Abstract
Objective
The objective of the study was to investigate medication usage patterns, health care resource utilization, and direct medical costs of patients with major depressive disorder (MDD) in Beijing, People’s Republic of China.
Methods
Data were extracted from a random sample of the Beijing Urban Employee Basic Medical Insurance database. Patients aged ≥18 years, with ≥1 primary diagnosis of MDD and 12-month continuous enrollment after their first observed MDD diagnosis between 2012 and 2013 were identified. Those with a diagnosis of schizophrenia, bipolar disorder, or cancer during the analysis period were excluded.
Results
In total 8,484 patients, with a mean age of 57.2 years, were included and 63% were female. The top three commonly observed comorbidities were hypertension (70.9%), anxiety disorder (68.6%), and coronary heart disease (65.1%). Furthermore, 71.4% of patients were treated with antidepressant medications, including 60.5% of patients treated with selective serotonin reuptake inhibitors, followed by noradrenergic and specific serotonergic antidepressants (9.0%) and serotonin–norepinephrine reuptake inhibitors (8.3%). The proportions of patients who discontinued their initial antidepressant within the first and second months after the index date were 45.4% and 77.0%, respectively. Concomitant medications were prescribed for 76.8% of patients. Only 0.42% of patients experienced ≥1 MDD-related hospitalization(s) during the 1-year follow-up, and the average annual number of hospitalization was 1.2 for those hospitalized. The mean length of stay was 33.4 days per hospitalization. All patients had ≥1 MDD-related outpatient visit(s). The mean annual number of outpatient visits per patient was 3.1. The mean annual direct medical costs per patient with MDD was RMB ¥1,694.1 (48.5% for antidepressant medications), and that for hospitalized patients was RMB ¥21,291.0 (15.0% for antidepressant medications).
Conclusion
In Beijing, the majority of patients with MDD were treated in the outpatient setting only and they received antidepressants. Selective serotonin reuptake inhibitors were the most commonly used antidepressants. However, the duration to antidepressant medication was short, and persistence was low. The economic burden of MDD-related hospitalization was considerable.
Keywords: depression, People’s Republic of China, antidepressants, health care resource, persistence
Introduction
Major depressive disorder (MDD) is a mental disorder characterized by a persistently low mood, accompanied by feelings of inferiority and loss of interest or pleasure in normally enjoyable activities.1 With a relatively high prevalence, MDD can afflict anyone at any age and is one of the top five major illnesses in terms of burden worldwide.2 A large survey conducted across ten countries showed that the lifetime prevalence of MDD was within the range of 8%–12%, with 6.7% of adults >18 years affected by MDD in the USA alone.3,4 In the People’s Republic of China, domestic research has shown that the prevalence of MDD is high.2 The point prevalence of MDD was found to be 3.31% and the life time prevalence was 6.87% in an epidemiological survey of 5,926 subjects conducted in Beijing.5
Both antidepressant medications and psychotherapy are recommended forms of treatment. According to the American Psychiatric Association (APA) Practice Guideline for The Treatment of Patients with MDD, for most patients, a selective serotonin reuptake inhibitor (SSRI), serotonin–norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion is recommended as a first-line treatment. Both the APA guidelines and Chinese guidelines recommend a minimum duration of treatment before concluding that the patient is partially responsive or unresponsive to a specific intervention (4–8 weeks in the APA guidelines and 4–6 weeks in the Chinese guidelines).2,6 To reduce the risk of relapse, patients who have been treated successfully with antidepressant medications in the acute phase after their initial episode of MDD should continue treatment with these agents for a period of time (4–9 months in APA guidelines and at least 4–6 months in Chinese guidelines). For patients who have suffered multiple episodes of MDD, longer courses of treatment with antidepressants are recommended. Hospitalization is also recommended in cases with associated self-neglect, a significant risk of harm to self or others, or strong suicide attempts.2,6 In addition, MDD is associated with significant public health problems and poses a substantial burden on patients, caregivers, and payers.7,8 In Europe, it has been estimated that the costs of MDD correspond to 1% of the total gross domestic product, with the health care costs corresponding to 5% of European health budgets.9 A systematic analysis of 188 countries between 1990 and 2013 found that low-back pain and MDD are the leading causes of years lived with disability and among the top causes of disability across the globe.10 It was estimated that mental disorders and suicide will rank in first place in terms of the overall disease burden in the People’s Republic of China by 2020.11
Although some studies12,13 have been conducted to assess the disease burden of MDD in the People’s Republic of China, studies on health care resource unitization, medication usage patterns, and the direct medical costs for patients with MDD in a tier-1 city, such as Beijing (the capital city of the People’s Republic of China), using recent real-world data, are scarce. The objective of this analysis is to investigate the medication usage patterns, health care resource utilization, and associated economic burden among patients diagnosed with MDD in Beijing, People’s Republic of China, using information between 2011 and 2013 from the Beijing Urban Employee Basic Medical Insurance (UEBMI) claims database.
Methods
Data source
In Beijing, >90% of the urban population is covered by the Urban Basic Health Insurance scheme. Among them, most are employees or retirees covered by the Beijing UEBMI scheme. In 2012, this scheme contained 13 million participants. Among them, five million participants used some form of health care service or clinical visits, and the associated clinical records were available in the database.
A retrospective analysis was conducted by using deidentified records from the Beijing UEBMI claims database from January 1, 2012, to December 31, 2013, by an approved third party organization (Beijing Brainpower Pharma Consulting Co, Ltd., Beijing, People’s Republic of China). Data extracted included the enrollment history and every claim for inpatient, outpatient, and pharmacy services received for the analysis sample, which was a simple random sample of 10% of the participants who had ≥1 MDD diagnosis in the insurance system.
Patients diagnosed with MDD were identified using the International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes F32–F33, and the first primary diagnosis date in 2012 was designated as the index date. Patients were required to be at least 18 years old without comorbid cancer, schizophrenia, or bipolar disorder and with at least 12 months of continuous enrollment after the index date in the claims database. Because the data was retrospectively collected and the approved third party organization (BrainPower) can only access de-identified records in the Beijing UEBMI claims database, ethical approval and written informed patient consent were waived for this study.
Analyzed outcomes
Medications of interest are shown in Table 1. They include the following:
Antidepressants: tricyclic antidepressants (TCAs), SSRIs, SNRIs, noradrenergic and specific serotonergic antidepressants (NaSSA), and other antidepressants.
Concomitant medications used in the treatment of patients with MDD: atypical antipsychotics, sedative–hypnotic drugs, and pain-related medications.
Other medications: maprotiline, mianserin, propion, trazodone, reboxetine, nefazodone, tianeptine, and neurostan.
Table 1.
List of antidepressants and concomitant medications
| Antidepressant medications | |
| TCAs | Imipramine, doxepin, amitriptyline, clomipramine |
| SSRIs | Fluoxetine, paroxetine, fluvoxamine, sertraline, citalopram, escitalopram |
| SNRIs | Duloxetine, venlafaxine |
| NaSSA | Mirtazapine |
| Other antidepressants | Maprotiline, mianserin, propion, trazodone, reboxetine, nefazodone, tianeptine, neurostan |
| Concomitant medications | |
| Atypical antipsychotics | Clozapine, risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, paliperidone |
| Sedative–hypnotic drugs | Anxiolytics, benzodiazepines, hypnotics, anticonvulsants, sedatives |
| Pain-related medications | |
| Muscle relaxants | Eperisone, baclofen |
| Analgesics | Acetaminophen, NSAIDs, tramadol, opioids |
Abbreviations: TCA, tricyclic antidepressant; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; NaSSA, noradrenergic and specific serotonergic antidepressant; NSAID, nonsteroidal anti-inflammatory drug.
The treatment use patterns of interest during the follow-up period were defined as follows14,15:
Stopped: pattern wherein patients had a gap of >30 days for the antidepressant medication initiated at the index date
Switched: pattern wherein patients switched to other antidepressant medications during the postindex period
Augmented: pattern wherein patients added antipsychotics or other concomitant medication listed herein while continuing use of the antidepressant initiated at the index date
Discontinued: pattern wherein patients either stopped or switched treatment
Treatment persistence: the duration of time from initiation to discontinuation of therapy.
In the Beijing UEBMI claims database, only the total prescribed dose (strength of the medication multiplied by the number of units [ie, tablets] dispensed) is collected. For the purposes of this analysis, we assumed that every patient used the international defined daily dose defined by the World Health Organization (the assumed average maintenance dose per day for a drug used for its main indication in adults) to calculate the number of days on individual antidepressant.16 Mental comorbidities of interest included anxiety disorders and obsessive compulsive disorder. Physical comorbidities of interest included hypertension, hyperlipidemia, diabetes, coronary heart disease, and cerebral vascular disease.
The use of the following health care resources were measured: inpatient services (number of hospitalizations during the 1-year follow-up, total length of stay per admission, total length of stay per patient per year, readmission rate, and number of days between the first discharge from hospital to the second hospitalization) and outpatient health care services (number of outpatient visits per patient per year, >1 outpatient visits rate, and days from the first outpatient visit to the second outpatient visit) related to MDD (the primary diagnosis was MDD) or for all cause.
The direct medical costs were medication-related costs (antidepressants, concomitant medications, and other medications) and nonmedication medical costs (including examination fees, registration fees, bed fees, and other service fees).
Statistical methods
Descriptive statistics (mean ± standard error of the mean [SEM] for continuous variables and percentage [%] for categorical variables) were used to describe patient demographics, medication usage patterns, health care resource utilization, and direct medical costs during the 1-year follow-up period after the index date. Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) 20.0 software (IBM SPSS, Armonk, NY, USA).
Results
Analysis population
A total of 15,008 patients with a diagnosis of MDD in 2012 were randomly sampled from the claims database. A total of 6,524 patients were excluded from the analysis for the following reasons: also had a diagnosis of cancer (n=1,071), schizophrenia (n=1,007), or bipolar disorder (n=512); did not have 12-month continuous insurance enrollment (n=396); or nonprimary diagnosis of MDD (n=3,538). A total of 8,484 (56.5%) patients were included in the analysis.
Patient demographics and clinical characteristics
The mean (± standard deviation) age was 57.2 years (±15.3 years), with 10.3% aged 18–34 years, 10.7% aged 35–44 years, 19.8% aged 45–54 years, 27.0% aged 55–64 years, and 32.2% aged ≥65 years. The majority of the analyzed patients were female (63.0%) and were treated in general hospitals (82.0%). During the 1-year follow-up period, the commonly observed (experienced by ≥50.0% patients) comorbidities were hypertension (70.9%), anxiety disorder (68.6%), coronary heart disease (65.1%), cerebral vascular disease (61.4%), and hyperlipidemia (53.0%) (data not shown).
Treatment use patterns
Medication usage
During the 1-year follow-up after the index date, 71.4% of patients received a prescription for at least one antidepressant. SSRIs were prescribed to 60.5% of patients, followed by 9% receiving an NaSSA, 8.3% receiving SNRIs, 2.3% receiving TCAs, and 4.9% receiving other antidepressants. Concomitant medications related with MDD were prescribed for 76.8% of patients, with 71.1% receiving analgesics, 60.0% sedative–hypnotic drugs, 11.6% atypical antipsychotics, and 7.8% muscle relaxants (data not shown).
Antidepressant treatment persistence
A total of 5,209 patients were initiated on antidepressants (TCA, SSRI, SNRI, and NaSSA) at the index date and were included in the analysis on antidepressant persistence. During the follow-up period, the proportions of patients who discontinued their initial antidepressant were 45.4%, 77.0%, 84.8%, 90.0%, and 91.5% by the end of months 1, 2, 3, 6, and 12, respectively. By the end of the 1-year follow-up, very few patients (1.3%) had had their medication augmented, and the proportion of patients who switched to another antidepressant medication from the initial antidepressant was only 16.3% (Table 2).
Table 2.
Antidepressant persistence in patients with MDD
| Follow-up period (N=5,209) | Stopped (%) | Switched (%) | Augmented (%) | Discontinued (%) |
|---|---|---|---|---|
| By 1 month since index date | 42.0 | 3.4 | 0.77 | 45.4 |
| By 2 months since index date | 70.7 | 6.7 | 1.0 | 77.0 |
| By 3 months since index date | 77.3 | 8.4 | 1.1 | 84.8 |
| By 6 months since index date | 80.7 | 12.3 | 1.2 | 90.0 |
| By 12 months since index date | 82.2 | 16.3 | 1.3 | 91.5 |
Note: The percentages are cumulative.
Abbreviations: MDD, major depressive disorder; N, number of patients.
Health care resource utilization
Table 3 shows the health care resource utilization of patients with MDD during the 1-year follow-up period after the index date.
Table 3.
Health care resource utilization of patients with MDD during 1-year follow-up postindex date
| MDD related | All cause | |
|---|---|---|
| With ≥1 inpatient visit(s) for MDD (N=36) | ||
| Number of hospitalizations per patient per year, mean (±SEM), median | 1.2 (±0.1), 1.0 | 1.8 (±0.2), 1.0 |
| Length of stay per admission (days), mean (±SEM), median | 33.4 (±5.1), 28.5 | 31.6 (±5.1), 26.7 |
| Total length of stay per patient per year (days), mean (±SEM), median | 36.6 (±6.7), 29.0 | 50.1 (±7.2), 40.0 |
| Readmission, N (%) | 5 (13.9%) | 15 (41.7%) |
| Days from first discharge of hospitalization to second hospitalization, mean (±SEM), mediana | 50.6 (±11.2), 44.0 | 98.3 (±20.4), 74.0 |
| With ≥1 outpatient visit(s) for MDD (N=8,484) | ||
| Number of outpatient visits per patient per year, mean (±SEM), median | 3.1 (±0.03), 2.0 | 31.5 (±0.23), 28.0 |
| >1 outpatient visits, N (%) | 4,895 (57.7%) | 8,384 (98.8%) |
| Days from first outpatient visit to second outpatient visit, mean (±SEM), medianb | 78.0 (±1.1), 49.0 | 17.8 (±0.33), 9.0 |
Notes:
For the patients with >1 hospitalizations;
for the patients with >1 outpatient visits.
Abbreviations: MDD, major depressive disorder; N, number of patients; SEM, standard error of the mean.
Among the total of 8,484 patients, only 0.42% experienced at least one MDD-related hospitalization during the 1-year follow-up. Those patients who were hospitalized had on average 1.2 MDD-related hospitalizations during the 1-year follow-up, while the frequency of hospitalizations for all cause was 1.8 over the same period. The mean total number of days in hospital for each MDD-related admission was 36.6 days per patient per year and that for all cause was 50.1 days. Of those patients hospitalized, 13.9% had >1 MDD-related hospitalizations during the 1-year follow-up, and 41.7% had >1 hospitalization for all cause.
All analyzed patients had at least one MDD-related outpatient visit. Patients on average had 3.1 MDD-related outpatient visits during the 1-year follow-up and 31.5 outpatient visits for all cause. There were 57.7% of patients who had >1 MDD-related outpatient visits, and 98.8% of patients had visited an outpatient clinic more than once for all cause. The mean numbers of days between the first and second MDD-related outpatient and inpatient visits were 78.0 days and 50.6 days, respectively, while those for the all-cause visits were 17.8 days and 98.3 days, respectively.
Direct medical costs
Table 4 and Figure 1 show the direct health care costs for patients with MDD during the 1-year follow-up period after the index date.
Table 4.
Mean (±SEM) annual health care direct medical costs of patients with MDD during 1-year follow-up postindex date
| Cost (unit: RMB ¥a) | Total (N=8,484) | With ≥1 MDD-related hospitalizations (N=36) | Without any MDD-related hospitalizations (N=8,448) |
|---|---|---|---|
| MDD related | |||
| Total cost | 1,694 (±27.3) | 21,291 (±2,687) | 1,611 (±20.7) |
| Medication cost | 1,569 (±20.9) | 7,144 (±808) | 1,545 (±20.3) |
| Antidepressant medications | 822 (±15.2) | 3,199 (±358) | 812 (±15.1) |
| SSRIs | 641 (±13.2) | 2,033 (±356) | 635 (±13.1) |
| SNRIs | 106 (±6.5) | 501 (±219) | 104 (±6.4) |
| Concomitant medications | 119 (±5.2) | 1,043 (±207) | 115 (±5.1) |
| Other medications | 627 (±11.4) | 2,901 (±577) | 618 (±11.1) |
| Nonmedication medical cost | 125 (±13.5) | 14,147 (±2,097) | 65 (±2.8) |
| All cause | |||
| Total cost | 20,105 (±226) | 40,719 (±4,798) | 19,999 (±226) |
| Medication cost | 14,724 (±131) | 19,744 (±2,468) | 14,703 (±131) |
| Antidepressant medications | 1,623 (±24.4) | 5,218 (±571) | 1,608 (±24.2) |
| Concomitant medications | 411 (±12.6) | 1,536 (±313) | 406 (±12.6) |
| Other medications | 12,690 (±128) | 12,991 (±2,287) | 12,689 (±127.9) |
| Nonmedication medical cost | 5,362 (±134) | 20,975 (±2,680) | 5,296 (±133) |
Note:
1 USD = RMB ¥6.23 at the 2012 currency exchange rate.
Abbreviations: MDD, major depressive disorder; N, number of patients; SEM, standard error of the mean; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor.
Figure 1.
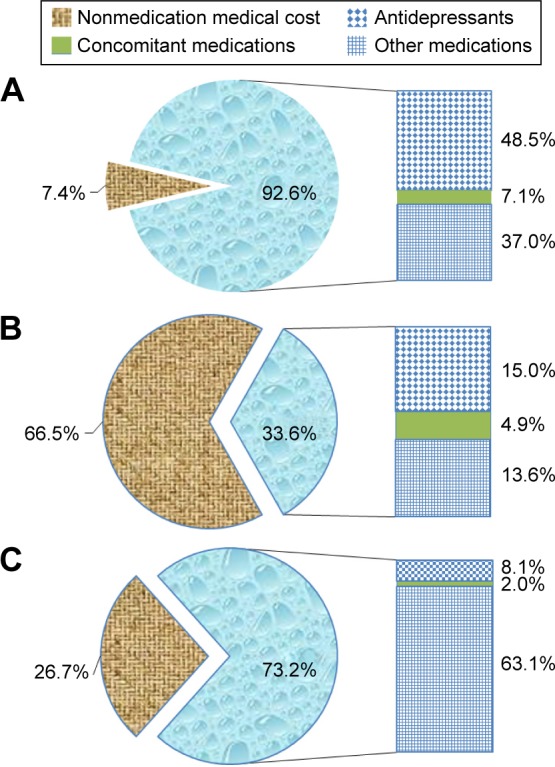
Annual direct medical costs for patients with MDD.
Notes: (A) MDD-related costs for overall patients (N=8,484). (B) MDD-related costs for patients with MDD-related hospitalization (N=36). (C) All-cause-related costs for overall patients (N=8,484).
Abbreviations: MDD, major depressive disorder; N, number of patients.
Annual health care costs
The mean of annual MDD-related direct medical costs per patient for all patients was RMB ¥1,694 (note: 1 USD = RMB ¥6.23 at the 2012 currency exchange rate).17 The nonmedication medical costs accounted for 7.4% of the total costs, whereas 92.6% of the total costs was attributed to medication costs, including 48.5% for antidepressant medications, 7.1% for concomitant medications, and 37.0% for other medications.
For patients who had at least one MDD-related hospitalization, the mean of annual MDD-related direct medical costs per patient was RMB ¥21,291, whereas for patients without any MDD-related hospitalization, it was considerably lower, at RMB ¥1,611 (Figure 1A and B).
The mean of annual all-cause-related direct medical costs per patient for all patients was RMB ¥20,105. Nonmedication medical costs accounted for 26.7% of the total costs, whereas 73.2% of the total costs were attributed to medication costs, including 8.1% for antidepressant medications, 2.0% for concomitant medications, and 63.1% for other medications (Figure 1C).
Discussion
This is the first analysis to investigate the treatment patterns, health care resource utilization, and direct medical costs for patients with MDD in the People’s Republic of China using real-world data from the Beijing UEBMI claims database. We found that the majority of patients with MDD in this claims database had at least one comorbidity of interest during the 1-year follow-up period; moreover, hypertension, coronary heart disease, cerebral vascular disease, and hyperlipidemia were commonly observed physical comorbidities. The high prevalence of physical comorbidities might also be in part associated with the relatively high mean age (57.2 years) of the patients included in this analysis. We recognize that MDD may aggravate some physical comorbidities; on the other hand, deterioration of physical illness may be associated with a relapse of MDD. In the People’s Republic of China, most psychiatric and general hospital services have been historically separated into different facilities. This may result in problems in terms of caring for patients with comorbid physical illnesses in the psychiatric system or caring for patients with comorbid psychiatric illnesses in the general hospital system.18 Closer collaboration between psychiatrists and general physicians has the potential to provide patients with more comprehensive treatment for both MDD and physical illness, which may help achieve better clinical outcomes and lower the overall burden on patients, caregivers, and society. So-called mainstreaming of psychiatric services within the general hospital system has occurred in many countries.19,20
Per the Chinese guidelines for the management of patients with MDD, antidepressant therapy is recommended as a mainstay in the treatment of patients with depressive disorders, and the newer generation of antidepressants, such as the SSRIs, SNRIs, and NaSSAs, are recommended as first-line treatments.2 In Beijing, we found that 71.4% of patients with MDD were treated with antidepressant medications, and within this group, 84.8% used one of the SSRIs. A previous analysis conducted in Tianjin, People’s Republic of China, found that the percentage of conventional antidepressants used (ie, TCA or monoamine oxidase inhibitors) compared to the use of the new-generation antidepressants was 45.1% and 54.9%, respectively, in 2006.21 Compared with conventional antidepressants, the newer antidepressants are considered safer, have fewer drug interactions with other medications commonly used, and are better tolerated by patients. In recent years, the newer-generation antidepressants have been widely adopted in clinical practice. This finding is consistent with the antidepressant-prescribing patterns in other countries, such as Singapore and New Zealand.22,23
Of note, 71.1% of patients in our analysis also used analgesics, suggesting that MDD-related pain symptoms or independent pain conditions frequently coexist in patients with MDD. To some extent, this finding is consistent with the analysis of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D)15 study in 2007, which showed that 77% of depressive patients suffered from pain symptoms. Similarly, a large study of Asian patients with MDD found high rates of concomitant painful physical symptoms in patients with MDD.24 Research has indicated that the presence of painful symptoms is a strong predictor of poor outcomes in Chinese patients with MDD.25 In the People’s Republic of China, because primary care is not well established, patients with such symptoms may be treated in general hospitals due to their pain symptoms or pain-related conditions; hence, it is likely that their MDD is underdiagnosed or treated in the general hospital setting. This may be one explanation for the mismatch between the recommended and actual treatment patterns seen in our analysis. During the first month after the index date in our analysis, we found that approximately half (42.0%) of the patients had stopped the antidepressant that had been started at the index date. By the end of 3 months, 77.3% of patients had stopped antidepressant treatment. Results are not in concordance with the majority of recommendations for the duration of medication treatment in patients with MDD. In contrast, an analysis from Belgium26 showed that 53% of patients terminated antidepressant treatment by the end of the 10-month follow-up period. A quantitative review reported that compared with nondepressed patients, the odds were three times greater that depressed patients would be noncompliant with medical treatment recommendations.27 It is well known that good medication adherence in patients with MDD is associated with improved controlled core mood symptoms, lower relapse rate, and decreased burden of disease.28,29 Some researchers have found that medication persistence in patients with MDD was determined by several factors, such as severity of disease, treatment-emergent adverse events, patients’ characteristics, and supports from physician and society.30 Patients’ perceived stigma about the illness also played an important role in treatment persistence.31 A previous Chinese analysis showed that ~10% of patients with a depressive disorder or neurosis were not willing to be treated in a psychiatric facility because they were afraid of being discriminated against by others.32 In addition, the poor treatment persistence might be due to low disease awareness. Bao et al33 pointed out that some patients had prejudice against mental health. These patients did not want to be diagnosed as having MDD by exaggerating physical symptoms and ignoring affective symptoms. They did not think MDD was a disease that could be mitigated by themselves. Due to the heavy workload of Chinese physicians, who may see 40–50 patients in a half-day clinic session, the barriers to communication between patients and physicians may result in patients not understanding the importance of medication treatment duration and persistence in achieving a good outcome. Patient education programs have an important opportunity to improve patient outcomes. The proportion of patients who switched from the initial antidepressant to another antidepressant within 3 months was 8.4%. This finding is consistent with that reported in an American analysis, which showed that the proportion of antidepressant switching in 3 months was 8.6%.34 But in our analysis, the medication augmentation rate in 3 months was much lower (1.1% vs 23.8%).35
During the 1-year follow-up period, compared with all-cause-related hospital visits (including outpatient and inpatient visits), MDD-related visits were much lower (ie, the percentage of patients with MDD-related hospitalization was 0.42%, and mean number of MDD-related outpatient visits was 3.1). This finding may be due to many reasons, including a lack of understanding about MDD, stigma, separate health care facilities for psychiatric care, a greater focus on physical symptoms compared to psychiatric symptoms, and fewer dedicated resources for mental health care in the People’s Republic of China.18 It was reported that ~90% of depression and neurosis were first treated in the nonpsychiatric departments.32 Although, in recent years, psychiatric services or psychological counseling services have been available in general hospitals in Beijing, a recent analysis found that health care professionals in general hospitals generally had limited knowledge about the diagnosis and treatment of psychiatric disorders. Only 20% of patients with depressive or psychotic disorders were appropriately diagnosed.32 ln our analysis, we found that 82.0% of patients were treated in the general hospital, which might underestimate the MDD-related health care resource use and direct medical costs.
The annual MDD-related and all-cause-related direct medical costs per patient were RMB ¥1,694 (RMB ¥822 for antidepressants) and RMB ¥20,105 (RMB ¥1,623 for antidepressants), respectively, showing that non-MDD-related health care costs were predominant.
Although the proportion of patients with MDD-related hospitalizations was extremely low, annual direct costs for those with MDD-related hospitalization were considerable (RMB ¥21,291), which was driven by nonmedication medical costs. The economic burden on MDD-related hospitalization was considerable, given that the gross domestic product per capita of Beijing area in 2012 was RMB ¥87,091.36
Limitations
There are some limitations in this analysis. In the Beijing UEBMI claims database, the information available is limited. Information on disease duration and severity, clinical effectiveness and reasons for changes to treatment, and side effects are not captured. The data are drawn from the medical insurance records of the urban employees in Beijing, so the findings may not be applicable to patients who are not enrolled in the Beijing UEBMI scheme or who live in regions outside of Beijing. Details on the medications dispensed outside of the hospital setting were not available, which may result in underestimation of the outpatient service and direct medical costs. Because of lack of information on the actual daily dose of antidepressants used by patients, we assumed that every patient used the international defined daily dose when calculating the number of days on antidepressant treatment for each patient. This may well be an overestimation. Not including data before 2012 may affect the results on duration of antidepressant use. If the individuals have initiated treatment before 2012, our estimations might underestimate the patients’ treatment compliance. The target patients were identified by using ICD-10 codes, which include the diagnosis of depression occurrence and recurrent depressive disorder. The key word “depression” was also used for identifying patients with MDD in the absence of the appropriate ICD-10 code to ensure that we did not underestimate the health care resource utilization and direct medical costs for treating MDD. Lack of a comparison group of individuals without an MDD primary diagnosis limits the comparison of difference between two cohorts. Future work will include this part.
Conclusion
This analysis using real-world data for Beijing, People’s Republic of China, found that most patients with MDD were treated in the outpatient setting only and received antidepressant medications. SSRIs were the most commonly used antidepressants. However, the duration to antidepressant medication was short and persistence was low in this study, which differs greatly from the recommendations in the recent Chinese guideline on the management of MDD. The economic burden of MDD-related hospitalization was considerable. Efforts to improve antidepressant treatment persistence and patient outcomes are strongly recommended.
Acknowledgments
The abstract of this paper was presented at the 17th Annual European Congress of the International Society for Pharmacoeconomics and Outcome Research (ISPOR), November 8–12, 2014, held in Amsterdam, the Netherlands, as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Value in Health (November 2014, Volume 17, Issue 7, Page A457: http://www.valueinhealthjournal.com/). The actual paper, however, has never been published. This analysis was sponsored by Lilly Suzhou Pharmaceutical Company, Ltd.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Ling Zhang reports no conflicts of interest in this work. Yun Chen, Lihua Zhi, and Wanqi Wang are employees of Lilly Suzhou Pharmaceutical Company, Ltd. Li Yue is an employee and minor stockholder of Lilly Suzhou Pharmaceutical Company, Ltd. Qingjing Liu is an employee of Beijing Brainpower Pharma Consulting Co, Ltd. William Montgomery is an employee and minor stockholder of Eli Lilly and Company. The authors report no other conflicts of interest in this work.
References
- 1.Brown CH. Pharmacotherapy of major depressive disorder. US Pharm. 2011;36(11):HS3–HS8. [Google Scholar]
- 2.Jiang K. Prevention and Treatment Guideline of Depression Disorder. 1st ed. Beijing: Peking University Medical Press; 2007. Chinese. [Google Scholar]
- 3.Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) surveys. Int J Methods Psychiatr Res. 2003;12(1):3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication (NCS-R) Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma X, Li S, Xiang Y. An epidemiological survey on depressive disorder in Beijing area. Chin J Psychiatry. 2007;40(2):100–103. [Google Scholar]
- 6.American Psychiatric Association (APA) Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. Arlington, VA: American Psychiatric Association (APA); 2010. [Google Scholar]
- 7.Freeman H, Arikian S, Lenox-Smith A. Pharmacoeconomic analysis of antidepressants for major depressive disorder in the United Kingdom. Pharmacoeconomics. 2000;18(2):143–148. doi: 10.2165/00019053-200018020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Berglund P, Demler O, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 9.Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. J Ment Health Policy Econ. 2006;9(2):87–98. [PubMed] [Google Scholar]
- 10.Global Burden of Disease Analysis 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Analysis 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X, Chen X. Researches on depression economic burden. Shanghai Med Pharm J. 2006;27(12):539–541. Chinese. [Google Scholar]
- 12.Zhai J, Zhao J, Chen M, Su Z. An investigation on economic costs of major depression in Shandong. Chin J Nerv Ment Dis. 2008;34(3):165–168. [Google Scholar]
- 13.Zhai J, Chen M, Zhao J, Yang H, Zhang Q, Wang K. An investigation on family burden of depressive disorder in Shandong province of China. J Int Psychiatry. 2012;39(1):5–9. [Google Scholar]
- 14.Cui Z, Faries D, Gelwicks S, Novick D, Liu X. Early discontinuation and suboptimal dosing of duloxetine treatment in patients with major depressive disorder: analysis from a US third-party payer perspective. J Med Econ. 2012;15(1):1–15. doi: 10.3111/13696998.2011.632043. [DOI] [PubMed] [Google Scholar]
- 15.Warden D, Rush AJ, Wisniewski SR, et al. What predicts attrition in second step medication treatments for depression?: a STAR*D report. Int J Neuropsychopharmacol. 2009;12(4):459–473. doi: 10.1017/S1461145708009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Collaborating Center for Drug Statistics Methodology [homepage on the Internet] Definition and General Considerations. [Accessed February 1, 2016]. Available from: http://www.whocc.no/ddd/definition_and_general_considera/
- 17.Wikipedia [homepage on the Internet] List of World Bank Nominal Exchange Rates. [Accessed February 1, 2016]. Available from: https://en.wikipedia.org/wiki/List_of_renminbi_exchange_rates.
- 18.Shan H, homepage on the Internet Main Direction of Mental Health Work in Contemporary China. [Accessed February 1, 2016]. Available from: http://www.myeducs.cn/mianfeilunwen/dangdaizhongguo/10110597/ Chinese.
- 19.Harvey CA, Fielding JM. The configuration of mental health services to facilitate care for people with schizophrenia. Med J Aust. 2003;178(9):49–52. doi: 10.5694/j.1326-5377.2003.tb05307.x. [DOI] [PubMed] [Google Scholar]
- 20.Lipsitt DR. Psychiatry and the general hospital in an age of uncertainty. World Psychiatry. 2003;2(2):87–92. [PMC free article] [PubMed] [Google Scholar]
- 21.Cui B, Du C, Wang Y, et al. Analysis on antidepressants used in Tianjin medical institutions in 2006. Tianjin Pharm. 2009;21(2):26–28. Chinese. [Google Scholar]
- 22.Soh TH, Lim L, Chan HN, Chan YH. Antidepressant prescribing patterns for depressive and anxiety disorders in a Singapore hospital. Open J Psychiatry. 2015;5:144–152. [Google Scholar]
- 23.Ministry of Health . Patterns of Antidepressant Drug Prescribing and Intentional Self-Harm Outcomes in New Zealand: An Ecological Analysis. Wellington: Ministry of Health; 2007. [Google Scholar]
- 24.Lee P, Zhang M, Hong JP, et al. Frequency of painful physical symptoms with major depressive disorder in Asia: relationship with disease severity and quality of life. J Clin Psychiatry. 2009;70(1):83–91. doi: 10.4088/jcp.08m04114. [DOI] [PubMed] [Google Scholar]
- 25.Novick D, Montgomery WS, Aguado J, Peng X, Brugnoli R, Haro JM. Which somatic symptoms are associated with an unfavorable course in Chinese patients with major depressive disorder? Asia Pac Psychiatry. 2015;7(4):427–435. doi: 10.1111/appy.12189. [DOI] [PubMed] [Google Scholar]
- 26.Emyttenaere K, Enzlin P, Dewe W, et al. Compliance with antidepressants in a primary care setting, 1: beyond lack of efficacy and adverse events. J Clin Psychiatry. 2001;62(suppl 22):30–33. [PubMed] [Google Scholar]
- 27.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 28.Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361(9358):653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 29.Cantrell CR, Eaddy MT, Shah MB, Regan TS, Sokol MC. Methods for evaluating patient adherence to antidepressant therapy: a real-world comparison of adherence and economic outcomes. Med Care. 2006;44(4):300–303. doi: 10.1097/01.mlr.0000204287.82701.9b. [DOI] [PubMed] [Google Scholar]
- 30.Ashton AK, Jamerson BD, Weinstein WL, Wagoner C. Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp. 2005;66(2):96–106. doi: 10.1016/j.curtheres.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Friedman SJ, Meyers BS. Stigma as a barrier to recovery: perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psychiatr Serv. 2001;52(12):1615–1620. doi: 10.1176/appi.ps.52.12.1615. [DOI] [PubMed] [Google Scholar]
- 32.Shi S, Lu Z, Zhu S, et al. Seeking behavior of patients with mental disorders and interfering factors. Natl Med J China. 2000;80(1):75–78. [PubMed] [Google Scholar]
- 33.Bao LJ, Hu ZH, Han DL, Jiang BS, Ying HF. Predictors of antidepressant adherence of discharged patients with major depressive disorder. J Neurosci Ment Health. 2014;14(5):469–473. [Google Scholar]
- 34.Marcus SC, Hassan M, Olfson M. Antidepressant switching among adherent patients treated for depression. Psychiatr Serv. 2009;60(5):617–623. doi: 10.1176/ps.2009.60.5.617. [DOI] [PubMed] [Google Scholar]
- 35.Soh TH, Lim L, Chan HN, Chan YH. Antidepressant prescribing patterns for depressive and anxiety disorders in a Singapore hospital. Open Journal of Psychiatry. 2015;5:144–152. [Google Scholar]
- 36.The Central People’s Government of the People’s Republic of China [homepage on the Internet] Beijing Gross Regional Product Growth 7.7% and GDP Per Capital 87,091 Yuan in 2012. [Accessed February 1, 2016]. Available from: http://www.gov.cn/gzdt/2013-01/21/content_2316558.htm. Chinese.


