Abstract
The study was performed to retrospectively analyze the correlation of dual specificity phosphatase 4 (DUSP4) expression with clinicopathological variables and BRAFV600E mutation to better characterize the potential role of DUSP4 as a biomarker in papillary thyroid cancer (PTC). Patients (n=120) who underwent surgery for PTC at Fudan University Shanghai Cancer Center (FUSCC) were enrolled in this study, and a validation cohort from The Cancer Genome Atlas (TCGA) database was identified to confirm the preliminary findings in our study. We investigated DUSP4 expression at the mRNA level in PTC tissues and adjacent normal tissues using real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR). BRAFV600E mutation analysis was also performed in PTC tissues using Sanger sequencing. Initially, we compared PTC tissues with paired normal tissues in DUSP4 expression using Student’s t-test, and then analyzed the correlation of DUSP4 with clinicopathological variables and BRAFV600E mutation in PTC using Mann–Whitney U, Kruskal–Wallis, χ2, and Fisher’s exact tests. Human-derived thyroid cell lines were also used to verify our findings. DUSP4 was significantly overexpressed in PTC tissues compared with the adjacent normal tissues (P<0.001). High DUSP4 expression showed a significant association with lymph node metastasis and extrathyroidal extension in both FUSCC and TCGA cohorts, and DUSP4 overexpression was an independent risk factor for lymph node metastasis in multivariate analysis. Additionally, DUSP4 expression was associated with BRAFV600E mutation in both the cohorts (FUSCC: P=0.002, TCGA: P<0.001) and PTC cell lines (P=0.023). In conclusion, DUSP4 was identified as a potential biomarker for aggressive behavior in PTC, and its overexpression was BRAFV600E mutation-related.
Keywords: DUSP4/MKP2, BRAFV600E, LNM, PTC
Introduction
Differentiated thyroid cancer comprises the vast majority of all thyroid cancers, which includes papillary thyroid and follicular cancer.1 Papillary thyroid cancer (PTC) is responsible for the rapid increase in the incidence of thyroid cancer over the last few decades.2 Though the prognosis for PTC is usually excellent with a 10-year survival rate exceeding 90%,3 many patients suffer disease recurrence, which in some cases proves to be incurable and fatal.4 Clinicopathological parameters such as older age at diagnosis, poor histological subtypes, extrathyroidal extension (ETE), lymph node metastasis (LNM), and advanced tumor–node–metastasis (TNM) stage are considered as prognostic factors for poor clinical outcomes. Identification of specific molecular markers for the prediction of these aggressive behaviors of PTC may assist us in evaluating disease status and prognosis for patients.
Alteration of genes encoding effectors in the mitogen-activated protein kinase (MAPK) signaling pathway is a critical characteristic of PTC.5 MAPK phosphatases (MKPs), representing a distinct subfamily within a large group of dual-specificity protein phosphatases, act as negative regulators of MAPK activity in mammalian cells. Dual specificity phosphatase 4 (DUSP4), also known as MAPK phosphatase 2 (MKP2), plays a critical role as an inducible nuclear phosphatase in regulating cellular proliferation and differentiation via inactivation of ERK1/2, P38, and JNK.6
The behavior of DUSP4 in carcinoma still remains a complicated puzzle. As a negative regulator of the MAPK signaling pathway via inhibition of ERK1/2, DUSP4 represents a logical tumor suppressor. Downregulation or loss of DUSP4 expression has been reported to be correlated with carcinogenesis in breast cancer, glioma, and lung cancer.7–9 However, there are some studies, by contrast, suggesting that the gain of DUSP4 expression plays a crucial role in cancer development and progression.10–12 It is also interesting to note recent studies showing that DUSP4 is related to drug resistance in breast cancer patients following chemotherapy.13,14 So far, the clinical significance and biological effects of DUSP4 have not been established in PTC. BRAFV600E mutation, the most common and specific gene alteration in PTC, has been considered as a poor prognostic factor for PTC.15–17 Recently, Cagnol and Rivard18 suggested that BRAFV600E mutation as well as KRAS mutation may be involved in the regulation of DUSP4 expression in colon cancer cell lines. To better characterize the potential role of DUSP4 as a biomarker, the present study was performed to analyze the correlation of DUSP4 expression with the above clinicopathological variables and BRAFV600E mutation in PTC.
Materials and methods
Patients and clinicopathological data
Consecutive samples were selected from 120 patients of thyroidectomy diagnosed with PTC by pathology at the Fudan University Shanghai Cancer Center (FUSCC) from March 2012 to June 2012. The data on patients’ clinical features including sex, age at diagnosis, maximum size of tumor, multifocality, Hashimoto’s thyroiditis, histological types, ETE, and cervical LNM were retrospectively abstracted from patient records. All the patients were staged using the 2009 TNM classification of the American Joint Committee on Cancer/International Union Against Cancer.19 The selected samples were subjected to repeated evaluation to confirm the diagnosis of the aforementioned histological characteristics. Each patient provided a written informed consent for his/her specimens and information to be used for research and stored in the hospital database; this study was approved by the Ethical Committee of FUSCC. All the procedures performed in our study were in accordance with the ethical standards of our institutional research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, a validation cohort from The Cancer Genome Atlas (TCGA) database was identified to confirm the preliminary findings at FUSCC. A total of 499 primary PTC patients with detailed DUSP4 expression, BRAFV600E mutation, and clinical data were collected from the updated TCGA database according to parameters in a previous study.20 The TCGA cohort data were available on the website of Cancer Genomics Brower of California Santa Cruz (UCSC) (https://genome-cancer.ucsc.edu/), and the gene expression dataset and clinical data were obtained from the file named TCGA_THCA_exp_HiSeqV2_PANCAN.21 The process of analysis of DUSP4 in the TCGA cohort in detail is shown in Figure S1.
Human PTC cell lines and cell culture
Human-derived Nthy-ori-3-1, TPC-1, K1, and B-CPAP cell lines were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific) at 37°C in a 5% CO2 chamber. The Nthy-ori-3-1 cell line was obtained from normal follicular thyroid cells, and the other cell lines were derived from human PTC. BRAFV600E mutation status in the present cell lines has been reported in a previous study,22 and the outcomes of BRAFV600E mutation in the cell lines were also found on the website of Cancer Cell Line Encyclopedia (http://www.broadinstitute.org/ccle/home). The B-CPAP and K1 cell lines were obtained from the cell bank at Chinese Academy of Sciences (Shanghai, People’s Republic of China) and the Cancer Research Institute of FUSCC (Shanghai, People’s Republic of China), respectively; the other cell lines were kindly provided by Professor Haixia Guan from China Medical University (Shenyang, People’s Republic of China).
RNA extraction and real-time qRT–PCR analysis
Total RNA was extracted from the surgical specimens and cell lines using the QIAamp RNA Mini Kit (Qiagen, Chatsworth, California, USA) according to the manufacturer’s instructions. The extracted RNA was reverse transcribed for cDNA, followed by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) as previously described.23 The primers for DUSP4 were as follows: AGGCGGCTATGAGGTTTT (sense) and CACTGCCGAGGTAGAGGAAG (antisense). GAPDH was used as a housekeeping gene. qRT–PCR assays were performed in triplicates for each sample, and the mean value was used for the calculation of mRNA expression levels. The relative mRNA expression levels of DUSP4 were determined by the comparative Ct (2−ΔCt) method. The amount of target gene expression levels was given as ratios to GAPDH mRNA level.
DNA and BRAFV600E mutation analysis
Genomic DNA was extracted from the aforementioned specimens using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. The DNA template was amplified for analysis of mutations in exon 15 of the BRAF gene using PCR protocol as previously mentioned,24 followed by a Big Dye (Thermo Fisher Scientific) reaction for Sanger sequencing. BRAFV600E mutation was recognized on sequencing electropherograms.
Statistical analysis
Categorical data were summarized with frequencies and percentages. The continuous results were expressed as mean ± standard deviation (SD). Paired and independent Student’s t-tests were used to compare continuous variables in two groups. Associations between continuous variables and categorical variables were evaluated using Mann–Whitney U-test for two groups and Kruskal–Wallis test for more than two groups. χ2 and Fisher’s exact tests were used for categorical variables. To verify the associations between DUSP4 and BRAFV600E mutation and other characteristics, patients were divided into two subgroups (low expression and high expression) according to the median value of DUSP4 expression at the mRNA level in each cohort. A nonparametric receiver operating characteristic (ROC) analysis was performed to calculate the best cutoff value for DUSP4 expression level that would be predictive of LNM, using the GraphPad Prism 6.0 for Windows (La Jolla, California, USA). Moreover, univariate and multivariate analyses were performed to determine the risk factors for LNM in PTC in the FUSCC and TCGA cohorts using a logistic regression calculated by odds ratio (OR) and 95% confidence interval (CI). The area under a receiver characteristic curve was used to measure the relative predictability of independent factors for LNM. A P-value <0.05 was considered significant. Statistical analyses were performed using the GraphPad Prism 6.0 and SPSS for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological data of patients in the FUSCC and TCGA cohorts
A total of 120 patients (29 males and 91 females; median age: 44.54±1.20; range: 16–84 years) from the FUSCC were enrolled in this study. The maximum size of a tumor, on an average, was 1.37±0.94 cm. The incidence of LNM and ETE was 64.71% (77 of 119 PTC patients) and 9.20% (11 of 120 PTC patients), respectively. Conventional PTC comprised the majority of patients (98.30%), and the follicular-variant PTC was present in only two patients (1.70%).
A validation cohort of 499 PTC patients (136 males and 363 females; median age: 47.13±15.86; range: 15–89 years) was collected from the TCGA database. LNM was present in 226 of 449 (50.33%) patients, and ETE occurred in 153 of 482 (31.74%) patients. The ratio of classic, follicular, and tall-cell PTC types was 71.54%, 19.24% and 7.41%, respectively.
Comparison of DUSP4 expression between PTC and adjacent normal tissues in the FUSCC cohort and in vitro outcomes
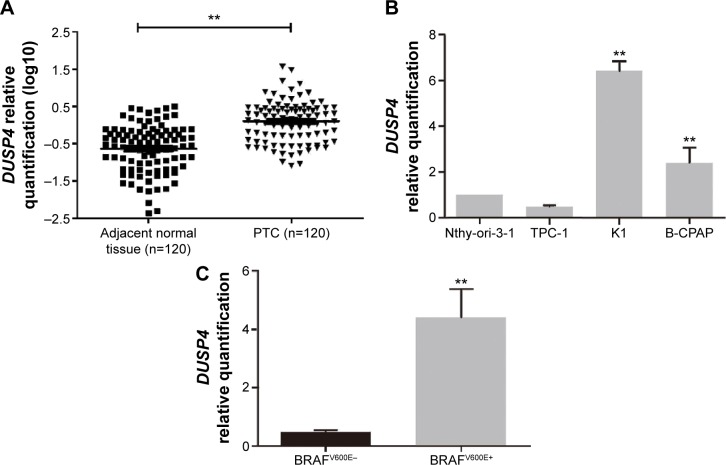
DUSP4 expression was detected in the carcinoma specimens and paired normal tissues from 120 PTC patients at FUSCC. As shown in Figure 1A, DUSP4 mRNA expression was significantly elevated in PTC compared with the level in the adjacent normal tissues (P<0.001). We also detected DUSP4 expression in human-derived Nthy-ori-3-1, TPC-1, K1, and B-CPAP cell lines. Increased DUSP4 expression was significant in PTC-derived K1 (P=0.036) and B-CPAP (P=0.042) cell lines in comparison to Nthy-ori-3-1 cell line (Figure 1B). There was no significant difference in DUSP4 expression between TPC-1 and Nthy-ori-3-1 cell lines (Figure 1B, P>0.05).
Figure 1.
A comparison of DUSP4 expression between PTC and adjacent normal tissues in the FUSCC cohort and in vitro outcomes.
Notes: (A) Shows Comparison of DUSP4 expression between PTC and adjacent normal tissues. DUSP4 mRNA expression was significantly elevated in PTC compared with the level in the adjacent normal tissues (**P<0.001). DUSP4 mRNA expression was normalized for the GAPDH mRNA level (2−ΔCt). The relative quantification of DUSP4 expression in PTC (n=120) and adjacent normal tissues (n=120) was measured as log10 values. (B) Shows DUSP4 expression in human-derived thyroid cell lines. Increased DUSP4 expression was significant in PTC-derived K1 (**P=0.036) and B-CPAP (**P=0.042) cell lines in comparison to Nthy-ori-3-1 cell line. DUSP4 expression difference between the TPC-1 and Nthy-ori-3-1 cell lines was not significant (P>0.05). The relative quantification of DUSP4 expression in PTC cell lines was measured as folds to Nthy-ori-3-1 cell line. (C) Shows DUSP4 expression in BRAFV600E wild-type and -mutated thyroid cell lines. A higher level expression of DUSP4 was present in BRAFV600E-mutated cell lines (**P=0.023). The relative quantification of DUSP4 expression in BRAFV600E-mutated cell lines was measured as folds to BRAFV600E wild-type cell line.
Abbreviations: DUSP4, dual specificity phosphatase 4; PTC, papillary thyroid cancer; FUSCC, Fudan University Shanghai Cancer Center.
Clinical significance of DUSP4 in PTC
The correlation of DUSP4 with the clinicopathological characteristics of PTC patients in the TCGA and FUSCC cohorts was analyzed, as shown in Table 1. High DUSP4 expression was associated with LNM and ETE in both the FUSCC and TCGA cohorts, showing a higher expression level in PTC with aggressive behavior. The TCGA cohort also indicated there was a significant difference in DUSP4 expression among different TNM stages (P<0.005). The other characteristics including sex, age, tumor size, multifocality, histological types, and Hashimoto’s thyroiditis failed to correlate with DUSP4 expression.
Table 1.
Correlation between DUSP4 expression and clinicopathological characteristics in PTC in the FUSCC and TCGA cohorts
| Variables | FUSCC cohort (N=120)
|
TCGA cohort (N=499)
|
||||||
|---|---|---|---|---|---|---|---|---|
| N | Low | High | P-value | N | Low | High | P-value | |
| Sex | 0.242 | 0.075 | ||||||
| Male | 29 | 12 (41.4%) | 17 (58.6%) | 136 | 77 (56.6%) | 59 (43.4%) | ||
| Female | 91 | 49 (53.8%) | 42 (46.2%) | 363 | 173 (47.7%) | 190 (52.3%) | ||
| Age (years) | 0.584 | 0.503 | ||||||
| <45 | 60 | 29 (48.3%) | 31 (51.7%) | 229 | 111 (48.5%) | 118 (51.5%) | ||
| ≥45 | 60 | 32 (53.3%) | 28 (46.7%) | 270 | 139 (51.5%) | 131 (48.5%) | ||
| Maximum size of tumor (cm) | 0.733 | 0.250 | ||||||
| ≤2 | 99 | 51 (51.5%) | 48 (48.5%) | 85 | 50 (58.8%) | 35 (41.2%) | ||
| 2–4 | 18 | 8 (44.4%) | 10 (55.6%) | 131 | 73 (55.7%) | 58 (44.3%) | ||
| >4 | 3 | 2 (66.7%) | 1 (33.3%) | 131 | 63 (48.1%) | 68 (51.9%) | ||
| Multifocality | 0.458 | 0.225 | ||||||
| Unifocal | 91 | 48 (52.7%) | 43 (47.3%) | 263 | 124 (47.1%) | 239 (52.9%) | ||
| Multifocal | 29 | 13 (44.8%) | 16 (55.2%) | 226 | 119 (52.7%) | 107 (47.3%) | ||
| Histological type | 0.981 | 0.901 | ||||||
| Classical PTC | 118 | 60 (50.8%) | 58 (49.2%) | 357 | 181 (50.7%) | 176 (49.3%) | ||
| Follicular PTC | 2 | 1 (50.0%) | 1 (50.0%) | 96 | 45 (46.9%) | 51 (53.1%) | ||
| Tall-cell PTC | – | – | – | 37 | 19 (51.4%) | 18 (48.6%) | ||
| Other types | – | – | – | 9 | 5 (55.6%) | 4 (44.4%) | ||
| Coexistent HT | 0.886 | 0.058 | ||||||
| Yes | 23 | 12 (52.2%) | 11 (47.8%) | 35 | 12 (34.3%) | 23 (65.7%) | ||
| No | 97 | 49 (50.5%) | 48 (49.5%) | 406 | 207 (51.0%) | 199 (49.0%) | ||
| ETE | 0.023* | 0.024* | ||||||
| Yes | 11 | 2 (18.2%) | 9 (81.8%) | 153 | 65 (42.5%) | 88 (57.5%) | ||
| No | 109 | 59 (54.1%) | 50 (45.9%) | 329 | 176 (53.5%) | 153 (46.5%) | ||
| LNM | 0.006* | 0.003* | ||||||
| N0 | 42 | 29 (69.0%) | 13 (31.0%) | 226 | 128 (56.6%) | 98 (43.4%) | ||
| N1 | 77 | 33 (42.9%) | 44 (57.1%) | 223 | 95 (42.6%) | 128 (57.4%) | ||
| Nx | 1 | 50 | ||||||
| T stage | 0.076 | 0.132 | ||||||
| T1–T2 | 106 | 57 (53.8%) | 49 (46.2%) | 307 | 162 (52.8%) | 145 (47.2%) | ||
| T3–T4 | 14 | 4 (28.6%) | 10 (71.4%) | 192 | 88 (45.8%) | 104 (54.2%) | ||
| TNM stage | 0.547 | 0.005* | ||||||
| I | 92 | 50 (54.3%) | 42 (45.7%) | 284 | 148 (52.1%) | 136 (47.9%) | ||
| II | 3 | 1 (33.3%) | 2 (66.7%) | 53 | 34 (64.2%) | 19 (35.8%) | ||
| III | 16 | 6 (37.5%) | 10 (62.5%) | 108 | 51 (47.2%) | 57 (52.8%) | ||
| IV | 9 | 4 (44.4%) | 5 (55.6%) | 54 | 17 (31.5%) | 37 (68.5%) | ||
| BRAFV600E | 0.002* | <0.001* | ||||||
| Mutation | 57 | 21 (36.8%) | 36 (63.2%) | 249 | 93 (37.3%) | 156 (62.7%) | ||
| Wild-type | 63 | 41 (65.1%) | 22 (34.9%) | 167 | 113 (67.7%) | 54 (32.3%) | ||
Note:
Statistically significant.
Abbreviations: DUSP4, dual specificity phosphatase 4; PTC, papillary thyroid cancer; FUSCC, Fudan University Shanghai Cancer Center; TCGA, The Cancer Genomics Atlas; HT, Hashimoto’s thyroiditis; ETE, extrathyroidal extension; LNM, lymph node metastasis; Nx, evaluation not available; TNM, tumor–node–metastasis.
A further analysis was performed to determine whether increased DUSP4 expression was an independent risk factor for LNM in PTC in the two cohorts. ROC analysis was used to identify the best cutoff of DUSP4 expression levels that were predictive of LNM. Table 2 shows tumor size between 2 and 4 cm (OR =3.113, 95% CI 1.035–22.375, P=0.045), and DUSP4 expression greater than the cutoff value (OR =4.064, 95% CI 1.632–10.124, P=0.003) was a risk factor for LNM in univariate analysis in the FUSCC cohort. In total, 29 (48.3%) of 60 patients aged ≥45 years had LNM in comparison with 48 (81.4%) of 59 patients less than 45 years, showing that age ≥45 years (OR =0.219, 95% CI 0.091–0.524, P=0.001) was a protective factor for LNM. After adjusting for age, tumor size, and DUSP4, DUSP4 expression greater than the cutoff value (OR =4.215, 95% CI 1.565–11.347, P=0.004) was found to be an independent risk factor for LNM, and age ≥45 years as a protective factor remained significant (OR =0.195, 95% CI 0.076–0.501, P=0.001) in multivariate analysis. The TCGA cohort was analyzed to confirm the significance of DUSP4 overexpression, which showed that DUSP4 expression greater than the cutoff value (OR =1.741, 95% CI 1.074–2.820, P=0.024), age ≥45 years (OR =0.456, 95% CI 0.287–0.724, P=0.001), and ETE (OR =3.214, 95% CI 1.948–5.303, P<0.001) were independent factors for LNM in a multivariate analysis. Though BRAFV600E mutation and female sex were shown to be correlated with LNM in a univariate analysis, they failed to be independent factors after adjusting sex, age, ETE, BRAFV600E, and DUSP4 (Table 3).
Table 2.
Clinicopathological and molecular factors associated with LNM in PTC in the FUSCC cohort
| Variables | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| P-value | OR | 95% CI for OR | P-value | OR | 95% CI for OR | |
| Female | 0.094 | 0.422 | 0.154–1.159 | |||
| Age ≥45 years | 0.001# | 0.219 | 0.091–0.524 | 0.001# | 0.195 | 0.076–0.501 |
| Tumor size (cm) | ||||||
| 2–4 | 0.045# | 3.113 | 1.035–22.375 | 0.062 | 4.740 | 0.927–24.290 |
| >4 | 0.841 | 1.283 | 0.112–14.702 | |||
| Multifocality | 0.431 | 1.484 | 0.556–3.960 | |||
| HT | 0.113 | 0.450 | 0.168–1.208 | |||
| ETE | 0.120 | 5.311 | 0.646–43.664 | |||
| BRAFV600E | 0.394 | 1.424 | 0.632–3.208 | |||
| DUSP4 (greater than cutoff)* | 0.003# | 4.064 | 1.632–10.124 | 0.004# | 4.215 | 1.565–11.347 |
Notes:
Statistically significant;
DUSP4 expression level greater than the best cutoff value for the prediction of LNM.
Abbreviations: LNM, lymph node metastasis; PTC, papillary thyroid cancer; FUSCC, Fudan University Shanghai Cancer Center; OR, odds ratio; CI, confidence interval; HT, Hashimoto’s thyroiditis; ETE, extrathyroidal extension; DUSP4, dual specificity phosphatase 4.
Table 3.
Clinicopathological and molecular factors associated with LNM in PTC in the TCGA cohort
| Variables | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| P-value | OR | 95% CI for OR | P-value | OR | 95% CI for OR | |
| Female | 0.037# | 0.643 | 0.424–0.974 | 0.064 | 0.626 | 0.381–1.027 |
| Age ≥45 years | 0.010# | 0.610 | 0.420–0.887 | 0.001# | 0.456 | 0.287–0.724 |
| Tumor size (cm) | ||||||
| 2–4 | 0.343 | 1.340 | 0.732–2.455 | |||
| >4 | 0.127 | 1.593 | 0.876–2.895 | |||
| Multifocality | 0.107 | 1.362 | 0.935–1.982 | |||
| HT | 0.058 | 1.987 | 0.976–4.043 | |||
| ETE | <0.001# | 2.919 | 1.919–4.440 | <0.001# | 3.214 | 1.948–5.303 |
| BRAFV600E | <0.001# | 2.168 | 1.415–3.321 | 0.076 | 1.549 | 0.955–2.511 |
| DUSP4 (greater than cutoff)* | <0.001# | 2.513 | 1.689–3.738 | 0.024# | 1.741 | 1.074–2.820 |
Notes:
Statistically significant;
DUSP4 expression level greater than the best cutoff value for the prediction of LNM.
Abbreviations: LNM, lymph node metastasis; PTC, papillary thyroid cancer; TCGA, The Cancer Genomics Atlas; OR, odds ratio; CI, confidence interval; HT, Hashimoto’s thyroiditis; ETE, extrathyroidal extension; DUSP4, dual specificity phosphatase 4.
Additionally, preoperative independent factors for LNM including age and DUSP4 were evaluated to see if DUSP4 could influence the predictive effect for LNM in the TCGA cohort. As shown in Figure 2, a comparison of predictability for LNM as measured by area under the ROC curve among age, DUSP4, and their combination showed that the predictive effect size for LNM increased from 0.571 to 0.643 after DUSP4 was added (P=0.014). The difference in predication between age (0.571) and DUSP4 (0.637) alone was not significant (P=0.075).
Figure 2.
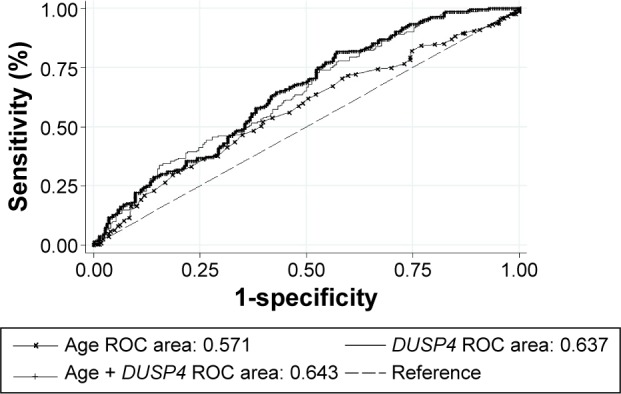
A comparison of predictability for LNM as measured by area under the ROC curve among age, DUSP4, and their combination in the TCGA cohort.
Notes: The predictive effect size for LNM increased from 0.571 to 0.643 after DUSP4 was added (P=0.014). The difference in predication between age (0.571) and DUSP4 (0.637) alone was not significant (P=0.075).
Abbreviations: ROC, receiver operating characteristic; DUSP4, dual specificity phosphatase 4; LNM, lymph node metastasis; TCGA, The Cancer Genome Atlas.
Relationship between DUSP4 and BRAFV600E mutation in PTC
The positive rates of BRAFV600E mutation in the FUSCC and TCGA cohorts were 47.50% (57/120) and 59.86% (249/416), respectively. DUSP4 expression was associated with the BRAFV600E mutation status in PTC in the two cohorts (FUSCC: P=0.002, TCGA: P<0.001), showing a higher expression level in BRAFV600E-mutated PTC than the level in BRAFV600E wild-type PTC (Table 1). DUSP4 expression analysis was also performed in the BRAFV600E wild-type cell line (TPC-1) and BRAFV600E-mutated cell lines (K1 and B-CPAP). As shown in Figure 1C, a higher level of DUSP4 expression was present in BRAFV600E-mutated cell lines (P=0.023). A multivariate logistic regression analysis was performed to investigate factors that could affect DUSP4 expression in both the FUSCC and TCGA cohorts, and the outcomes indicated that BRAFV600E mutation was an independent risk factor with strong effect size (FUSCC: OR =3.366, 95% CI 1.377–8.228, P=0.008; TCGA: OR =2.794, 95% CI 1.753–4.452, P<0.001, Table 4).
Table 4.
Multivariate analysis of factors that could affect DUSP4 expression in PTC in the FUSCC and TCGA cohorts
| Variables | FUSCC
|
TCGA
|
||||
|---|---|---|---|---|---|---|
| P-value | OR | 95% CI for OR | P-value | OR | 95% CI for OR | |
| Female | 0.337 | 0.485 | 0.111–2.126 | 0.066 | 1.589 | 0.970–2.602 |
| Age ≥45 years | 0.533 | 0.727 | 0.267–1.980 | 0.012* | 0.399 | 0.195–0.816 |
| ETE | 0.069 | 4.768 | 0.883–25.743 | 0.867 | 0.956 | 0.566–1.616 |
| LNM | 0.417 | 1.714 | 0.467–6.292 | 0.555 | 1.163 | 0.704–1.921 |
| TNM stage | 0.276 | 1.480 | 0.731–2.999 | 0.007* | 1.632 | 1.147–2.323 |
| BRAFV600E | 0.008* | 3.366 | 1.377–8.228 | <0.001* | 2.794 | 1.753–4.452 |
Note:
Statistically significant.
Abbreviations: DUSP4, dual specificity phosphatase 4; PTC, papillary thyroid cancer; FUSCC, Fudan University Shanghai Cancer Center; TCGA, The Cancer Genomics Atlas; OR, odds ratio; CI, confidence interval; ETE, extrathyroidal extension; LNM, lymph node metastasis; TNM, tumor–node–metastasis.
Discussion
DUSP4 is a mitogen- and stress-inducible nuclear MKP, which displays a substrate preference for ERK, p38, and JNK; it actually binds to ERK and p38 with higher affinity than to JNK.25 Several authors have shown in studies of the ERK pathway how DUSP4 may cooperate to regulate MAPK signaling. Caunt et al26 indicated that DUSP4 is employed in a sustained MEK stimulus-specific manner and regulates nuclear dephosphorylation and accumulation of ERK in loss-of-functional experiments using siRNA knockdown of MKPs. Cagnol and Rivard18 reported that DUSP4 is induced in response to oncogenic activation of the ERK pathway in colon-derived cancer cells, which correlates with nuclear accumulation of dephosphorylated ERK, and revealed it as a potential regulator of ERK-driven tumor cell proliferation. The correlation between DUSP4 and cancer progression has been established regardless of the loss or gain of DUSP4 expression in other cancer types. Saigusa et al23 showed that decreased DUSP4 expression is associated with tumor progression, especially distant metastasis, in colorectal cancer. Balko et al27 reported that the activation of the MAPK pathway due to DUSP4 loss promotes cancer stem cell-like phenotype in basal-like breast cancer. By contrast, Kim et al12 suggested that DUSP4 is frequently upregulated in breast malignancy and may be a marker of adverse prognosis. The effects of DUSP4 in cancer progression still remain a puzzle. There is no adequate evidence and research report regarding the role of DUSP4 in PTC.
In the present study, DUSP4 expression was significantly elevated in PTC tissues compared with adjacent normal tissues, which was consistent with the findings in previous studies.5,28 In addition, our report has suggested for the first time that increased DUSP4 expression is associated with aggressive behavior of PTC such as LNM and ETE. It was also indicated in the TCGA cohort that high DUSP4 expression correlates with advanced TNM stage. In the FUSCC cohort, DUSP4 expression was high in 15 (60.0%) of 25 patients in the stage III and stage IV compared with 44 (46.3%) of 95 patients in the stage I and stage II, but the outcome failed to be statistically significant. The effect size could be affected by the limited sample size and big difference in sample distribution from stage I to stage IV in our cohort.
LNM is a critical parameter for physicians in determining whether performing lymph node dissection and providing the following I131 therapy, and it also assists in evaluating the regional recurrence and prognosis for PTC patients. Cervical LNM is very common in PTC, and the sensitivity of preoperative detection of LNM by ultrasound imaging and computerized tomography is relatively low.29 Though the presence of BRAFV600E mutation identifies a part of PTC patients with LNM, the BRAFV600E mutation status taken in isolation cannot specifically identify PTC with extrathyroidal spread.30 Therefore, the identification of specific predictors for LNM in PTC is crucial for surgeons to increase preoperative sensitivity of LNM detection. We performed a further analysis of LNM risk factors. DUSP4 overexpression was confirmed to be an independent risk factor for LNM in PTC, and DUSP4 combined with age could improve predictive value for LNM. The abovementioned outcomes may support that DUSP4 plays a positive role in promoting the development and progression of PTC.
Moreover, our findings also showed that BRAFV600E mutation significantly correlated with increased DUSP4 expression. Oncogenic RAS and BRAFV600E mutations have been reported to activate the MEK/ERK pathway, resulting in DUSP4 expression upregulation and further ERK1/2 inhibition in colorectal cancer cells.18 A previous study also suggested that BRAFV600E mutation can drive intensive downstream signaling of the MAPK pathway via blocking a feedback loop from ERK to BRAF, but actually BRAFV600E-mutated tumor cells do not have higher levels of ERK activity than cells of other ERK-dependent tumor types.31 A promising explanation suggests that BRAFV600E-mutated tumors are likely to have higher levels of MKPs, which could to some extent compensate for the loss of ERK to BRAF feedback.31 Integrated genomic characterization of PTC5 suggests that PTC could be divided into two subgroups based on molecular landscape: BRAFV600E-variant-like PTC and RAS-variant-like PTC; the overexpression of DUSP4, 5, and 6 is relatively exclusive for BRAFV600E-variant-like PTCs, which is consistent with our findings. These findings may reveal that DUSP4 is induced by the BRAFV600E mutation-activated MAPK pathway in PTC.
Despite an observation of the role of DUSP4 overexpression in PTC in this study, the mechanism of the effects of DUSP4 has not yet been elucidated. According to the study by Cancer Genome Atlas Research Network, DUSP4 overexpression is interpreted to represent the high output of the ERK transcriptional program caused by robust activation of MAPK pathway signaling in BRAFV600E-mutated PTC cases.5 Hasegawa et al32 found that the inhibition of DUSP4 attenuates the in vitro and in vivo proliferation of thyroid cancer cells from transgenic mice, which is mediated by the suppression of cyclin B1 expression. Furthermore, Lee et al33 confirmed elevated expression of DUSP4 in thyroid cancer tissues, and their observation on the methylation status of DUSP4 suggests that this tumor-suppressor gene does not actually suppress tumor growth. The logical interpretations here may be that elevated expression of DUSP4 in PTC enables it to play a positive role in tumor progression rather than a suppressor in a manner dependent on the upstream oncogenic mutation.
Conclusion
In conclusion, we identify DUSP4 as a potential biomarker for aggressive behavior, especially for LNM, in PTC and that its overexpression is BRAFV600E mutation-related.
Supplementary material
A flow graph of analysis of DUSP4 expression in the TCGA cohort.
Abbreviations: TCGA, The Cancer Genome Atlas; DUSP4, dual specificity phosphatase 4; LNM, lymph node metastasis; PTC, papillary thyroid cancer.
Acknowledgments
The research was supported by the Science and Technology Commission of Shanghai Municipality (14ZR1407300).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338(5):297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 4.Lin JD, Hsueh C, Chao TC. Long-term follow-up of the therapeutic outcomes for papillary thyroid carcinoma with distant metastasis. Medicine (Baltimore) 2015;94(26):e1063. doi: 10.1097/MD.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27(2):253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 7.Armes JE, Hammet F, de Silva M, et al. Candidate tumor-suppressor genes on chromosome arm 8p in early-onset and high-grade breast cancers. Oncogene. 2004;23(33):5697–5702. doi: 10.1038/sj.onc.1207740. [DOI] [PubMed] [Google Scholar]
- 8.Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene. 2009;28(31):2773–2783. doi: 10.1038/onc.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waha A, Felsberg J, Hartmann W, et al. Epigenetic downregulation of mitogen-activated protein kinase phosphatase MKP-2 relieves its growth suppressive activity in glioma cells. Cancer Res. 2010;70(4):1689–1699. doi: 10.1158/0008-5472.CAN-09-3218. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa T, Enomoto A, Kato T, et al. Roles of induced expression of MAPK phosphatase-2 in tumor development in RET-MEN2A transgenic mice. Oncogene. 2008;27(43):5684–5695. doi: 10.1038/onc.2008.182. [DOI] [PubMed] [Google Scholar]
- 11.Lawan A, Al-Harthi S, Cadalbert L, et al. Deletion of the dual specific phosphatase-4 (DUSP-4) gene reveals an essential non-redundant role for MAP kinase phosphatase-2 (MKP-2) in proliferation and cell survival. J Biol Chem. 2011;286(15):12933–12943. doi: 10.1074/jbc.M110.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Jang SM, Ahn H, et al. Clinicopathological significance of dual-specificity protein phosphatase 4 expression in invasive ductal carcinoma of the breast. J Breast Cancer. 2015;18(1):1–7. doi: 10.4048/jbc.2015.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balko JM, Cook RS, Vaught DB, et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012;18(7):1052–1059. doi: 10.1038/nm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Du F, Chen W, Yao M, Lv K, Fu P. Knockdown of dual specificity phosphatase 4 enhances the chemosensitivity of MCF-7 and MCF-7/ADR breast cancer cells to doxorubicin. Exp Cell Res. 2013;319(20):3140–3149. doi: 10.1016/j.yexcr.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 16.Guerra A, Fugazzola L, Marotta V, et al. A high percentage of BRAFV600E alleles in papillary thyroid carcinoma predicts a poorer outcome. J Clin Endocrinol Metab. 2012;97(7):2333–2340. doi: 10.1210/jc.2011-3106. [DOI] [PubMed] [Google Scholar]
- 17.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cagnol S, Rivard N. Oncogenic KRAS and BRAF activation of the MEK/ERK signaling pathway promotes expression of dual-specificity phosphatase 4 (DUSP4/MKP2) resulting in nuclear ERK1/2 inhibition. Oncogene. 2013;32(5):564–576. doi: 10.1038/onc.2012.88. [DOI] [PubMed] [Google Scholar]
- 19.Sobin LH, Gospodarowicz M, Wittekind C. UICC: TNM Classification of Malignant Tumors. 7th ed. New York: Wiley-Liss; 2009. [Google Scholar]
- 20.Jiang YZ, Yu KD, Zuo WJ, Peng WT, Shao ZM. GATA3 mutations define a unique subtype of luminal-like breast cancer with improved survival. Cancer. 2014;120(9):1329–1337. doi: 10.1002/cncr.28566. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Wan F, Zhang H, Shi G, Ye D. ITGA2B and ITGA8 are predictive of prognosis in clear cell renal cell carcinoma patients. Tumour Biol. 2015 Jul 22; doi: 10.1007/s13277-015-3792-5. Epub. [DOI] [PubMed] [Google Scholar]
- 22.Saiselet M, Floor S, Tarabichi M, et al. Thyroid cancer cell lines: an overview. Front Endocrinol (Lausanne) 2012;3:133. doi: 10.3389/fendo.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saigusa S, Inoue Y, Tanaka K, et al. Decreased expression of DUSP4 is associated with liver and lung metastases in colorectal cancer. Med Oncol. 2013;30(3):620. doi: 10.1007/s12032-013-0620-x. [DOI] [PubMed] [Google Scholar]
- 24.Lang BH, Chai YJ, Cowling BJ, Min HS, Lee KE, Youn YK. Is BRAFV600E mutation a marker for central nodal metastasis in small papillary thyroid carcinoma? Endocr Relat Cancer. 2014;21(2):285–295. doi: 10.1530/ERC-13-0291. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Hutter D, Yang X, Gorospe M, Davis RJ, Liu Y. Discordance between the binding affinity of mitogen-activated protein kinase subfamily members for MAP kinase phosphatase-2 and their ability to activate the phosphatase catalytically. J Biol Chem. 2001;276(31):29440–29449. doi: 10.1074/jbc.M103463200. [DOI] [PubMed] [Google Scholar]
- 26.Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem. 2008;283(39):26612–26623. doi: 10.1074/jbc.M801500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balko JM, Schwarz LJ, Bhola NE, et al. Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res. 2013;73(20):6346–6358. doi: 10.1158/0008-5472.CAN-13-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Degl’Innocenti D, Romeo P, Tarantino E, et al. DUSP6/MKP3 is overexpressed in papillary and poorly differentiated thyroid carcinoma and contributes to neoplastic properties of thyroid cancer cells. Endocr Relat Cancer. 2013;20(1):23–37. doi: 10.1530/ERC-12-0078. [DOI] [PubMed] [Google Scholar]
- 29.Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18(4):411–418. doi: 10.1089/thy.2007.0269. [DOI] [PubMed] [Google Scholar]
- 30.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratilas CA, Taylor BS, Ye Q, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106(11):4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa T, Enomoto A, Kato T, et al. Roles of induced expression of MAPK phosphatase-2 in tumor development in RET-MEN2A transgenic mice. Oncogene. 2008;27(43):5684–5695. doi: 10.1038/onc.2008.182. [DOI] [PubMed] [Google Scholar]
- 33.Lee EK, Chung KW, Yang SK, et al. DNA methylation of MAPK signal-inhibiting genes in papillary thyroid carcinoma. Anticancer Res. 2013;33(11):4833–4839. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A flow graph of analysis of DUSP4 expression in the TCGA cohort.
Abbreviations: TCGA, The Cancer Genome Atlas; DUSP4, dual specificity phosphatase 4; LNM, lymph node metastasis; PTC, papillary thyroid cancer.