Abstract
Arabidopsis thaliana has proven a powerful system for developmental genetics, but identification of gametophytic genes with developmental mutants can be complicated by factors such as gametophyte-lethality, functional redundancy, or poor penetrance. These issues are exemplified by the Plant Intracellular Ras-group LRR (PIRL) genes, a family of nine genes encoding a class of leucine-rich repeat proteins structurally related to animal and fungal LRR proteins involved in developmental signaling. Previous analysis of T-DNA insertion mutants showed that two of these genes, PIRL1 and PIRL9, have an essential function in pollen formation but are functionally redundant. Here, we present evidence implicating three more PIRLs in gametophyte development. Scanning electron microscopy revealed that disruption of either PIRL2 or PIRL3 results in a low frequency of pollen morphological abnormalities. In addition, molecular analysis of putative pirl6 insertion mutants indicated that knockout alleles of this gene are not represented in current Arabidopsis mutant populations, suggesting gametophyte lethality may hinder mutant recovery. Consistent with this, available microarray and RNA-seq data have documented strongest PIRL6 expression in developing pollen. Taken together, these results now implicate five PIRLs in gametophyte development. Systematic reverse genetic analysis of this novel LRR family has therefore identified gametophytically active genes that otherwise would likely be missed by forward genetic screens.
Keywords: Arabidopsis, gametophyte, knockout mutant, leucine-rich repeat, pollen, reverse-genetics
1. Introduction
The proper development of gametophytes is critical for plant reproduction. An integrative understanding of gametophyte formation and function requires identification of genes active in the haploid phase and elucidation of their roles and functional relationships. With a complete and well-annotated genome sequence and well-established genetic resources, Arabidopsis thaliana has proven a valuable model system for identifying genes with functions in gametophyte development.
Forward genetic approaches have proven effective for identifying loci with gametophytic functions [1,2,3,4,5]. One strategy for forward genetic screens has been to look for distorted segregation of mutagen-encoded marker phenotypes, such as T-DNA encoded antibiotic resistance, to identify plants with reduced transmission of marker phenotypes [6,7]. Forward genetic screens have also targeted mutants exhibiting abnormal gametophtye development or altered expression of gametophyte-specific reporter constructs [8,9,10,11,12]. Forward screens have been especially successful in pollen, leading to identification of mutants with specific cellular defects in male gametophyte development (e.g., [13,14]). Taking into account these studies and others, Muralla et al. [15] compiled a dataset of 173 loci genetically confirmed to be essential for gametophyte development. Complementing genetic efforts, transcriptome studies have successfully cataloged many gametophytically expressed loci, particularly in pollen, providing a starting point for further functional analyses and a resource for confirming gametophytic expression of genes identified by other methods [16,17,18,19].
A number of factors can complicate identification of gametophyte genes by forward genetics. Some are issues that can hinder any genetic screen. One of these is functional redundancy among gene family members, which can interfere with the expression of a mutant phenotype in homozygous mutants defective in just one locus [20,21,22]. Another factor is incomplete penetrance or poor expressivity of mutant alleles, which can result in subtle or low-frequency defects that can potentially be missed in large-scale genetic screens. These factors can be related because partial redundancy can be one potential cause for incomplete penetrance or variable expressivity; however this need not always be the case, as non-essential genes or genes needed only under certain environmental conditions may also exhibit subtle or variable phenotypes.
With the popularity of insertion mutagenesis in model systems such as Arabidopsis, another factor that complicates gametophyte developmental genetics is the haploid nature of the gametophyte phase, which can prevent recovery of knockout mutant alleles of genes essential in both the male and female gametophyte [4]. Gametophytic developmental genes account for only about 8% of documented Arabidopsis loss-of-function phenotypes [22], and thus may be underrepresented in mutant collections [5,23,24]. Indeed, despite the generation of over 325,000 sequence-tagged Arabidopsis insertion mutant lines, insertion alleles have been persistently elusive for approximately 12% of loci [25]. It is likely that at least a portion of these are gametophyte-essential genes for which bona fide knockout alleles cannot be recovered. However, the exact numbers of such genes is difficult to determine because of their very nature-mutant lethality stands as a barrier to identification by insertion mutation [4,23]. What is clear is that, despite the successful genetic identification of numerous gametophytic developmental genes in recent years, others are likely to go undetected in many mutant screens, due to the various factors described above.
The Arabidopsis PIRL1 and PIRL9 genes are a case in point. PIRLs encode a plant-specific class of leucine-rich repeat proteins related to Ras-interacting LRRs that take part in developmental signaling in animals and fungi [26]. PIRLs are distinct from larger, well-characterized classes of plant LRR proteins such as NBS-LRR pathogen response proteins [27] or LRR-receptor-like kinases, many of which have well-established functions in development [28,29,30]. Characterization of Arabidopsis T-DNA insertion mutants demonstrated that PIRL1 and PIRL9 have an essential role early in pollen development, but are functionally redundant. Plants homozygous for single mutations in either gene do not display obvious defects in pollen development or transmission, but pirl1;pirl9 double mutant microspores undergo consistent developmental arrest and are inviable [31,32]. Thus, detailed reverse genetic analysis revealed a functional context for these two genes whose gametophyte phenotypes would otherwise have remained masked by functional redundancy.
Here, we provide evidence implicating three additional members of this novel LRR family in gametophyte development. We have examined pollen produced by plants homozygous for T-DNA knockout mutations in PIRL2 and PIRL3, and observed a low frequency of abnormal pollen in pirl2 and pirl3 mutants, revealing that these genes act in pollen development, but by themselves do not likely have a large enough effect to be detected in forward genetic screens. Furthermore, analysis of another locus, PIRL6, provided two lines of indirect evidence that this gene may function in gametophytes: (1) transcripts are detected primarily in gametophtyes and reproductive tissues, especially pollen; and (2) plants homozygous for putative knockout alleles still express PIRL6 mRNA, indicating that they are not bona fide knockout mutants and suggesting that gametophyte lethality may be preventing recovery of null mutant alleles. Taken together with prior analysis of PIRL1 and PIRL9, these finding now suggest that five of the nine Arabidopsis PIRLs may be involved in gametophyte development.
2. Results and Discussion
2.1. The Arabidopsis PIRL Family and Insertion Mutants
The PIRLs were originally recognized in the completed Arabidopsis genome sequence as encoding a distinct plant-specific class of LRRs related to animal and fungal Ras-group LRRs [26]. Homologs have since been identified in rice [33]. Based on sequence similarity and intron/exon positions, the nine Arabidopsis PIRLs group into three subfamilies, with PIRL1, PIRL2, PIRL3, and PIRL9 constituting the largest [26]. Candidate T-DNA insertion alleles have been identified for all members of the gene family by PCR screening of original Wisconsin mutant pools [34] or by mining publically available sequence-tagged insertion mutant collections [35,36,37,38]. Allele and polymorphism designations and sources for mutants used in this study are listed in Table 1. With one exception (pirl6-2), inserts were located within transcription units. The pirl1 and pirl9 alleles were previously confirmed to be true knockouts by RT-PCR [31]. pirl2, pirl3 and candidate pirl6 mutants are described below in Section 2.3 and Section 2.4.
Table 1.
Plant Intracellular Ras-group LRR (PIRL) genes and mutant alleles included in this study.
| PIRL (sub-family) | AGI Locus | Mutant alleles in this study | Insert position (nucleotide position) |
|---|---|---|---|
| PIRL1 (I) | At5g05850 | pirl1-1 a | Intron I (+1107) |
| PIRL9 (I) | At3g11330 | pirl9-1 a | Exon I (+357) |
| PIRL2 (I) | At3g26500 | pirl2-1 a | Exon I (+526) |
| pirl2-2 [SALK_138743] b | Exon III (+1147) | ||
| PIRL3 (I) | At1g12970 | pirl3-1 a | Exon III (+1640) |
| pirl3-2 [SALK_033703] b | Exon I (+514) | ||
| PIRL6 (II) | At2g19330 | pirl6-1 [SAIL574A05] c; | Exon I (+182) |
| pirl6-2 [WISCDSLOX393-396L14] c | Promoter region (-272) |
2.2. PIRL1 and PIRL9 Illustrate Functional Redundancy in Pollen
Disruption of both PIRL1 and PIRL9 together results in pollen lethality [31]. Figure 1 illustrates developmental arrest in pollen produced by pirl1/PIRL1;pirl9 plants, which segregates 50% for the double mutant genotype. Mature pollen from dehisced anthers was approximately 50% inviable based on Alexander’s staining, in which inviable grains appear shrunken and blue-green (Figure 1A). Nomarski DIC microscopy of developing anthers showed that segregating pirl1;pirl9 microspores were not distinguishable in tetrads just after meiosis (Figure 1B), but became clearly evident later, first appearing in mitotic phase anthers as smaller, arrested grains and later appearing shrunken and inviable (Figure 1C,D). These observations are consistent with prior genetic and fluorescence microscopy results that demonstrated that PIRL1 and PIRL9 act post-meiosis to affect pollen formation [31,32].
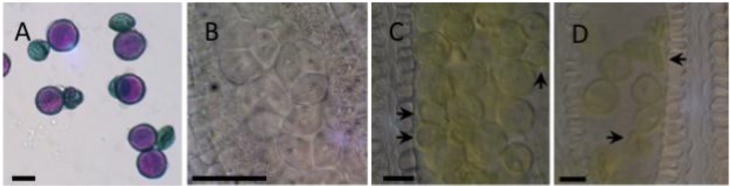
Figure 1.
Developmental arrest in pirl1, pirl9 pollen. (A) Alexander-stained dehisced pollen from a pirl1/PIRL1, pirl9 plant, segregating at 50% for the pirl1, pirl9 mutant phenotype. Purple stain indicates viable pollen; (B) Nomarski DIC microscopy of post-meiotic tetrads in a pirl1/PIRL1, pirl9 anther in which double mutant microspores are segregating but cannot be clearly identified; (C) A pirl1/PIRL1, pirl9 anther with mitotic stage pollen; arrows indicate developmentally arrested pollen; (D) A mature pirl1/PIRL1, pirl9 anther; arrows indicate shrunken inviable double mutants segregating within the pollen population. Scale bars = 20 µm
2.3. PIRL2 and PIRL3 Affect Pollen Morphology
To determine if other members of PIRL sub-family I are involved in pollen development, we identified two independent T-DNA insertion mutants for each of PIRL2 and PIRL3 (Figure 2). T-DNA inserts in the pirl2-1, pirl2-2, pirl3-1 and pirl3-2 alleles were located within exon sequences at positions upstream of or within the region encoding the highly conserved PIRL LRR domain (Figure 2A; see Table 1 for nucleotide positions). The knockout status for all of these lines was verified by RT-PCR on homozygous mutants, using gene specific primers that targeted the full-length PIRL2 or PIRL3 ORFs (Figure 2B). The exon positions of the pirl2 and pirl3 T-DNA inserts make it highly unlikely these alleles give rise to undetected truncated mRNAs with any residual functional potential, especially given their disruption of the conserved LRR domain, the most prominent feature of the PIRL gene products [26].
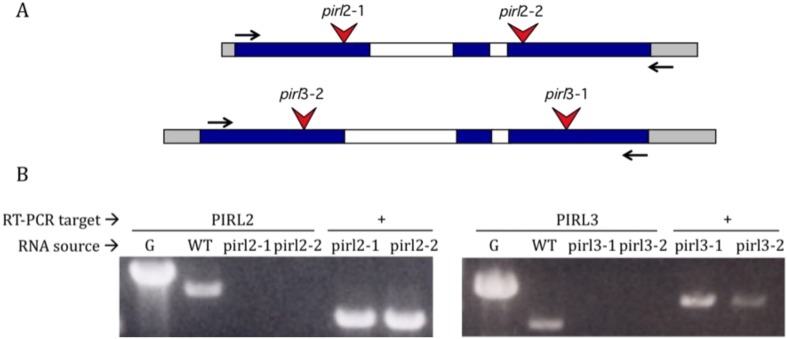
Figure 2.
PIRL2 and PIRL3 T-DNA insertion alleles and knockout status. (A) PIRL2 and PIRL3 gene maps, with exons shaded and introns shown in white; light gray shading indicates UTR regions. Positions of T-DNA insertions in pirl2-1, pirl2-2, pirl3-1, pirl3-2 alleles are indicated by red arrows. Horizontal arrows indicate positions of RT-PCR primers used for part B; (B) Confirmation of knockout status in pirl2 and pirl3 DNA insertion lines. RT-PCR was carried out with gene-specific primers on total RNA isolated from flowers or leaves of homozygous mutant (pirl2-1, pirl2-2, pirl3-1, or pirl3-2, as indicated) or wild-type (WT) controls. G, PIRL gene (with introns) amplified from genomic DNA.(+) lanes contain RT-PCR products for PIRL9 or PIRL1 amplified from pirl2 or pirl3 mutants, respectively, as positive controls for RNA quality and RT-PCR efficacy.
We examined pollen produced by pirl2 and pirl3 mutant homozygotes. Dehisced pollen were collected and subjected to scanning electron microscopy (SEM). Abnormal pollen was observed both in pirl2 and in pirl3 mutant lines (Figure 3). Abnormal pollen varied from shrunken to large, irregular, and angular in shape. This contrasts to what was observed for pirl1;pirl9 pollen, which were consistently severely shrunken and inviable (Figure 1A and [31]). Penetrance was determined by light microscopy of Alexander-stained pollen from multiple plants from each mutant line and from wild-type controls. The pirl2 and pirl3 phenotypes were poorly penetrant but were reproducibly and consistently observed for both mutant alleles of each gene (Figure 3F). The penetrance values in Figure 3 are conservative estimates because they were derived using light microscopy, which cannot detect modest morphological defects due to the fact that pollen are hydrated and viewed at much lower magnification than with SEM. However, this method had the advantage of allowing for much larger sample sizes than did SEM.
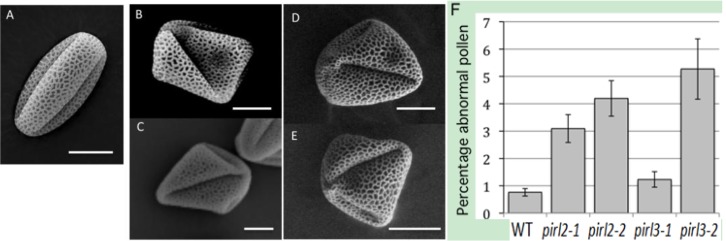
Figure 3.
Mutations in pirl2 or pirl3 result in abnormal pollen. Dehisced pollen produced by wild-type Arabidopsis (A), or by pirl2 (B,C) or pirl3 (D,E) homozygotes was viewed by scanning electron microscopy. Scale bars, 10 µm. (F) Percentages of abnormal pollen estimated by light microscopy. Values are means derived from pollen obtained from four to nine plants of each genotype; standard errors are shown. Mean values and total sample sizes: Wild type (WT), 0.76% (n = 2826); pirl2-1, 3.09% (n = 4913); pirl2-2, 4.20% (n = 2872); pirl3-1, 1.23% (n = 3588); pirl 3-2, 5.27% (n = 1441).
Other features of the PIRL2 and PIRL3 genes are consistent with a role in pollen. While both genes are broadly expressed throughout plant development, based on RT-PCR [26], available microarray and RNA-seq data show PIRL3 expression is at its highest level in pollen [19,40,41]. Microarray data for PIRL2 are not available because it is unfortunately among the loci not represented on most Arabidopsis microarrays. Recent RNA-seq data suggest a very low level of PIRL2 expression in dehisced pollen, however earlier stages of pollen development that would be the most likely to affect pollen morphology were not tested [19]; major changes in the pollen transcriptome occur after pollen morphogenesis as pollen prepare for dehiscence and later germination [17]. Notably, based on sequence, PIRL2 appears to be an ortholog of SF17, a sunflower gene expressed predominantly in pollen [42].
The morphological defects observed in pirl2 and pirl3 pollen strongly suggest that these genes act after meiosis, in microspores or pollen. This is based on their relatively robust appearance in comparison to pirl1, pirl9 microspores, which also act after meiosis but arrest just prior to pollen mitosis I. Hypothetically, crossing pirl2 and pirl3 into a qrt1 background could confirm post-meiotic activity of pirl2 and pirl3 [3]; however, because of the low penetrance, phenotype segregation within qrt1 tetrads would not provide meaningful results, as tetrads would rarely feature more than one defective pollen grain.
While the pirl2 and pirl3 mutations affect pollen they apparently do not disrupt development sufficiently to affect efficacy of transmission. Therefore, these genes would not be classified as essential [4], and their involvement in pollen development would not likely be detectable in forward-genetic screens relying on segregation distortion. We considered the possibility that pirl2 and pirl3 phenotypes might be largely masked by redundancy in this gene pair, as was the case for pirl1 and pirl9 [31]. However, this does not appear to be the case, as Alexander’s staining of pirl2;pirl3 double mutant pollen indicated a phenotype frequency no more severe than that observed for the single mutants.
2.4. Analysis of Putative pirl6 Knockout Mutants and PIRL6 mRNA Expression.
We extended reverse genetic analysis to PIRL subfamily II by identifying and characterizing putative knockout mutations in PIRL6. We identified only two candidate insertion mutations in this locus, which we labeled pirl6-1 (SAIL574A05) and pirl6-2 (WISCDSLOX393-396L14). Analysis of junction sites confirmed the position of the insertions in the first exon and the presumed promoter region, respectively (Figure 4A). However, RT-PCR of RNA from flowers of mutant homozygotes clearly indicated the expression of intact PIRL6 transcripts, demonstrating that neither allele was a null mutation (Figure 4B). In the case of pirl6-2, the residual gene expression may be explained by the position of the insertion outside the transcription unit. The exact reason for PIRL6 transcript expression in pirl6-1 cannot easily be determined, but it is likely that this line contains some type of T-DNA associated rearrangement or duplication, such that an intact copy of the locus remains, possibly at a different chromosomal location. Such duplications and rearrangements are not uncommon in T-DNA mutagenized populations [25,43,44].
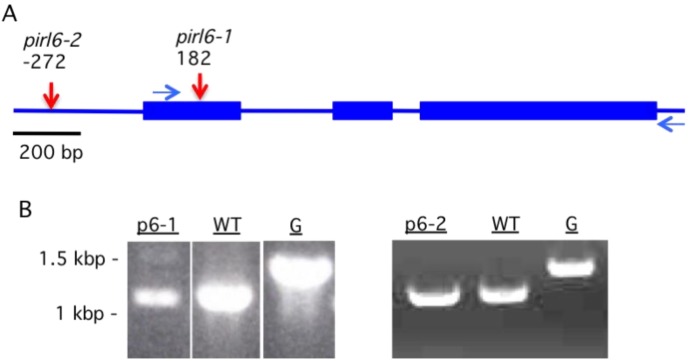
Figure 4.
PIRL6 gene structure, T-DNA insertion alleles, and knockout status. (A) The PIRL6 locus (At2g19330), with exons shown as blue rectangles, introns and flanking regions as lines. Positions of T-DNA insertions in putative knockout mutants pirl6-1 (SAIL574A05) and pirl6-2 (WISCDSLOX393-396L14) are indicated by arrows. Horizontal arrows indicate positions of RT-PCR primers used for part B; (B) Detection of PIRL6 transcripts in pirl6-1 and pirl6-2 putative T-DNA knockout lines. RT-PCR was carried out with PIRL6-specific primers on total RNA isolated from flowers of homozygous mutant (p6-) or wild-type (WT) plants. G, PIRL6 gene (with introns) amplified from genomic DNA. RT-PCR specificity was confirmed by sequencing of gel purified reaction products.
The presence of only two candidate pirl6 knockout alleles, and the finding that neither is really a bona fide knockout, suggest that the gene could be gametophyte-essential. We acknowledge that this is negative evidence, and we cannot rule out that the dearth of pirl6 candidate alleles is just due to chance. For example, the gene could lie in a chromosomal region not prone to Agrobacterial T-DNA insertion. However, we investigated the neighboring locus, At2g19340, which lies immediately adjacent to PIRL6, and identified six candidate insertion lines [39]. Thus, this region of chromosome 2 is readily accessible to T-DNA insertion mutagenesis. It therefore appears that some other feature of PIRL6, such as an essential gametophyte function, may be the reason knockout alleles have not been generated.
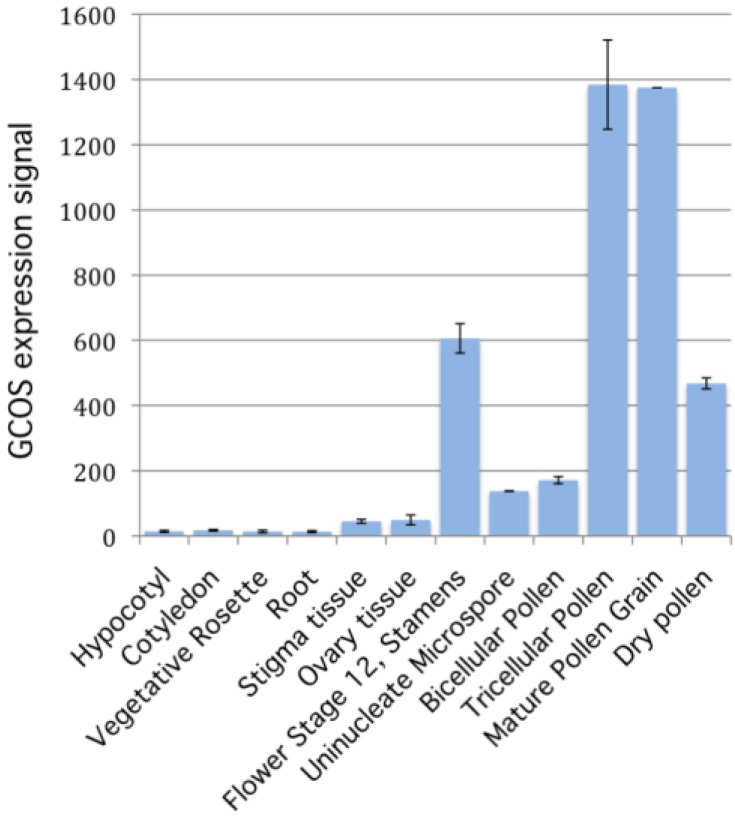
To determine if PIRL6 expression was consistent with a gametophyte function, we investigated PIRL6 transcript levels in existing microarray databases and a pollen RNA-seq dataset. Our aim was not to determine whether PIRL6 expression is gametophyte specific, because essential genes need not exhibit expression at one specific location or developmental stage. Rather, we sought confirmation that PIRL6 was expressed in male and female gametophytes—a prerequisite for gametophytic function. Multiple transcriptome studies accessed via both the eFP browser [41] and Genevestigator [45,46] suggested that PIRL6 transcripts are indeed expressed primarily in male and female reproductive structures and gametophytes (Figure 5; Table 2). Expression levels were very low throughout vegetative development, but were high in microspores and developing pollen, strongly suggesting a function in the male gametophyte. These microarray findings were recently confirmed by a large-scale RNA-seq study comparing mature pollen and seedling transcriptomes [19]. Female reproductive organs also expressed PIRL6 above background levels seen in vegetative tissues, although expression was much lower than in pollen. Taken together with the negative evidence from the knockout analysis described above, these results are consistent with a gametophytic role for PIRL6. Direct genetic evidence for PIRL6 gametophyte function will require targeted gametophyte-specific gene knockdown in transgenic plants, tilling for non-lethal point mutations, or fortuitous identification of less severe mutant alleles in a forward genetic screen.
Figure 5.
Detection of PIRL6 transcripts in reproductive tissues, gametophytes, and representative vegetative organs. Data originated from multiple published microarray experiments (see Table 2) and was accessed via the Arabidopsis eFP browser [41].
Table 2.
Multiple microarray studies as well as RNA-seq document PIRL6 expression in gametophytes and reproductive organs.
| Expression database or dataset | Primary reference | |
|---|---|---|
| Microspores, pollen, stamens | eFP browser, developmental map [41] | [47] |
| eFP browser, tissue specific map | [17] | |
| Genevestigator [46] | [16] | |
| Genevestigator | [48] | |
| Pollen RNA-seq dataset | [19] | |
| Ovaries, ovules, carpels | Genevestigator | [49] |
| eFP browser, developmental map | [47] | |
| eFP browser, tissue specific map | [50] |
3. Experimental
3.1. Mutant Identification and Confirmation of T-DNA Insert Position
Seeds for prospective mutant lines were obtained from the Arabidopsis stock center at Ohio State University. Plants were grown in climate-controlled growth chambers under a 16 h light/8 hours dark regime as previously described [44]. pirl1-1, pirl9-1, pirl2-1, and pirl3-1 alleles were identified by manual PCR screening of T-DNA mutagenized mutant pools from the Wisconsin collection [34], and insert position and segregation confirmed by PCR of T-DNA:plant DNA junction fragments as described by Forsthoefel et al. [26]. pirl2-2 and pirl3-2 were identified in the SALK mutant collection [35], and T-DNA insert positions and allele segregation were confirmed using the forward and reverse gene specific primers listed below in combination with T-DNA-specific primers specific for the SALK collection T-DNA border sequences [35]. All mutant lines were subjected to serial backcrossing to wild type prior to phenotype analysis. Candidate pirl6 insertion mutants were identified as At2g19330 polymorphisms at TAIR [39]. In combination with insert-specific border primers appropriate for either the SAIL mutant collection [36] (primer LB3, for pirl6-1) or the WISCDSLOX collection [37] (primer p745, for pirl6-2), PIRL6 locus-specific primers were used for genomic DNA analyses to assess the presence of inserts and identify homozygous individuals: forward (F), TAGTGAGAACAATCCTTTTGATTTGGTCA; reverse (R), AGAAATAGTCAAATA-GGGACCTGGTGCAA. In the absence of a pirl6 phenotype and with residual gene activity in putative knockout lines, homozygotes lines were unambiguously identified by presence of the insert in 100% of F1 progeny from self-fertilized individuals.
Knockout status of putative homozygous mutants was determined by using RT-PCR on total flower RNA to detect the presence or lack of target mRNA. RNA preparations and RT-PCR reactions were carried out as described by Cushing et al. [51]. Gene specific forward and reverse primer combinations targeted full-length transcripts: PIRL2 F, GCCGCCGTCGCCGCCGTCTAT GGCCGCGC; PIRL2 R, CGGAGTTTGTTCAGCTGG; PIRL3 F, CTCTCCTACGTCCTCCACCA; PIRL3 R, GCTTCTTAGCTGCACCACCA; PIRL6 F, ATGCGAGGAGGCATATCATC; PIRL6 R, CGACGTGGAGAGAACATTCC. PIRL gene sequences are sufficiently divergent to allow design of gene-specific primers without cross-reactivity to other gene family members [26]. Specificity for all RT-PCR primer pairs was verified by prior sequencing of gel-purified RT-PCR reaction products. Products were isolated with the QIAquick gel extraction kit (Qiagen, Valencia, CA, USA) and sequenced by the by the University of Arizona DNA sequencing core facility (Tucson, AZ, USA).
3.2 Microscopy
Anthers of selected developmental stages [52] were microdissected from developing buds and immediately cleared on slides in Hoyer’s solution (100 g chloral hydrate, 5 mL glycerin, 7.5 g gum Arabic, 30 mL ddH2O) for 15 min. Cover slips were added and samples were viewed under an Olympus BX60 microscope equipped with Nomarski DIC optics. For light microscopy pollen were stained and viewed using the procedure described in Forsthoefel et al. [31]. For penetrance determination, samples were obtained from 4–9 plants of each genotype. The percentage of defective pollen was determined for each sample; mean percentages for each genotype were plotted along with standard error. Total N values are provided in Figure 3.
For scanning electron microscopy, dehisced pollen from plants homozygous for the indicated genotypes were transferred by lightly touching flowers onto carbon tape adhered to a sample stub. Stubs were air-dried for 24–72 hours and either viewed directly under high vacuum conditions at 1 KV, or gold coated with a Cressington 108 sputter coater for 20 seconds and viewed under high vacuum settings at 12.5–15 KV, using an FEI Quanta200 scanning electron microscope.
4. Conclusions
Systematic reverse genetic analysis has now implicated five of the nine Arabidopsis PIRLs in gametophyte development. For differing reasons, it is unlikely that any of these genes would have been implicated in gametophyte development by forward genetics. In the cases of PIRL1 and PIRL9, essential pollen function was masked by almost complete redundancy, while for both PIRL2 and PIRL3, loss of function under laboratory growth conditions does not generate a phenotype of adequate scope to be detected in a forward screen. In the case of PIRL6, we can offer only indirect evidence for a gametophytic role: molecular characterization of available insertion mutants reveals that bona fide knockouts are not present in current sequence-tagged Arabidopsis mutant populations, and flower specific mRNA expression and high pollen expression support a possible gametophyte function. Now that the biological contexts of these PIRLs’ functions have been defined, future work can focus on the cellular and biochemical roles of these novel, previously uncharacterized LRR genes, to better understand their contributions to plant reproductive development.
The value of reverse genetic strategies for identifying developmental genes were recognized by Berg et al. [23], who suggested that some genes with functions in reproductive development will escape identification, even as Arabidopsis mutagenesis efforts approach genome saturation. The results reported here support that point and underscore the value of detailed reverse genetics for achieving a full understanding of the genetic components of gametophyte formation and function.
Acknowledgments
This work was supported by NSF award 0616166 to D.M.V. Whitman College’s SEM facility was made possible by NSF MRI Grant 0922978. K.A.K and R.R. were supported in part by S. A. Abshire awards from Whitman College.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.McCormick S. Control of male gametophyte development. Plant Cell. 2004;16:S142–S153. doi: 10.1105/tpc.016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadegari R., Drews G.N. Female gametophyte development. Plant Cell. 2004;16:S133–S141. doi: 10.1105/tpc.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson-Brousseau S.A., McCormick S. A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J. 2004;39:761–775. doi: 10.1111/j.1365-313X.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- 4.Meinke D., Muralla R., Sweeney C., Dickerman A. Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci. 2008;13:483–491. doi: 10.1016/j.tplants.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bolle C., Schneider A., Leister D. Perspectives on systematic analyses of gene function in Arabidopsis thaliana: New tools, topics and trends. Curr. Genomics. 2011;12:1–14. doi: 10.2174/138920211794520187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann K.A., Coury D.A., Christianson M.L. Exceptional segregation of a selectable marker (kanr) in Arabidopsis identifies genes important for gametophytic growth and development. Genetics. 1997;147:1411–1422. doi: 10.1093/genetics/147.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howden R., Park S.K., Moore J.M., Orme J., Grossniklaus U., Twell D. Selection of t-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics. 1998;149:621–631. doi: 10.1093/genetics/149.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen C.A., Subramanian S., Drews G.N. Identification of gametophytic mutations affecting female gametophyte development in arabidopsis. Dev. Biol. 1998;202:136–151. doi: 10.1006/dbio.1998.8980. [DOI] [PubMed] [Google Scholar]
- 9.Procissi A., de Laissardiere S., Ferault M., Vezon D., Pelletier G., Bonhomme S. Five gametophytic mutations affecting pollen development and pollen tube growth in Arabidopsis thaliana. Genetics. 2001;158:1773–1783. doi: 10.1093/genetics/158.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson M.A., von Besser K., Zhou Q., Smith E., Aux G., Patton D., Levin J.Z., Preuss D. Arabidopsis hapless mutations define essential gametophytic functions. Genetics. 2004;168:971–982. doi: 10.1534/genetics.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagnussat G.C., Yu H.J., Ngo Q.A., Rajani S., Mayalagu S., Johnson C.S., Capron A., Xie L.F., Ye D., Sundaresan V. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development. 2005;132:603–614. doi: 10.1242/dev.01595. [DOI] [PubMed] [Google Scholar]
- 12.Boavida L.C., Shuai B., Yu H.J., Pagnussat G.C., Sundaresan V., McCormick S. A collection of Ds insertional mutants associated with defects in male gametophyte development and function in Arabidopsis thaliana. Genetics. 2009;181:1369–1385. doi: 10.1534/genetics.108.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalanne E., Twell D. Genetic control of male germ unit organization in Arabidopsis. Plant Physiol. 2002;129:865–875. doi: 10.1104/pp.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durbarry A., Vizir I., Twell D. Male germ line development in Arabidopsis. Duo pollen mutants reveal gametophytic regulators of generative cell cycle progression. Plant Physiol. 2005;137:297–307. doi: 10.1104/pp.104.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muralla R., Lloyd J., Meinke D. Molecular foundations of reproductive lethality in Arabidopsis thaliana. PLoS One. 2011;6:e28398. doi: 10.1371/journal.pone.0028398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pina C., Pinto F., Feijo J.A., Becker J.D. Gene family analysis of the arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol. 2005;138:744–756. doi: 10.1104/pp.104.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honys D., Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Y., Leydon A.R., Manziello A., Pandey R., Mount D., Denic S., Vasic B., Johnson M.A., Palanivelu R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 2009;5:e1000621. doi: 10.1371/journal.pgen.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loraine A., McCormick S., Estrada A., Patel K., Qin P. High-throughput sequencing of Arabidopsis thaliana pollen cdna uncovers novel transcription and alternative splicing. Plant Physiol. 2013 doi: 10.1104/pp.112.211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouche N., Bouchez D. Arabidopsis gene knockout: Phenotypes wanted. Curr. Opin. Plant Biol. 2001;4:111–117. doi: 10.1016/S1369-5266(00)00145-X. [DOI] [PubMed] [Google Scholar]
- 21.Cutler S., McCourt P. Dude, where’s my phenotype? Dealing with redundancy in signaling networks. Plant Physiol. 2005;138:558–559. doi: 10.1104/pp.104.900152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd J., Meinke D. A comprehensive dataset of genes with a loss-of-function mutant phenotype in Arabidopsis. Plant Physiol. 2012;158:1115–1129. doi: 10.1104/pp.111.192393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg M., Rogers R., Muralla R., Meinke D. Requirement of aminoacyl-tRNA synthetases for gametogenesis and embryo development in Arabidopsis. Plant J. 2005;44:866–878. doi: 10.1111/j.1365-313X.2005.02580.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonhomme S., Horlow C., Vezon D., de Laissardiere S., Guyon A., Ferault M., Marchand M., Bechtold N., Pelletier G. T-DNA mediated disruption of essential gametophytic genes in Arabidopsis is unexpectedly rare and cannot be inferred from segregation distortion alone. Mol. Gen. Genet. 1998;260:444–452. doi: 10.1007/s004380050915. [DOI] [PubMed] [Google Scholar]
- 25.O’Malley R.C., Ecker J.R. Linking genotype to phenotype using the arabidopsis unimutant collection. Plant J. 2010;61:928–940. doi: 10.1111/j.1365-313X.2010.04119.x. [DOI] [PubMed] [Google Scholar]
- 26.Forsthoefel N.R., Cutler K., Port M.D., Yamamoto T., Vernon D.M. Pirls: A novel class of plant intracellular leucine rich repeat proteins. Plant Cell Physiol. 2005;46:913–922. doi: 10.1093/pcp/pci097. [DOI] [PubMed] [Google Scholar]
- 27.McHale L., Tan X., Koehl P., Michelmore R.W. Plant NBS-LRR proteins: Adaptable guards. Genome Biol. 2006;7:212. doi: 10.1186/gb-2006-7-4-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nodine M.D., Bryan A.C., Racolta A., Jerosky K.V., Tax F.E. A few standing for many: Embryo receptor-like kinases. Trends Plant Sci. 2011;16:211–217. doi: 10.1016/j.tplants.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Morillo S.A., Tax F.E. Functional analysis of receptor-like kinases in monocots and dicots. Curr. Opin. Plant Biol. 2006;9:460–469. doi: 10.1016/j.pbi.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 30.De Smet I., Voss U., Jürgens G., Beeckman T. Receptor-like kinases shape the plant. Nat. Cell Biol. 2009;11:1166–1173. doi: 10.1038/ncb1009-1166. [DOI] [PubMed] [Google Scholar]
- 31.Forsthoefel N.R., Dao T.P., Vernon D.M. PIRL1 and PIRL9, encoding members of a novel family of plant leucine-rich repeat proteins, are essential for differentiation of microspores into pollen. Planta. 2010;232:1101–1114. doi: 10.1007/s00425-010-1242-6. [DOI] [PubMed] [Google Scholar]
- 32.Forsthoefel N.R., Vernon D.M. Effect of sporophytic PIRL9 genotype on post-meiotic expression of the Arabidopsis pirl1;pirl9 mutant pollen phenotype. Planta. 2011;233:423–431. doi: 10.1007/s00425-010-1324-5. [DOI] [PubMed] [Google Scholar]
- 33.You C., Dai X., Li X., Wang L., Chen G., Xiao J., Wu C. Molecular characterization, expression pattern, and functional analysis of the osirl gene family encoding intracellular ras-group-related LRR proteins in rice. Plant Mol. Biol. 2010;74:617–629. doi: 10.1007/s11103-010-9704-6. [DOI] [PubMed] [Google Scholar]
- 34.Sussman M.R., Amasino R.M., Young J.C., Krysan P.J., Austin-Phillips S. The Arabidopsis knockout facility at the University of Wisconsin-Madison. Plant Physiol. 2000;124:1465–1467. doi: 10.1104/pp.124.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso J.M., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 36.Sessions A., Burke E., Presting G., Aux G., McElver J., Patton D., Dietrich B., Ho P., Bacwaden J., Ko C., et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woody S.T., Austin-Phillips S., Amasino R.M., Krysan P.J. The wiscdslox t-DNA collection: An Arabidopsis community resource generated by using an improved high-throughput t-DNA sequencing pipeline. J. Plant Res. 2007;120:157–165. doi: 10.1007/s10265-006-0048-x. [DOI] [PubMed] [Google Scholar]
- 38.Rhee S.Y., Beavis W., Berardini T.Z., Chen G., Dixon D., Doyle A., Garcia-Hernandez M., Huala E., Lander G., Montoya M., et al. The Arabidopsis information resource (TAIR): A model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res. 2003;31:224–228. doi: 10.1093/nar/gkg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Arabidopsis information resource. [(accessed on 10 December 2012)]. Available online: http://www.arabidopsis.org.
- 40.Winter D., Vinegar B., Nahal H., Ammar R., Wilson G.V., Provart N.J. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Arabidopsis eFP browser. [(accessed on 1 March 2013)]. Available online: http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi/
- 42.Reddy J.T., Dudareva N., Evrard J.-L., Krauter R., Steinmetz A., Pillay D.T.N. A pollen-specificgene from sunflower encodes a member of the leucine-rich-repeat protein superfamily. Plant Sci. 1995;111:81–93. doi: 10.1016/0168-9452(95)04233-K. [DOI] [Google Scholar]
- 43.Clark K.A., Krysan P.J. Chromosomal translocations are a common phenomenon in Arabidopsis thaliana t-DNA insertion lines. Plant J. 2010;64:990–1001. doi: 10.1111/j.1365-313X.2010.04386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tax F.E., Vernon D.M. T-DNA-associated duplication/translocations in Arabidopsis. Implications for mutant analysis and functional genomics. Plant Physiol. 2001;126:1527–1538. doi: 10.1104/pp.126.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genevestigator. [(accessed on 3 March 2013)]. Available online: https://www.genevestigator.com/gv/plant.jsp/
- 47.Schmid M., Davison T.S., Henz S.R., Pape U.J., Demar M., Vingron M., Scholkopf B., Weigel D., Lohmann J.U. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 48.Mandaokar A., Thines B., Shin B., Lange B.M., Choi G., Koo Y.J., Yoo Y.J., Choi Y.D., Choi G., Browse J. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006;46:984–1008. doi: 10.1111/j.1365-313X.2006.02756.x. [DOI] [PubMed] [Google Scholar]
- 49.Boavida L.C., Borges F., Becker J.D., Feijo J.A. Whole genome analysis of gene expression reveals coordinated activation of signaling and metabolic pathways during pollen-pistil interactions in Arabidopsis. Plant Physiol. 2011;155:2066–2080. doi: 10.1104/pp.110.169813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swanson R., Clark T., Preuss D. Expression profiling of Arabidopsis stigma tissue identifies stigma-specific genes. Sex. Plant. Reprod. 2005;18:163–171. doi: 10.1007/s00497-005-0009-x. [DOI] [Google Scholar]
- 51.Cushing D.A., Forsthoefel N.R., Gestaut D.R., Vernon D.M. Arabidopsis emb175 and other ppr knockout mutants reveal essential roles for PPR proteins in plant embryogenesis. Planta. 2005;222:424–436. doi: 10.1007/s00425-004-1452-x. [DOI] [PubMed] [Google Scholar]
- 52.Sanders P.M., Bui A.Q., Weterings K., McIntire K.N., Hsu Y.C., Lee P.Y., Truong M.T., Beals T.P., Goldberg R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant. Reprod. 1999;11:297–322. doi: 10.1007/s004970050158. [DOI] [Google Scholar]