Abstract
Beginning with Vale and Colleagues in 1981, corticotropin releasing factor (CRF) also called corticotropin releasing hormone (CRH) has repeatedly been identified as an important contributor to fear and anxiety behavior. These findings have proven useful to further our understanding of disorders that have significant fear-dysregulation, such as post-traumatic stress, as well as other stress- and anxiety-related disorders. Unfortunately, the data are not all in agreement. In particular the role of CRF in fear learning is controversial, with studies pointing to contradictory effects from CRF manipulation even within the same brain structure. Further, very few studies address the potentially promising role of CRF manipulation in fear extinction behavior. Here, we briefly review the role of CRF in anxiety, fear learning and extinction, focusing on recent cell-type and neurotransmitter-specific studies in the amygdala and bed nucleus of the stria terminalis (BNST) that may help to synthesize the available data on the role of CRF in fear and anxiety-related behaviors.
Keywords: CRF, GABA, NMDA, Fear, Extinction, Amygdala, BNST, Anxiety, PTSD, r121919
Introduction
CRF background
Corticotropin releasing factor CRH, also known as corticotropin releasing hormone (CRH), is a 41 amino acid peptide discovered by Vale and colleagues in 1981. CRF is largely expressed in stress responsive areas of the brain. In the brain, including the paraventricular nucleus of the hypothalamus (PVN), the central nucleus of amygdala (CeA) and the bed nucleus of stria terminalis (BNST) (Merchenthaler, 1984; Palkovits et al., 1985). CRF is widely studied due to its role in activating the hypothalamic–pituitary–adrenal (HPA) axis in response to a perceived threat and coordinating the autonomic and behavioral response to stress. While CRF is critical for responding to a threatening situation, hyper-activation of the CRF pathway is associated with severe emotional dysregulation including post-traumatic stress and other stress-related disorders (Bale, 2005; Nemeroff et al., 2006).
Our ability to study CRF is made more difficult because it is produced in many different types of cells (Kapcala and Dicke, 1992) and it is colocalized with numerous neurotransmitters and neuropeptides (Sawchenko and Swanson, 1985; Honkaniemi et al., 1992). One way to interrogate CRFergic cells directly is to utilize a transgenic mouse that allows CRF containing cells to be identified and manipulated apart from neighboring cells (Martin et al., 2010). After originally creating a mouse line in which cells expressing CRF also express Cre recombinase (CRF–Cre) (Martin et al., 2010), work in our lab utilized these CRF–Cre mice and crossed them with either “floxed” GABAAα1 (Gafford et al., 2012) or “floxed” NMDA (N-methyl-d-aspartate (NMDA) glutamate) receptor 1 (NR1) (Gafford et al., 2014) genes. The floxed gene of interest is surrounded (flanked) by LoxP sites which are recognized by Cre as the site of gene excision. The result of crossing CRF–Crewith floxed GABAAα1 or floxed NR1 mice is disruption of CRF producing-GABAAα1 or CRF producing-NR1 neurons throughout development. One concern particularly with manipulation of GABAergic neurons during development is that GABAergic neurons gradually shift from excitatory to inhibitory over the course of development, providing a basis for activity dependent neural circuit formation and for critical developmental periods (Hensch, 2005). Some work has shown that genetic disruption of GABAergic transmission early in postnatal life effects anxiety- and depression-related behaviors in adulthood (Shen et al., 2012). Therefore, it is possible that some of our behavioral effects were due to tuning of GABAergic neurons during these critical developmental periods even though we do see normal GABA receptor responses in electrophysiological recordings from these neurons (Gafford et al., 2012). These manipulations offer insight into inhibitory (GABAergic) and excitatory (glutamatergic) control over CRFergic cells in fear and anxiety behavior. This review will focus on these and other findings contributing to what is known regarding CRF in fear and anxiety in the BNST and CeA.
CRF and anxiety
The CeA and the BNST are highly interconnected (Alheid et al., 1998), receive information from and project to similar structures (Gray and Magnuson, 1987; Rosen et al., 1991; Gray and Magnuson, 1992; Dong et al., 2001) are both largely GABAergic (Sun and Cassell, 1993), receive glutamatergic projections from the lateral amygdala (LA) (Krettek and Price, 1978; Pitkanen et al., 1995; Dong et al., 2001) and express a wide variety of neuropeptides including CRF (Roberts et al., 1982;Woodhams et al., 1983). Even with the similarities between BNST and CeA, manipulation of CRF has vastly different effects on fear and anxiety behavior dependent on which structure is targeted as detailed below (Walker and Davis, 1997; Sullivan et al., 2004; Waddell et al., 2006; Sink et al., 2011).
CRF, anxiety and the BNST
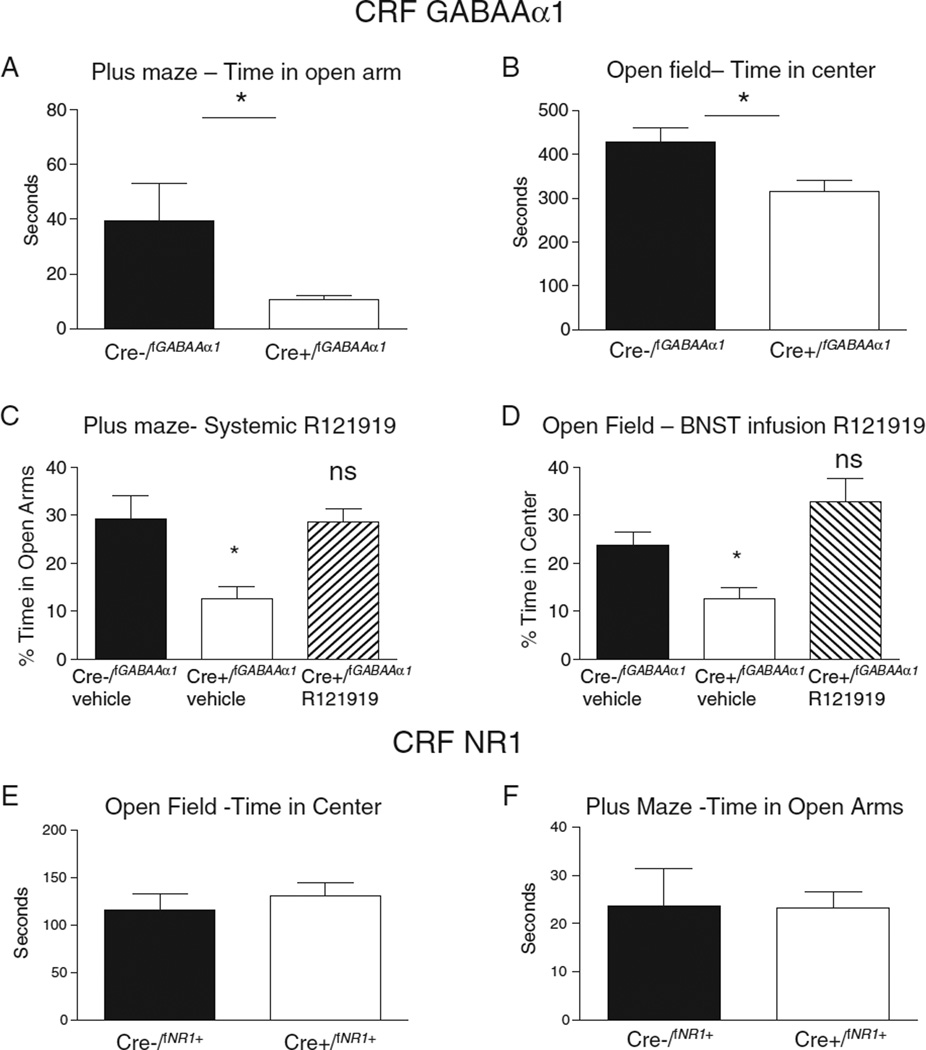
CRF in the BNST has been implicated in mediation of anxiety-like responses (Sahuque et al., 2006; Lee et al., 2008). Within the BNST, work has shown that introduction of stressful stimuli increases CRF expression (Choi et al., 2006; Funk et al., 2006; Kim et al., 2006; Shepard et al., 2006) and overexpression of CRF1 or direct activation of CRF1 and CRF2 receptors with urocortin 1 enhances anxiety (Lee et al., 2008; Sink et al., 2013). Moreover, blockade of CRF with an antagonist infused into the BNST blocks sustained fear behavior that resembles anxiety (Davis et al., 2010). Recent work has implicated a specific subtype of CRF neuron in the BNST that may contribute strongly to the anxiety phenotype. Specifically, transgenic deletion of the GABAAα1 receptor only within CRF producing neurons was found to enhance anxiety as measured across a variety of different tasks (Figs. 1A, B), and this effect was reversed with systemic (Fig. 1C) or BNST (Fig. 1D) infusion of CRFR1 receptor antagonist R121919 (Gafford et al., 2012). These findings suggest that hyperactivation of CRFergic cells, through deletion of GABA receptors, is associated with increased anxiety in agreement with work showing increases in CRF enhance anxiety (Britton et al., 1982; Liang and Lee, 1988; Takahashi et al., 1989). Further, when CRFR1 activation is disrupted with administration of R121919 anxiety behavior is normalized.
Fig. 1.
CRF GABAAα1 deficient mice show enhanced anxiety while CRF NR1 deficient mice show no difference from controls in anxiety. (A) CRF GABAAα1 deficient mice (Cre+/fGABAAα1) spent significantly less time in the open arm of the plus maze than Cre−/fGABAα1 mice. (B) Cre+/fGABAAα1 mice also spent significantly less time in the center of an open field compared to Cre−/fGABAAα1 mice. Both (C) systemic administration prior to the plus maze test and (D) BNST infusion prior to the open field of the CRF receptor 1 antagonist R121919 rescued the anxious phenotype in Cre+/f fGABAAα1 mice. However, in CRF NMDAR1 deficient mice (Cre+/fNR1+) there were no differences in anxiety behavior in either (E) open field or (F) plus maze test compared to Cre−/fNR1+. Figures are adapted from recent work by Gafford et al. (2012, 2014).
CRF, anxiety and the CeA
In contrast to the effects of CRF manipulation in the BNST on anxiety, disruption of CRF in the CeA has not been shown to affect anxiety behavior (Lee and Davis, 1997; Callahan et al., 2013). One study took advantage of the finding that intracerebroventricular administration of CRF facilitates anxiety and directly tested whether this CRF mediated increase in anxiety can be blocked by NMDA lesions of the CeA or the BNST (Lee and Davis, 1997). The authors found that lesions of the BNST blocked CRF enhanced anxiety while CeA lesions had no effect, further demonstrating the important role of CRF in the BNST on anxiety behavior. Another recent study used RNA interference of CRF in the CeA to locally knock down CRF expression and found no effect on anxiety (Callahan et al., 2013). Interestingly, we have also tested the effect of disruption of CRF producing NR1 neurons on anxiety behavior. When we disrupted glutamatergic input onto CRF neurons we found no effect on anxiety measures (Gafford et al., 2014) (Figs. 1E, F). This may be due to the difference in expression of NR1 and GABAAα1 in the BNST since GABAAα1 is so heavily expressed in the BNST (Heldt and Ressler, 2007) compared to NR1 (Lein et al., 2007).
CRF and fear learning
CRF, BNST and fear learning
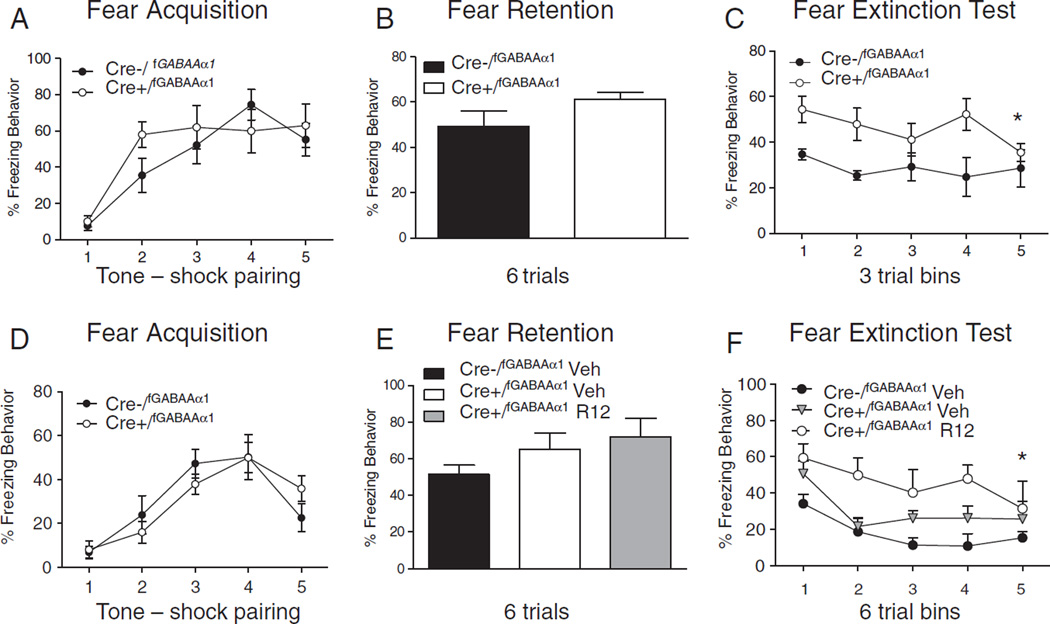
While the role of the BNST in anxiety behavior has been well established, there is little evidence to date that the BNST is engaged during fear learning (LeDoux et al., 1988; Hitchcock and Davis, 1991). However, a recent study showed that virally mediated overexpression of CRF in the BNST 2 weeks before fear conditioning resulted in attenuation of associative fear learning (Sink et al., 2013). In this same study when CRF was overexpressed after fear conditioning fear expression was significantly enhanced. Since intracranial administration of CRF has been shown to enhance stress responding (Cole and Koob, 1988; Sherman and Kalin, 1988) it is possible that CRF overexpression during fear consolidation enhanced anxiety resulting in enhanced fear memory consolidation, as shown previously (Cahill et al., 2003; Rau et al., 2005; Hui et al., 2006). Our work using transgenic disruption of CRF in GABAAα1 neurons enhanced CRF expression in the BNST, PVN and CeA (Gafford et al., 2012) however, we show no effect on fear acquisition (Figure 3A, B). We do find significant disruption of fear extinction with disruption of CRF in GABAAα1 neurons. Since we did not specifically manipulate the BNST or amygdala during the fear conditioning experiments we cannot attribute the effects on extinction to a specific structure.
Fig. 3.
Disruption of CRF GABAAα1 neurons has no effect on fear acquisition but significantly disrupts fear extinction. (A) CRF GABAAα1 deficient mice (Cre+/fGABAAα1) show no difference from Cre−/fGABAAα1 mice during fear conditioning or when tested for (B) fear retention. However, during the (C) fear extinction retention test they shown significant deficits. A follow-up experiment again shows no difference in freezing behavior during (D) fear acquisition or (E) fear retention for CRF GABAAα1 deficient mice (Cre+/fGABAAα1). However, Cre+/f GABAAα1 mice given a systemic infusion of a CRF receptor 1 antagonist (R121919) prior to fear extinction show a (E) partial rescue of fear extinction behavior during the (F) fear extinction retention test compared to vehicle injected controls. Asterisk (*) indicates significant group difference.
Figures are adapted from Gafford et al. (2012).
CRF, amygdala and fear learning
The amygdala is a structure critically engaged in fear learning (LeDoux, 2000). The major subnuclei of the amygdala include LA, basal (B), accessory basal (AB) and CeA. The term basolateral amygdala (BLA) is often used to refer to LA and B together (LeDoux, 2000). Evidence strongly supports the BLA as a structure critical for formation and storage of fear memory (LeDoux, 2000; Johansen et al., 2011). Studies have shown that the CeA is also required for the acquisition, consolidation, and expression of fear memories (Campeau and Davis, 1995; Goosens and Maren, 2003;Wilensky et al., 2006; Zimmerman and Maren, 2010) and potentially does so in parallel with the BLA (Pape and Pare, 2010). CRF has been shown to play a critical role within the amygdala in fear learning processes. The BLA contains a high density of CRF1 receptors (Baram and Hatalski, 1998; Chen et al., 2000) while the CeA has many CRF expressing neurons but lacks strong expression of CRF receptors (Sakanaka et al., 1986; Potter et al., 1994; Van Pett et al., 2000). Both the BLA and CeA are critical for fear memory (LeDoux et al., 1985; Wilensky et al., 2006). Infusion of a CRF receptor antagonist into the BLA disrupts contextual fear conditioning (Hubbard et al., 2007) and inhibitory avoidance learning (Roozendaal et al., 2002, 2008). Increases in CRF in the BLA facilitate performance in a variety of learning tasks (Liang and Lee, 1988; Roozendaal et al., 2008), but also see Isogawa et al. (2013) which found CRF infusion in LA impairs fear memory.
Interestingly, some investigators manipulated CRF around the time of auditory fear conditioning (Isogawa et al., 2013), after inhibitory avoidance training (Roozendaal et al., 2002) or at different times after contextual fear conditioning and found no disruption of learning tasks, even though other work has shown CRF expression is increased in the CeA after contextual fear conditioning (Thompson et al., 2004). In fact, other studies have found that CeA infusion of a CRF antagonist prior to contextual fear conditioning (Swiergiel et al., 1993) or CRF antisense at different time points after contextual fear conditioning (Pitts et al., 2009; Pitts and Takahashi, 2011) is effective at modulating fear memory. The lack of effectiveness of CRF receptor antagonists in the CeA in some studies may be partially explained by the relative expression of CRF receptors in the BLA compared to the CeA. The BLA strongly expresses CRF receptors (Baram and Hatalski, 1998; Chen et al., 2000) while the CeA has many CRF expressing neurons but fewer CRF receptors (Sakanaka et al., 1986; Potter et al., 1994; Van Pett et al., 2000) making the BLA a more target-rich environment for CRF receptor antagonists. A summary of these findings and further details from the infusion studies related to CRF regulation of fear behavior are detailed in Table 1.
Table 1.
Findings from amygdala infusion studies related to CRF regulation of fear behavior.
| Citation | Structure targeted |
Drug infused | Drug action | Timing of infusion | Behavior tested | Results |
|---|---|---|---|---|---|---|
| Liang and Lee (1988) | Amygdala | CRH | Increases CRF | Immediately post training |
Inhibitory avoidance | Improved retention |
| Roozendaal et al. (2002) | BLA | [9–41]-α-helical CRF | CRF receptor antagonist | Immediately post training |
Inhibitory avoidance | Impairs retention. |
| 3 hour post training | No effect | |||||
| CeA | Immediately post training |
No effect | ||||
| Roozendaal et al. (2008) | BLA | CRF6–33 | CRF-binding protein ligand inhibitor | Immediately post training |
Inhibitory avoidance | Enhancement of retention |
| CRF6–33 + α-helical CRF9–41 |
CRF-binding protein ligand inhibitor + CRF receptor antagonist |
Immediately post training |
Blockade of retention enhancement |
|||
| Swiergiel et al. (1993) | CeA | CRF9–41, | CRF antagonist | Pre training | Contextual fear conditioning |
Disrupted post shock freezing |
| Pre testing | Disrupted freezing during test |
|||||
| Hubbard et al. (2007) | BLA | DMP696 | CRF1 receptor antagonist | 5 min post training | Contextual fear | disrupted retention |
| 3 hour post training | conditioning | disrupted retention | ||||
| 9 hour post training | No effect | |||||
| CeA | DMP696 | CRF1 receptor antagonist | 5 min post training | No effect | ||
| 3 hour post training | No effect | |||||
| 9 hour post training | No effect | |||||
| Pitts et al. (2009) | CeA | CRF antisense | Inhibits CRF | Pre training | Contextual fear | Disrupted retention |
| Pre testing | conditioning | No effect | ||||
| Pitts and Takahashi (2011) | CeA | CRF antisense | Inhibits CRF | 5 m post training | Contextual fear | Disrupted retention |
| 9 hour post training | conditioning | Disrupted retention | ||||
| 24 hour post training |
Disrupted retention | |||||
| 96 hour post training |
No effect | |||||
| Isogawa et al. (2013) | LA | CRH | Increases CRF | One hour pre training |
Auditory fear conditioning |
Disrupts retention |
| Immediately post training |
Disrupts retention | |||||
| 1 hour pre testing | Disrupted retention | |||||
| CeA | One hour pre training |
No effect | ||||
| Immediately post training |
No effect | |||||
| 1 hour pre testing | No effect |
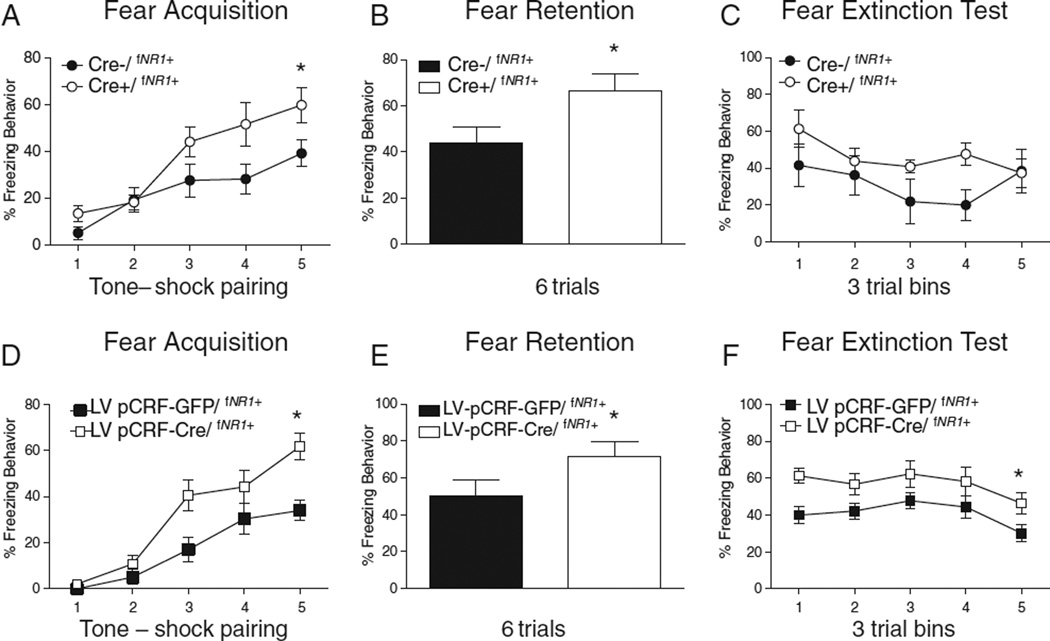
Recent work from our lab has additionally manipulated specific subtypes of CRF containing neurons (CRF/NMDAR1 containing versus CRF/GABAAα1 containing, as described above) to examine whether cell-type and receptor specificity could help to explain some of the above discrepancies in fear-related behaviors. We find either enhancement of fear conditioning with the transgenic (Fig. 2A) or lentiviral (Fig. 2D) mediated NMDAR1 deletion in CRF neurons compared to no effect on fear conditioning with the transgenic deletion of GABAAα1 in CRF neurons (Figs. 3A, D) (Gafford et al., 2012, 2014). The discrepancy in findings may again be due to the difference in the brain area affected by the manipulation as noted earlier in this review. The stronger expression of CRF containing NMDAR1 neurons within the CeA, a structure engaged in fear acquisition (Wilensky et al., 2006) may indicate a more critical role for these CRF containing neurons in fear conditioning, while CRF containing GABAAα1 neurons are more strongly expressed in the BNST which is less engaged in fear memory formation (Walker et al., 2009).
Fig. 2.
Disruption of CRF NMDAR1 (NR1) neurons significantly disrupts fear acquisition without effects on fear extinction retention. (A) CRF NR1 deficient mice (Cre+/fNR1+) show significantly enhanced fear acquisition and (B) fear retention compared to Cre−/fNR1+ mice with no significant difference during the (C) fear extinction test. A follow-up experiment infused CRF promoter driven lentivirus into the CeA of fNR1 mice resulting in disrupted CRF NR1 neurons only within the CeA. We again show significantly enhanced freezing behavior during (D) fear acquisition for mice with disruption of CRF NR1 neurons in the CeA (LV pCRF-Cre/fNR1+) compared to control virus infused mice (LV pGFP-Cre/fNR1+). LV pCRF-Cre/fNR1+ mice showed significantly enhanced (E) fear retention and (F) impaired fear extinction retention. Asterisk (*) indicates significant group difference.
Figures are adapted from Gafford et al. (2014).
CRF and fear extinction
Fear extinction involves learning to no longer fear a previously fearful stimulus. Extinction is achieved by exposing a fear conditioned organism to multiple presentations of the feared stimulus without the previously paired aversive event (e.g., footshock) resulting in an eventual decrease of the fear behavior. This process is thought to be comprised of new inhibitory learning mechanisms (Myers and Davis, 2007). Fear extinction is a complex learning event that is reliant upon a broad network of structures including the amygdala (Pare and Duvarci, 2012; Furini et al., 2014), and multiple cellular networks within these structures mediate extinction behavior (Pare and Duvarci, 2012; Duvarci and Pare, 2014).
Methods to facilitate fear extinction has been proposed as a behavioral treatment for fear and anxiety disorders such as posttraumatic stress disorder (PTSD) (VanElzakker et al., 2014) since work has shown that those with PTSD have impairments in extinction (Orr et al., 2000; Peri et al., 2000; Rothbaum and Davis, 2003). Therapeutic outcome of those with PTSD who undergo fear extinction can be improved with administration of a partial agonist of the NMDA receptor (Walker et al., 2002; Difede et al., 2014; Rothbaum et al., 2014) and possibly impaired with administration of a benzodiazepine that potentiates GABAergic inhibition (Rothbaum et al., 2014). Work in humans has shown there is also a role for CRF in PTSD. Specifically, individuals with PTSD have been found to have enhanced CRF levels in their cerebrospinal fluid (CSF) (Bremner et al., 1997; Baker et al., 1999; Sautter et al., 2003; Risbrough and Stein, 2006) suggesting dysregulation of CRF may contribute to PTSD. It is unknown whether the increase in CRF is a predetermining factor in PTSD or a result of the development of PTSD.
Little research has directly manipulated CRF in fear extinction in the animal model. One recent study examined CRF in fear extinction by infusing either CRF, CRF binding protein which increases endogenous levels of free CRF or a CRF receptor antagonist into the BLA prior to fear extinction (Abiri et al., 2014). Abiri and colleagues found that endogenously increasing CRF in the BLA just prior to fear extinction with either infusion of CRF or CRF binding protein resulted in impaired fear extinction consolidation while application of a CRF receptor antagonist facilitated fear extinction (Abiri et al., 2014). These findings are in agreement with the work in humans with PTSD showing that increased CRF concentration resulted in disrupted fear extinction.
Work in our lab has also investigated the role of CRF in fear extinction learning. We assessed extinction with disruption of either GABAAα1 or NMDAR1 gene expression within CRFergic neurons (Gafford et al., 2012, 2014). We found that transgenic disruption of GABAAα1 within CRFergic neurons did not affect fear conditioning (as noted previously) or retention behavior (Figs. 3A, B) but resulted in a significant and prolonged deficit in fear extinction (Gafford et al., 2012) (Fig. 3C). In a follow-up experiment we again show that transgenic disruption of GABAAα1 gene expression within CRF–containing neurons does not affect fear acquisition or retention of fear (Figs. 3D, E), but systemic infusion of the CRFR1 receptor antagonist R121919 could partially rescue the fear extinction deficit (Fig. 3F). In contrast, disruption of CRF NR1 neurons significantly facilitated fear acquisition (as noted previously) (Fig. 2A) and significantly enhanced fear retention tested the following day (Fig. 2B) without significantly effecting long term extinction retention (Fig. 2C). We replicated the finding of enhanced fear learning (Fig. 2D) and fear retention (Fig. 2E) with virus infusions that knocked down NR1 containing CRF neurons specifically within the CeA. However, with viral manipulation of CeA CRF NR1 neurons we show impaired fear extinction over 15 trials of extinction (Fig. 2F). The difference between disruption of CRFergic NR1 neurons in the transgenic mouse compared to the same disruption delivered via virus infusion directed at the CeA may be due to the targeted nature of the disruption. Altogether, our data may suggest that different CRF neuronal subpopulations selectively contribute to accelerated fear acquisition or disrupted fear extinction behavior. Specifically, transgenic disruption of inhibitory input onto CRFergic neurons strongly impairs fear extinction while transgenic of virus-mediated disruption of CRFergic NR1 neurons strongly enhances fear conditioning and fear retention. Since disorders such as PTSD have been linked to enhanced fear conditioning as well as disrupted fear extinction (Orr et al., 2000; Lissek et al., 2005; Blechert et al., 2007; Glover et al., 2011; VanElzakker et al., 2014), these data may highlighting a potential mechanism for further investigation of this dissociation.
Conclusion
In summary, CRF plays a critical, yet complex role in anxiety and fear memory. Both the BNST and CeA have similar connections to upstream and from downstream targets, receive highly processed sensory information and are rich with neuropeptides including CRF (Krettek and Price, 1978; Roberts et al., 1982; Woodhams et al., 1983; Gray and Magnuson, 1987; Rosen et al., 1991; Gray and Magnuson, 1992; Sun and Cassell, 1993; Pitkanen et al., 1995; Dong et al., 2001). However, a good deal of evidence shows that CRF in the BNST contributes to anxiety behavior while CRF in the amygdala contributes to fear memory processing. One reason for the difference in responsivity to fearful stimuli may lie in the different subtypes of CRF neurons in the BNST compared to the CeA. Recent technological innovations such as optogenetics and chemogenetic techniques may illuminate this question by allowing for specific manipulation of CRF neurons in the BNST or CeA. Further technological innovations such as FACS (Fluorescence Activated Cell Sorting) and TRAP (Translating Ribosome Affinity Purification) will allow genetic profiling of cell subtypes so that we may begin to determine differential genetic contributions responsible for normal and dysfunctional fear and anxiety behavior. These studies hold the potential to uncover novel, more directed and effective treatments for debilitating disorders of fear and anxiety such as PTSD.
Acknowledgments
Funding for work in the Ressler lab was provided by the National Institutes of Mental Health (MH096764 to K.J.R.; F32MH090785 to G.M.G.).
References
- Abiri D, Douglas CE, Calakos KC, Barbayannis G, Roberts A, Bauer EP. Fear extinction learning can be impaired or enhanced by modulation of the CRF system in the basolateral nucleus of the amygdala. Behav. Brain Res. 2014;271:234–239. doi: 10.1016/j.bbr.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998;84:967–996. doi: 10.1016/s0306-4522(97)00560-5. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm. Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav. Res. Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn. Mem. (Cold Spring Harb. N. Y.) 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan LB, Tschetter KE, Ronan PJ. Inhibition of corticotropin releasing factor expression in the central nucleus of the amygdala attenuates stress-induced behavioral and endocrine responses. Front. Neurosci. 2013;7:195. doi: 10.3389/fnins.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J. Comp. Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol. Behav. 2006;89:301–310. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J. Pharmacol. Exp. Ther. 1988;247:902–910. [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, Altemus M. d-Cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology. 2014;39:1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Brain Res. Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Furini C, Myskiw J, Izquierdo I. The learning of fear extinction. Neurosci. Biobehav. Rev. 2014;47C:670–683. doi: 10.1016/j.neubiorev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford G, Jasnow AM, Ressler KJ. Grin1 receptor deletion within CRF neurons enhances fear memory. PLoS One. 2014;9:e111009. doi: 10.1371/journal.pone.0111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety. 2011;28:1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J. Comp. Neurol. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Forebrain and midbrain distribution of major benzodiazepine-sensitive GABAA receptor subunits in the adult C57 mouse as assessed with in situ hybridization. Neuroscience. 2007;150:370–385. doi: 10.1016/j.neuroscience.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav. Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Pelto-Huikko M, Rechardt L, Isola J, Lammi A, Fuxe K, Gustafsson JA, Wikstrom AC, Hokfelt T. Colocalization of peptide and glucocorticoid receptor immunoreactivities in rat central amygdaloid nucleus. Neuroendocrinology. 1992;55:451–459. doi: 10.1159/000126156. [DOI] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui IR, Hui GK, Roozendaal B, McGaugh JL, Weinberger NM. Posttraining handling facilitates memory for auditory-cue fear conditioning in rats. Neurobiol. Learn. Mem. 2006;86:160–163. doi: 10.1016/j.nlm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Isogawa K, Bush DE, LeDoux JE. Contrasting effects of pretraining, posttraining, and pretesting infusions of corticotropin-releasing factor into the lateral amygdala: attenuation of fear memory formation but facilitation of its expression. Biol. Psychiatry. 2013;73:353–359. doi: 10.1016/j.biopsych.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapcala LP, Dicke JA. Brain corticotropin-releasing hormone receptors on neurons and astrocytes. Brain Res. 1992;589:143–148. doi: 10.1016/0006-8993(92)91174-d. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Park SH, Choi SH, Moon BH, Lee KJ, Kang SW, Lee MS, Choi SH, Chun BG, Shin KH. Effects of repeated tianeptine treatment on CRF mRNA expression in non-stressed and chronic mild stress-exposed rats. Neuropharmacology. 2006;50:824–833. doi: 10.1016/j.neuropharm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J. Comp. Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Iwata J, Reis DJ. Auditory emotional memories: establishment by projections from the medial geniculate nucleus to the posterior neostriatum and/or dorsal amygdala. Ann. N. Y. Acad. Sci. 1985;444:463–464. doi: 10.1111/j.1749-6632.1985.tb37611.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J. Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Liang KC, Lee EH. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology (Berl.) 1988;96:232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res. Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, Nemeroff CB, Owens MJ. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol. Psychiatry. 2010;67:1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl. 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J. Psychiatr. Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J. Abnorm. Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Vale W. Distribution of corticotropin-releasing factor in rat brain. Fed. Proc. 1985;44:215–219. [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr. Opin. Neurobiol. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J. Comp. Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol. Learn. Mem. 2011;95:86–91. doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J. Neurosci. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm. Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Woodhams PL, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the amygdaloid complex. Neuroscience. 1982;7:99–131. doi: 10.1016/0306-4522(82)90156-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J. Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJ, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav. Neurosci. 1991;105:817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of posttrauma reactions. Ann. N. Y. Acad. Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Duncan EJ, Rizzo A, Ressler KJ. A randomized, double-blind evaluation of d-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am. J. Psychiatry. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl.) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol. Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed. Proc. 1985;44:221–227. [PubMed] [Google Scholar]
- Shen Q, Fuchs T, Sahir N, Luscher B. GABAergic control of critical developmental periods for anxiety- and depression-related behavior in mice. PLoS One. 2012;7:e47441. doi: 10.1371/journal.pone.0047441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav. Brain Res. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Kalin NH. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol. Biochem. Behav. 1988;30:801–807. doi: 10.1016/0091-3057(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J. Neurosci. 2011;31:1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol. Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J. Comp. Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav. Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav. Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav. Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams PL, Roberts GW, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the bed nucleus of the stria terminalis, septum and preoptic area. Neuroscience. 1983;8:677–703. doi: 10.1016/0306-4522(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur. J. Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]