Abstract
The vacuole is by far the largest intracellular Ca2+ store in most plant cells. Here, the current knowledge about the molecular mechanisms of vacuolar Ca2+ release and Ca2+ uptake is summarized, and how different vacuolar Ca2+ channels and Ca2+ pumps may contribute to Ca2+ signaling in plant cells is discussed. To provide a phylogenetic perspective, the distribution of potential vacuolar Ca2+ transporters is compared for different clades of photosynthetic eukaryotes. There are several candidates for vacuolar Ca2+ channels that could elicit cytosolic [Ca2+] transients. Typical second messengers, such as InsP3 and cADPR, seem to trigger vacuolar Ca2+ release, but the molecular mechanism of this Ca2+ release still awaits elucidation. Some vacuolar Ca2+ channels have been identified on a molecular level, the voltage-dependent SV/TPC1 channel, and recently two cyclic-nucleotide-gated cation channels. However, their function in Ca2+ signaling still has to be demonstrated. Ca2+ pumps in addition to establishing long-term Ca2+ homeostasis can shape cytosolic [Ca2+] transients by limiting their amplitude and duration, and may thus affect Ca2+ signaling.
Keywords: vacuole, ligand-gated Ca2+ channel, SV channel, cyclic nucleotide-gated channel, Ca2+:H+ exchanger, P-type Ca2+-ATPase
1. The Vacuole—A Huge Intracellular Ca2+ Store
The large central vacuole of a typical mature plant cell is by far the largest intracellular Ca2+ store. It is reasonable to assume that this huge Ca2+ store contributes to changes in cytosolic free Ca2+ concentrations, [Ca2+]cyt, during Ca2+-mediated intracellular signaling. Usually, the volume of the large central vacuole is about an order of magnitude larger compared to the volume of the cytosol, and the free Ca2+ concentration is about three orders of magnitude higher inside the vacuole, compared to the cytosol. The cytosolic free Ca2+ concentration at rest, as recorded with ion-selective microelectrodes or fluorescent dyes, is around 200 nM, and may increase to low micromolar concentrations during transient [Ca2+]cyt increase [1,2,3,4]. The few published values for vacuolar free Ca2+ concentrations, based on measurements with ion-selective microelectrodes, range from 2.3 and 1.5 mM for rhizoids of the liverwort Riccia fluitans and root corpus of corn (Zea mays), respectively [1], to 200 μM for red beet (Beta vulgaris) taproots [5], and the green alga Eremosphaera viridis [2]. Total vacuolar Ca2+ concentrations can be much higher, more than 60 mM in mesophyll cells of Eudicots [6]. Very little is known about the dynamics of vacuolar free Ca2+ concentrations, but the observation that the vacuolar SV channel (see below) is regulated by physiological concentrations of vacuolar Ca2+ [7,8] indicates that vacuolar free Ca2+ concentrations may change and that these changes probably have physiological effects.
About a thousand-fold gradient in free Ca2+ concentration plus an electric potential difference across the vacuolar membrane in the range of 0 to −30 mV [2,9] (negative on the cytosolic side [10]) add up to an electrochemical driving force of roughly −100 mV, pushing Ca2+ from the vacuole towards the cytosol. As a result, Ca2+ release from the vacuole into the cytosol is passive and can be mediated by ion channels, while Ca2+ uptake from the cytosol into the vacuole requires energy input and is performed by either ATPases or H+-antiporters (Figure 1). The latter use the existing pH gradient of about two pH units [2,11] across the vacuolar membrane for secondary active transport. The opening of vacuolar Ca2+ channels increases [Ca2+]cyt, making these channels good candidates to start or amplify a cytosolic Ca2+ signal. This review will summarize our current knowledge of vacuolar Ca2+ channels and their involvement in Ca2+ signaling. Vacuolar Ca2+-ATPases and Ca2+:H+ exchangers maintain the Ca2+ concentration gradient between cytosol and vacuole, and can contribute to restoring low [Ca2+]cyt. The last part of this review will summarize recently emerging knowledge about how vacuolar Ca2+ pumps may shape cytosolic [Ca2+] transients. For further information the reader is referred to a number of excellent reviews that have been published recently, summarizing vacuolar ion transport [12,13,14], plant Ca2+ transporter [15,16,17,18,19], organellar Ca2+ transport [20], or vacuolar Ca2+ transport [21,22].
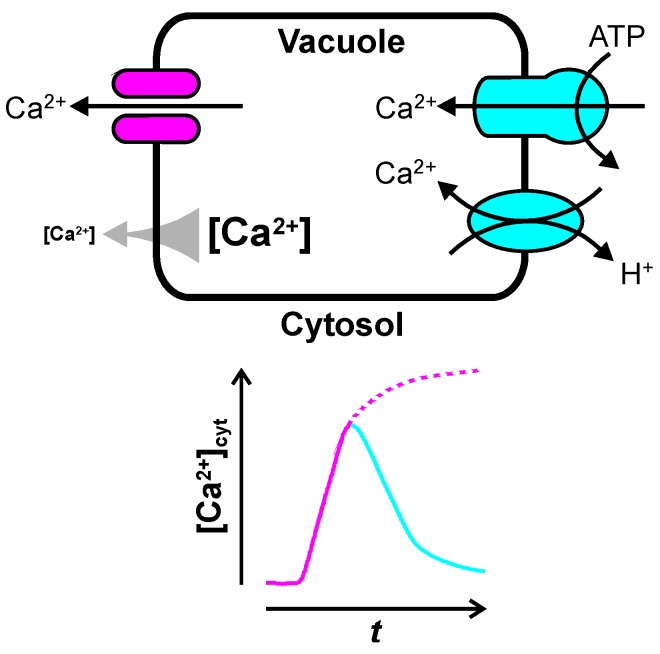
Figure 1.
How the vacuole may shape transient changes in cytosolic free Ca2+ concentration, [Ca2+]cyt. A large (≈100 mV) electrochemical potential gradient (gray arrow) pushes Ca2+ from the vacuole into the cytosol. As a result, transient opening of vacuolar Ca2+ channels (in magenta) elevates [Ca2+]cyt (magenta line). Ca2+ is pumped back into the vacuole by Ca2+-ATPases and Ca2+:H+ exchangers (in cyan), resulting in a decrease of [Ca2+]cyt back to resting levels (cyan line).
2. Ca2+ Release from the Vacuole
Vacuolar Ca2+ release seems a well-established step of intracellular signal transduction in plant cells. In plant physiology textbooks for example, ABA-induced stomatal closure is usually depicted as being mediated by the opening of vacuolar Ca2+ channels. However, there is a lack of solid evidence for a physiological stimulus triggering release of Ca2+ from the large central vacuole of an intact cell. ABA-induced increase in guard cell [Ca2+]cyt, visualized by digital ratio imaging of fluorescent Ca2+ indicators, has been reported to be more pronounced close to the vacuole [23,24,25]. Similar reports exist for transient increases in guard cell [Ca2+]cyt triggered by high external Ca2+ concentrations [26] or by plasma membrane hyperpolarization [27]. Yet as some of these reports mention, better spatial and temporal resolution is required before definitive statements about the contributing Ca2+ stores can be made. Even though the experimental techniques for intracellular Ca2+ imaging have improved in recent years, no progress has been reported in imaging vacuolar Ca2+ release.
Another approach to detect vacuolar Ca2+ release was to record luminescence from transgenic Arabidopsis thaliana seedlings expressing the Ca2+-sensitive photoprotein aequorin either in the cytosol or anchored to the cytosolic side of the vacuolar membrane. [Ca2+]cyt spikes were induced by cooling [28] or addition of mannitol [29], and slightly different kinetics in luminescence of cytosolic versus vacuole-bound aequorin were interpreted as indication for vacuolar Ca2+ release. While these recordings are compatible with vacuolar Ca2+ release, alternative explanations cannot be ruled out. Only 24% of aequorin activity could be detected on isolated vacuoles [28], raising the possibility that other subcellular domains than the “vacuolar microdomain” contributed to recorded signals. Ca2+-dependent luminescence was recorded from intact A. thaliana seedlings, six to seven days old, and different cell types may have contributed to recorded signals to a varying degree in different transgenic lines. Repeating the pioneering work with aequorin using other recombinant Ca2+ indicators could provide important new insights into vacuolar Ca2+ release.
2.1. Inositol Trisphosphate-Dependent Vacuolar Ca2+ Release
In animal cells D-myo-inositol 1,4,5-trisphosphate (InsP3) binds to a family of Ca2+ channels, the inositol trisphosphate receptor, located in the ER, resulting in Ca2+ release into the cytosol [30,31]. In plant cells different stimuli elicit an increase in InsP3, and InsP3 has been shown to cause various physiological responses [32,33]. These physiological responses to InsP3 are probably mediated by [Ca2+]cyt. Increase of cytosolic InsP3 by release from a caged photoactivatable derivative [34,35] or by microinjection [36] result in a transient increase in [Ca2+]cyt. However, in plant cells the molecular mechanisms of InsP3-induced [Ca2+]cyt increase are not well understood, since the typical InsP3 receptor Ca2+ channel found in animals seems to be missing in land plants (Embryophyta). None of the more than 50 sequenced land plant genomes seems to encode an ortholog of the InsP3 receptor (Figure 2). To reconcile in vivo evidence for InsP3-mediated signal transduction in plant cells, with the seeming lack of an InsP3 receptor homolog two explanations have been offered. First, the similarity between land plant and animal InsP3 receptor Ca2+ channels might be too low to be detected due to early evolutionary divergence [37]. Alternatively, there might be no InsP3 receptor ortholog in land plants, and an InsP3-dependent Ca2+ release mechanism might have evolved from different membrane transport proteins [38].
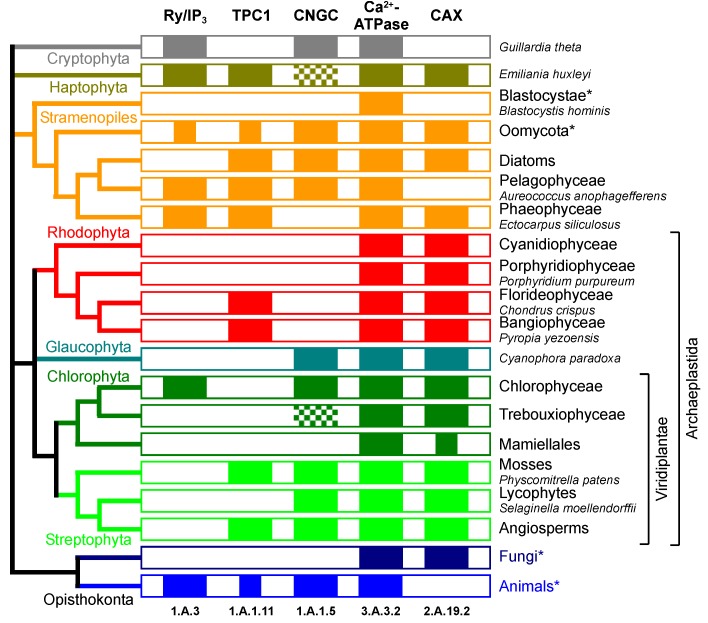
Figure 2.
Phylogenetic distribution of Ca2+ transporters in photosynthetic eukaryotes. Colored blocks indicate the presence of a certain Ca2+ transport protein (top and bottom labels) in a specific clade (labels on left and right). Abbreviations used for Ca2+ transport proteins: Ry/IP3, ryanodine-InsP3 receptor Ca2+ channel (TC 1.A.3); TPC1, two-pore Ca2+ channel (TC 1.A.1.11.13/18/19/22); CNGC, cyclic nucleotide-gated cation channel (TC 1.A.1.5); Ca2+-ATPase (TC 3.A.3.2); CAX, Ca2+:H+ exchanger (TC 2.A.19.2). Smaller block indicates that not all species in this clade seem to contain the Ca2+ transporter (see text). Checkered blocks indicate that it is currently not clear whether Haptophyta and Trebouxiphyceae contain cyclic nucleotide-gated cation channels or a similar cation channel from a different family of the voltage-gated ion channel superfamily (TC 1.A.1). The phylogenetic relationship (left [39]) of major eukaryotic photosynthetic clades (listed right) is presented and color coded (according to labels on the left). Clades marked with an asterisk are non-photosynthetic. For clades containing only one species with a fully sequenced genome, the species is given. Ca2+ transporters were identified by comparing established members from each Ca2+ transporter family to proteoms from selected eukaryotes with completely sequenced genomes using BLAST, followed by verification of each candidate by BLAST at TCDB [40,41].
Surprisingly, an InsP3 receptor was detected as a very abundant component of the flagellar proteome of Chlamydomonas reinhardtii [42]. The genomes of both Chlorophyceae sequenced so far, namely C. reinhardtii [43] and Volvox carteri [44], encode a single InsP3 receptor homolog. Pharmacological studies indicate that InsP3 is involved in Ca2+-dependent deflagellation observed in C. reinhardtii upon application of pH, osmotic or temperature shock [33,45]. The existence of InsP3 receptors in Chlorophyceae gave rise to speculation that this Ca2+ channel has been present in the common ancestor of green algae and land plants, and was lost in land plants [46]. However, InsP3 receptor homologs are not detected in other classes of green alga, such as Prasinophyceae (Mamiellales) or Trebouxiophyceae (Figure 2). For E. viridis, a Trebouxiophyceae, injection of InsP3 has been shown to trigger the opening of Ca2+-dependent plasma membrane K+ channels, which together with pharmacological evidence indicates the existence of an InsP3-dependent Ca2+ release mechanism in this green alga [47]. Homologs of animal InsP3 receptor Ca2+ channels also do not seem to be present in the glaucophyte Cyanophora paradoxa [48] or in any of the five red algae (Rhodophyta) with sequenced genomes. Currently, it seems as if Chlorophyceae are the only class within Archaeplastida (red algae & green plants & glaucophytes) containing InsP3 receptor homologs (Figure 2). While this patchy distribution of InsP3 receptors in Archaeplastida could be the result of multiple losses in different lineages, acquisition via horizontal gene transfer [49], seems more likely. In embryos of the brown alga (Phaeophyceae) Fucus serratus, photolysis of caged InsP3 caused an increase in [Ca2+]cyt [50]. The genome of the brown alga Ectocarpus siliculosus [51] does seem to encode an InsP3 receptor homolog, as do some other Stramenopile genomes (Figure 2).
Most attempts to identify the nature of the InsP3-sensitive Ca2+ store in plant cells produced results that point to the vacuole [32]. InsP3-dependent Ca2+ release was observed with tonoplast-enriched vesicles from oat (Avena sativa) roots [52] and from beet taproots [53], and with isolated intact vacuoles from sycamore (Acer pseudoplatanus) [54] and Chenopodium album [55] suspension culture cells. Yet some binding studies with [3H]InsP3 [56], or with antibodies raised against human InsP3 receptor Ca2+ channels [57] indicate that InsP3 binds to other membranes than the tonoplast.
Two groups in the early 1990s reported patch-clamp recordings of InsP3-dependent ion currents with intact isolated vacuoles from red beet taproots [58,59,60]. Whole vacuole currents induced by application of 1 μM InsP3 were comparable (when applying the same sign convention [10]), and estimated (single channel current) reversal potentials came close to the Nernst potential of Ca2+ indicating Ca2+-selectivity. Yet single channel characteristics greatly differed. Single channel I/V curves were linear in one publication [58] while they increased non-linearly at more negative voltages in the other [60]. Moreover, neither channel gating nor open channel conductance levels, reported by the two groups did match [58,59,60]. Surprisingly, InsP3-induced whole vacuole currents did not seem to be affected by the Ca2+ concentration on the cytosolic side [60]. This is different from animal InsP3 receptors, which are activated by cytosolic Ca2+ [30,31]. Attempts by several other groups to record InsP3-dependent vacuolar ion currents were unsuccessful [61,62,63]. With Ca2+-selective microelectrodes, no InsP3-induced Ca2+ release was detected with isolated intact vacuoles from beet taproots [64]. It has been suggested that a hyperosmolar incubation of red beet tissue before vacuole isolation is essential to activate InsP3-dependent vacuolar ion channels [60]. This seems to be in line with observations that a decrease of bath osmolarity increases InsP3-induced current density in patch-clamp recordings [60] as well as vacuolar Ca2+ efflux rates recorded with the fluorescent indicator Quin 2 [54]. Moreover, for vacuoles isolated from sycamore suspension culture cells a clear dependence of InsP3-dependent Ca2+ release on culture age was observed, increasing in parallel to cell fresh weight over time [54]. It therefore seems possible that InsP3-depedent vacuolar Ca2+ release may only be detected with cells at a certain developmental stage and when a slight hypoosmolar stress is applied to isolated vacuoles. Since the pioneering work on InsP3-dependent vacuolar Ca2+ release in the late 1980s and early 1990s little new experimental work on this important topic has been reported. The answer to the question if there is InsP3-dependent vacuolar Ca2+ release, is still that there are several evidences for it, but the molecular mechanisms are unclear.
2.2. Ca2+ Mobilization by Inositol Hexakisphosphate
Studies with guard cells from potato (Solanum tuberosum) and broad bean (Vicia faba) indicated that ABA-induced [Ca2+]cyt increase and stomatal closure might be mediated by myo-inositol hexakisphosphate (InsP6, a.k.a. phytate) [65]. Corroborating these observations, photoactivation of caged InsP6 in broad bean guard cells resulted in a transient increase in [Ca2+]cyt [66]. Application of 5 μM InsP6 to isolated broad bean guard cell vacuoles increased whole-vacuole current amplitudes [66]. These InsP6-dependent vacuolar currents are different from vacuolar currents induced by 1 μM InsP3 [58,59,60]. Even when recorded with a 106-fold [Ca2+] gradient (10 mM in the vacuole and ≈2 nM outside), InsP6-dependent vacuolar currents did not show strong rectification, indicating that ions other than Ca2+ were conducted, and current reversal potentials were close to 0 mV, indicating a lack of Ca2+-selectivity. Therefore, there seems to be no electrophysiological evidence for Ca2+ mobilization by InsP6 from the large central vacuole.
2.3. Cyclic Adenosine Diphosphoribose (cADPR)-Induced Vacuolar Ca2+ Release
Cyclic adenosine diphosphoribose (cADPR) is generated from NAD+ by ADP-ribosyl cyclases and functions as a Ca2+ mobilizing second messenger in different eukaryotic clades [67]. In animal cells, cADPR controls Ca2+ release from the ER by activating ryanodine receptor Ca2+ channels [68,69]. While InsP3 receptors (see above) are activated by direct binding of InsP3, the mechanism(s) by which cADPR activates ryanodine receptors is unclear [70,71]. Isolated, intact ryanodine receptor Ca2+ channels reconstituted into planar lipid bilayers are not affected by cADPR, indicating that cADPR does not directly bind to ryanodine receptors [72,73,74]. Instead, cADPR might bind to a secondary protein, which regulates ryanodine receptor activity [71,75], or cADPR might elevate luminal [Ca2+], which is known to activate ryanodine receptors [74].
Even though no homolog of ryanodine receptor Ca2+ channels can be detected in land plants (Figure 2), there are evidence that cADPR does function as a Ca2+ mobilizing second messenger in plant cells—a situation comparable to InsP3. Early work from Dale Sanders’ group demonstrated cADPR-dependent Ca2+ release from vacuolar-enriched microsomes of red beet taproots [53,76]. Later work indicated that systemin-induced elevation of [Ca2+]cyt observed after wounding tomato (Lycopersicon esculentum) leaves was in part caused by cADPR-dependent Ca2+ release from internal stores [77]. In A. thaliana circadian [Ca2+]cyt oscillations were reported to be the result of circadian oscillations of cytosolic cADPR concentrations [78]. Yet this is a matter of debate [79,80], and a regulation of circadian [Ca2+]cyt oscillations in A. thaliana by InsP3 has been reported as well [81]. Intracellular Ca2+ release by cADPR seems to be an essential step of ABA signal transduction [82,83]. ABA activates ADP-ribosyl cyclases and elevates cADPR concentrations triggering the expression of ABA-responsive genes [82,84]. During ABA-induced stomatal closure, cADPR causes an increase in [Ca2+]cyt resulting in potassium salt release and guard cell turgor loss [83,85].
In the unicellular green alga E. viridis, pharmacological experiments with ryanodine, ruthenium red, and different caffeine analogs indicated a ryanodine receptor-like Ca2+ release mechanism [86,87]. However, no ryanodine receptor homolog can be detected in sequenced genomes of two Trebouxiophyceae (Figure 2), the class of green alga to which E. viridis belongs. It should be mentioned that the Ca2+ channels in Chlorophyceae and brown algae (Phaeophyceae), mentioned above as possible InsP3 receptors, belong to the same ion channel family (TC 1.A.3) as ryanodine receptors. Due to low sequence conservation, phylogenetic studies do currently not allow a clear assignment of these algal Ca2+ channels to either InsP3 or ryanodine receptor subfamily [88].
Early studies provided evidence that cADPR acts on Ca2+ channels in the vacuolar membrane [53,76]. Later studies from the same group indicated that in addition to the vacuole, the ER might also function as cADPR-sensitive Ca2+ store in plant cells [89]. Patch-clamp recordings with isolated vacuoles from red beet tap roots [53] or from broad bean guard cells [83] detected currents elicited by cADPR. Current reversal potentials indicated Ca2+ selectivity of cADPR-dependent whole vacuole currents [83]. As mentioned above, isolated ryanodine receptor Ca2+ channels from animals are not gated open by cADPR [72,73,74]. This seems to be in contrast to the cADPR-dependent currents recorded from plant vacuoles. Moreover, cADPR-dependent vacuolar currents were largest at low [Ca2+]cyt (≈10 nM) and decreased at increasing [Ca2+]cyt, almost disappearing at 1 μM [83]. In contrast, animal ryanodine receptors are involved in Ca2+-induced Ca2+ release, showing increasing open probabilities at increasing [Ca2+]cyt [69,70]. The inhibition of cADPR-dependent vacuolar currents by micromolar [Ca2+]cyt might explain why recordings with Ca2+-selective microelectrodes in media containing [Ca2+] ≈ 2 μM failed to detect a cADPR-dependent Ca2+ efflux from isolated vacuoles [64]. A more detailed characterization of cADPR-elicited vacuolar currents is urgently needed. Results so far suggest that cADPR-dependent vacuolar Ca2+ release is regulated in a different way than animal ryanodine receptors are.
2.4. The Slow Vacuolar / Two Pore Channel (SV/TPC1)
The first patch-clamp studies with isolated plant vacuoles resulted in the description of a voltage-dependent, slowly activating, Ca2+ regulated, non-selective cation channel—the slow vacuolar (SV) channel [90,91]. It soon became clear that this channel is present in all land plants (Embryophyta) and in each cell type containing a large central vacuole [92,93]. Almost 20 years after these initial patch-clamp studies, the SV channel was shown to be encoded by the TPC1 (two pore channel) gene [94], which is present in one or two copies in all Embryophyta. This finally allowed the application of genetic manipulations to study vacuolar Ca2+ release. Only the aspect of vacuolar Ca2+ release will be covered here. For other possible physiological functions of the SV/TPC1 channel the reader is referred to a recent comprehensive review [95].
2.4.1. Is There Ca2+-Induced Ca2+ Release by the SV Channel?
It took several years before it was realized that the SV channel is Ca2+ permeable and might thus function as vacuolar Ca2+ channel [96]. Earlier reports of voltage-dependent vacuolar Ca2+ channels [62,97] probably were the result of SV channel recordings, and it was proposed [98] that the voltage-gated Ca2+ channel described in vacuoles from sugar beet (Beta vulgaris) tap roots [99,100] does reflect SV channel activity as well. Detailed biophysical studies confirmed a selectivity of SV channels for divalent cations [101,102]. Yet SV channels show a strong rectification allowing cation uptake into the vacuole but allowing little, if any, cation release from the vacuole. In other words, SV currents are usually recorded at positive membrane potentials, while the vacuole has a slightly negative potential [2,9]. To allow vacuolar Ca2+ release, some factor has to open the SV channel at physiological potentials (or the membrane potential has to change).
When Ward and Schroeder [96] discovered that the SV channel is permeable for Ca2+, they combined this with the fact that increasing [Ca2+]cyt increases the open probability and postulated Ca2+-induced Ca2+ release by the SV channel. In animal cells, an initial small [Ca2+]cyt increase is amplified via Ca2+-induced Ca2+ release by ER ryanodine and InsP3 receptor Ca2+ channels [31,69]. It was tempting to assume that the SV channel might mediate Ca2+-induced Ca2+ release in plant cells. However, a detailed investigation on how SV channel open probability is regulated by cytosolic [Ca2+], vacuolar [Ca2+], and trans-tonoplast electrical potential seemed to exclude Ca2+-induced Ca2+ release. As long as the electrochemical potential gradient for Ca2+, ΔμCa, would drive Ca2+ efflux, SV channel open probability was extremely low, and only at unphysiological conditions creating a ΔμCa driving Ca2+ into the vacuole, higher open probabilities were observed [103]. It almost seemed as if the SV channel were regulated by cytosolic [Ca2+], vacuolar [Ca2+], and membrane potential in a way to prevent vacuolar Ca2+ release. In the following years several studies presented evidence for [104,105,106] or against [5,7,107] Ca2+-induced Ca2+ release by the SV channel. As a result of these studies and others it was realized just how complex the regulation of SV channels is. In addition to Ca2+ and membrane potential, reducing agents increase SV channel activity, while oxidizing agents abolish it [108]; cytosolic Mg2+ activates SV channels, comparable to Ca2+ [105,106], while vacuolar Mg2+-comparable to vacuolar Ca2+-inhibits SV channel activity [7]; likewise vacuolar Na+ inhibits SV channel activity [107].
Measurement of SV channel activity by whole vacuole patch-clamp recording reports net charge movement and does not provide direct information about Ca2+ fluxes. Yet direct measurements of Ca2+ fluxes with isolated vacuoles resulted in seemingly conflicting results. Using Ca2+-selective microelectrodes, an increase in vacuolar Ca2+ efflux was recorded upon addition of 5 or 10 μM Ca2+ as well as upon addition of ABA, calmodulin, cAMP, or ATP [64]. Yet due to technical limitations, these recordings were performed at [Ca2+]cyt ≈ 2 μM, and one could question the physiological relevance of a vacuolar Ca2+ release brought about by an elevation of [Ca2+]cyt from 2 μM to 5 μM. For comparison, in animals cells the InsP3 receptor has maximum activity at [Ca2+]cyt ≈ 300 nM [31], mediating Ca2+-induced Ca2+ release from submicromolar to low micromolar concentrations. In another study, combining patch-clamp with ratiometric fluorescence imaging of Ca2+, no SV-mediated Ca2+ release from the vacuole could be recorded, while Ca2+ flux into the vacuole was recorded [109]. Remarkably, at −10 mV negative currents, i.e., net cation (K+) efflux from the vacuole was recorded electrophysiologically, while fura-2 fluorescence indicated opposite Ca2+ flux-into the vacuole. This is in line with a multi-ion single-file permeation mechanism proposed for the SV channel [110], where different cations get alternating access to the same pore allowing movement of different cations in opposite directions. These elegant recordings with fura-2 loaded into the tip of a patch pipette [109] demonstrate that negative whole vacuole currents cannot be equated with Ca2+ efflux from the vacuole.
The author of this review has always been skeptical of Ca2+-induced Ca2+ release by the SV channel and would argue that vacuolar Ca2+ release by the SV channel under physiologically relevant conditions still has to be demonstrated.
2.4.2. Is TPC1 Involved in Ca2+ Signaling?
The SV channel consists of a dimer of the TPC1 (two pore channel) protein [94]. Each TPC1 has two pore domains, as indicated by the name, and four pore domains form a functional voltage-dependent ion channel. A single TPC1 gene is found in most land plants (Embryophyta), others have two genes, likely due to recent gene duplication. In the genome of the lycophyte Selaginella moellendorffii [111], no TPC1 gene has been annotated. TPC1 shows a patchy distribution among photosynthetic eukaryotes (Figure 2) [95]. It seems to be lacking from Chlorophyta [46]. Yet patch-clamp recordings with isolated vacuoles from the green alga E. viridis resulted in whole vacuole currents reminiscent of SV currents, showing outward rectification, slow activation, Ca2+ dependence, inhibition by acidification, and blockage by Zn2+ [112]. There are no sequence data available for E. viridis, but the genomes of two other Trebouxiophyceae (Coccomyxa subellipsoidea [113] and Chlorella variabilis [114]) do not seem to encode TPC1. Among the five red algal genomes sequenced, only Chondrus crispus [115] and Pyropia yezoensis [116] seem to contain a TPC1 gene. As other Ca2+ channels, TPC1 has a patchy distribution among Archaeplastida (Figure 2), and it is hard to decide whether the last common ancestor of Archaeplastida had a TPC1 gene, which was lost in several lineages, or whether two lineages of the Archaeplastida acquired a TPC1 gene via horizontal gene transfer [49]. TPC1 channels were originally discovered in rats [117], and are wide spread in vertebrates, while absent in insects and fungi.
In mammalian cells, TPC’s (there are more subfamilies than just TPC1) are localized in acidic organelles, the endolysosomal compartment, where they are activated by NAADP (nicotinic acid adenine dinucleotide phosphate) causing Ca2+ release [118,119,120]. NAADP, which is produced from NADP+ by ADP-ribosyl cyclases (the same enzymes converting NAD+ into cADPR [121]) had been known as a Ca2+-mobilizing second messenger for several years [122]. NAADP-elicited Ca2+ release by mammalian TPC channels does not behave as a Ca2+-induced Ca2+ release system [123,124]. Instead, the different Ca2+ pools and messengers in mammalian cells are supposed to work together in the following way: NAADP triggers Ca2+ release from endolysosomes via TPC channels resulting in a small (and possibly localized) increase in [Ca2+]cyt; this activates (nearby) InsP3 and ryanodine receptors, and Ca2+-induced Ca2+ release from the ER causes an effective (and widespread) [Ca2+]cyt elevation [120,125]. However, the function of mammalian TPC channels as NAADP-dependent Ca2+ channels has recently been questioned. It has been reported that TPC proteins are activated by the phosphoinositide PIns(3,5)P2 instead, and that they function as Na+ channels, mediating Na+ efflux to depolarize lysosome membranes [126]. Moreover, it has been questioned whether NAADP binds directly to the TPC channel or to an accessory component [127]. Little is known about the NAADP or PIns(3,5)P2 in plants. A study with microsomal vesicles from red beet and cauliflower (Brassica oleracea) observed NAADP-dependent Ca2+ release from ER vesicles but not from vacuolar vesicles [128]. Still, it might be worthwhile to investigate the effect of NAADP or PIns(3,5)P2 on SV/TPC1 channel activity.
With the coding gene identified, mutants of TPC1 should give insight into the physiological function of SV/TPC1 channels. Surprisingly, an A. thaliana T-DNA-knockout line, attpc1-2, lacking the TPC1 protein does not show obvious phenotypic differences compared to wild-type plants, even though AtTPC1 is a single copy gene [94]. However, some more ‘subtle’ differences were observed. For attpc1-2 mutants, ABA was a less effective inhibitor of seed germination, and TPC1-overexpresing lines were ABA-hypersensitive. ABA-induced stomatal closure was not affected by the absence or presence of TPC1, yet stomatal closure by high external Ca2+ was not observed in tpc1-2 mutants in contrast to wild-type plants. These results were taken as an indication for vacuolar Ca2+ release by the SV/TPC1 channel [94]. However, as shown later, [Ca2+]cyt changes induced by high external Ca2+ were identical in attpc1-2 and wild-type plants, indicating a role of AtTPC1 in stomatal closure in a signal pathway downstream of [Ca2+]cyt [129,130]. To further elucidate the function of AtTPC1 in stress-induced [Ca2+]cyt increase, [Ca2+]cyt was recorded in attpc1-2 knockout, wild-type, and TPC1-overexpressing lines during the application of abiotic stresses (cold, hyperosmotic, salt, and oxidative) or biotic factors (elf18, flg22) [130]. For all stresses and biotic factors tested there was no difference in amplitude or time course of recorded [Ca2+]cyt transients between lines lacking or overexpressing AtTPC1. Moreover, expression of stress-induced genes known to require [Ca2+]cyt increase did not show any dependence on TPC1 either. This seems to rule out an involvement of AtTPC1 in [Ca2+]cyt increase induced by the different tested stresses and elicitors [130]. It is important to emphasize that some of those stresses (cold, hyperosmotic, and salt) have been reported to cause vacuolar Ca2+ release based on recordings with vacuole-bound aequorin (see above) [28,29].
While the loss-of-function mutant attpc1-2 has a wild-type phenotype, in the gain-of-function fou2 (fatty acid oxygenation upregulated 2) mutant older leaves have shorter petioles, show slight epinasty, and accumulate more anthocyanins, comparable to other mutants overaccumulating the plant hormone jasmonic acid [131]. In fou2, a point mutation (D454N) reduces the sensitivity of SV/TPC1 channels against inhibition by vacuolar Ca2+ and H+ [132]. This is expected to result in increased channel activity in the mutant. Yet somewhat counter-intuitive, fou2 mutants compared to wild-type plants accumulate higher vacuolar [Ca2+] and lower vacuolar [K+] [132], and display a transcript profile reminiscent of a K+ starvation transcriptome [133]. This has been explained by loss of K+ from vacuoles due to an overactive SV/TPC1 channel in the fou2 mutant, being compensated by Ca2+ uptake into the vacuole by the Ca2+:H+ antiporter CAX3 [132], which is upregulated under K+ deficiency [134]. However, comparing different cell types within A. thaliana leaves, epidermal cells with a higher AtTPC1 transcript level, compared to mesophyll cells, have lower vacuolar [Ca2+], which increases in Attpc1-2 mutants [135]. SV/TPC1 channels seem to influence vacuolar [Ca2+]. However, whether this indicates that the SV/TPC1 channel functions as a vacuolar K+ efflux channel [95,132], or as a Ca2+ efflux channel [135,136], or as a vacuolar Ca2+ sensor [8,136] seems currently not possible to differentiate.
While studies mentioned so far seem to provide little support for a function of TPC1 in Ca2+ signaling, there are a few observations that still might support such a function. Application of high concentrations of sucrose induce a transient increase in [Ca2+]cyt in plant cells [137]. This sucrose-induced [Ca2+]cyt increase was enhanced in plants overexpressing TPC1 and reduced in plant lines with suppressed TPC1 expression [138,139]. In tobacco (Nicotiana tabacum) BY-2 cells, a transient increase in [Ca2+]cyt induced by the fungal elicitor cryptogein was reduced in cell lines with suppressed NtTPC1 expression, as were defense-related gene expression and programmed cell death [139]. Similar, in rice (Oryza sativa) a transient increase in [Ca2+]cyt induced by the fungal elicitor TvX was reduced in Ostpc1 knockout mutants [140]; elicitor-induced defense responses were reduced in Ostpc1 mutants and enhanced in OsTPC1 overexpressing lines [141]. In this context it has to be mentioned that the intracellular localization of TPC1 in monocots, and especially in rice, has been debated until recently. In A. thaliana and other dicots the vacuolar localization of TPC1 is well established [94,95,142], and recordings of typical SV channel currents from vacuoles of different monocots [93,143,144,145] strongly suggest a vacuolar localization as well. However, in rice, anti-OsTPC1 antibodies seem to bind to the plasma membrane of intact protoplasts and to plasma membrane-enriched subcellular fractions from suspension-cultured rice cells [140,146]. Patch-clamp recording of characteristic SV channel activity from vacuolar membranes of wild-type rice, but not from Ostpc1 knock-out lines recently clarified that rice OsTPC1 forms the vacuolar SV channel—as in other land plants [147].
It is currently not understood why the large central vacuole of plant cells contains thousands of SV/TPC1 channels. Would all these cation channels open, the resulting huge fluxes would probably collapse existing cation gradients within 10 to 20 s, with disastrous consequences for the cell. But then, these channels seem to be closed (almost?) all the time, and the suggestion that “Arabidopsis is better off without” TPC1 [95] seems to hold for plants growing in a green house. In a computational model for the guard cell based on existing knowledge about transport proteins, it was observed that the SV/TPC1 channel “is unsuited to the task of Ca2+ release using any of the range of gating and permeation parameters that are reported in the literature” [148]. To describe vacuolar Ca2+ release in this guard cell computational model, a Ca2+ channel similar to animal InsP3 or ryanodine receptors was included, with properties so far not observed for vacuolar Ca2+ channels [149].
2.5. Cyclic Nucleotide-Gated Channels
Cyclic nucleotide-gated channels AtCNGC19 and AtCNGC20 were recently reported to be targeted to the vacuolar membrane [150]. However, another group at the same time reported AtCNGC20 to be targeted to the plasma membrane [151]. CNGCs are nonselective cation channels, some conducting Ca2+, regulated by cAMP, cGMP, and calmodulin [152,153,154]. While nothing is currently known about the ion selectivity of AtCNGC19 and AtCNGC20, they might potentially be involved in vacuolar Ca2+ release. These two CNGC’s from A. thaliana seem to be involved in ion homeostasis and nutrient signaling. Expression of AtCNGC19 and AtCNGC20 is upregulated within hours after applying salt stress (200 mM NaCl) [155]. Expression of AtCNGC19 is upregulated within hours after applying boron deficiency (transfer from 2 μM to 0 μM B) in parallel to an increase in [Ca2+]cyt [156]. Cyclic nucleotide-gated cation channels seem to be present in most photosynthetic eukaryotes with the exception of red algae (Rhodophyta) (Figure 2). Yet most of these cyclic nucleotide-gated channels are probably not located in the vacuole but in other membranes—as in A. thaliana.
2.6. Transient Receptor Potential Ca2+ Channels
In fungi, Ca2+ is released from the large central vacuole by a Ca2+ channel belonging to the transient receptor potential (TRP) family (Yvc1 in Saccharomyces cerevisiae; TC 1.A.4.4.1) [157,158]. No genes encoding orthologs of TRP Ca2+ channels have been detected in genomes of higher plants, while some green algae may contain TRP Ca2+ channels [46]. Yet no TRP Ca2+ channel outside Opisthokonta has been characterized so far, which is why this Ca2+ channel family is not included in Figure 2.
3. Ca2+ Uptake into the Vacuole
Ca2+ uptake into the large central vacuole is an essential part of cytosolic Ca2+ homeostasis and—in contrast to Ca2+ release—the molecular mechanisms of vacuolar Ca2+ uptake are well understood. P-type Ca2+-ATPases and Ca2+:H+ exchanger move Ca2+ against its electrochemical potential gradient from the cytosol into the vacuole (Figure 1). These Ca2+ pumps establish and maintain the electrochemical potential gradient driving Ca2+ release via vacuolar Ca2+ channels (see above). Most work on vacuolar Ca2+ pumps has concentrated on the aspect of ion homeostasis, which will not be covered here. In addition to establishing a Ca2+ gradient, vacuolar Ca2+ pumps are likely to contribute to shaping [Ca2+]cyt transients in the cytosol. High activity of Ca2+ pumps, e.g., is expected to decrease amplitude and duration of [Ca2+]cyt transients (Figure 1). Especially in signal transduction pathways where [Ca2+]cyt transients do not just function as a simple switch [159], but where the shape of the [Ca2+]cyt transients is important [160], the activity of vacuolar Ca2+ pumps is likely to play a role. Different stimuli have been shown to result in [Ca2+]cyt transients of different shape and duration in plants cells [28,160,161], and the same stimulus, such as salt stress (NaCl), can cause [Ca2+]cyt transients that increase in amplitude with stimulus intensity [162]. Attempts to describe [Ca2+]cyt oscillations by mathematical models nicely show that amplitude and frequency depend on both, Ca2+ influx into the cytosol and Ca2+ removal from the cytosol by Ca2+ pumps [86]. In guard cells it has been shown that [Ca2+]cyt oscillations with different frequencies have different effects on stomatal closure [163]. How vacuolar Ca2+ pumps may affect intracellular signal transduction by shaping [Ca2+]cyt transients (Figure 1) is slowly beginning to emerge [15,17].
3.1. P-Type Ca2+-ATPases
P-type Ca2+-ATPases function as high affinity low turnover Ca2+ pumps, operating as ATP-driven Ca2+:H+ exchanger. Flowering plants (angiosperms) seem to contain more than a dozen Ca2+-ATPase genes, and plasma membrane and each organelle seem to contain at least one Ca2+-ATPase [164,165]. Ca2+-ATPases, as P-type ATPases in general, are phylogenetically very old and have been detected in all eukaryotic clades [166] (Figure 2). In A. thaliana, two Ca2+-ATPases are targeted to the vacuolar membrane, with ACA4-GFP detected in small vacuoles of transiently transformed protoplasts [167] and ACA11-GFP detected in the large central vacuole of root cells [168]. Both vacuolar Ca2+-ATPases contain an N-terminal autoinhibitory domain with a calmodulin binding site.
Knockout mutants, lacking either ACA4 or ACA11, did not show obvious phenotypic differences compared to wild-type A. thaliana plants, while double knockout lines developed hypersensitive-like lesions [169]. Total leaf Ca2+ was not changed by the double knockout, and lesions could be suppressed by blocking salicylic acid production or accumulation, indicating that the lack of vacuolar Ca2+-ATPases resulted in hyperactivity of the salicylic acid-dependent programmed cell death pathway. It was suggested that vacuolar Ca2+-ATPases shape [Ca2+]cyt transients that trigger programmed cell death, but no [Ca2+]cyt transients were recorded [169]. The first direct evidence that lack of a vacuolar Ca2+-ATPase can change the shape of a [Ca2+]cyt transient came from the moss Physcomitrella patens. When the Ca2+-ATPase PCA1, which is localized in small vacuoles and not in the large central vacuole, was lacking, salt-induced (250 mM NaCl) [Ca2+]cyt transients were more than two-fold enhanced and dramatically prolonged. Moreover, knockout plants displayed reduced expression of stress-induced genes and decreased salt tolerance [170]. Similarly, in tobacco (Nicotiana benthamiana) lacking the ER localized Ca2+-ATPase NbCA1, elicitor-induced (cryptogein) [Ca2+]cyt transients were enhanced and programmed cell death was accelerated [171]. These studies indicate that organellar Ca2+-ATPases are shaping stimulus-induced [Ca2+]cyt transients and thereby may play a role in Ca2+-mediated signaling.
However, when interpreting the phenotype of knockout mutants lacking specific Ca2+ pumps, it will always be difficult to differentiate what is a result of altered [Ca2+]cyt transients and what is a result of changes in ion homeostasis [15]. Subtle changes in [Ca2+] in a certain compartment may have unexpected results. Moreover, since Ca2+-ATPases transport both, Ca2+ and H+, pH homeostasis might be affected as well, as shown for Ca2+:H+ exchanger of the CAX family [172].
3.2. Ca2+:H+ Exchanger (CAX)
Ca2+:H+ exchanger function as low affinity high turnover vacuolar Ca2+ pumps, driven by the electrochemical potential gradient of H+ (Figure 1). Flowering plants (angiosperms) have been reported to contain five to fourteen Ca2+:H+ exchanger genes. Ca2+:H+ exchanger of the CAX family are part of the Ca2+:cation antiporter (CaCA) superfamily, and have been detected in most eukaryotic clades; they were lost in some Mamiellales and animals (Figure 2). Plant CAXs are not selective for Ca2+, but transport other divalent cations, such as Mn2+ or Cd2+, as well [173,174].
Most work on Ca2+:H+ exchangers of the CAX family has concentrated on ion homeostasis, and single and double knockout mutants show varying defects not only in Ca2+ homeostasis but also changes in compartmentalization of other divalent cations, such as Mg2+ and Mn2+ [175,176]. Resulting phenotypes can be rather complex. An A. thaliana double knockout lacking both AtCAX1 and AtCAX3 had three-fold higher apoplastic [Ca2+], resulting in reduced cell wall extensibility, transpiration, and leaf growth [177]. In another study with the same double knockout (Atcax1 & Atcax3) higher apoplastic pH was detected resulting in a defect in auxin transport [178]. Overexpression of GhCAX3, a Ca2+:H+ exchanger from cotton (Gossypium hirsutum), in tobacco (N. benthamiana) reduced ABA sensitivity of transgenic seedlings and affected cold stress responses, which was interpreted as an indication for a regulatory role of GhCAX3 in ABA and cold signaling [179]. Yet no [Ca2+]cyt transients were recorded. A. thaliana knockout lines lacking either AtCAX1 or AtCAX3 display an increased ABA sensitivity of seed germination [180], and these knockout lines are known to have altered ion homeostasis [175,176]. For CAX, gain of function or loss of function mutants direct experimental evidence for changes in stimulus-induced [Ca2+]cyt transients are still awaited.
4. Conclusions
Most work on the involvement of the vacuole in Ca2+ signaling in plant cells (Figure 1) has concentrated on vacuolar Ca2+ channels. Available data provide evidence for InsP3 receptor and ryanodine receptor Ca2+ channels, the voltage-dependent SV/TPC1 channel, and most recently for vacuolar cyclic nucleotide-gated cation channels. The phylogenetic distribution of these Ca2+ channels among photosynthetic eukaryotes is patchy (Figure 2) indicating a complex evolutionary history. Since no gene for InsP3 or ryanodine receptors has been identified in land plants, after a very promising start in the 1990s, little progress has been made in our understanding of second messenger-mediated vacuolar Ca2+ release. As TPC1 was identified as encoding the SV channel, research on this field has seen a renaissance, and the SV/TPC1 channel is probably the best described ion channel in plant cells. However, we still do not understand the in vivo function of this channel; it is probably not Ca2+-induced vacuolar Ca2+ release. Progress in identification of genes encoding vacuolar Ca2+ pumps resulted in a better understanding of their physiological functions. In addition to maintaining Ca2+ gradients and ion homeostasis, P-type Ca2+-ATPases have been shown to affect the shape of [Ca2+]cyt transients, indicating that they might contribute more to Ca2+ signaling then just providing the driving force for vacuolar Ca2+ release.
Acknowledgments
Work in the author’s lab has been supported by the National Science Foundation (MCB-0925298).
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Felle H. Cytoplasmic free calcium in Riccia fluitans L. and Zea mays L.: Interaction of Ca2+ and pH? Planta. 1988;176:248–255. doi: 10.1007/BF00392452. [DOI] [PubMed] [Google Scholar]
- 2.Bethmann B., Thaler M., Simonis W., Schönknecht G. Electrochemical potential gradients of H+, K+, Ca2+, and Cl− across the tonoplast of the green alga Eremosphaera viridis. Plant Physiol. 1995;109:1317–1326. doi: 10.1104/pp.109.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felle H.H., Hepler P.K. The cytosolic Ca2+ concentration gradient of Sinapis alba root hairs as revealed by Ca2+-selective microelectrode tests and fura-dextran ratio imaging. Plant Physiol. 1997;114:39–45. doi: 10.1104/pp.114.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plieth C. Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- 5.Perez V., Wherrett T., Shabala S., Muniz J., Dobrovinskaya O., Pottosin I. Homeostatic control of slow vacuolar channels by luminal cations and evaluation of the channel-mediated tonoplast Ca2+ fluxes in situ. J. Exp. Bot. 2008;59:3845–3855. doi: 10.1093/jxb/ern225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conn S., Gilliham M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010;105:1081–1102. doi: 10.1093/aob/mcq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pottosin I.I., Martínez-Estévez M., Dobrovinskaya O.R., Muñiz J., Schönknecht G. Mechanism of luminal Ca2+ and Mg2+ action on the vacuolar slowly activating channels. Planta. 2004;219:1057–1070. doi: 10.1007/s00425-004-1293-7. [DOI] [PubMed] [Google Scholar]
- 8.Dadacz-Narloch B., Beyhl D., Larisch C., López-Sanjurjo E.J., Reski R., Kuchitsu K., Müller T.D., Becker D., Schönknecht G., Hedrich R. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell. 2011;23:2696–2707. doi: 10.1105/tpc.111.086751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker D.J., Leigh R.A., Miller A.J. Potassium homeostasis in vacuolate plant cells. Proc. Natl. Acad. Sci. USA. 1996;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertl A., Blumwald E., Coronado R., Eisenberg R., Findlay G.P., Gradmann D., Hille B., Köhler K., Kolb H.A., MacRobbie E.A.C., et al. Electrical measurements on endomembranes. Science. 1992;258:873–874. doi: 10.1126/science.1439795. [DOI] [PubMed] [Google Scholar]
- 11.Taiz L. The plant vacuole. J. Exp. Biol. 1992;172:113–122. doi: 10.1242/jeb.172.1.113. [DOI] [PubMed] [Google Scholar]
- 12.Martinoia E., Meyer S., de Angeli A., Nagy R. Vacuolar transporters in their physiological context. Annu. Rev. Plant Biol. 2012;63:183–213. doi: 10.1146/annurev-arplant-042811-105608. [DOI] [PubMed] [Google Scholar]
- 13.Hedrich R. Ion channels in plants. Physiol. Rev. 2012;92:1777–1811. doi: 10.1152/physrev.00038.2011. [DOI] [PubMed] [Google Scholar]
- 14.Isayenkov S., Isner J.C., Maathuis F.J.M. Vacuolar ion channels: Roles in plant nutrition and signalling. FEBS Lett. 2010;584:1982–1988. doi: 10.1016/j.febslet.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Spalding E.P., Harper J.F. The ins and outs of cellular Ca2+ transport. Curr. Opin. Plant Biol. 2011;14:715–720. doi: 10.1016/j.pbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jammes F., Hu H.-C., Villiers F., Bouten R., Kwak J.M. Calcium-permeable channels in plant cells. FEBS J. 2011;278:4262–4276. doi: 10.1111/j.1742-4658.2011.08369.x. [DOI] [PubMed] [Google Scholar]
- 17.Bose J., Pottosin I., Shabala S.S., Palmgren M.G., Shabala S. Calcium efflux systems in stress signalling and adaptation in plants. Front. Plant Sci. 2011 doi: 10.3389/fpls.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swarbreck S., Colaco R., Davies J. Plant calcium-permeable channels. Plant Physiol. 2013 doi: 10.1104/pp.113.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd A.N., Kudla J., Sanders D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 20.Stael S., Wurzinger B., Mair A., Mehlmer N., Vothknecht U.C., Teige M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2011;433:1–9. doi: 10.1093/jxb/err394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiter E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium. 2011;50:120–128. doi: 10.1016/j.ceca.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Pittman J.K. Vacuolar Ca2+ uptake. Cell Calcium. 2011;50:139–146. doi: 10.1016/j.ceca.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Webb A.A.R., Larman M.G., Montgomery L.T., Taylor J.E., Hetherington A.M. The role of calcium in ABA-induced gene expression and stomatal movements. Plant J. 2001;26:351–362. doi: 10.1046/j.1365-313X.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 24.Gilroy S., Fricker M.D., Read N.D., Trewavas A.J. Role of calcium in signal transduction of Commelina guard cells. Plant Cell. 1991;3:333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAinsh M.R., Brownlee C., Hetherington A.M. Visualizing changes in cytosolic-free calcium during the response of stomatal guard cells to abscisic acid. Plant Cell. 1992;4:1113–1122. doi: 10.1105/tpc.4.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAinsh M.R., Webb A.A.R., Taylor J.E., Hetherington A.M. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabov A., Blatt M.R. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight H., Trewavas A.J., Knight M.R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight H., Trewavas A.J., Knight M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 30.Foskett J.K., White C., Cheung K.-H., Mak D.-O.D. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 32.Krinke O., Novotna Z., Valentova O., Martinec J. Inositol trisphosphate receptor in higher plants: is it real? J. Exp. Bot. 2007;58:361–376. doi: 10.1093/jxb/erl220. [DOI] [PubMed] [Google Scholar]
- 33.Coté G.G., Crain R.C. Why do plants have phosphoinositides? BioEssays. 1994;16:39–46. doi: 10.1002/bies.950160106. [DOI] [Google Scholar]
- 34.Gilroy S., Read N.D., Trewavas A.J. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro D., Liu Q., Lisboa S., Scherer G.E.F., Quader H., Malhó R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J. Exp. Bot. 2005;56:1665–1674. doi: 10.1093/jxb/eri163. [DOI] [PubMed] [Google Scholar]
- 36.Tucker E.B., Boss W.F. Mastoparan-induced intracellular Ca2+ fluxes may regulate cell-to-cell communication in plants. Plant Physiol. 1996;111:459–467. doi: 10.1104/pp.111.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maathuis F.J. Ligand-gated ion channels. In: Blatt M.R., editor. Membrane Transport in Plants. Blackwell Publishing; Oxford, UK: 2004. pp. 193–220. [Google Scholar]
- 38.Bothwell J.H.F., Ng C.K.-Y. The evolution of Ca2+ signalling in photosynthetic eukaryotes. New Phytol. 2005;166:21–38. doi: 10.1111/j.1469-8137.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 39.Burki F., Okamoto N., Pombert J.-F., Keeling P.J. The evolutionary history of haptophytes andcryptophytes: Phylogenomic evidence for separate origins. Proc. Biol. Sci. 2012;279:2246–2254. doi: 10.1098/rspb.2011.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saier M.H., Jr., Yen M.R., Noto K., Tamang D.G., Elkan C. The Transporter Classification Database: recent advances. Nucleic Acids Res. 2009;37:D274–D278. doi: 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saier M.H., Jr., Tran C.V., Barabote R.D. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34:D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pazour G.J., Agrin N., Leszyk J., Witman G.B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merchant S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G.B., Terry A., Salamov A., Fritz-Laylin L.K., Marechal-Drouard L., et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prochnik S.E., Umen J., Nedelcu A.M., Hallmann A., Miller S.M., Nishii I., Ferris P., Kuo A., Mitros T., Fritz-Laylin L.K., et al. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science. 2010;329:223–226. doi: 10.1126/science.1188800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuin H., Koerten H., Ghijsen W.E.J.M., Munnik T., van den Ende H., Musgrave A. Chlamydomonas contains calcium stores that are mobilized when phospholipase C is activated. Planta. 2000;210:286–294. doi: 10.1007/PL00008136. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler G.L., Brownlee C. Ca2+ signalling in plants and green algae—Changing channels. Trends Plant Sci. 2008;13:506–514. doi: 10.1016/j.tplants.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Förster B. Injected inositol 1,4,5-trisphosphate activates Ca2+-sensitive K+ channels in the plasmalemma of Eremosphaera viridis. FEBS Lett. 1990;269:197–201. doi: 10.1016/0014-5793(90)81153-F. [DOI] [PubMed] [Google Scholar]
- 48.Price D.C., Chan C.X., Yoon H.S., Yang E.C., Qiu H., Weber A.P.M., Schwacke R., Gross J., Blouin N.A., Lane C., et al. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science. 2012;335:843–847. doi: 10.1126/science.1213561. [DOI] [PubMed] [Google Scholar]
- 49.Keeling P.J., Palmer J.D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 2008;9:605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- 50.Coelho S.M., Taylor A.R., Ryan K.P., Sousa-Pinto I., Brown M.T., Brownlee C. Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. Plant Cell. 2002;14:2369–2381. doi: 10.1105/tpc.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cock J.M., Sterck L., Rouze P., Scornet D., Allen A.E., Amoutzias G., Anthouard V., Artiguenave F., Aury J.M., Badger J.H., et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature. 2010;465:617–621. doi: 10.1038/nature09016. [DOI] [PubMed] [Google Scholar]
- 52.Schumaker K.S., Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J. Biol. Chem. 1987;262:3944–3946. [PubMed] [Google Scholar]
- 53.Allen G.J., Muir S.R., Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- 54.Ranjeva R., Carrasco A., Boudet A.M. Inositol trisphosphate stimulates the release of calcium from intact vacuoles isolated from Acer cells. FEBS Lett. 1988;230:137–141. doi: 10.1016/0014-5793(88)80657-4. [DOI] [Google Scholar]
- 55.Lommel C., Felle H.H. Transport of Ca2+ across the tonoplast of intact vacuoles from Chenopodium album L suspension cells: ATP-dependent import and inositol-1,4,5-trisphosphate-induced release. Planta. 1997;201:477–486. doi: 10.1007/s004250050092. [DOI] [Google Scholar]
- 56.Martinec J., Feltl T., Scanlon C.H., Lumsden P.J., Machackova I. Subcellular localization of a high affinity binding site for D-myo-inositol 1,4,5-trisphosphate from Chenopodium rubrum. Plant Physiol. 2000;124:475–483. doi: 10.1104/pp.124.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muir S.R., Sanders D. Inositol 1,4,5-trisphosphate-sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiol. 1997;114:1511–1521. doi: 10.1104/pp.114.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexandre J., Lassalles J.P., Kado R.T. Opening of Ca2+ channels in isolated red beet vacuole membranes by inositol 1,4,5-trisphosphate. Nature. 1990;343:567–570. doi: 10.1038/343567a0. [DOI] [Google Scholar]
- 59.Alexandre J., Lassalles J.P. Intracellular Ca2+ release by InsP3 in plants and effect of buffers on Ca2+ diffusion. Phil. Trans. R. Soc. Lond. B. 1992;338:53–61. doi: 10.1098/rstb.1992.0128. [DOI] [Google Scholar]
- 60.Allen G.J., Sanders D. Osmotic stress enhances the competence of Beta vulgaris vacuoles to respond to inositol 1,4,5-trisphosphate. Plant J. 1994;6:687–695. [Google Scholar]
- 61.Chasan R., Schroeder J.I. Excitation in plant membrane biology. Plant Cell. 1992;4:1180–1188. [Google Scholar]
- 62.Ping Z., Yabe I., Muto S. Identification of K+, Cl−, and Ca2+ channels in the vacuolar membrane of tobacco cell suspension cultures. Protoplasma. 1992;171:7–18. doi: 10.1007/BF01379275. [DOI] [Google Scholar]
- 63.Gelli A., Blumwald E. Calcium retrieval from vacuolar pools. Characterization of a vacuolar calcium channel. Plant Physiol. 1993;102:1139–1146. doi: 10.1104/pp.102.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pottosin I., Wherrett T., Shabala S. SV channels dominate the vacuolar Ca2+ release during intracellular signaling. FEBS Lett. 2009;583:921–926. doi: 10.1016/j.febslet.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Lemtiri-Chlieh F., MacRobbie E.A., Brearley C.A. Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells. Proc. Natl. Acad. Sci. USA. 2000;97:8687–8692. doi: 10.1073/pnas.140217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lemtiri-Chlieh F., MacRobbie E.A.C., Webb A.A.R., Manison N.F., Brownlee C., Skepper J.N., Chen J., Prestwich G.D., Brearley C.A. Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc. Natl. Acad. Sci. USA. 2003;100:10091–10095. doi: 10.1073/pnas.1133289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guse A.H. Biochemistry, biology, and pharmacology of cyclic adenosine diphosphoribose (cADPR) Curr. Med. Chem. 2004;11:847–855. doi: 10.2174/0929867043455602. [DOI] [PubMed] [Google Scholar]
- 68.Clapper D.L., Walseth T.F., Dargie P.J., Lee H.C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- 69.Galione A., Lee H.C., Busa W.B. Ca2+-induced Ca2+ release in sea urchin egg homogenates:modulation by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- 70.Galione A., Chuang K.-T. Pyridine nucleotide metabolites and calcium release from intracellular stores. In: Islam M.S., editor. Calcium Signaling. Volume 740. Springer; Dordrecht, The Netherlands: 2012. pp. 305–323. [DOI] [PubMed] [Google Scholar]
- 71.Venturi E., Pitt S., Galfré E., Sitsapesan R. From eggs to hearts: What is the link between cyclic ADP-ribose and ryanodine receptors? Cardiovasc. Ther. 2012;30:109–116. doi: 10.1111/j.1755-5922.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- 72.Copello J.A., Qi Y., Jeyakumar L.H., Ogunbunmi E., Fleischer S. Lack of effect of cADP-ribose and NAADP on the activity of skeletal muscle and heart ryanodine receptors. Cell Calcium. 2001;30:269–284. doi: 10.1054/ceca.2001.0235. [DOI] [PubMed] [Google Scholar]
- 73.Fruen B.R., Mickelson J.R., Shomer N.H., Velez P., Louis C.F. Cyclic ADP-ribose does not affect cardiac or skeletal muscle ryanodine receptors. FEBS Lett. 1994;352:123–126. doi: 10.1016/0014-5793(94)00931-7. [DOI] [PubMed] [Google Scholar]
- 74.Lukyanenko V., Györke I., Wiesner T.F., Györke S. Potentiation of Ca2+ release by cADP-ribose in the heart is mediated by enhanced SR Ca2+ uptake into the sarcoplasmic reticulum. Circ. Res. 2001;89:614–622. doi: 10.1161/hh1901.098066. [DOI] [PubMed] [Google Scholar]
- 75.Zheng J., Wenzhi B., Miao L., Hao Y., Zhang X., Yin W., Pan J., Yuan Z., Song B., Ji G. Ca2+ release induced by cADP-ribose is mediated by FKBP12.6 proteins in mouse bladder smooth muscle. Cell Calcium. 2010;47:449–457. doi: 10.1016/j.ceca.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Muir S.R., Sanders D. Pharmacology of Ca2+ release from red beet microsomes suggests the presence of ryanodine receptor homologs in higher plants. FEBS Lett. 1996;395:39–42. doi: 10.1016/0014-5793(96)01000-9. [DOI] [PubMed] [Google Scholar]
- 77.Moyen C., Hammond-Kosack K.E., Jones J., Knight M.R., Johannes E. Systemin triggers an increase of cytoplasmic calcium in tomato mesophyll cells: Ca2+ mobilization from intra- and extracellular compartments. Plant Cell Environ. 1998;21:1101–1111. doi: 10.1046/j.1365-3040.1998.00378.x. [DOI] [Google Scholar]
- 78.Dodd A.N., Gardner M.J., Hotta C.T., Hubbard K.E., Dalchau N., Love J., Assie J.M., Robertson F.C., Jakobsen M.K., Goncalves J., et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science. 2007;318:1789–1792. doi: 10.1126/science.1146757. [DOI] [PubMed] [Google Scholar]
- 79.Dodd A.N., Gardner M.J., Hotta C.T., Hubbard K.E., Dalchau N., Robertson F.C., Love J., Sanders D., Webb A.A.R. Response to comment on “The Arabidopsis circadian clock incorporates a cADPR-based feedback loop”. Science. 2009;326:230. doi: 10.1126/science.1146757. [DOI] [PubMed] [Google Scholar]
- 80.Xu X., Graeff R., Xie Q., Gamble K.L., Mori T., Johnson C.H. Comment on “The Arabidopsis circadian clock incorporates a cADPR-based feedback loop”. Science. 2009;326:230. doi: 10.1126/science.1169503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang R.H., Han S., Zheng H., Cook C.W., Choi C.S., Woerner T.E., Jackson R.B., Pei Z.M. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science. 2007;315:1423–1426. doi: 10.1126/science.1134457. [DOI] [PubMed] [Google Scholar]
- 82.Wu Y., Kuzma J., Marechal E., Graeff R., Lee H.C., Foster R., Chua N.H. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- 83.Leckie C.P., McAinsh M.R., Allen G.J., Sanders D., Hetherington A.M. Abscisic acid-inducedstomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez J.P., Duque P., Chua N.H. ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. Plant J. 2004;38:381–395. doi: 10.1111/j.1365-313X.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- 85.MacRobbie E.A.C. ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+(Rb+) release. Proc. Natl. Acad. Sci. USA. 2000;97:12361–12368. doi: 10.1073/pnas.220417197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bauer C.S., Plieth C., Bethmann B., Popescu O., Hansen U.-P., Simonis W., Schönknecht G. Strontium-induced repetitive calcium spikes in a unicellular green alga. Plant Physiol. 1998;117:545–557. doi: 10.1104/pp.117.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauer C.S., Simonis W., Schönknecht G. Different xanthines cause membrane potential oscillations in a unicellular green alga pointing to a ryanodine/cADPR receptor Ca2+ channel. Plant Cell Physiol. 1999;40:453–456. doi: 10.1093/oxfordjournals.pcp.a029563. [DOI] [Google Scholar]
- 88.Mackrill J.J. Ryanodine receptor calcium release channels: An evolutionary perspective. In: Islam M.S., editor. Calcium Signaling. Volume 740. Springer; Dordrecht, The Netherlands: 2012. pp. 159–182. [DOI] [PubMed] [Google Scholar]
- 89.Navazio L., Mariani P., Sanders D. Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiol. 2001;125:2129–2138. doi: 10.1104/pp.125.4.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hedrich R., Flügge U.-I., Fernandez J.M. Patch-clamp studies of ion transport in isolated plant vacuoles. FEBS Lett. 1986;204:228–232. doi: 10.1016/0014-5793(86)80817-1. [DOI] [Google Scholar]
- 91.Hedrich R., Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–835. doi: 10.1038/329833a0. [DOI] [Google Scholar]
- 92.Colombo R., Cerana R., Lado P., Peres A. Voltage-dependent channels permeable to K+ and Na+ in the membrane of Acer pseudoplantanus vacuoles. J. Membr. Biol. 1988;103:227–236. doi: 10.1007/BF01993982. [DOI] [Google Scholar]
- 93.Hedrich R., Barbier-Brygoo H., Felle H., Flügge U.-I., Lüttge U., Maathuis F.J.M., Marx S., Prins H.B.A., Raschke K., Schnabl H., et al. General mechanisms for solute transport across the tonoplast of plant vacuoles: A patch-clamp survey of ion channels and proton pumps. Bot. Acta. 1988;101:7–13. [Google Scholar]
- 94.Peiter E., Maathuis F.J.M., Mills L.N., Knight H., Pelloux J., Hetherington A.M., Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 95.Hedrich R., Marten I. TPC1—SV channels gain shape. Mol. Plant. 2011;4:428–441. doi: 10.1093/mp/ssr017. [DOI] [PubMed] [Google Scholar]
- 96.Ward J.M., Schroeder J.I. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pantoja O., Gelli A., Blumwald E. Voltage-dependent calcium channels in plant vacuoles. Science. 1992;255:1567–1570. doi: 10.1126/science.255.5051.1567. [DOI] [PubMed] [Google Scholar]
- 98.Pottosin I.I., Schönknecht G. Vacuolar calcium channels. J. Exp. Bot. 2007;58:1559–1569. doi: 10.1093/jxb/erm035. [DOI] [PubMed] [Google Scholar]
- 99.Johannes E., Sanders D. The voltage-gated Ca2+ release channel in the vacuolar membrane of sugar beet resides in two activity states. FEBS Lett. 1995;365:1–6. doi: 10.1016/0014-5793(95)00424-8. [DOI] [PubMed] [Google Scholar]
- 100.Johannes E., Sanders D. Lumenal calcium modulates unitary conductance and gating of a plant vacuolar calcium release channel. J. Membr. Biol. 1995;146:211–224. doi: 10.1007/BF00238010. [DOI] [PubMed] [Google Scholar]
- 101.Allen G.J., Sanders D. Calcineurin, a type 2B protein phosphatase, modulates the Ca2+-permeable slow vacuolar ion channel of stomatal guard cells. Plant Cell. 1995;7:1473–1483. doi: 10.1105/tpc.7.9.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pottosin I.I., Dobrovinskaya O.R., Muniz J. Conduction of monovalent and divalent cations in the slow vacuolar channel. J. Membr. Biol. 2001;181:55–65. doi: 10.1007/s0023200100073. [DOI] [PubMed] [Google Scholar]
- 103.Pottosin I.I., Tikhonova L.I., Hedrich R., Schönknecht G. Slowly activating vacuolar channels can not mediate Ca2+-induced Ca2+ release. Plant J. 1997;12:1387–1398. [Google Scholar]
- 104.Bewell M.A., Maathuis F.J.M., Allen G.J., Sanders D. Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 1999;458:41–44. doi: 10.1016/S0014-5793(99)01109-6. [DOI] [PubMed] [Google Scholar]
- 105.Pei Z.M., Ward J.M., Schroeder J.I. Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cell vacuoles. Plant Physiol. 1999;121:977–986. doi: 10.1104/pp.121.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carpaneto A., Cantu A.M., Gambale F. Effects of cytoplasmic Mg2+ on slowly activating channels in isolated vacuoles of Beta vulgaris. Planta. 2001;213:457–468. doi: 10.1007/s004250100519. [DOI] [PubMed] [Google Scholar]
- 107.Ivashikina N., Hedrich R. K+ currents through SV-type vacuolar channels are sensitive to elevated luminal sodium levels. Plant J. 2005;41:606–614. doi: 10.1111/j.1365-313X.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- 108.Carpaneto A., Cantu A.M., Gambale F. Redox agents regulate ion channel activity in vacuoles from higher plant cells. FEBS Lett. 1999;442:129–132. doi: 10.1016/S0014-5793(98)01642-1. [DOI] [PubMed] [Google Scholar]
- 109.Gradogna A., Scholz-Starke J., Gutla P.V.K., Carpaneto A. Fluorescence combined with excised patch: Measuring calcium currents in plant cation channels. Plant J. 2009;58:175–182. doi: 10.1111/j.1365-313X.2008.03762.x. [DOI] [PubMed] [Google Scholar]
- 110.Gambale F., Bregante M., Stragapede F., Cantu A.M. Ionic channels of the sugar beet tonoplast are regulated by a multi-ion single-file permeation mechanism. J. Membr. Biol. 1996;154:69–79. doi: 10.1007/s002329900133. [DOI] [PubMed] [Google Scholar]
- 111.Banks J.A., Nishiyama T., Hasebe M., Bowman J.L., Gribskov M., dePamphilis C., Albert V.A., Aono N., Aoyama T., Ambrose B.A., et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science. 2011;332:960–963. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Linz K.W., Köhler K. Vacuolar ion currents in the primitive green alga Eremosphaera viridis: The electrical properties are suggestive of both the Characeae and higher plants. Protoplasma. 1994;179:34–45. doi: 10.1007/BF01360735. [DOI] [Google Scholar]
- 113.Blanc G., Agarkova I., Grimwood J., Kuo A., Brueggeman A., Dunigan D., Gurnon J., Ladunga I., Lindquist E., Lucas S., et al. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 2012;13:R39. doi: 10.1186/gb-2012-13-5-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blanc G., Duncan G., Agarkova I., Borodovsky M., Gurnon J., Kuo A., Lindquist E., Lucas S., Pangilinan J., Polle J., et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell. 2010;22:2943–2955. doi: 10.1105/tpc.110.076406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collén J., Porcel B., Carré W., Ball S.G., Chaparro C., Tonon T., Barbeyron T., Michel G., Noel B., Valentin K., et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA. 2013;110:5247–5252. doi: 10.1073/pnas.1221259110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura Y., Sasaki N., Kobayashi M., Ojima N., Yasuike M., Shigenobu Y., Satomi M., Fukuma Y., Shiwaku K., Tsujimoto A., et al. The first symbiont-free genome sequence of marine red alga, Susabi-nori Pyropia yezoensis. PLoS One. 2013;8:e57122. doi: 10.1371/journal.pone.0057122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishibashi K., Suzuki M., Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 118.Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T., et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rybalchenko V., Ahuja M., Coblentz J., Churamani D., Patel S., Kiselyov K., Muallem S. Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J. Biol. Chem. 2012;287:20407–20416. doi: 10.1074/jbc.M112.359612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu M.X., Ma J., Parrington J., Galione A., Mark Evans A. TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010;584:1966–1974. doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee H.C. A unified mechanism of enzymatic synthesis of two calcium messengers: Cyclic ADP-ribose and NAADP. Biol. Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- 122.Lee H.C., Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 123.Genazzani A.A., Galione A. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem. J. 1996;315:721–725. doi: 10.1042/bj3150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chini E.N., Dousa T.P. Nicotinate-adenine dinucleotide phosphate-induced Ca2+-release does not behave as a Ca2+-induced Ca2+-release system. Biochem. J. 1996;316:709–711. doi: 10.1042/bj3160709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cancela J.M., Churchill G.C., Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 126.Wang X., Zhang X., Dong X.-P., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin-Moshier Y., Walseth T.F., Churamani D., Davidson S.M., Slama J.T., Hooper R., Brailoiu E., Patel S., Marchant J.S. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Navazio L., Bewell M.A., Siddiqua A., Dickinson G.D., Galione A., Sanders D. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc. Natl. Acad. Sci. USA. 2000;97:8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Islam M.M., Munemasa S., Hossain M.A., Nakamura Y., Mori I.C., Murata Y. Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol. 2010;51:302–311. doi: 10.1093/pcp/pcq001. [DOI] [PubMed] [Google Scholar]
- 130.Ranf S., Wunnenberg P., Lee J., Becker D., Dunkel M., Hedrich R., Scheel D., Dietrich P. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 2008;53:287–299. doi: 10.1111/j.1365-313X.2007.03342.x. [DOI] [PubMed] [Google Scholar]
- 131.Bonaventure G., Gfeller A., Proebsting W.M., Hortensteiner S., Chetelat A., Martinoia E., Farmer E.E. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 2007;49:889–898. doi: 10.1111/j.1365-313X.2006.03002.x. [DOI] [PubMed] [Google Scholar]
- 132.Beyhl D., Hörtensteiner S., Martinoia E., Farmer E.E., Fromm J., Marten I., Hedrich R. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 2009;58:715–723. doi: 10.1111/j.1365-313X.2009.03820.x. [DOI] [PubMed] [Google Scholar]
- 133.Bonaventure G., Gfeller A., Rodriguez V.M., Armand F., Farmer E.E. The fou2 gain-of-function allele and the wild-type allele of Two Pore Channel 1 contribute to different extents or by different mechanisms to defense gene expression in Arabidopsis. Plant Cell Physiol. 2007;48:1775–1789. doi: 10.1093/pcp/pcm151. [DOI] [PubMed] [Google Scholar]
- 134.Armengaud P., Breitling R., Amtmann A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004;136:2556–2576. doi: 10.1104/pp.104.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gilliham M., Athman A., Tyerman S.D., Conn S.J. Cell-specific compartmentation of mineral nutrients is an essential mechanism for optimal plant productivity—Another role for TPC1? Plant Signal. Behav. 2011;6:1656–1661. doi: 10.4161/psb.6.11.17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conn S.J., Berninger P., Broadley M.R., Gilliham M. Exploiting natural variation to uncover candidate genes that control element accumulation in Arabidopsis thaliana. New Phytol. 2012;193:859–866. doi: 10.1111/j.1469-8137.2011.03977.x. [DOI] [PubMed] [Google Scholar]
- 137.Furuichi T., Mori I.C., Takahashi K., Muto S. Sugar-induced increase in cytosolic Ca2+ in Arabidopsis thaliana whole plants. Plant Cell Physiol. 2001;42:1149–1155. doi: 10.1093/pcp/pce150. [DOI] [PubMed] [Google Scholar]
- 138.Furuichi T., Cunningham K.W., Muto S. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001;42:900–905. doi: 10.1093/pcp/pce145. [DOI] [PubMed] [Google Scholar]
- 139.Kadota Y., Furuichi T., Ogasawara Y., Goh T., Higashi K., Muto S., Kuchitsu K. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem. Biophys. Res. Commun. 2004;317:823–830. doi: 10.1016/j.bbrc.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 140.Hamada H., Kurusu T., Okuma E., Nokajima H., Kiyoduka M., Koyano T., Sugiyama Y., Okada K., Koga J., Saji H., et al. Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured rice cells. J. Biol. Chem. 2012;287:9931–9939. doi: 10.1074/jbc.M111.337659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kurusu T., Yagala T., Miyao A., Hirochika H., Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005;42:798–809. doi: 10.1111/j.1365-313X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- 142.Larisch N., Schulze C., Galione A., Dietrich P. An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic. 2012;13:1012–1022. doi: 10.1111/j.1600-0854.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 143.Amodeo G., Escobar A., Zeiger E. A cationic channel in the guard cell tonoplast of Allium cepa. Plant Physiol. 1994;105:999–1006. doi: 10.1104/pp.105.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carpaneto A., Cantu A.M., Busch H., Gambale F. Ion channels in the vacuoles of the seagrass Posidonia oceanica. FEBS Lett. 1997;412:236–240. doi: 10.1016/S0014-5793(97)00786-2. [DOI] [PubMed] [Google Scholar]
- 145.Wherrett T., Shabala S., Pottosin I. Different properties of SV channels in root vacuoles from near isogenic Al-tolerant and Al-sensitive wheat cultivars. FEBS Lett. 2005;579:6890–6894. doi: 10.1016/j.febslet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 146.Hashimoto K., Saito M., Hidetoshi I., Matsuoka H. Evidence for the plasma membrane localization of a putative voltage-dependent Ca2+ channel, OsTPC1, in rice. Plant Biotechnol. 2005;22:235–239. doi: 10.5511/plantbiotechnology.22.235. [DOI] [Google Scholar]
- 147.Dadacz-Narloch B., Kimura S., Kurusu T., Farmer E.E., Becker D., Kuchitsu K., Hedrich R. On the cellular site of two-pore channel TPC1 action in the Poaceae. New Phytol. 2013 doi: 10.1111/nph.12402. [DOI] [PubMed] [Google Scholar]
- 148.Chen Z.-H., Hills A., Bätz U., Amtmann A., Lew V.L., Blatt M.R. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol. 2012;159:1235–1251. doi: 10.1104/pp.112.197350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hills A., Chen Z.-H., Amtmann A., Blatt M.R., Lew V.L. OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol. 2012;159:1026–1042. doi: 10.1104/pp.112.197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuen C.C.Y., Christopher D.A. The group IV-A cyclic nucleotide-gated channels, CNGC19 and CNGC20, localize to the vacuole membrane in Arabidopsis thaliana. AoB Plants. 2013;5:plt012. doi: 10.1093/aobpla/plt012. [DOI] [Google Scholar]
- 151.Fischer C., Kugler A., Hoth S., Dietrich P. An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol. 2013;54:573–584. doi: 10.1093/pcp/pct021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dietrich P., Anschütz U., Kugler A., Becker D. Physiology and biophysics of plant ligand-gated ion channels. Plant Biol. 2010;12:80–93. doi: 10.1111/j.1438-8677.2010.00362.x. [DOI] [PubMed] [Google Scholar]
- 153.Chin K., Moeder W., Yoshioka K. Biological roles of cyclic-nucleotide-gated ion channels in plants: What we know and dont know about this 20 member ion channel family. Botany. 2009;87:668–677. doi: 10.1139/B08-147. [DOI] [Google Scholar]
- 154.Zelman A.K., Dawe A., Berkowitz G.A., Gehring C. Evolutionary and structural perspectives of plant cyclic nucleotide gated cation channels. Front. Plant Sci. 2012 doi: 10.3389/fpls.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kugler A., Kohler B., Palme K., Wolff P., Dietrich P. Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 2009;9:140. doi: 10.1186/1471-2229-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Quiles-Pando C., Rexach J., Navarro-Gochicoa M.T., Camacho-Cristóbal J.J., Herrera-Rodríguez M.B., González-Fontes A. Boron deficiency increases the levels of cytosolic Ca2+ and expression of Ca2+-related genes in Arabidopsis thaliana roots. Plant Physiol. Biochem. 2013;65:55–60. doi: 10.1016/j.plaphy.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 157.Palmer C.P., Zhou X.L., Lin J., Loukin S.H., Kung C., Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cunningham K.W. Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium. 2011;50:129–138. doi: 10.1016/j.ceca.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scrase-Field S.A., Knight M.R. Calcium: Just a chemical switch? Curr. Opin. Plant Biol. 2003;6:500–506. doi: 10.1016/S1369-5266(03)00091-8. [DOI] [PubMed] [Google Scholar]
- 160.McAinsh M.R., Pittman J.K. Shaping the calcium signature. New Phytol. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- 161.Zhu X., Feng Y., Liang G., Liu N., Zhu J.-K. Aequorin-based luminescence imaging reveals stimulus- and tissue-specific Ca2+ dynamics in Arabidopsis plants. Mol. Plant. 2013;6:444–455. doi: 10.1093/mp/sst013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tracy F.E., Gilliham M., Dodd A.N., Webb A.A.R., Tester M. NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ. 2008;31:1063–1073. doi: 10.1111/j.1365-3040.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- 163.Allen G.J., Chu S.P., Harrington C.L., Schumacher K., Hoffmann T., Tang Y.Y., Grill E., Schroeder J.I. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1057. doi: 10.1038/35082575. [DOI] [PubMed] [Google Scholar]
- 164.Bonza M.C., de Michelis M.I. The plant Ca2+-ATPase repertoire: Biochemical features and physiological functions. Plant Biol. 2011;13:421–430. doi: 10.1111/j.1438-8677.2010.00405.x. [DOI] [PubMed] [Google Scholar]
- 165.Huda K.M.K., Banu M.S.A., Tuteja R., Tuteja N. Global calcium transducer P-type Ca2+-ATPases open new avenues for agriculture by regulating stress signalling. J. Exp. Bot. 2013;64:3099–3109. doi: 10.1093/jxb/ert182. [DOI] [PubMed] [Google Scholar]
- 166.Pedersen C.N.S., Axelsen K.B., Harper J.F., Palmgren M.G. Evolution of plant P-type ATPases. Front. Plant Sci. 2012;3:31. doi: 10.3389/fpls.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Geisler M., Frangne N., Gomes E., Martinoia E., Palmgren M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000;124:1814–1827. doi: 10.1104/pp.124.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee S.M., Kim H.S., Han H.J., Moon B.C., Kim C.Y., Harper J.F., Chung W.S. Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Lett. 2007;581:3943–3949. doi: 10.1016/j.febslet.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 169.Boursiac Y., Lee S.M., Romanowsky S., Blank R., Sladek C., Chung W.S., Harper J.F. Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol. 2010;154:1158–1171. doi: 10.1104/pp.110.159038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qudeimat E., Faltusz A.M.C., Wheeler G., Lang D., Brownlee C., Reski R., Frank W. A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc. Natl. Acad. Sci. USA. 2008;105:19555–19560. doi: 10.1073/pnas.0800864105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu X., Caplan J., Mamillapalli P., Czymmek K., Dinesh-Kumar S.P. Function of endoplasmic reticulum calcium ATPase in innate immunity-mediated programmed cell death. EMBO J. 2010;29:1007–1018. doi: 10.1038/emboj.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pittman J. Multiple transport pathways for mediating intracellular pH homeostasis: The contribution of H+/ion exchangers. Front. Plant Sci. 2012;3:11. doi: 10.3389/fpls.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Emery L., Whelan S., Hirschi K.D., Pittman J.K. Protein phylogenetic analysis of Ca2+/cation antiporters and insights into their evolution in plants. Front. Plant Sci. 2012;3:1–19. doi: 10.3389/fpls.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Manohar M., Shigaki T., Hirschi K.D. Plant cation/H+ exchangers (CAXs): Biological functions and genetic manipulations. Plant Biol. 2011;13:561–569. doi: 10.1111/j.1438-8677.2011.00466.x. [DOI] [PubMed] [Google Scholar]
- 175.Cheng N.-H., Pittman J.K., Shigaki T., Lachmansingh J., LeClere S., Lahner B., Salt D.E., Hirschi K.D. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005;138:2048–2060. doi: 10.1104/pp.105.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Connorton J.M., Webster R.E., Cheng N., Pittman J.K. Knockout of multiple Arabidopsis cation/H+ exchangers suggests isoform-specific roles in metal stress response, germination and seed mineral nutrition. PLoS One. 2012;7:e47455. doi: 10.1371/journal.pone.0047455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Conn S.J., Gilliham M., Athman A., Schreiber A.W., Baumann U., Moller I., Cheng N.-H., Stancombe M.A., Hirschi K.D., Webb A.A.R., et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell. 2011;23:240–257. doi: 10.1105/tpc.109.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cho D., Villiers F., Kroniewicz L., Lee S., Seo Y.J., Hirschi K.D., Leonhardt N., Kwak J.M. Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol. 2012;160:1293–1302. doi: 10.1104/pp.112.201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu L., Zahid K.R., He L., Zhang W., He X., Zhang X., Yang X., Zhu L. GhCAX3 Gene, a novel Ca2+/H+ exchanger from cotton, confers regulation of cold response and ABA induced signal transduction. PLoS One. 2013;8:e66303. doi: 10.1371/journal.pone.0066303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao J., Barkla B., Marshall J., Pittman J., Hirschi K. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta. 2008;227:659–669. doi: 10.1007/s00425-007-0648-2. [DOI] [PubMed] [Google Scholar]