Abstract
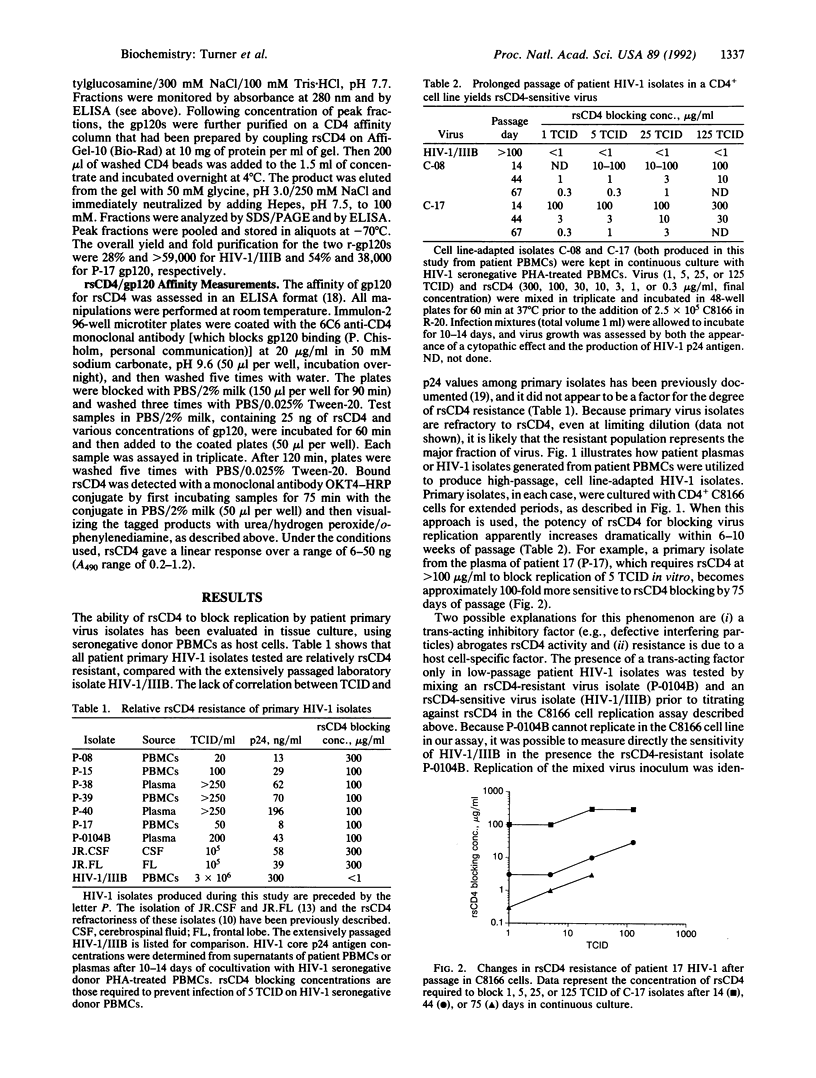
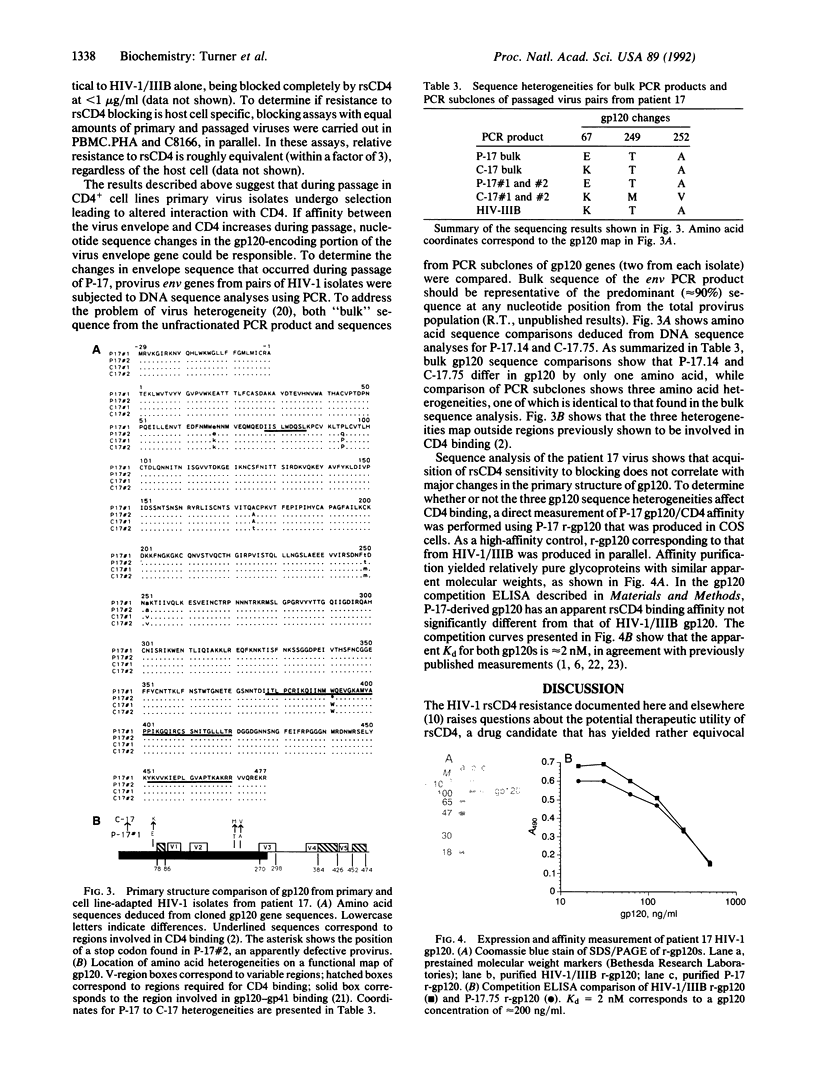
Recombinant soluble CD4 (rsCD4) has potent antiviral activity against cell line-adapted isolates of the human immunodeficiency virus type 1 (HIV-1) but low activity toward HIV-1 primary isolates from patients. A simple hypothesis proposed to explain this discrepancy, which questions the therapeutic utility of soluble CD4-based approaches, is that the major envelope glycoprotein, gp120, of patient virus has lower affinity for CD4 than does gp120 from laboratory viruses. To test this hypothesis, we have produced pairs of low- and high-passage HIV-1 isolates which, depending on culture passage history, display dramatically different sensitivities to neutralization by rsCD4. Here, we present evidence that the HIV-1 major envelope glycoprotein cDNAs cloned from one such isolate pair show only minor differences in their deduced gp120 primary structures, and these occur outside regions previously shown to be involved in CD4 interactions. In addition, recombinant gp120 from a low-passage rsCD4-resistant patient virus binds rsCD4 with high affinity, equal to that previously measured for recombinant gp120 from high-passage cell line-adapted virus isolates. These data indicate that differences in CD4-gp120 affinity do not account for rsCD4 resistance in HIV-1 recently isolated from patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S. Receptor-mediated activation of immunodeficiency viruses in viral fusion. Science. 1991 May 31;252(5010):1322–1323. doi: 10.1126/science.1925547. [DOI] [PubMed] [Google Scholar]
- Allan J. S., Strauss J., Buck D. W. Enhancement of SIV infection with soluble receptor molecules. Science. 1990 Mar 2;247(4946):1084–1088. doi: 10.1126/science.2309120. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Smith D. H., Marsters S. A., Riddle L., Gregory T. J., Ho D. D., Capon D. J. Resistance of primary isolates of human immunodeficiency virus type 1 to soluble CD4 is independent of CD4-rgp120 binding affinity. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7056–7060. doi: 10.1073/pnas.88.16.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighty D. W., Rosenberg M., Chen I. S., Ivey-Hoyle M. Envelope proteins from clinical isolates of human immunodeficiency virus type 1 that are refractory to neutralization by soluble CD4 possess high affinity for the CD4 receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7802–7805. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrn R. A., Sekigawa I., Chamow S. M., Johnson J. S., Gregory T. J., Capon D. J., Groopman J. E. Characterization of in vitro inhibition of human immunodeficiency virus by purified recombinant CD4. J Virol. 1989 Oct;63(10):4370–4375. doi: 10.1128/jvi.63.10.4370-4375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Ward R. H. The CD4-gp120 interaction and AIDS pathogenesis. Annu Rev Immunol. 1991;9:649–678. doi: 10.1146/annurev.iy.09.040191.003245. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Li X. L., Moudgil T., Ho D. D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Furman C., Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991 Apr;65(4):2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Culp J. S., Chaikin M. A., Hellmig B. D., Matthews T. J., Sweet R. W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. A., Barlow M. A., Chou T. C., Fisher R. A., Walker B. D., Hirsch M. S., Schooley R. T. Synergistic inhibition of human immunodeficiency virus type 1 (HIV-1) replication in vitro by recombinant soluble CD4 and 3'-azido-3'-deoxythymidine. J Infect Dis. 1989 May;159(5):837–844. doi: 10.1093/infdis/159.5.837. [DOI] [PubMed] [Google Scholar]
- Kahn J. O., Allan J. D., Hodges T. L., Kaplan L. D., Arri C. J., Fitch H. F., Izu A. E., Mordenti J., Sherwin J. E., Groopman J. E. The safety and pharmacokinetics of recombinant soluble CD4 (rCD4) in subjects with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase 1 study. Ann Intern Med. 1990 Feb 15;112(4):254–261. doi: 10.7326/0003-4819-112-4-. [DOI] [PubMed] [Google Scholar]
- Kirsh R., Hart T. K., Ellens H., Miller J., Petteway S. A., Jr, Lambert D. M., Leary J., Bugelski P. J. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1209–1212. doi: 10.1089/aid.1990.6.1209. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Spouge J. L., Dembo M. Quantifying the infectivity of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4644–4648. doi: 10.1073/pnas.86.12.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J. A., McKnight A., Moore J. P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991 Feb;65(2):852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Norton W. A., Sattentau Q. J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991 Mar;65(3):1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Moore J. P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990 Apr;4(4):297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Qureshi N. M., Coy D. H., Garry R. F., Henderson L. A. Characterization of a putative cellular receptor for HIV-1 transmembrane glycoprotein using synthetic peptides. AIDS. 1990 Jun;4(6):553–558. doi: 10.1097/00002030-199006000-00009. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooley R. T., Merigan T. C., Gaut P., Hirsch M. S., Holodniy M., Flynn T., Liu S., Byington R. E., Henochowicz S., Gubish E. Recombinant soluble CD4 therapy in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase I-II escalating dosage trial. Ann Intern Med. 1990 Feb 15;112(4):247–253. doi: 10.7326/0003-4819-112-4-247. [DOI] [PubMed] [Google Scholar]
- Stamm S., Longo F. M. Direct sequencing of PCR products using the Maxam-Gilbert method. Genet Anal Tech Appl. 1990 Sep;7(5):142–143. doi: 10.1016/0735-0651(90)90021-7. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Reimann K. A., DeLong P. A., Liu T., Fisher R. A., Letvin N. L. Effect of recombinant soluble CD4 in rhesus monkeys infected with simian immunodeficiency virus of macaques. Nature. 1989 Jan 19;337(6204):267–270. doi: 10.1038/337267a0. [DOI] [PubMed] [Google Scholar]
- Williams L. M., Cloyd M. W. Polymorphic human gene(s) determines differential susceptibility of CD4 lymphocytes to infection by certain HIV-1 isolates. Virology. 1991 Oct;184(2):723–728. doi: 10.1016/0042-6822(91)90442-e. [DOI] [PubMed] [Google Scholar]




