Abstract
Many plants can establish symbioses with nitrogen-fixing bacteria, some of which lead to nodulation, including legumes. Indeed, in the rhizobium/legume symbiosis, new root organs, called nodules, are formed by the plant in order to host the rhizobia in protective conditions, optimized for nitrogen fixation. In this way, these plants can benefit from the reduction of atmospheric dinitrogen into ammonia by the hosted bacteria, and in exchange the plant provides the rhizobia with a carbon source. Since this symbiosis is costly for the plant it is highly regulated. Both legume nodule and lateral root organogenesis involve divisions of the root inner tissues, and both developmental programs are tightly controlled by plant hormones. In fact, most of the major plant hormones, such as auxin, cytokinins, abscisic acid, and strigolactones, control both lateral root formation and nodule organogenesis, but often in an opposite manner. This suggests that the sensitivity of legume plants to some phytohormones could be linked to the antagonism that exists between the processes of nodulation and lateral root formation. Here, we will review the implication of some major phytohormones in lateral root formation in legumes, compare them with their roles in nodulation, and discuss specificities and divergences from non-legume eudicot plants such as Arabidopsis thaliana.
Keywords: auxin, peptides, lateral root, local/systemic regulation, miRNA, Medicago truncatula
1. Legume Plants and Root Development
Nitrogen is indispensable for life and is required in many biochemical processes such as nucleic acid and amino acid biosynthesis. Despite the huge quantity of dinitrogen (N2) present in the air, mineral forms of nitrogen (nitrate and ammonium) are often limiting in the soil. Although four different orders of flowering plants (Rosales/Fagales/Cucurbitales/Fabales) are able to associate with micro-organisms that fix dinitrogen, the Leguminosae has the highest proportion of species able to nodulate [1]. This nodulation capability is thought to have favored the success and radiation of the Leguminosae. Indeed, the Leguminosae (or Fabaceae) is the third largest family of flowering plants and most of its 20,000 species are able to nodulate. Nodulation implies the formation of a new organ, the nodule, that hosts soil bacteria, called rhizobia, in conditions optimal for nitrogen fixation. To some extent, nodules resemble lateral roots and they originate from the same root cell layers. Two types of nodules can be discriminated depending on the persistence or not of their meristematic activity. Hence, most temperate legumes, like Medicago ssp., Pisum sativum (pea) and Trifolium sp. (clover), produce indeterminate nodules whose meristems persist over the entire nodule lifespan while other legumes, like Lotus japonicus (Lotus) or Glycine max (soybean), produce determinate nodules, whose meristematic activity stops early on during nodule development giving rise to small, round shaped nodules. In legume species, both the onset of nodulation and rhizobial entry into the root are dependent on a specific molecular dialog involving the production by the rhizobia of Nodulation Factors (NF) which also control notably host specificity [2]. Downstream of NF perception, signal transduction notably involves calcium spiking ([3] for review).
Hormones are regulatory signals produced within an organism and usually active at very low concentrations and at a distance from their site of production. Plants, like other eukaryotes, produce different types of hormones. In contrast to animals, it is more difficult to specify which cells produce phytohormones and distinguish them from cells that perceive the hormone signals. However, hormonal control of plant growth is well established and was first studied with auxin at the beginning of the 20th century [4]. In Leguminosae, many phytohormones control both root and nodule development. Interestingly, Liang and Harris [5] have studied the ability of various legumes and non-legumes plants to respond to the phytohormone abscisic acid (ABA) by increasing their lateral root (LR) density and hypothesized that the common predisposition of plants to form nodules may be linked to a difference in ABA sensitivity. Hence Leguminosae may have evolved specific hormonal sensitivities that enable them to control two types of “lateral” root organs, i.e., LR and nodules.
In this review, we will concentrate on nodulating legumes from the Papilionoideae clade that have been more widely studied and especially on Medicago truncatula (a diploid model close to alfalfa, M. sativa), soybean (Glycine max), Lotus (Lotus japonicus), pea (Pisum sativum) and clover (Trifolium repens). We will review the ability of endogenous and exogenous signals to modulate the root development of these plants and show how this modulation is linked to hormonal control. When relevant, we will highlight the dual hormonal controls on root and nodule development and possible differences between hormonal control of root development in legumes and Arabidopsis thaliana (that does not nodulate), as summarized in Table 1.
Table 1.
Summary of the action of the main phytohormones on root development and nodulation in legumes and root development in Arabidopsis or other non-legume dicot species (when specified).
| Hormone | Action on Root Development in Legumes | Action on Nodulation | Action on Root Development in Arabidopsis or Other Dicots | References |
|---|---|---|---|---|
| Auxin | + on LRF at low doses, − at higher doses | + at low doses on indeterminate nodules | + on LRF at low doses, − at higher doses | [6,7,8,9] |
| Cytokinins | − on LRF | + on nodulation | − on LRF | [10,11,12] |
| Abscisic acid (ABA) | + on LRF at low doses (≤ 10−7 M), − at higher doses + on intermediate LRF stages and meristematic activity |
− on nodule development and NF signaling | − on LRF at 10−7 M − on LRP emergence and meristematic activity |
[5,13,14,15,16] |
| Ethylene | + on LRF at low doses, − at higher doses. Inhibits primary root length |
− | − on LRF and primary root growth through auxin interaction | [17,18,19,20,21] |
| Nitric oxide | Present in LRP. Necessary downstream of auxin for LRF? |
Necessary for early infection and nodule primordia formation. Accelerates nodule senescence | + on LRF through reactivation of cell cycle genes in tomato, downstream of auxin. + on LRF in sunflower seedlings | [8,22,23,24,25,26,27] |
| Jasmonic acid | + on LRF, − on primary root length | − on nodulation by acting on NF signaling | + on LRF and − on primary root length | [28,29] |
| Brassinosteroids | − on primary root length and LRF | ± (cf. [30], for review) | Promote LR emergence at low doses (10−8 M) Promote LRF and root apical meristem maintenance |
[31,32] |
| Gibberelic acid | Pea biosynthetic mutants are dwarf with fewer LR | May require an optimum concentration + on pea, − on lotus [33] |
Inhibits LRF (in poplar) | [31,34] |
| Strigolactones | Reduce LRF from 10−7 M | + at low doses in M. truncatula and in pea, lotus; − at higher doses | − on LRF (phosphate dependent conditions) | [35,36,37,38] |
| CLE/CLV1 | − on LRF (dependent on nitrate status in the shoot) | − on nodulation through the AON pathway | − on LRF in nitrate limiting conditions | [39,40,41,42] |
| CEP/LRR-RLK | Reduces LRF | Enhances nodulation in a systemic pathway | Reduces LRF in nitrate limiting conditions (systemic action) | [43,44,45] |
Data is compiled for legumes from literature in M. truncatula, L. japonicus, Glycine max (soybean) and Pisum sativum (pea). LRF: Lateral Root Formation. CLE: CLAVATA3/EMBRYO-SURROUNDING REGION peptides; CLV1: Clavata 1; CEP: C-terminally Encoded Peptide; LRR-RLK: Leucine Rich Repeat Receptor Like Kinase.
2. Root Anatomy: Cell Layers and Definitions
The root system plays a major role in plants as it ensures the anchorage of plants in the soil and their nutrition. Roots are also highly reactive to exogenous “signals” such as nutrient availability or biotic stresses (pathogens or symbionts) and their development is, therefore, highly plastic. The root system of eudicot species is classically composed of a primary root (which directly originates from the radicle formed in the embryo) and LR that are formed post embryonically and are thus very responsive to environmental cues [46]. When talking about root development, one generally takes into account both the primary root growth and LR formation (LRF), these two parameters can be recapitulated by the LR density (number of LR per cm of primary root) [47].
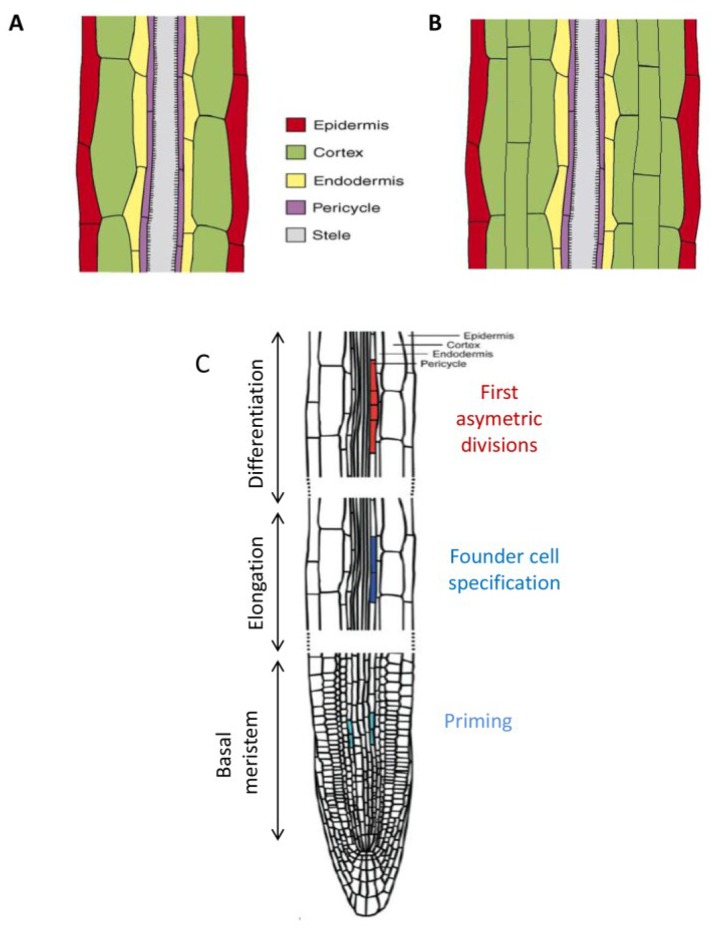
The primary root of Angiosperms can be divided into different developmental zones along the proximo-distal axis, following a differentiation gradient [48]. Hence, close to the root tip is the root apical meristem, whose cells are dividing and will give rise to the different cell layers of the root. Above the root tip is the elongation zone, where the cells stop dividing and start elongating. In between these two zones is the basal meristem, which has a critical role in specifying LR founder cells [49]. Above the elongation zone (towards the shoot) is the differentiation zone where the cells differentiate, for example to form root hairs (cf. Figure 1C).
Figure 1.
Schematic organization of the primary root. (A) The different cell layers in a longitudinal section along the primary root in Arabidopsis, note that there is only one cell per root cell layer; (B) the different cell layers in a longitudinal section along the primary root of M. truncatula, showing several cortical cells (only three are represented whereas four to five layers are usually observed); (C) Longitudinal organization of the primary root in the younger region of the root (close to the root tip), showing the different zones of the LR specification steps. (A) and (B) are adapted from [50]; (C) is adapted from [49].
On the radial axis, the root is formed of several concentric cell layers (cf. Figure 1A,B). The innermost layer of the root is named the stele and comprises the vessels (xylem and phloem) that are responsible for the movement of water and nutrients inside the plant. The stele is surrounded by the pericycle, a tissue that is notably able to dedifferentiate to initiate the formation of new LR. Next is the endodermis, that is generally suberified (in the older part of the root) and forms a natural barrier between the inner part of the root and the cortex. The root cortex is generally composed of several cell layers, although Arabidopsis has only one cell layer for each of these root tissues (cf. Figure 1A,B). The outermost cell layer is the root epidermis (or rhizodermis) whose cells can form root hairs that are privileged sites of water and nutrient uptake.
3. Auxin, a Major Regulator of LRF and Nodule Development
Molecular root development studies have mostly been conducted on the model species Arabidopsis thaliana. This plant is a small dicot species from the Brassicaceae family which has only a single cell per root cell layer (Figure 1A). Thanks in particular to its simple root architecture, Arabidopsis has been a very powerful model to unravel the early steps of LRF and the role of the phytohormone auxin in this process.
Hence, it has been shown that LR originate from divisions in the pericycle. However, LR are formed sequentially and not all the cells from the pericycle are competent to form LR ([51,52] and references therein). Indeed, it was shown that only some of these pericycle cells are “primed” to become LR founder cells. In Arabidopsis, these LR founder cells are always found opposite a protoxylem pole and are characterized by an increased auxin accumulation [53]. Moreover, De Smet and collaborators have shown that priming is linked to a pulsatile auxin response in protoxylem cells in the basal meristem of the root [54]. Following the priming event, founder cell specification occurs within a developmental window that is located in a well-defined zone along the primary root axis where auxin content and response are minimal [55].
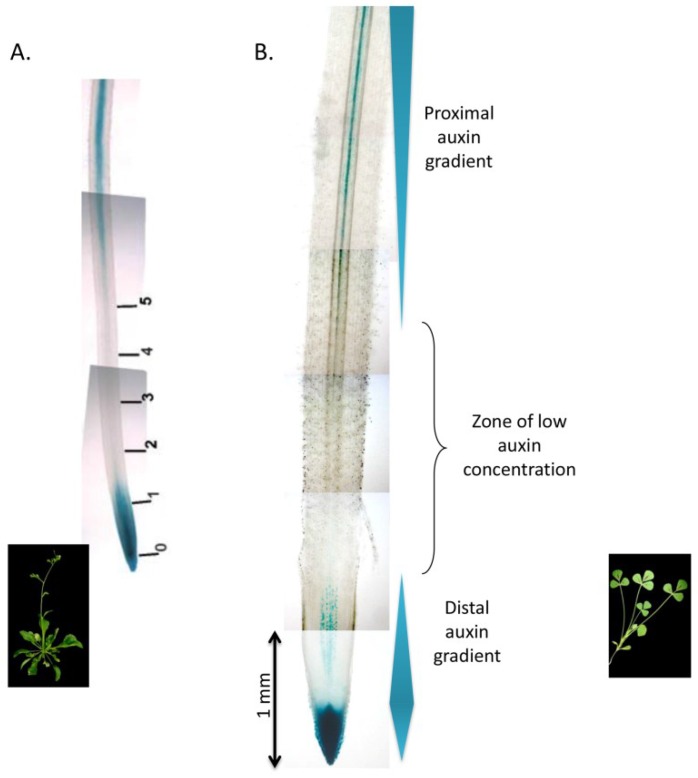
Auxin is the major phytohormone governing LRF and primary root growth in all plants so far studied, although most of the molecular evidence has been shown on Arabidopsis. For instance, it has been shown that local auxin accumulation in pericycle cells is sufficient to trigger LRF in Arabidopsis [53]. Auxin is also involved at later steps of LRF, such as the preparation of LR emergence from the main root [56]. Auxin is mainly synthesized in the shoot, but a portion is also locally produced in the root [57]. Auxin is thus moving from the shoot to the root (in an acropetal movement) and from the root to the shoot (mainly recycled at the root tip via auxin efflux transporters) in a basipetal movement [58]. Hence, two auxin gradients are formed in the root (cf. Figure 2). Auxin perception can be monitored by a reporter system, consisting of the auxin sensitive synthetic promoter DR5 fused to a GUS or GFP reporter gene. DR5 is a sevenfold tandem repeat of auxin responsive elements originally found in an auxin responsive GH3 gene from soybean [59]. Thus, DR5 reporters are suitable for legumes and have been used to assess auxin perception during root and nodule development [60,61]. As shown in Figure 2, M. truncatula displays the same auxin gradients as Arabidopsis as revealed by the DR5:GUS promoter, and notably the same zone of auxin minimum above the basal meristem. We have also shown that the LR initiation zone is located at the beginning of the differentiation zone in M. truncatula [61] as shown in Arabidopsis [62].
Figure 2.
DR5:GUS reporter gene activity reflecting auxin gradients in M. truncatula and A. thaliana primary roots. (A) DR5:GUS expression gradient in Arabidopsis, as described in [55]; (B) DR5:GUS expression gradient in M. truncatula [63]. GUS activity appears in blue.
Using the same DR5:GUS transgenic plants, we have described zones of auxin perception during LRF in M. truncatula [61]. We have thus shown that, as in Arabidopsis, DR5:GUS activity precedes the first divisions of pericycle cells. Auxin perception also precedes and possibly triggers the divisions of other cell layers contributing to LRF in M. truncatula, namely the endodermis and inner cortex [61].
Indeed, unlike Arabidopsis but similarly to many other plants, LRF in M. truncatula does not only involve pericycle cell divisions but also a large contribution of the endodermis and innermost layers of the cortex (cf. Figure 3) [61,64]. Endodermal divisions were also described during white clover, arachis, and pea LRF [65,66,67,68], as well as in non-legumes [65,66,67]. Cortical cell divisions and contribution to the formation of the LR primordium (LRP) varies between species, even within legume species. For example, cortical cell divisions were shown to accompany LRF in Lotus but, in that case, a more important contribution of outer cortical cell layers was observed [64], reminiscent of the important contribution of the outer cortex layer for determinate nodule development; whereas inner cortical cell divisions are contributing more significantly to LRP formation in M. truncatula, that forms indeterminate nodules [61,64]. However, very little inner cortical cell divisions and contribution to the LRP was described in white clover [68]. Cortical cell divisions can contribute either to the formation of the LRP itself or facilitate LRP emergence [56]. Hence, endodermis and cortical contribution to the LRP are not specific to legumes but, interestingly, endodermal and cortical cell divisions are also involved in nodule formation [64,69,70].
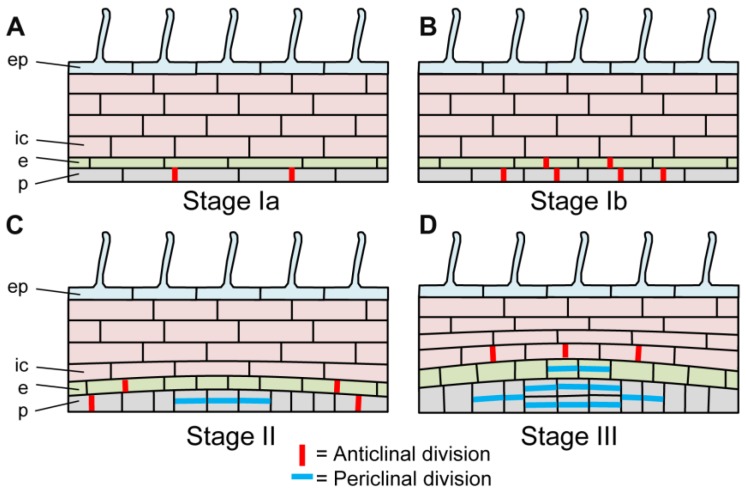
Figure 3.
Schematic representation of the early developmental stages of lateral root formation in Medicago truncatula. Scheme of longitudinal sections in the main root of M. truncatula during LRF, showing the main type of cell divisions but not the precise number of dividing cells. (A). Stage Ia. Anticlinal divisions in the pericycle; (B). Stage Ib. Anticlinal divisions in the endodermis and the pericycle; (C). Stage II. Periclinal divisions in the pericycle and anticlinal divisions in the endodermis and the pericycle; (D). Stage III. Periclinal divisions in the endodermis (two cell layers) and the pericycle (four cell layers), and anticlinal divisions in the inner cortex. p: pericycle; e: endodermis; ic: inner cortex, ep: epidermis. From [61].
Auxin perception zones have been extrapolated from DR5 or GH3 reporter systems both during LRF and nodule development in legumes. Hence, Mathesius et al. noticed a local increase in GH3:GUS expression in cortical cells after compatible rhizobium spot inoculation in white clover [71] and Pacios-Bras et al., noticed GH3:GUS and GH3:GFP up-regulation more specifically in outer cortical cells after spot inoculation in L. japonicus reporter lines [72]. In the same way, Suzaki and co-workers observed a local high expression of DR5:GFP reporter upon nodule development in L. japonicus [60]. In the study of white clover, Mathesius et al. also noticed high GH3:GUS expression in dividing pericycle cells during LRF as well as strong GH3:GUS expression in cortical cells in front of developing LRP [71]. We have also noticed DR5:GUS expression in cortical cells above developing LRP in M. truncatula [61].
These evidences point at a role for auxin perception in nodule development as well as in preparing LR emergence in legumes, as observed in Arabidopsis [56].
As in Arabidopsis and many other plants, auxin plays the same major role for root development in legumes. We will briefly summarize some studies that have addressed the significance of auxin and particularly auxin perception and signaling, in LRF as well as in nodulation in legumes. Indeed, on top of its role in cell division and nodule development, auxin was also recently shown to be important for rhizobium infection as well [73].
3.1. Auxin Perception and Signaling
Auxin accumulation has been shown to be necessary for both LRF and nodule development. For example, application of auxin transport inhibitors such as NPA or TIBA results in pseudo-nodules formation in Medicago ssp. [6,74] and disruption of auxin transport by knocking down efflux transporter of the PIN family resulted in a reduced number of nodules in M. truncatula [75]. Moreover, a low concentration of auxin (<10−7 M of IAA) has been shown to enhance nodulation in M. truncatula [7]. Some rhizobium strains engineered to produce more auxin (Indole-3-Acetic Acid, IAA) can enhance nodule and LR development in Medicago truncatula—that forms indeterminate nodules—but has no effect on nodule number in Phaseolus vulgaris, which forms determinate nodules [8]. Turner and collaborators have down-regulated auxin perception by overexpressing the micro-RNA miR393 that induces mRNA degradation of auxin receptors, both in soybean and M. truncatula [76]. In both species, this down-regulation of auxin perception led to a decreased sensitivity to auxin for primary root elongation and LRF but opposite effects were observed on nodulation between these two species. Whereas miR393 over-expression resulted in less LR, it had no effect on nodulation in soybean [76], however, the same miR393 overexpression reduced both LR density and nodule number in M. truncatula [77]. This supports the hypothesis that determinate and indeterminate nodules do not have the same auxin requirement for their development (even if auxin perception is required for LRF in both species as shown by the reduced LR density observed in the miR393 overexpressing lines).
Other auxin signaling pathways components have been targeted using microRNA overexpression. Thus, overexpressing miR160, which targets the ARF10/16/17 family of auxin signaling repressors enhanced auxin sensitivity and drastically reduced nodule primordia development in soybean [76] and Medicago [78]. Another transcription factor (TF) that is regulated by auxin and miRNA is AtNAC1, that has been shown to positively regulate LRF in Arabidopsis [79]. Overexpression of miR164, that targets NAC1 reduced LRF but had no significant effect on nodulation in soybean [77] whereas it reduced nodule number without any effect on LRF in M. truncatula [80]. However, mutation in the MtNAC1 gene was not sufficient to recapitulate this nodulation effect in Medicago [80]. This suggests again that auxin sensitivity required for determinate (as in soybean) and inderterminate (as in Medicago) nodule development is different.
Ta-siRNAs are also known to regulate auxin signaling pathway components such as the ARF3/4 transcription factors. Li et al. recently identified a mutant (rel3) involved in ta-siRNA production in L. japonicus and showed that this mutant displayed higher levels of ARF3 and ARF4 transcripts [81]. Interestingly, this mutant displayed a reduced primary root length and a reduced number of both LR and nodules. Rel3 roots showed reduced auxin sensitivity and enhanced ethylene perception or synthesis since a normal nodule number could be restored by exogenous application of ethylene antagonists (AVG or Ag+) [81].
Altogether, this supports a positive role of auxin in nodulation and LRF in legumes.
An important cellular role of auxin is notably to control cell cycle progression (and hence cell divisions). Interestingly, Kuppusamy et al. identified a component of the APC/C degradation complex (that controls notably cyclins turnover), named MtCDC16, which is induced upon nodulation. MtCDC16 is also expressed in root tips and LRP and is induced by auxin. MtCDC16 knock-down using an RNAi construct reduces root sensitivity to auxin as well as root length and LRF but increases nodule numbers in Medicago [82].This points to a possible opposite control of some auxin downstream elements on nodulation and LRF in legumes. Whether this opposite effect is due to a control of the balance between LRP and nodule primordia or to something else need to be elucidated.
3.2. Auxin Transport and Biosynthesis
In addition to its signaling pathway, auxin can be regulated by its transport. The PIN family of auxin efflux carriers has for instance been shown to redirect auxin flow in different cell layers [58]. Huo et al. have silenced PIN2/3/4 in M. truncatula and shown a negative effect on nodulation, although effect on LRF was not assessed [75]. Interestingly, some flavonoids, which are known to be produced by the host plant to establish nodulation, were also shown to act as auxin transport inhibitors [71]. Wasson et al. have silenced the flavonoid biosynthetic enzyme Chalcone Synthase (CHS) in M. truncatula and observed a decrease in nodule formation but no LRF default [83]. However, the same type of experiment (silencing of CHS) in soybean did not result in any major nodulation defects [84]. Once again, this points to differences between determinate and indeterminate nodules. The hypernodulation mutant sunn (super numeric nodules) was also shown to be affected in the long range shoot to root auxin transport modification induced upon rhizobial infection [7]. Indeed, sunn mutants did not show the reduction in auxin acropetal transport observed in wild type plants 24 h post rhizobial inoculation and this was hypothesized to be due to the lack of inhibition of auxin loading in the shoot following rhizobium inoculation in the sunn mutant [7]. This suggests that systemic control of nodulation [85] could also involve the regulation of shoot to root auxin loading and regulation of auxin concentration in the root. For a more complete view of the importance of auxin in nodulation see [86].
4. Endogenous Control of LRF by Other Hormones
Many other plant hormones control LRF, often in direct relation with auxin. Among these different hormones, we will review the major hormones for which root development data in legumes are available.
4.1. Cytokinins, Strigolactones, Gibberellic Acid, and Brassinosteroids
The main antagonists of auxin action are cytokinins (CK). These hormones are principally synthesized in the root but also in the aerial part of the plant [10]. CK have a promoting action on shoot branching but negatively regulate LRF, notably by negatively regulating auxin transport [10,87]. Held and collaborators have monitored CK response in both primary root and during nodule development in Lotus [88] using the synthetic TCS promoter [89]. In that way, they showed that CK response is high at the base of the LRP and later on in the root apex and transition zone. The TCS:GUS construct was also active throughout nodule development and in the Lotus root epidermis upon interaction with Mesorhizobium loti [88]. Loss of function in the cytokinin receptor genes MtCRE1 and LjLHK1 both reduces nodulation and enhances LRF [11,90], suggesting that the function of CK as negative regulators of LRF is conserved in legumes, where CK have evolved a positive role in nodule organogenesis [12]. Indeed, a gain of function mutation in the kinase domain of LjLHK1 results in spontaneous nodule development in the absence of rhizobium inoculation, pointing at the crucial role of CK perception in the onset of nodule development [91]. Downstream of CK perception are positive and negative regulators, named “response regulators” or RR genes. Type B-RRs are rapidly phosphorylated upon CK perception and regulate the transcription of type A-RRs that lack a DNA binding domain and are usually considered as negative regulators of CK signaling, as well as early and sensitive markers of CK signaling [10]. For example, Plet et al. have shown a rapid induction of the type A-RR MtRR4 by CK, in a MtCRE1 dependent manner, and shown that MtRR4 was expressed both during early stages of nodule development and later on in the apical zone of mature nodules. Interestingly, MtCRE1 mutants show a more important acropetal auxin transport in their root tip, both under symbiotic (rhizobium inoculation) and non-symbiotic condition. This was accompanied by an increased expression of some PIN genes and an accumulation of PIN proteins in Mtcre1 mutant roots [92]. Altogether, this suggests a negative feedback of CK signaling on auxin transport in M. truncatula, as observed in Arabidopsis. Lohar et al. have also shown, using an Arabidopsis promoter reporter fusion proARR5:GUS in L. japonicus roots, that this early target gene of CK signaling is not expressed in the pericycle undergoing cell divisions during early steps of LRF [93]. In the same way, down regulating CK levels of roots by overexpressing a cytokinin oxidase gene (that degrades CK) leads to an increase in LR and a decrease in nodule numbers in Lotus [93].
In an attempt to find nodulation specific RRs, Op den Camp et al. have looked at type A-RR genes that originated after the whole genome duplication that occurred in the Papilionoideae subfamily, with the hypothesis that these RRs could have been neo-functionalized to act during nodule development [64]. They found one pair of such genes, which they called MtRR9 and MtRR11 in M. truncatula. MtRR9 and MtRR11 expression was induced upon purified NF application, as well as during nodule development. However, knocking down the expression of MtRR9 using an RNAi strategy reduced both LR and nodule numbers and over-expression of MtRR9 led to the development of “nodule like” structure that were sharing both some of the nodule and LR development features, making it difficult to discriminate if MtRR9 had any specific role in nodule development compared to LR development [64].
Thus, CKs do have a negative role on LRF in legumes and a positive role on nodulation (and especially on nodule organogenesis). How this dual regulation is achieved is not fully understood, although it may rely on a tissue specific action with a difference balance of auxin/cytokinins in pericycle and cortical tissues for instance.
Strigolactones (SGL) were identified as hormones that control shoot branching but also control root branching. Although the action of SGL seems dependent on phosphate concentrations in the growth medium, they seem to negatively regulate LRF in Arabidopsis, with a direct connection to auxin transport [35]. In M. truncatula, exogenous application of the SGL analog GR24 at 10−7 M reduced LRF and, although a transient stimulation of nodule number can be observed using 10−7 M GR24, application in the micromolar range strongly inhibited nodule development [36]. In contrast, Foo et al. reported that nodulation is reduced in SGL Psccd7 and Psccd8 biosynthesis mutants in pea (but enhanced in the rms4 signaling mutant) [37,94]. The effect of GR24 on LRF in M. truncatula was observed on 19 day old plants in phosphate limiting conditions. Using the same growth conditions but younger (7 day old) plants, we observed a transient stimulation of LRF by 10−7 M GR24 application [95]. This may be linked to a different phosphate starvation status in younger plants that can benefit from cotyledon storage. However, a negative role for SGL in LRF in legumes is also suggested by the phenotype of the SGL biosynthesis mutants Mtccd7 and Mtccd8 [96]. Indeed, when grown under phosphate limiting conditions, both these mutants displayed a significantly higher number of LR than their corresponding wild type plants [97]. As found in Arabidopsis, SGL and auxin pathways are probably closely linked in legumes as well. Hence the semi-dominant mutant bushy (bsh) of pea shows a reduced auxin content (of approx 12-fold in the shoot and 3-fold in the root) and displays a root developmental phenotype and notably reduced LR root length and number ([98] or therein). Interestingly, the bsh mutant also shows reduced expression of the SGL biosynthetic gene PsCCD8, revealing a positive action of auxin on SGL biosynthesis [98]. Taken together, both genetic and pharmacological data suggests that SGL, in legumes as in Arabidopsis, are closely linked to auxin responses and that SGL are overall negative regulators of both LRF and nodulation in legumes, although their action may depend on the overall phosphate status of the plant.
Gibberellic acid (GA) and Brassinosteroids (BR) are phytohormones that control plant growth. Most of the legume mutants available so far in the GA and BR pathways were found in pea [31,99]. In pea, biosynthetic mutants of GA and BR form fewer LR and fewer nodules [31,99], although it may be difficult to quantify their effect exactly since they display a global dwarf phenotype. However, Maekawa et al. reported a negative role of GA on nodulation in L. japonicus, specifically, overexpression of a gain of function allele of SLEEPY, a F-box protein that acts as a positive regulator of GA signaling, resulted in reduced number of nodules but no overall effect on LR number in Lotus hairy roots [100]. Overall, the knowledge of the contribution of GA in the regulation of LRF and nodule development in legumes is still poor but should increase with the development of new genetic tools, and especially dominant negative regulators of GA signaling, as already done to test their role in mycorrhization [101].
For BR, Bazin et al. have shown that the miRNA miR396—that targets several growth-regulating factor genes (MtGRF) and two bHLH79—is induced by short BR treatment in M. truncatula. Overexpression of miR396 in M. truncatula affects primary root length and reduces arbuscular mycorrhizal colonization but not nodulation [102].
Other endogenous factors involved in LRF but with no obvious link to hormonal control have been documented in legumes. For instance, Boualem and collegues have shown that miR166 that targets class III HD-ZIP transcription factors (with known developmental roles in Arabidopsis) can control root and nodule development in M. truncatula [103]. Thus, overexpression of miR166 led to a decreased capability to form LR and nodules in hairy roots. Although miR166 has a link with auxin in Arabidopsis and despite a strong miR166 expression in LRP, nodule primordia and vascular tissues in M. truncatula, no link between the action of miR166 and auxin was shown in this study.
4.2. Stress Related Hormones
Although most plant hormones can be involved in stress responses, some of them have been often described as “stress related” and linked to abiotic (such as drought, salt…) or biotic (pathogen interaction) stresses.
One major hormone involved in abiotic (and biotic) stress and root development is absicisic acid (ABA) which has been shown to control both nodulation in several legumes [15,104] and, more recently, arbuscular mycorrhizal interactions in M. truncatula [105]. The ability of legumes to respond to ABA application, in a range of 10−7 to 10−6 M, by stimulating LRF and increasing LR density—instead of an inhibitory action in non-legumes and non-nodulating plants such as Arabidopsis—has been suggested to be a prerequisite for the ability to nodulate [5]. Interestingly, as summarized in Table 1, ABA has an antagonistic role in the regulation of LR and nodule development since the same concentration range that stimulates LRF actually inhibits nodulation [15]. This negative role of ABA was shown to be via an action on early NF signaling through inhibition of calcium spiking and, later on, by an action on nodule organogenesis through interaction with CK [15], and even on nodule senescence through the nitric oxide pathway [106]. During LRF, we have shown that 10−7 M ABA stimulates pre-emergence stages in M. truncatula [13]. Interestingly, ABA was also shown to rescue root meristem maintenance in the latD mutant of M. truncatula, mutated in a NRT1 member of the nitrate transporter family important both for nodule and root meristem maintenance [107,108]. These data point to another difference between legumes and Arabidopsis, in which ABA prevents LR emergence and does not promote meristematic activity [14]. However, this difference from Arabidopsis is lost at higher ABA concentrations. Indeed, Ariel et al. have shown that ABA was implicated in repressing LR emergence when applied in the 50 µM range [16].
Ariel et al. suggested that in M. truncatula, upon salt stress, ABA can induce the expression of the HD- ZIP transcription factor HB1 that, in turn, acts as a repressor of the LBD1 transcription factor that would mediates auxin induced LRF [16]. Another TF induced by salt stress and related to hormonal control is MtNAC969 [109]. Overexpression of this TF leads to less branched root systems while down regulation using an RNAi strategy leads to increased LRF. However, no effect on nodule number was observed [109]. Interestingly, MtNAC969 was rapidly induced by various hormone treatments such as CK, ABA and ethylene application in M. truncatula roots and by nitrate application in nodules. These data suggest that root development responses to salt stress could notably be mediated by ABA, through its action on the inhibition of LR emergence.
Nitric Oxide (NO) and ABA have been shown to cross-talk in many biological processes, including drought and other developmental responses [110], but the importance of NO in controlling root development in legumes is still poorly understood. Interestingly, NO accumulation was observed in LRP and nodule primordia in legumes [24]. Moreover, the increase in nodule number observed using a S. meliloti strain expressing the auxin biosynthesis genes iaaM and tms2 was shown to be dependent on NO production since the NO scavenger cPTIO caused a significant decrease in nodule number of the Medicago plants inoculated with this strain [8]. Therefore, NO could be, as shown for other dicot species [22,23,25,26,27] a positive regulator of cell cycle reactivation, acting downstream of the auxin pathway in both LRP and nodule development in legumes.
Ethylene is another gaseous hormone involved in biotic stress and plant development. In legumes, ethylene is a negative regulator of nodulation since application of the ethylene precursor aminocyclopropane 1-carboxylic acid (ACC) prevents Nod Factor (NF) induced calcium spiking and nodule formation [111]. Moreover, the ethylene biosynthesis inhibitor aminoethoxyvinylglycine (AVG) or the ethylene receptor antagonist silver ion (Ag+) both enhance nodulation in several species [112,113]. In the same way, Mtsickle, a mutant in the EIN2 gene, a component of the ethylene signaling pathway, shows a strongly enhanced number of nodules in Medicago as well as a better NF signaling [19,20,114]. The effect of the Mtsickle mutation on nodulation was shown to be related to a lack of auxin transport regulation triggered upon rhizobial inoculation [115]. For root developmental aspects, Mtsickle mutants show a more rapid primary root growth, which renders the measurement of LRF difficult to assess. However, studies in Lotus have shown that transgenic plants expressing the dominant negative form of the Arabidopsis ethylene receptor etr1 were insensitive to ethylene and displayed fewer LR [21], a phenotype also found in the Arabidopsis etr1-3 mutant [17], whereas the Ljein2a mutant “enigma” displayed higher ethylene content and a significant increase in LR number [112]. We have also addressed the effect of ACC on LRF in M. truncatula [116] and shown a dose dependent effect of ACC on LRF. Hence, concentrations up to 10−7 M ACC stimulated LRF of young M. truncatula seedlings whereas higher doses negatively regulated LR number. Altogether, this suggests that low ethylene concentrations stimulate LRF while high ethylene concentrations repress LRF. In Arabidopsis and tomato, 10−6 M ACC reduced both root elongation and LR number and 10−7 M ACC did not seem to positively affect LRF [17,18]. So, although high ethylene concentrations appear as a negative regulator of root elongation, LRF and nodulation in legumes, it may well be that sensitivity to low ethylene concentrations for LRF is slightly different between legume and non-legume plants but this requires further investigation.
The last “stress” related hormone we will discuss here is jasmonic acid (JA). This volatile molecule, and its biologically active derivative Methyl-Jasmonate (MeJA), is involved in pathogen interactions but has also been studied in a symbiotic context. Hence, Sun et al. have shown that JA inhibits NF induced calcium spiking and nodulation in M. truncatula, in a dose dependent manner and in a parallel and antagonistic manner to ethylene [29]. Sun et al. also noticed that, as for Arabidopsis, JA application (from 10−7 M) reduced primary root length of M. truncatula. Studies performed in soybean by over-expressing a MetJA biosynthesis gene, NTR1, showed that increased MetJA production resulted in an inhibition of nodulation and increased LR density [28]. These data suggest that JA has an antagonistic role on LRF and nodulation in legumes (cf. Table 1).
4.3. Small Regulatory Peptides
Peptides are also considered as hormones since they are endogenous products that can act at very low doses and at a distance from their site of production. In legumes, many of the regulatory peptides actions have been linked with nitrate—that is an important element controlling root development in all plants and nodulation in legumes—or with the negative systemic feedback loop known as “autoregulation of nodulation” that controls nodule numbers. We will review some of the effects of these small regulatory peptides relevant in legumes and their link with auxin when described.
Mutants impaired in the “autoregulation of nodulation” (AON) pathway often display root development phenotypes. For instance, different alleles of the super numeric nodule (sunn) mutant display reduced root length in non-symbiotic conditions [40]. The Lotus ortholog of SUNN, HAR1, shows a higher number of LR and a shorter primary root length in non-inoculated conditions [117], mutation in the orthologous gene of soybean, nts1 also enhances LRF [42]. SUNN encodes a homolog of the Clavata1 LRR-RLK from Arabidopsis [40]. In Lotus, another LRR-RLK (unrelated to CLV1) named Klavier (KLV) was identified as being involved in AON [41]. Interestingly, a klv mutation is not additive to har1 for nodulation indicating that both genes are involved in the same pathway for AON [118] but klv plants display shorter primary roots and a reduced number of LR in non-inoculated conditions [41]. In M. truncatula, Jin et al. found that 2.5 mM nitrate reduced LR density in wild type plants but not in sunn mutants. In this study, the authors also describe that LR density in the wild type plant is highly dependent on the nitrate content in the shoot and strongly correlated with shoot to root auxin transport. This link with nitrate content of the shoot and auxin transport is lost in the sunn-1 mutant, suggesting that SUNN controls the LRF response to nitrate by integrating the nitrate content in the shoot and linking it with auxin shoot to root transport [119]. This is reminiscent of the shoot controlled regulation of AON by sunn [19]. LRR-RLKs usually have peptidic ligands. CLAVATA3/EMBRYO-SURROUNDING REGION (CLE) are small secreted peptides, some of which were shown to be CLV1 ligands. Some CLE peptides were also shown to be produced in the root and seem to mediate the AON signal from the site of nodulation in roots to systemic perception in shoots [120]. Recently, 35S:LjCLE-RS2 was shown to reduce LR number as well as nodule number in Lotus [121]. In M. truncatula, overexpression of CLE14 diminishes primary root length [122] but no effect on LRF was described. Interestingly a similar role for the CLE-CLV-1 pathway was recently demonstrated in systemic regulation of LRF in response to nitrate starvation in Arabidopsis [39]. Hence, overexpression of any of the four CLE peptides CLE 1, 3, 4 and 7 reduced LR growth and emergence, especially under high nitrate concentrations in Arabidopsis. This effect was lost in the clv1 mutant [39]. Two of the closest homologs of these CLE peptides, named MtCLE12 and MtCLE13 are up-regulated in nodulated M. truncatula plants [122] and are closely related to LjCLE-RS1 and LjCLE-RS2. Interestingly, overexpression of MtCLE13 led to a strong reduction in nodule number (in a SUNN dependent manner) and MtCLE13 is regulated by CK [123]. 35S:MtCLE12 in M. truncatula inhibits nodulation, primary root growth and enhances GH3:GUS staining in the root, probably reflecting a change in auxin content/transport [124]. This suggests that the systematic regulation of nitrate status response through CLE-CLV1 signalling pathways is conserved in Arabidopsis and legumes, although CLE peptides are expressed in response to nitrate deprivation in Arabidopsis but in nitrate replete (during nodulation) conditions in legumes.
Some other peptides have been shown to mediate root developmental response to nitrate starvation. C-terminally Encoded Peptides (CEP) are small secreted peptides of 15 amino acids whose expression is induced by nitrate starvation (0 mM) or low (0.25 mM) nitrate concentration both in M. truncatula and in Arabidopsis [44,125]. Among the 11 CEP peptide genes found in Medicago, MtCEP1 was further characterized by Imin et al. [44]. MtCEP1 is expressed in root tips, vascular tissue, and young lateral organs and negatively regulates LRF but positively regulates nodulation. MtCEP1 expression is not responsive to a 24 h treatment of 1 µM ABA/ACC/GA/GR24/BAP (synthetic CK)/NAA (synthetic auxin)/MeJA. External application of 1 µM of synthetic MtCEP1 significantly reduced LRF of several legume plants [44]. In contrast to the effect observed in Arabidopsis root development [43], MtCEP1 did not affect primary root elongation in M. truncatula. Further studies have shown that MtCEP1 activity depends on conserved post translational modifications such as hydroxylation of conserved proline residues. Hence the peptide encoded by the first coding region of MtCEP1 (D1) is predominantly hydroxylated at the Pro 4 and Pro 11 residues. In M. truncatula D1:HyP4,11 or D1:HyP4,7,11 have the most potent action on LRF at 0 mM nitrate. D2:HyP11 increased pre-emergence stages but not emergence of M. truncatula LR at 5 mM nitrate. This inhibition was not released by low (10−10 M) auxin external application [45]. NMR studies have shown that the conformational plasticity of CEP peptide is greatest when both P4 and P11 are post-translationally modified to hydroxy-proline residues [126].
Interestingly, two CEP receptors have recently been identified in Arabidopsis and were shown to be involved in a systemic response of root development to nitrate starvation in a shoot dependent manner [125]. Hence, CEP peptides applied to Arabidopsis roots can induce nitrate transporter expression in another part of the root, but only if the shoot of the plant is not mutated in the two CEP receptor genes. These receptors are two Leucine-Rich-Repeat Receptor Like Kinases (LRR-RLK), including XIP1 that is involved in vasculature development in Arabidopsis [127]. Strikingly, the ortholog of XIP1 in M. truncatula is the compact root architecture 2 (CRA2) gene, which is involved in systemic regulation of nodulation (in a nitrate independent pathway) and, antagonistically in the local regulation of LRF. cra2 mutants display a strong increase in LRF-independent of the nitrate concentration of the growth medium- that is locally regulated in a root dependent manner; whereas cra2 mutants display a strong reduction in nodule number dependent on the shoot genotype [128]. Hence the same LRR-RLK is involved in systemic regulation in M. truncatula and Arabidopsis, but has a role for nodule number regulation in Medicago and root developmental response to nitrate starvation in Arabidopsis. Interestingly, the antagonistic role of this pathway on LRF and nodule regulation in M. truncatula depends on two different (local versus systemic) regulation processes. Studies on the CEP peptides in M. truncatula have not addressed a systemic regulation effect yet. It is thus possible that CEP peptides have a local regulatory role on LRF and a potential systemic effect on nodulation in legumes.
Strikingly, the same peptidic (CLE and CEP) systemic regulatory pathways are conserved between Arabidopsis and legumes but have been directed toward LRF regulation by nitrate in Arabidopsis and regulation of nodulation in legumes.
Finally, a new family of small regulatory peptides, called miPEPs, has very recently been shown to regulate root development in both M. truncatula and Arabidopsis, although no possible links to nitrate or hormonal control have yet been studied [129].
5. Concluding Remarks
5.1. What Specificities for Legume LRF?
Legume root development, and especially LRF, is not fundamentally different from that of many plants from a cellular point of view. Even if endodermal and cortical contributions to the formation of LRP occur in several legumes [65,67,68] and if the ontogeny of nodules in legumes correlates with that of LR [64,69], this ability of endodermal and cortical cells to divide to form the LRP is not specific to legumes but is shared by many other plants [65,66,67]. In this respect, the pericycle limited origin of LRP in Arabidopsis appears to be the exception rather than the rule.
However, both determinate and indeterminate legume nodules do resemble LR, they originate from the same root cell layers and have their development controlled by phytohormones.
5.2. Dual Hormonal Control of LRF/Nodulation in Legumes
Interestingly, some hormones do have the same action on LR and nodulation while others seem to have evolved antagonistic action on these two lateral organs. For example, auxin, although maybe not with the same level of sensitivity, is a positive regulator of both LR and nodule development and its accumulation and transport is crucial for the onset of these two developmental programs. Other hormones, like ABA, CK, JA, and ethylene play opposite roles between LRF and nodulation in legumes. ABA, JA, and ethylene are positive regulators of LRF and negative regulators of nodulation while CK are negative regulators of LRF and positive regulators of nodulation.
5.3. Comparison with LRF Hormonal Control in Other Dicots
Auxin, CK, JA, and strigolactones seem to play similar roles in controlling LRF in legumes and other dicots such as Arabidopsis or tomato (see Table 1). However, low doses of ABA and ethylene stimulate LRF in legumes, but not in non-nodulating plants for ABA and at least not in Arabidopsis or tomato for ethylene [5,17,18]. It is thus possible, as already suggested by Liang and colleagues for ABA [5], that different hormonal sensitivities have been a pre-requisite for plants to develop nodulation, maybe thanks to this capability to display antagonistic control of two different lateral organs with the same hormones. More extensive studies on nodulating vs non-nodulating plants should be conducted to clarify if root development sensitivity to other hormones than ABA could account for a general feature of nodulating vs. non-nodulating plants.
5.4. Future Directions
Interestingly, non-legume actinorhizal plants (that form a symbiotic interaction with the nitrogen fixing bacteria Frankia) and Parasponia (the only non-legume plant to establish a root endosymbiosis with rhizobia) do form a different type of nodule, much more developmentally related to LR. For instance, actinorhizal and Parasponia nodules originate from the pericycle and have a central vasculature, as for LR, whereas legume nodules have a peripheral vasculature ([130] for review). Molecular comparison of LR and nodule developmental programs in legumes, actinorhizal plants and Parasponia could be an interesting way to understand the specificities and overlaps of these developmental pathways.
Another issue that needs clarification is the possible balance that exists between LR and nodule numbers. So far, contradictory evidence exists and it is not clear if increasing nodule number reduces LRF or vice versa. The fact that some components of the auxin pathway (like MtCDC16 in Medicago) can play antagonistic roles in LRF and nodule development or that the level of auxin sensitivity required for LRF and determinate nodule development seems slightly different, for example, opens a way towards understanding how a difference in auxin sensitivity or signaling could possibly balance LR and nodule numbers.
In contrast to Arabidopsis, and apart from the extensive SGL pathway mutant collection in pea, little genetic dissection of the hormonal pathways has been performed in legumes, mainly because of the lack of extensive appropriate mutant collections. For instance, auxin perception/signaling/transport/biosynthesis mutants are mostly still missing. With the completion of many legume genomes and the development of mutant libraries, this gap is starting to be filled slowly and systematic studies of hormonal pathways are emerging [131,132]. Although these recent studies are still mainly focused on symbiotic interactions such as nodulation or mycorrhization, this focus will provide new tools to better address and dissect the roles of these signaling pathways on root development as well. Moreover, a systematic approach to address the effects of these hormones, alone or in interaction, in both root development and symbiotic responses remains a real challenge that needs to be addressed in order to better understand the regulatory complexity of these different developmental outcomes occurring in legume roots.
Acknowledgments
I am grateful to Clare Gough and Frédéric Debellé for critical reading of the manuscript and to Violaine Herrbach for some of the figures used to illustrate that review. Research conducted by SB at LIPM is partly supported by the “Laboratoire d’Excellence” (LABEX) entitled TULIP (ANR-10-LABX-41; ANR-11-IDEX-0002). I apologize for colleagues whose work may not have been cited in this manuscript due to space limitation or focus on root development aspects only.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Doyle J.J. Phylogenetic perspectives on the origins of nodulation. Mol. Plant Microbe Interact. 2011;24:1289–1295. doi: 10.1094/MPMI-05-11-0114. [DOI] [PubMed] [Google Scholar]
- 2.Dénarié J., Debellé F., Promé J.C. Rhizobium lipo-chitooligosaccharide nodulation factors: Signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- 3.Gough C., Cullimore J. Lipo-chitooligosaccharide signaling in endosymbiotic plant-microbe interactions. Mol. Plant Microbe Interact. 2011;24:867–878. doi: 10.1094/MPMI-01-11-0019. [DOI] [PubMed] [Google Scholar]
- 4.Thimann K.V., Went F.W. On the chemical nature of the root-forming hormone. Proc. R. Acad. Amst. 1934;37:456–458. [Google Scholar]
- 5.Liang Y., Harris J.M. Response of root branching to abscisic acid is correlated with nodule formation both in legumes and non legumes. Am. J. Bot. 2005;92:1675–1683. doi: 10.3732/ajb.92.10.1675. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch A.M., Bhuvaneswari T.V., Torrey J.G., Bisseling T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc. Natl. Acad. Sci. USA. 1989;86:1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Noorden G.E., Ross J.J., Reid J.B., Rolfe B.G., Mathesius U. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 2006;140:1494–1506. doi: 10.1104/pp.105.075879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pii Y., Crimi M., Cremonese G., Spena A., Pandolfini T. Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol. 2007;7:21. doi: 10.1186/1471-2229-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overvoorde P., Fukaki H., Beeckman T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010;2:a001537. doi: 10.1101/cshperspect.a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieber J.J., Schaller G.E. Cytokinins. Arabidopsis Book. 2014;12:e0168. doi: 10.1199/tab.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Rizzo S., Crespi M., Frugier F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell. 2006;18:2680–2693. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frugier F., Kosuta S., Murray J.D., Crespi M., Szczyglowski K. Cytokinin: Secret agent of symbiosis. Trends Plant Sci. 2008;13:115–120. doi: 10.1016/j.tplants.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez A.A., Agbévénou K., Herrbach V., Gough C., Bensmihen S. Abscisic acid promotes pre-emergence stages of lateral root development in Medicago truncatula. Plant Signal. Behav. 2015;10:e977741. doi: 10.4161/15592324.2014.977741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Smet I., Zhang H., Inzé D., Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y., Kalo P., Yendrek C., Sun J., Liang Y., Marsh J.F., Harris J.M., Oldroyd G.E. Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell. 2008;20:2681–2695. doi: 10.1105/tpc.108.061739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariel F., Diet A., Verdenaud M., Gruber V., Frugier F., Chan R., Crespi M. Environmental regulation of lateral root emergence in Medicago truncatula requires the HD-Zip I transcription factor HB1. Plant Cell. 2010;22:2171–2183. doi: 10.1105/tpc.110.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negi S., Ivanchenko M.G., Muday G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negi S., Sukumar P., Liu X., Cohen J.D., Muday G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010;61:3–15. doi: 10.1111/j.1365-313X.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 19.Penmetsa R.V., Frugoli J.A., Smith L.S., Long S.R., Cook D.R. Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 2003;131:998–1008. doi: 10.1104/pp.015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penmetsa R.V., Uribe P., Anderson J., Lichtenzveig J., Gish J.C., Nam Y.W., Engstrom E., Xu K., Sckisel G., Pereira M., et al. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008;55:580–595. doi: 10.1111/j.1365-313X.2008.03531.x. [DOI] [PubMed] [Google Scholar]
- 21.Lohar D., Stiller J., Kam J., Stacey G., Gresshoff P.M. Ethylene insensitivity conferred by a mutated Arabidopsis ethylene receptor gene alters nodulation in transgenic Lotus japonicus. Ann. Bot. 2009;104:277–285. doi: 10.1093/aob/mcp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagnussat G.C., Lanteri M.L., Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanz L., Albertos P., Mateos I., Sánchez-Vicente I., Lechón T., Fernández-Marcos M., Lorenzo O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015;66:2857–2868. doi: 10.1093/jxb/erv213. [DOI] [PubMed] [Google Scholar]
- 24.Del Giudice J., Cam Y., Damiani I., Fung-Chat F., Meilhoc E., Bruand C., Brouquisse R., Puppo A., Boscari A. Nitric oxide is required for an optimal establishment of the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytol. 2011;191:405–417. doi: 10.1111/j.1469-8137.2011.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa-Aragunde N., Graziano M., Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 26.Correa-Aragunde N., Graziano M., Chevalier C., Lamattina L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 2006;57:581–588. doi: 10.1093/jxb/erj045. [DOI] [PubMed] [Google Scholar]
- 27.Corti Monzón G., Pinedo M., di Rienzo J., Novo-Uzal E., Pomar F., Lamattina L., de la Canal L. Nitric oxide is required for determining root architecture and lignin composition in sunflower. Supporting evidence from microarray analyses. Nitric Oxide. 2014;39:20–28. doi: 10.1016/j.niox.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Xue R., Zhang B. Increased endogenous methyl jasmonate altered leaf and root development in transgenic soybean plants. J. Genet. Genomics. 2007;34:339–346. doi: 10.1016/S1673-8527(07)60036-8. [DOI] [PubMed] [Google Scholar]
- 29.Sun J., Cardoza V., Mitchell D.M., Bright L., Oldroyd G., Harris J.M. Crosstalk between jasmonic acid, ethylene and nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 2006;46:961–970. doi: 10.1111/j.1365-313X.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson B.J., Mathesius U. Phytohormone regulation of legume-rhizobia interactions. J. Chem. Ecol. 2014;40:770–790. doi: 10.1007/s10886-014-0472-7. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson B.J., Ross J.J., Reid J.B. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 2005;138:2396–2405. doi: 10.1104/pp.105.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A., Singh M., Laxmi A. Interaction between glucose and brassinosteroid during the regulation of lateral root development in Arabidopsis. Plant Physiol. 2015;168:307–320. doi: 10.1104/pp.114.256313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi S., Gresshoff P.M., Ferguson B.J. Mechanistic action of gibberellins in legume nodulation. J. Integr. Plant Biol. 2014;56:971–978. doi: 10.1111/jipb.12201. [DOI] [PubMed] [Google Scholar]
- 34.Gou J., Strauss S.H., Tsai C.J., Fang K., Chen Y., Jiang X., Busov V.B. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell. 2010;22:623–639. doi: 10.1105/tpc.109.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng X., Ruyter-Spira C., Bouwmeester H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 2013;4:199. doi: 10.3389/fpls.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Cuyper C., Fromentin J., Yocgo R.E., de Keyser A., Guillotin B., Kunert K., Boyer F.D., Goormachtig S. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. J. Exp. Bot. 2015;66:137–146. doi: 10.1093/jxb/eru404. [DOI] [PubMed] [Google Scholar]
- 37.Foo E., Davies N.W. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–1081. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- 38.Kapulnik Y., Koltai H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014;166:560–569. doi: 10.1104/pp.114.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araya T., Miyamoto M., Wibowo J., Suzuki A., Kojima S., Tsuchiya Y.N., Sawa S., Fukuda H., von Wirén N., Takahashi H. CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc. Natl. Acad. Sci. USA. 2014;111:2029–2034. doi: 10.1073/pnas.1319953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnabel E., Journet E.P., de Carvalho-Niebel F., Duc G., Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol. Biol. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- 41.Oka-Kira E., Tateno K., Miura K., Haga T., Hayashi M., Harada K., Sato S., Tabata S., Shikazono N., Tanaka A., et al. Klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J. 2005;44:505–515. doi: 10.1111/j.1365-313X.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- 42.Searle I.R., Men A.E., Laniya T.S., Buzas D.M., Iturbe-Ormaetxe I., Carroll B.J., Gresshoff P.M. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2003;299:109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- 43.Delay C., Imin N., Djordjevic M.A. Regulation of Arabidopsis root development by small signaling peptides. Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imin N., Mohd-Radzman N.A., Ogilvie H.A., Djordjevic M.A. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot. 2013;64:5395–5409. doi: 10.1093/jxb/ert369. [DOI] [PubMed] [Google Scholar]
- 45.Mohd-Radzman N.A., Binos S., Truong T.T., Imin N., Mariani M., Djordjevic M.A. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. J. Exp. Bot. 2015 doi: 10.1093/jxb/erv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian H., de Smet I., Ding Z. Shaping a root system: Regulating lateral versus primary root growth. Trends Plant Sci. 2014;19:426–431. doi: 10.1016/j.tplants.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 47.De Smet I., White P.J., Bengough A.G., Dupuy L., Parizot B., Casimiro I., Heidstra R., Laskowski M., Lepetit M., Hochholdinger F., et al. Analyzing lateral root development: How to move forward. Plant Cell. 2012;24:15–20. doi: 10.1105/tpc.111.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishikawa H., Evans M.L. Specialized zones of development in roots. Plant Physiol. 1995;109:725–727. doi: 10.1104/pp.109.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Smet I. Lateral root initiation: One step at a time. New Phytol. 2012;193:867–873. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- 50.Péret B., de Rybel B., Casimiro I., Benkova E., Swarup R., Laplaze L., Beeckman T., Bennett M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Lavenus J., Goh T., Roberts I., Guyomarc’h S., Lucas M., de Smet I., Fukaki H., Beeckman T., Bennett M., Laplaze L. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci. 2013;18:450–458. doi: 10.1016/j.tplants.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Malamy J.E., Benfey P.N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- 53.Dubrovsky J.G., Sauer M., Napsucialy-Mendivil S., Ivanchenko M.G., Friml J., Shishkova S., Celenza J., Benkova E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Smet I., Tetsumura T., De Rybel B., Frey N.F., Laplaze L., Casimiro I., Swarup R., Naudts M., Vanneste S., Audenaert D., et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- 55.Dubrovsky J.G., Napsucialy-Mendivil S., Duclercq J., Cheng Y., Shishkova S., Ivanchenko M.G., Friml J., Murphy A.S., Benkova E. Auxin minimum defines a developmental window for lateral root initiation. New Phytol. 2011;191:970–983. doi: 10.1111/j.1469-8137.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 56.Péret B., Larrieu A., Bennett M.J. Lateral root emergence: A difficult birth. J. Exp. Bot. 2009;60:3637–3643. doi: 10.1093/jxb/erp232. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrásek J., Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- 59.Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzaki T., Yano K., Ito M., Umehara Y., Suganuma N., Kawaguchi M. Positive and negative regulation of cortical cell division during root nodule development in lotus japonicus is accompanied by auxin response. Development. 2012;139:3997–4006. doi: 10.1242/dev.084079. [DOI] [PubMed] [Google Scholar]
- 61.Herrbach V., Remblière C., Gough C., Bensmihen S. Lateral root formation and patterning in Medicago truncatula. [(accessed on 20 October 2013)];J. Plant Physiol. 2014 171:301–310. doi: 10.1016/j.jplph.2013.09.006. Available online: http://dx.doi. org/310.1016/j.jplph.2013.1009.1006. [DOI] [PubMed] [Google Scholar]
- 62.Dubrovsky J.G., Gambetta G.A., Hernández-Barrera A., Shishkova S., González I. Lateral root initiation in Arabidopsis: Developmental window, spatial patterning, density and predictability. Ann. Bot. 2006;97:903–915. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrbach V., Remblière C., Bensmihen S. LIPM, UMR CNRS/INRA 2594/441, Castanet-Tolosan, France. 2010. Unpublished work.
- 64.Op den Camp R.H., de Mita S., Lillo A., Cao Q., Limpens E., Bisseling T., Geurts R. A phylogenetic strategy based on a legume-specific whole genome duplication yields symbiotic cytokinin type-a response regulators. Plant Physiol. 2011;157:2013–2022. doi: 10.1104/pp.111.187526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lloret P.G., Casero P.J., Pulgarin A., Navascues J. The behaviour of two cell populations in the pericycle of Allium cepa, Pisum sativum, and Daucus carota during early lateral root development. Ann. Bot. 1989;63:465–475. [Google Scholar]
- 66.Casero P.J., Casimiro I., Lloret P.G. Lateral root initiation by asymmetrical transverse divisions of pericycle cells in 4 plant-species—Raphanus sativus, Helianthus annuus, Zea mays, and Daucus carota. Protoplasma. 1995;188:49–58. doi: 10.1007/BF01276795. [DOI] [Google Scholar]
- 67.Mallory T.E., Chiang S.-H., Cutter E.G., Gifford E.M., Jr. Sequence and pattern of lateral root formation in five selected species. Am. J. Bot. 1970;57:800–809. doi: 10.2307/2441337. [DOI] [Google Scholar]
- 68.Lotocka B., Kopcinska J., Golinowski W. Morphogenesis of root nodules in white clover. I. Effective root nodules induced by the wild type Rhizobium leguminosarum biovar. Trifolii. Acta Soc. Bot. Pol. 1997;66:273–292. [Google Scholar]
- 69.Xiao T.T., Schilderink S., Moling S., Deinum E.E., Kondorosi E., Franssen H., Kulikova O., Niebel A., Bisseling T. Fate map of Medicago truncatula root nodules. Development. 2014;141:3517–3528. doi: 10.1242/dev.110775. [DOI] [PubMed] [Google Scholar]
- 70.Timmers A.C., Auriac M.C., Truchet G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development. 1999;126:3617–3628. doi: 10.1242/dev.126.16.3617. [DOI] [PubMed] [Google Scholar]
- 71.Mathesius U., Schlaman H.R., Spaink H.P., Of Sautter C., Rolfe B.G., Djordjevic M.A. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 72.Pacios-Bras C., Schlaman H.R., Boot K., Admiraal P., Langerak J.M., Stougaard J., Spaink H.P. Auxin distribution in lotus japonicus during root nodule development. Plant Mol. Biol. 2003;52:1169–1180. doi: 10.1023/B:PLAN.0000004308.78057.f5. [DOI] [PubMed] [Google Scholar]
- 73.Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E., et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rightmyer A.P., Long S.R. Pseudonodule formation by wild-type and symbiotic mutant Medicago truncatula in response to auxin transport inhibitors. Mol. Plant Microbe Interact. 2011;24:1372–1384. doi: 10.1094/MPMI-04-11-0103. [DOI] [PubMed] [Google Scholar]
- 75.Huo X., Schnabel E., Hughes K., Frugoli J. RNAi phenotypes and the localization of a protein::GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J. Plant Growth Regul. 2006;25:156–165. doi: 10.1007/s00344-005-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner M., Nizampatnam N.R., Baron M., Coppin S., Damodaran S., Adhikari S., Arunachalam S.P., Yu O., Subramanian S. Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiol. 2013;162:2042–2055. doi: 10.1104/pp.113.220699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mao G., Turner M., Yu O., Subramanian S. miR393 and miR164 influence indeterminate but not determinate nodule development. Plant Signal. Behav. 2013;8 doi: 10.4161/psb.26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bustos-Sanmamed P., Mao G., Deng Y., Elouet M., Khan G.A., Bazin J., Turner M., Subramanian S., Yu O., Crespi M., et al. Overexpression of miR160 affects root growth and nitrogen-fixing nodule number in Medicago truncatula. Funct. Plant Biol. 2013;40:1208–1220. doi: 10.1071/FP13123. [DOI] [PubMed] [Google Scholar]
- 79.Guo H.S., Xie Q., Fei J.F., Chua N.H. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell. 2005;17:1376–1386. doi: 10.1105/tpc.105.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.D’haeseleer K., Den Herder G., Laffont C., Plet J., Mortier V., Lelandais-Brière C., de Bodt S., de Keyser A., Crespi M., Holsters M., et al. Transcriptional and post-transcriptional regulation of a NaCl transcription factor in Medicago truncatula roots. New Phytol. 2011;191:647–661. doi: 10.1111/j.1469-8137.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 81.Li X., Lei M., Yan Z., Wang Q., Chen A., Sun J., Luo D., Wang Y. The REL3-mediated TAS3 ta-siRNA pathway integrates auxin and ethylene signaling to regulate nodulation in Lotus japonicus. New Phytol. 2014;201:531–544. doi: 10.1111/nph.12550. [DOI] [PubMed] [Google Scholar]
- 82.Kuppusamy K.T., Ivashuta S., Bucciarelli B., Vance C.P., Gantt J.S., Vandenbosch K.A. Knockdown of CELL DIVISION CYCLE16 reveals an inverse relationship between lateral root and nodule numbers and a link to auxin in Medicago truncatula. Plant Physiol. 2009;151:1155–1166. doi: 10.1104/pp.109.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wasson A.P., Pellerone F.I., Mathesius U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell. 2006;18:1617–1629. doi: 10.1105/tpc.105.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Subramanian S., Stacey G., Yu O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006;48:261–273. doi: 10.1111/j.1365-313X.2006.02874.x. [DOI] [PubMed] [Google Scholar]
- 85.Mortier V., Holsters M., Goormachtig S. Never too many? How legumes control nodule numbers. Plant Cell Environ. 2012;35:245–258. doi: 10.1111/j.1365-3040.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 86.Mathesius U. Auxin: At the root of nodule development? Funct. Plant Biol. 2008;35:651–668. doi: 10.1071/FP08177. [DOI] [PubMed] [Google Scholar]
- 87.Laplaze L., Benkova E., Casimiro I., Maes L., Vanneste S., Swarup R., Weijers D., Calvo V., Parizot B., Herrera-Rodriguez M.B., et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Held M., Hou H., Miri M., Huynh C., Ross L., Hossain M.S., Sato S., Tabata S., Perry J., Wang T.L., et al. Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. Plant Cell. 2014;26:678–694. doi: 10.1105/tpc.113.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zürcher E., Tavor-Deslex D., Lituiev D., Enkerli K., Tarr P.T., Müller B. A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol. 2013;161:1066–1075. doi: 10.1104/pp.112.211763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murray J.D., Karas B.J., Sato S., Tabata S., Amyot L., Szczyglowski K. A cytokinin perception mutant colonized by rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–104. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- 91.Tirichine L., Sandal N., Madsen L.H., Radutoiu S., Albrektsen A.S., Sato S., Asamizu E., Tabata S., Stougaard J. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–107. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 92.Plet J., Wasson A., Ariel F., Le Signor C., Baker D., Mathesius U., Crespi M., Frugier F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011;65:622–633. doi: 10.1111/j.1365-313X.2010.04447.x. [DOI] [PubMed] [Google Scholar]
- 93.Lohar D.P., Schaff J.E., Laskey J.G., Kieber J.J., Bilyeu K.D., Bird D.M. Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J. 2004;38:203–214. doi: 10.1111/j.1365-313X.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- 94.Foo E., Yoneyama K., Hugill C.J., Quittenden L.J., Reid J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant. 2013;6:76–87. doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- 95.Terrats L., Bensmihen S. LIPM, UMR CNRS/INRA 2594/441, Castanet-Tolosan, France. 2014. Unpublished work.
- 96.Lauressergues D., André O., Peng J., Wen J., Chen R., Ratet P., Tadege M., Mysore K.S., Rochange S.F. Strigolactones contribute to shoot elongation and to the formation of leaf margin serrations in Medicago truncatula R108. J. Exp. Bot. 2015;66:1237–1244. doi: 10.1093/jxb/eru471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terrats L., Herrbach V., Rochange S., Bensmihen S. LIPM, UMR CNRS/INRA 2594/441, Castanet-Tolosan, France. 2014. Unpublished work.
- 98.Foo E. Auxin influences strigolactones in pea mycorrhizal symbiosis. J. Plant Physiol. 2013;170:523–528. doi: 10.1016/j.jplph.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 99.Ferguson B.J., Foo E., Ross J.J., Reid J.B. Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytol. 2011;189:829–842. doi: 10.1111/j.1469-8137.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 100.Maekawa T., Maekawa-Yoshikawa M., Takeda N., Imaizumi-Anraku H., Murooka Y., Hayashi M. Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J. 2009;58:183–194. doi: 10.1111/j.1365-313X.2008.03774.x. [DOI] [PubMed] [Google Scholar]
- 101.Floss D.S., Levy J.G., Lévesque-Tremblay V., Pumplin N., Harrison M.J. DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA. 2013;110:E5025–E5034. doi: 10.1073/pnas.1308973110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bazin J., Khan G.A., Combier J.P., Bustos-Sanmamed P., Debernardi J.M., Rodriguez R., Sorin C., Palatnik J., Hartmann C., Crespi M., et al. miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J. 2013;74:920–934. doi: 10.1111/tpj.12178. [DOI] [PubMed] [Google Scholar]
- 103.Boualem A., Laporte P., Jovanovic M., Laffont C., Plet J., Combier J.P., Niebel A., Crespi M., Frugier F. MicroRNA166 controls root and nodule development in Medicago truncatula. Plant J. 2008;54:876–887. doi: 10.1111/j.1365-313X.2008.03448.x. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki A., Akune M., Kogiso M., Imagama Y., Osuki K., Uchiumi T., Higashi S., Han S.Y., Yoshida S., Asami T., et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004;45:914–922. doi: 10.1093/pcp/pch107. [DOI] [PubMed] [Google Scholar]
- 105.Charpentier M., Sun J., Wen J., Mysore K.S., Oldroyd G.E. Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the PROTEIN PHOSPHATASE 2A complex. Plant Physiol. 2014;166:2077–2090. doi: 10.1104/pp.114.246371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tominaga A., Nagata M., Futsuki K., Abe H., Uchiumi T., Abe M., Kucho K., Hashiguchi M., Akashi R., Hirsch A.M., et al. Enhanced nodulation and nitrogen fixation in the abscisic acid low-sensitive mutant enhanced nitrogen fixation1 of Lotus japonicus. Plant Physiol. 2009;151:1965–1976. doi: 10.1104/pp.109.142638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bright L.J., Liang Y., Mitchell D.M., Harris J.M. The LATD gene of Medicago truncatula is required for both nodule and root development. Mol. Plant Microbe Interact. 2005;18:521–532. doi: 10.1094/MPMI-18-0521. [DOI] [PubMed] [Google Scholar]
- 108.Liang Y., Mitchell D.M., Harris J.M. Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev. Biol. 2007;304:297–307. doi: 10.1016/j.ydbio.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 109.De Zélicourt A., Diet A., Marion J., Laffont C., Ariel F., Moison M., Zahaf O., Crespi M., Gruber V., Frugier F. Dual involvement of a Medicago truncatula NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J. 2012;70:220–230. doi: 10.1111/j.1365-313X.2011.04859.x. [DOI] [PubMed] [Google Scholar]
- 110.León J., Castillo M.C., Coego A., Lozano-Juste J., Mir R. Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J. Exp. Bot. 2014;65:907–921. doi: 10.1093/jxb/ert454. [DOI] [PubMed] [Google Scholar]
- 111.Oldroyd G.E., Engstrom E.M., Long S.R. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001;13:1835–1849. doi: 10.1105/tpc.13.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chan P.K., Biswas B., Gresshoff P.M. Classical ethylene insensitive mutants of the Arabidopsis ein2 orthologue lack the expected “hypernodulation” response in Lotus japonicus. J. Integr. Plant Biol. 2013;55:395–408. doi: 10.1111/jipb.12040. [DOI] [PubMed] [Google Scholar]
- 113.Mohd-Radzman N.A., Djordjevic M.A., Imin N. Nitrogen modulation of legume root architecture signaling pathways involves phytohormones and small regulatory molecules. Front. Plant Sci. 2013;4 doi: 10.3389/fpls.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Combier J.P., Küster H., Journet E.P., Hohnjec N., Gamas P., Niebel A. Evidence for the involvement in nodulation of the two small putative regulatory peptide-encoding genes MtRALFL1 and MtDVL1. Mol. Plant Microbe Interact. 2008;21:1118–1127. doi: 10.1094/MPMI-21-8-1118. [DOI] [PubMed] [Google Scholar]
- 115.Prayitno J., Rolfe B.G., Mathesius U. The Ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiol. 2006;142:168–180. doi: 10.1104/pp.106.080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herrbach V., Agbévénou K., Bensmihen S. LIPM, UMR CNRS/INRA 2594/441, Castanet-Tolosan, France. 2010. Unpublished work.
- 117.Nishimura R., Hayashi M., Wu G.J., Kouchi H., Imaizumi-Anraku H., Murakami Y., Kawasaki S., Akao S., Ohmori M., Nagasawa M., et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 118.Miyazawa H., Oka-Kira E., Sato N., Takahashi H., Wu G.J., Sato S., Hayashi M., Betsuyaku S., Nakazono M., Tabata S., et al. The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus. Development. 2010;137:4317–4325. doi: 10.1242/dev.058891. [DOI] [PubMed] [Google Scholar]
- 119.Jin J., Watt M., Mathesius U. The autoregulation gene SUNN mediates changes in root organ formation in response to nitrogen through alteration of shoot-to-root auxin transport. Plant Physiol. 2012;159:489–500. doi: 10.1104/pp.112.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reid D.E., Ferguson B.J., Hayashi S., Lin Y.H., Gresshoff P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011;108:789–795. doi: 10.1093/aob/mcr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sasaki T., Suzaki T., Soyano T., Kojima M., Sakakibara H., Kawaguchi M. Shoot-derived cytokinins systemically regulate root nodulation. Nat. Commun. 2014;5 doi: 10.1038/ncomms5983. [DOI] [PubMed] [Google Scholar]
- 122.Mortier V., Den Herder G., Whitford R., Van de Velde W., Rombauts S., D’Haeseleer K., Holsters M., Goormachtig S. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010;153:222–237. doi: 10.1104/pp.110.153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mortier V., De Wever E., Vuylsteke M., Holsters M., Goormachtig S. Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling. Plant J. 2012;70:367–376. doi: 10.1111/j.1365-313X.2011.04881.x. [DOI] [PubMed] [Google Scholar]
- 124.Saur I.M., Oakes M., Djordjevic M.A., Imin N. Crosstalk between the nodulation signaling pathway and the autoregulation of nodulation in Medicago truncatula. New Phytol. 2011;190:865–874. doi: 10.1111/j.1469-8137.2011.03738.x. [DOI] [PubMed] [Google Scholar]
- 125.Tabata R., Sumida K., Yoshii T., Ohyama K., Shinohara H., Matsubayashi Y. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science. 2014;346:343–346. doi: 10.1126/science.1257800. [DOI] [PubMed] [Google Scholar]
- 126.Bobay B.G., DiGennaro P., Scholl E., Imin N., Djordjevic M.A., Mck Bird D. Solution NMR studies of the plant peptide hormone CEP inform function. FEBS Lett. 2013;587:3979–3985. doi: 10.1016/j.febslet.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 127.Bryan A.C., Obaidi A., Wierzba M., Tax F.E. XYLEM INTERMIXED WITH PHLOEM1, a leucine-rich repeat receptor-like kinase required for stem growth and vascular development in Arabidopsis thaliana. Planta. 2012;235:111–122. doi: 10.1007/s00425-011-1489-6. [DOI] [PubMed] [Google Scholar]
- 128.Huault E., Laffont C., Wen J., Mysore K.S., Ratet P., Duc G., Frugier F. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genet. 2014;10:e1004891. doi: 10.1371/journal.pgen.1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lauressergues D., Couzigou J.M., Clemente H.S., Martinez Y., Dunand C., Bécard G., Combier J.P. Primary transcripts of microRNAs encode regulatory peptides. Nature. 2015;520:90–93. doi: 10.1038/nature14346. [DOI] [PubMed] [Google Scholar]
- 130.Pawlowski K., Bisseling T. Rhizobial and actinorhizal symbioses: What are the shared features? Plant Cell. 1996;8:1899–1913. doi: 10.1105/tpc.8.10.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen C., Yue R., Yang Y., Zhang L., Sun T., Xu L., Tie S., Wang H. Genome-wide identification and expression profiling analysis of the Aux/IAA gene family in Medicago truncatula during the early phase of Sinorhizobium meliloti infection. PLoS ONE. 2014;9:e107495. doi: 10.1371/journal.pone.0107495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shen C., Yue R., Sun T., Zhang L., Xu L., Tie S., Wang H., Yang Y. Genome-wide identification and expression analysis of auxin response factor gene family in Medicago truncatula. Front. Plant Sci. 2015;6:73. doi: 10.3389/fpls.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]