplanted with HLA-matched-related donors, four with HLA-MUDs, and one with an HLA-mMUD. All patients were transplanted with T-cell replete peripheral stem cell grafts (median CD34 number 4.9 × 106/kg). Patient, donor, and graft characteristics are shown in Table 1.
Table 1.
Patient, donor, and graft characteristics
| Median age (yr) | 47 (25–60) |
| Diagnosis | |
| DLBCL | 2 (18.2%) |
| T-NHL | 2 (18.2%) |
| MM | 2 (18.2%) |
| HL | 2 (18.2%) |
| MCL | 2 (18.2%) |
| MZL | 1 (9%) |
| Prior therapies (median) | 4 (2–9) |
| Prior auto-SCT | 10 (91%) |
| Auto to allo-SCT (median days) | 375 (82–920) |
| Disease state pre-allo-SCT | |
| PR | 9 (82%) |
| SD | 1 (9%) |
| CR | 1 (9%) |
| Donor | |
| MRD | 6 (55%) |
| MUD | 4 (36%) |
| mMUD | 1 (9%) |
| Median CD34 dose (x106/kg) | 4.9 (4, 1–13) |
MUD, matched unrelated donor; mMRD, mismatched related donor; mMUD, mismatched unrelated donor.
Transplant outcomes
All patients engrafted and the median time to engraftment was 18, 21, and 18 d for leukocytes, neutrophils, and platelets, respectively (Table 2). All patients developed complete donor chimerism in the bone marrow by day 30 after allo-SCT (data not shown). Acute GvHD grade II–IV was reported in five of the patients (45%) and severe acute GvHD grade III–IV occurred in three patients (27%, intestinal and skin GvHD in two patients, intestinal and hepatic GvHD in one patient). Median time to onset of acute GvHD was 32 d with a range from 16 to 38 d (Table 2). One patient developed chronic GvHD after cessation of IS following lymphoma relapse and one after DLI-administration for the treatment of lymphoma relapse (both de novo onset and NIH mild grade).
Table 2.
Clinical outcomes
| Engraftment (median days) | |
| Leukocyte | 18(14–24) |
| Neutrophils | 21 (17–33) |
| Platelets | 18(10–30) |
| Acute GvHD | |
| II-IV | 5 (45%) |
| III-IV | 3 (27%) |
| Time to aGvHD (median days) | 32 (16–38) |
| Chronic GvHD | |
| NIH mild | 2 (1 post-DLI) |
| Time to cGvHD (median days) | 124 (122–126) |
| NRM | |
| Day+100 | 2 (18%) |
| 1 yr | 4 (36%) |
| 2 yr | 4 (36%) |
| 1 yr OS | 64% |
| 2 yr OS | 64% |
| 1 yr EFS | 45% |
| 2 yr EFS | 34% |
| Time to relapse (median days) | 140 (77–511) |
| Follow-up (median days) | 927 (348–1131) |
GvHD, graft-versus-host disease; NRM, non-relapse mortality; OS, overall survival; EFS, relapse-free survival.
After a median follow-up of 927 d (348–1131 d), seven patients are still alive (64%). One patient died due to intracranial hemorrhage occurring on day+23, one due to a late septic complication, and two died due to sepsis in the context of steroid-refractory stage IV intestinal GvHD. Causes of death and non-fatal toxicities occurring in the first 100 d after transplantation are listed in Table 3. The most common non-hematological toxicities were mucositis, diarrhea, and fever.
Table 3.
Adverse events
| Grade III–IV CTC 4.0 (n) | |
|---|---|
| Non-fatal toxicities | |
| Mucositis | 3 |
| Diarrhea | 4 (GvHD) |
| Fever | 8 |
| Microangiopathy | 3 |
| Rash other than GvHD | 1 |
| Gastrointestinal hemorrhage | 2 |
| Duodenal ulcers | 1 |
| Fatal toxicities | |
| Sepsis | 1 |
| Refractory GvHD/Sepsis | 2 |
| Subarachnoidal hemorrhage | 1 |
GvHD, graft-versus-host disease.
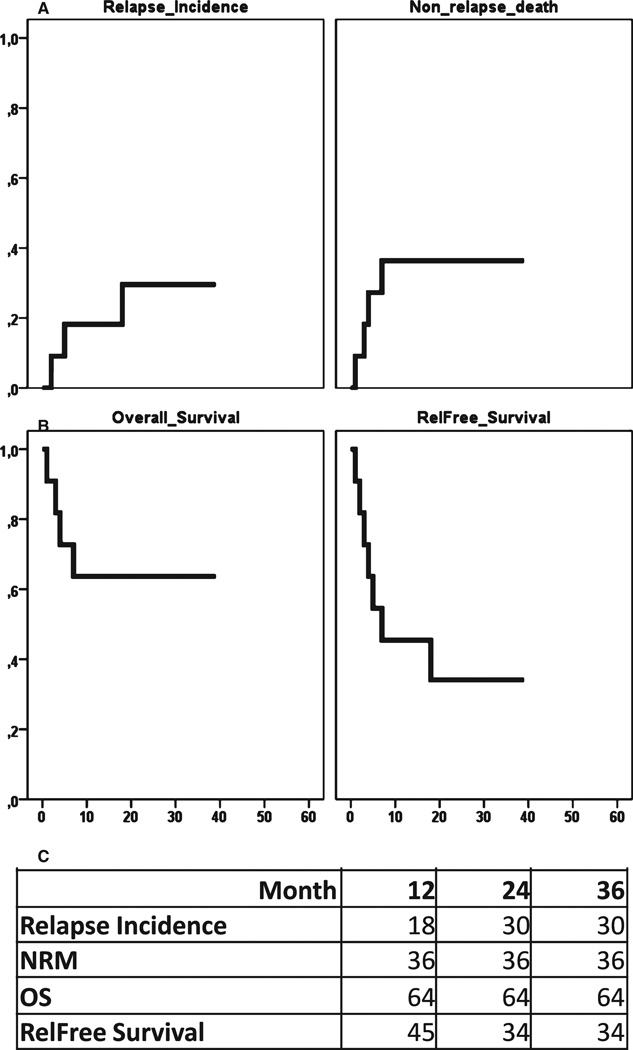
Using competing risk analysis, the cumulative incidence of NRM was 36% at 1 and 2 yr after transplant. The cumulative incidence of relapse was 18% and 30%, and the EFS was 45% at one and 34% at 2 yr after transplant (Table 2 and Fig. 1).
Figure 1.
Transplant outcomes. (A) Depicts cumulative incidence of relapse and non-relapse death. (B) Kaplan-Meier curves of overall and relapse-free survival (EFS). (C) Percentages of relapse, NRM, OS and EFS at 12, 24, and 36 months.
Immune reconstitution
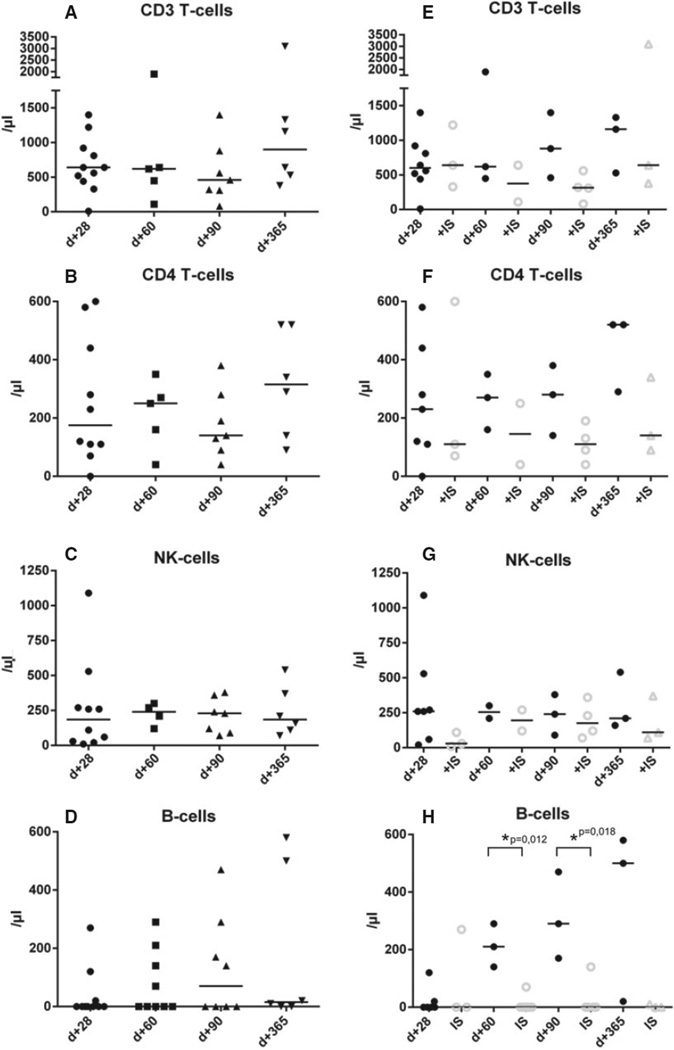
Absolute numbers of CD3 and CD4 T cells as well as B cells and NK cells were analyzed on day+28, +60, +90, and +365 after transplant (Table 4 and Fig. 2). Timely recovery could be documented for T cells, T-helper cells, and NK cells in all patients with higher counts in patients not receiving additional IS at the given time points, which however did not reach statistical significance (Table 4 and Fig. 2A–C, E–G). B cells recovered significantly faster in patients receiving post-transplant cyclophosphamide as sole IS (210 vs. 0/µL, P = 0.012 and 290 vs. 0/µL, 0.018 at day+60 and day+90, respectively, 500 vs. 0/µL on day+365 P = 0.1, Mann–Whitney test; Table 4 and Fig. 2D,H).
Table 4.
Immune reconstitution of CD3/CD4 T cells, NK cells, and B cells
| Day+28 | Day+60 | Day+90 | Day+365 | |
|---|---|---|---|---|
| CD3 T cells | ||||
| No IS | 600 | 620 | 880 | 1160 |
| IS | 640 | 375 | 315 | 640 |
| CD4 Tcells | ||||
| No IS | 230 | 270 | 280 | 520 |
| IS | 110 | 145 | 110 | 140 |
| NK cells | ||||
| No IS | 260 | 255 | 240 | 210 |
| IS | 30 | 195 | 175 | 110 |
| B cells | ||||
| No IS | 0 | 210* | 290* | 500 |
| IS | 0 | 0 | 0 | 0 |
IS, immunosuppression.
Median absolute numbers/µL.
P<0.05 Mann–Whitney test.
Figure 2.
Immune reconstitution of CD3/CD4 T cells, NK cells, and B cells. Figure depicts absolute numbers/µL of (A) CD3 T cells, (B) CD4 T cells, (C) NK cells, and (D) B cells at day+28, day+60, day+90, and day+365 after transplantation. Patients under continuous immunosuppression (IS) are compared to patients without continuous IS for absolute numbers of (E) CD3 T cells, (F) CD4 T cells, (G) NK cells, and (H) B cells for the respective time points. A P-value < 0.05 was considered statistically significant.
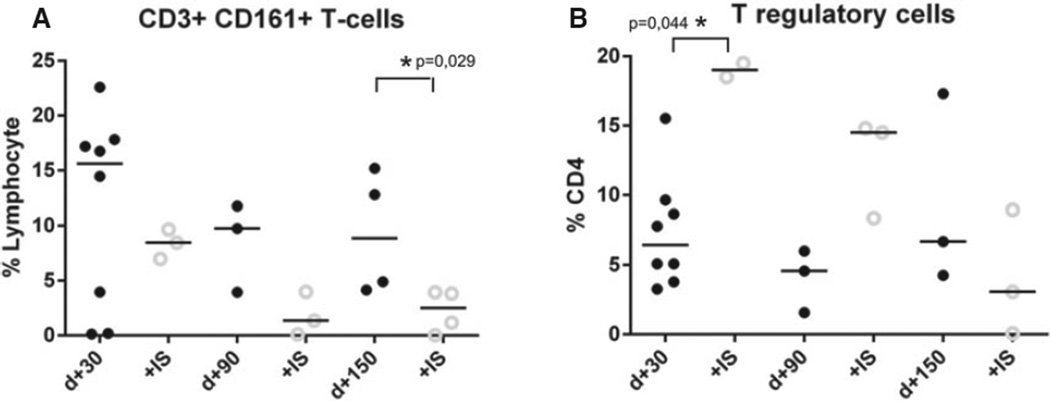
To further assess recovery of the immune response in patients treated with post-transplant cyclophosphamide, relative numbers of T-regulatory cells, defined as CD25 high CD127 low CD4+ T cells, and CD3+ CD161+ T cells were assessed on day+30, +90, and +150. At all time points analyzed, relative percentages of CD3+ CD161+ T cells were higher in patients without additional IS, but statistical significance was reached only on day+150 (8.9% vs. 2.5%, P = 0.029, Mann–Whitney test; Fig. 3A). For the median relative percentages of T-regulatory cells, no advantage could be detected for patients not receiving additional IS (Fig. 3B).
Figure 3.
Recovery of CD3+ CD161 + T cells and T-regulatory cells. Figure depicts relative percentages of (A) CD3+ CD161+ T cells and (B) T-regulatory cells on day+28, day+90, and day+150: Patients under continuous immunosuppression (IS) are compared to patients without continuous IS: A P-value < 0.05 was considered statistically significant.
Discussion
We here report the feasibility of high-dose post-transplant cyclophosphamide as sole IS in the context of PBSCT after reduced-intensity conditioning with fludarabine and busulfan in the non-haploidentical transplantation setting. In three of 11 patients, cyclophosphamide was sufficient to prevent the appearance of GvHD until day+100 and thus the primary endpoint of the study was met. Overall and EFS rates were 64% and 34% at 2 yr. However, the relatively high incidence of severe grade III–IV acute intestinal GvHD (27%) largely contributed to the NRM rate of 36% at 12 up to 48 months. Chronic GvHD occurred in two of the surviving patients and was induced by donor lymphocyte infusion or cessation of IS due to disease recurrence.
Engraftment and chimerism kinetics are comparable to those previously reported by a large study of 117 patients undergoing matched related or unrelated bone marrow transplantation (37). Despite the higher incidence of grade III–IV acute GvHD and NRM described in our study, the overall and EFS is similar to patients receiving bone marrow as stem cell source (OS 55%, EFS 39% at 2 yr). This could be due to a lower relapse rate (cumulative incidence of relapse PB 30% vs. BM 44% at 2 yr). Our study population consisted of heavily pretreated lymphoma and myeloma patients with a median number of four prior therapies including autologous transplantation in ten of 11 patients, and most patients had substantial tumor burden prior to transplant. Although our study is limited by the small patient number, the concept of PBSCT with post-transplant cyclophosphamide may be of particular interest for this group of patients, as myeloma and lymphoma patients with persisting disease prior to allogeneic stem cell transplantation are at very high risk of early relapse within the first 6–12 months after transplant (38–40). The additional role of high-dose cyclophosphamide as cytoreductive therapy in addition to its immunosuppressive properties may also at least in part contribute to these results.
Finally, early reconstitution of T cells, NK cells, and particularly of B cells (particularly in patients not requiring any additional continuous IS, i.e., calcineurin inhibitors) is very encouraging. As previously reported, post-transplant cyclophosphamide can promote the recovery of T-regulatory cells (32) potentially accounting for the low chronic GvHD incidence. In our study, we furthermore demonstrated higher relative numbers of T cells expressing the natural killer cell c-type lectin receptor (CD161). This subset characterizes a new, heterogeneous population comprising activated T cells, natural killer T-cell-like cells, and T-helper 17 (TH17) cells with immunomodulatory properties (41, 42). This subset with memory phenotype was previously linked to graft vs. tumor reactivity (43), and a fast recovery may also contribute to a reduced relapse risk. Recently at ASH, the MD Anderson group reported a matched controlled analysis of post-transplant cyclophosphamide vs. tacrolimus and mini-dose methotrexate in matched sibling and unrelated donor transplant recipients receiving reduced-intensity conditioning (26). Forty-nine patients were treated with post-transplant cyclophosphamide, one-third of whom were transplanted with peripheral blood stem cells. As compared to the tacrolimus group, a higher incidence of acute GvHD and NRM as well as worse OS was noted for the cyclophosphamide group [26% vs. 46%, HR 1.8 (0.9–3.3, P = 0.08)]. However, 59% of patients in both groups received ATG prior to transplant. We are confident that ATG administration significantly hampers the alloreactive T-cell depleting effect of post-transplant cyclophosphamide as it significantly interferes with the required early activation and subsequent proliferation of alloreactive donor-derived T cells. Thus, ATG depletes the targets for the cyclophosphamide effect (20).
In conclusion, post-transplantation cyclophosphamide is feasible and well tolerated in the setting of PBSCT following reduced-intensity conditioning in the non-haplo setting. Fast immune recovery and the chance of sparing continuous IS makes this approach attractive for conditions with high relapse risk. The beneficial effects of these properties have to be weighed against the potentially higher risk for severe acute GvHD, which may be associated with post-transplant cyclophosphamide in the context of peripheral blood transplants. Future studies should evaluate whether combination of post-transplantation cyclophosphamide in combination with short-term IS, for example, by mTor-inhibitors such as sirolimus (27), may help to mitigate the risk for severe acute GvHD in the early phase after transplant while maintaining the beneficial immune recovery.
Acknowledgments
This study was supported by a grant from the ‘Forschung-spool Klinische Studien’, Faculty of Medicine, University of Cologne and the ZKS Köln (Clinical Trials Center Cologne, BMBF grant 01KN1106).
Footnotes
Conflict of interest
The authors declare no conflict of interest with regard to the submitted manuscript.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 3.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 4.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 5.Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, Kalaycio M. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–625. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 6.Kansu E, Gooley T, Flowers ME, et al. Administration of cyclosporine for 24 months compared with 6 months for prevention of chronic graft-versus-host disease: a prospective randomized clinical trial. Blood. 2001;98:3868–3870. doi: 10.1182/blood.v98.13.3868. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald KP, Shlomchik WD, Reddy P. Biology of graft-versus-host responses: recent insights. Biol Blood Marrow Transplant. 2013;19(1 Suppl):S10–S14. doi: 10.1016/j.bbmt.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayumi H, Good RA. The necessity of both allogeneic antigens and stem cells for cyclophosphamide-induced skin allograft tolerance in mice. Immunobiology. 1989;178:287–304. doi: 10.1016/S0171-2985(89)80053-1. [DOI] [PubMed] [Google Scholar]
- 9.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayumi H, Himeno K, Tanaka K, Tokuda N, Fan JL, Nomoto K. Drug-induced tolerance to allografts in mice. XII. The relationships between tolerance, chimerism, and graft-versus-host disease. Transplantation. 1987;44:286–290. doi: 10.1097/00007890-198708000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 12.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, Brodsky RA. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, Morris LE, Solomon SR. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 16.Solomon SR, Jacobson S, Sanacore M, Zhang X, Sizemore C, Brown S, Holland HK, Morris LE, Bashey A. Myeloablative Conditioning with PBSC grafts for T-replete haploidentical donor hematopoietic cell transplantation using post-transplant cyclophosphamide results in universal engraftment, low rates of Gvhd, NRM and excellent survival outcomes: an analysis of two consecutive phase II studies from a single center. Blood. 2013;122:3351. [Google Scholar]
- 17.Perez-Corral AM, Gayoso J, Anguita J, et al. Early and favourable immune reconstitution after unmanipulated haploidentical stem cell transplantation with high dose post-transplant cyclophosphamide regardless intensity of conditioning regimen. Blood. 2013;122:4620. [Google Scholar]
- 18.Castagna L, Crocchiolo R, Furst S, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20:724–729. doi: 10.1016/j.bbmt.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Raj K, Pagliuca A, Bradstock K, et al. Peripheral blood hematopoietic stem cells for transplantation of hematological diseases from related, haploidentical donors after reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:890–895. doi: 10.1016/j.bbmt.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanakry CG, Tsai HL, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817–3827. doi: 10.1182/blood-2014-07-587477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.StemCellTrialists Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtick U, Albrecht M, Chemnitz JM, Theurich S, Skoetz N, Scheid C, von Bergwelt-Baildon M. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD010189.pub2. CD010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alousi AM, Saliba RM, Chen J, et al. A matched controlled analysis of post-transplant cyclophosphamide (CY) versus tacrolimus and mini-dose methotrexate in matched sibling and unrelated donor transplant recipients receiving reduced-intensity conditioning: post-transplant CY is associated with higher rates of acute Gvhd. Blood. 2012;120 4200 abstract 4200. [Google Scholar]
- 27.Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE, Bashey A. Calcineurin inhibitor-free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1828–1834. doi: 10.1016/j.bbmt.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, Fuchs EJ, Jones RJ, Hess AD, Luznik L. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2014;5 doi: 10.1126/scitranslmed.3006960. 211ral57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–693. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peccatori J, Oliveira G, Greco R, Marktel S, Lunghi F, Ciceri F, Bonini C. Tracking T cell dynamics in the first month after Haplo-HSCT with post-transplant cyclophosphamide reveals a predominant contribution of memory stem T cells to the early phase of immune reconstitution. Blood. 2013;122:4615. [Google Scholar]
- 31.Ross D, Jones M, Komanduri K, Levy RB. Antigen and lymphopenia-driven donor T cells are differentially diminished by post-transplantation administration of cyclophosphamide after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1430–1438. doi: 10.1016/j.bbmt.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luznik L, Fuchs EJ, Chen AR, Kaup M, Bright EC, Bolanos-Meade J, Hess AD, Jones RJ. Post-transplantation high-Dose cyclophosphamide (Cy) is effective single agent GvHD prophylaxis that permits prompt immune reconstitution after myeloablative HLA-matched related and unrelated bone marrow transplantation (BMT) Biol Blood Marrow Transplant. 2007;13(2 Suppl. 1):4. [Google Scholar]
- 33.Greco R, Morelli M, Giglio F, et al. Post-transplant cyclophosphamide Haplo-HSCT revised: peripheral blood stem cell graft and sirolimus to enhance immune reconstitution and graft versus leukemia effect in patients with active leukemia. Blood. 2013;122:2118. [Google Scholar]
- 34.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 35.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 36.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroger N. Mini-Midi-Maxi? How to harness the graft-versus-myeloma effect and target molecular remission after allogeneic stem cell transplantation. Leukemia. 2007;21:1851–1858. doi: 10.1038/sj.leu.2404775. [DOI] [PubMed] [Google Scholar]
- 39.Armand P, Kim HT, Ho VT, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14:418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ringden O, Shrestha S, da Silva GT, et al. Effect of acute and chronic GVHD on relapse and survival after reduced-intensity conditioning allogeneic transplantation for myeloma. Bone Marrow Transplant. 2012;47:831–837. doi: 10.1038/bmt.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fergusson JR, Fleming VM, Klenerman P. CD161-expressing human T cells. Front Immunol. 2011;2:36. doi: 10.3389/fimmu.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jukes JP, Jones ND. Immunology in the clinic review series; focus on host responses: invariant natural killer T cell activation following transplantation. Clin Exp Immunol. 2012;167:32–39. doi: 10.1111/j.1365-2249.2011.04500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theurich S, Malcher J, Wennhold K, et al. Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol. 2013;31:e59–e63. doi: 10.1200/JCO.2012.43.6832. [DOI] [PubMed] [Google Scholar]