Abstract
Background
Erythroderma is an uncommon and severe dermatological manifestation of a variety of diseases. It is commonly challenging to find the underlying cause. Objective: The aim of this study was to analyze the causes of the disease in patients with erythroderma.
Patients and Methods
Data including the clinical symptoms, laboratory examinations, histopathology and follow-up information were collected from patients with erythroderma admitted to our department between 2000 and 2010.
Results
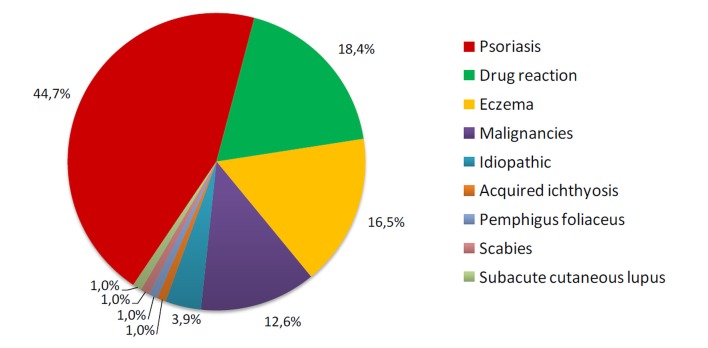
One-hundred and three patients diagnosed with erythroderma were identified during this period (11.9% of all hospitalized patients; hospital incidence = 9.4 cases/year). The mean age of onset was 54.4 years (range: 17-89 years) with a male:female ratio of 1.5:1. The most frequent cause of erythroderma was exacerbation of preexisting dermatoses (65.0%), including psoriasis (44.7%) and eczema (16.5%). Drugs (18.4%) and cutaneous T-cell lymphomas (11.7%) induced most of the remaining cases. No cause could be identified in four cases (3.9%). Apart from erythema and scaling, that were present in all patients, clinical findings were dominated by pruritus (97.1%), followed by edema (56.3%), fever (54.4%), palmoplantar keratoderma (50.5%), nail changes (42.7%), liver or spleen enlargement (41.7%) and lymphadenopathy (40.8%).
Conclusions
Although numerous clinical features and laboratory values were abnormal, most findings were non-specific. The skin biopsy yielded a positive clinical correlation in most cases. Our study had a high percentage of erythroderma secondary to preexisting skin disease and a relatively low percentage of idiopathic erythroderma.
Keywords: dermatitis, exfoliative, erythroderma, psoriasis
Background
Erythroderma, or generalized exfoliative dermatitis, is a rare inflammatory disorder characterized by generalized erythema, involving more than 90% of the body surface area accompanied by a variable degree of scaling. It is the consequence of several conditions, mainly skin diseases, drug consumption and more rarely, secondary to some malignancies. Therefore, determining the exact etiology is important to facilitate its management. Because most patients are elderly and the skin involvement is widespread, it is a potentially life-threatening disease.
To date, there are few published studies on the frequency of underlying causes and prognosis of erythroderma from European countries.[1-3] Our study aimed to examine the causes, as well as the clinical and laboratory profiles of patients with erythroderma in our department. Finally, our data are discussed and compared with previously published series.
Patients/Materials and Methods
We conducted a retrospective analysis of the clinical and laboratory profile of all patients with erythroderma admitted to the Dermatology and Venereology Department of Centro Hospitalar São João EPE, between January 2000 and December 2010. Our department is one of the largest dermatology teaching units in Portugal, integrated in a tertiary referral hospital in the northern region of the country.
All the patients diagnosed with erythroderma had developed erythema involving more than 90% of the body surface area. Due to the risks that erythroderma implies for the patient’s life and to study the cause in each case, they are always treated as inpatients.
The records of these patients were carefully reviewed and the following data recorded: personal data, past medical history (including history of skin diseases), drug consumption history, previous episodes of erythroderma, onset and evolution of erythroderma, symptoms, and physical examination information (with special emphasis placed on the assessment of the skin, mucosas, hair, nails, lymph nodes, temperature, presence of edema as well as hepatic or splenic enlargement). Laboratory investigations including complete hematological parameters, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum protein levels, serum electrolytes, blood sugar, liver and kidney function tests, urine test, chest radiography, abdominal ultrasound and electrocardiogram, were performed as part of the routine investigation for all erythrodermic patients in our dermatology ward. Whenever indicated, further investigations such as skin biopsies, lymph node biopsies, bone marrow investigation, flow-cytometry, immunophenotyping, patch tests and CT-scans were performed. We also examined follow-up data concerning the management, outcome and relapses when such information was available.
Data were compiled electronically and analyzed using SPSS® (Statistical Package for the Social Sciences, v. 19, SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P<0.05. We analyzed the clinical and laboratory data using Fisher’s exact test and Kruskal-Wallis non-parametric test to compare mean values.
Results
Epidemiology/Demographics
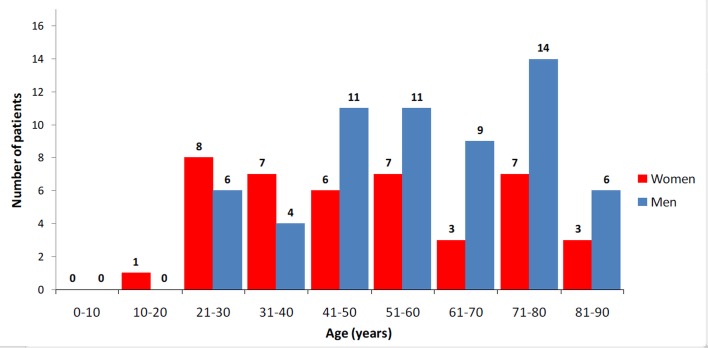
During the 11-year study period 866 patients were admitted to our department. Erythroderma was diagnosed in 103 patients (11.9% of all inpatients), corresponding to an incidence of 9.4 cases/year. Men outnumbered women by 1.5:1 (n= 61:42). The age of these patients ranged from 17 to 89 years old, with a mean age of 54.4 years. The erythrodermic patients were on average 14.5 years younger than the remaining 763 inpatient cases (p=0.01). Patient distribution according to age is shown in Figure 1. Erythroderma occurred later in men (mean 57.8 years, range: 22-89 years) than in women (mean 49.4 years, range: 17-86 years) (p=0.01).
Figure 1.
Incidence arranged by age groups.
As shown in Table 1, patients were admitted in our department at a mean of 30 days (range: 1 day-5 months) after the onset of erythroderma. A shorter duration before admission was observed in patients with drug-induced erythroderma, and a longer one with psoriasis-related erythroderma. Six patients (5.8%) had a history of previous episodes of erythroderma, all of them related caused by psoriasis (five patients had one previous hospitalization each, and one patient had two previous admissions).
Table 1. Epidemiological, clinical and laboratory features of the 103 patients with erythroderma according to etiology.
| Etiology | Psoriasis n=46, 44.7% | Drug reaction n=19, 18.4% | Eczema n=17, 16.5% | Malignancies n=13, 12.6% | Idiopathic n=4, 3.9% | Others* n=4, 3.9% | Total n=103 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at admission (years) |
Mean | 50.9 | 61.2 | 46.0 | 69.4 | 51.8 | 51.0 | 54.4 | |||||||
| Range | (17-87) | (22-89) | (22-71) | (37-86) | (36-80) | (28-76) | (17-89) | ||||||||
| Male:Female ratio | 1.6 : 1 | 1 : 1.4 | 2.4 : 1 | 1 : 1 | 1 : 1 | 4 : 0 | 1.5 : 1 | ||||||||
| Erythroderma’s duration before admission (days) | Mean | 40 | 10 | 25 | 20 | 20 | 30 | 12 | |||||||
| Range | 4-150 | 1-20 | 8-90 | 15-60 | 8-45 | 4-40 | (1-150) | ||||||||
| Hospital stay (days) | Mean | 20 | 16 | 14 | 20 | 14 | 14 | 18 | |||||||
| Range | 5-40 | 5-24 | 4-20 | 10-45 | 5-20 | 4-18 | (4-45) | ||||||||
| Clinical findings | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Pruritus | 46 | 100.0 | 17 | 89.5 | 17 | 100.0 | 13 | 100.0 | 4 | 100.0 | 3 | 75.0 | 100 | 97.1 | |
| Peripheral edema | 23 | 50.0 | 12 | 63.2 | 6 | 35.3 | 12 | 92.3 | 3 | 75.0 | 2 | 50.0 | 58 | 56.3 | |
| Fever | 22 | 47.8 | 17 | 89.5 | 5 | 29.4 | 7 | 53.8 | 3 | 75.0 | 2 | 50.0 | 56 | 54.4 | |
| Palmoplantar keratoderma | 32 | 69.6 | 2 | 10.5 | 7 | 41.2 | 10 | 76.9 | 0 | 0.0 | 1 | 25.0 | 52 | 50.5 | |
| Nail changes | 27 | 58.7 | 1 | 5.3 | 4 | 23.5 | 10 | 76.9 | 2 | 50.0 | 0 | 0.0 | 44 | 42.7 | |
| Liver and/or spleen enlargement | 16 | 34.8 | 3 | 15.8 | 9 | 52.9 | 13 | 100.0 | 1 | 25.0 | 1 | 25.0 | 43 | 41.7 | |
| Lymphadenopathy | 14 | 30.4 | 7 | 36.8 | 4 | 23.5 | 13 | 100.0 | 2 | 50.0 | 2 | 50.0 | 42 | 40.8 | |
| Psoriatic arthritis | 19 | 41.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 18.4 | |
| Alcohol dependence | 12 | 26.1 | 0 | 0 | 1 | 5.9 | 0 | 0 | 1 | 25.0 | 1 | 25.0 | 15 | 14.6 | |
| Hypertension | 7 | 15.2 | 2 | 10.5 | 2 | 11.8 | 2 | 15.4 | 1 | 25.0 | 1 | 25.0 | 15 | 14.6 | |
| Diabetes | 5 | 10.9 | 1 | 5.3 | 1 | 5.9 | 2 | 15.4 | 1 | 25.0 | 0 | 0 | 10 | 9.7 | |
| Congestive heart failure | 5 | 10.9 | 1 | 5.3 | 0 | 0 | 2 | 15.4 | 0 | 0 | 1 | 25.0 | 9 | 8.7 | |
| Dyslipidemia | 4 | 8.7 | 1 | 5.3 | 0 | 0 | 1 | 7.7 | 1 | 25.0 | 0 | 0 | 7 | 6.8 | |
| Chronic kidney disease | 2 | 4.3 | 1 | 5.3 | 0 | 0 | 2 | 15.4 | 0 | 0 | 0 | 0 | 5 | 4.9 | |
| Epilepsy | 0 | 0 | 5 | 26.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 4.9 | |
| Laboratory data | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Elevated ESR or CRP | 44 | 95.7 | 19 | 100.0 | 16 | 94.1 | 13 | 100.0 | 4 | 100.0 | 3 | 75.0 | 99 | 96.1 | |
| Leukocytosis | 20 | 43.5 | 13 | 68.4 | 4 | 23.5 | 8 | 61.5 | 2 | 50.0 | 3 | 75.0 | 50 | 48.5 | |
| Eosinophilia | 11 | 23.9 | 8 | 42.1 | 9 | 52.9 | 10 | 76.9 | 2 | 50.0 | 1 | 25.0 | 41 | 39.8 | |
| Anemia | 10 | 21.7 | 7 | 36.8 | 3 | 17.6 | 9 | 69.2 | 2 | 50.0 | 0 | 0.0 | 31 | 30.1 | |
| * Subacute cutaneous lupus (n=1); acquired ichthyosis (n=1); pemphigus foliaceus (n=1); scabies (n=1). | |||||||||||||||
We did not find differences in the causes of erythroderma between genders. Patients diagnosed with malignancy-related erythroderma were older (mean 69.4 years, range: 37-86 years) than those from other etiological groups (p=0.02). In contrast, patients suffering from eczema-related erythroderma were the youngest (mean 46.0 years, range: 22-71 years) (p=0.02).
Clinical findings
The most common clinical symptoms, associated disorders and their incidence can be seen in Table 1. In addition to erythema, all patients had some degree of scaling. Pruritus, the most common complaint, was recorded in 100 patients (97.1%). Fifty-six patients (54.4%) had fever during the episode (temperature ≥ 38°C) and peripheral pitting edema was found in 58 patients (56.3%). Palmar/plantar hyperkeratosis and nail changes (including Beau’s lines, onychodystrophy, pitting, discoloration, subungueal hyperkeratosis, onycholysis and paronychia) were observed in 52 (50.5%) and 44 (42.7%) patients, respectively. Forty-three patients (41.7%) had liver and/or spleen enlargement detected by abdominal palpation or imaging techniques. Fortytwo patients (40.8%) had enlarged lymph nodes detected by palpation or imaging techniques.
We identified several associated disorders in the patient’s medical records. Thirty patients (29.1%) had previous known history of plaque psoriasis and 19 (18.4%) suffered from psoriatic arthritis. Fifteen patients (17.5%) had ongoing alcohol dependence (as defined by the DSM-IV-TR diagnostic criteria), and most of them (n=12) were psoriatic patients. Five patients suffering from epilepsy were diagnosed with drug-induced erythroderma, and in every case an anticonvulsant drug was implicated (carbamazepine: 4 cases, lamotrigine: 1 case). We did not find differences between etiological groups on the prevalence of arterial hypertension (14.6%), type-2 diabetes (9.7%), congestive heart failure (8.7%), dyslipidemia (6.8%) or chronic kidney disease (4.9%). However, some etiologies were found to be significantly associated with certain clinical signs, as follows:
Fever was mostly found in patients with drug-induced erythroderma (p=0.01).
Palmar/plantar keratoderma, nail changes, psoriatic arthritis as well as alcohol dependence were more prevalent among patients with psoriasis (p=0.01, p=0.02, p=0.02 and p=0.02, respectively).
Lymphadenopathy as well as liver/spleen enlargement were clinical features significantly associated with malignancy-related erythroderma cases (p=0.01 and p=0.02, respectively).
Epilepsy was found exclusively in patients with drug-induced erythroderma (p=0.02).
Laboratory findings
The most commonly identified laboratory abnormalities are described in Table 1. They included an increased ESR/CRP (96.1%), decreased hemoglobin (30.1%) and leukocytosis (48.5%).
Skin biopsy was performed in 95 (92.2%) patients. In the remaining cases a cutaneous biopsy was not performed because the cause of erythroderma was clear from the start, namely: history of severe psoriasis (n=6); crusted scabies confirmed by microscopy (n=1); generalized allergic contact eczema two days after exposure to an iodine compound applied during wound care of a chronic venous leg ulcer (n=1). In most instances, the biopsies were performed during the first 2 days after the patient was admitted to the hospital. In those cases where a skin biopsy was performed, the histopathology identified features that contributed to the final diagnosis in 66.3% of cases (n=63), including psoriasis (n=35), drug reaction (n=11), eczema (n=8), mycosis fungoides (MF) (n=6), subacute cutaneous lupus (n=1); acquired ichthyosis (n=1) and pemphigus foliaceus (n=1). Non-specific histopathological features were observed in the remaining 32 cases.
Six out of the seven lymph node biopsies performed were non-specific (showing dermatopathic lymphadenopathy). Only one of them suggested Sézary syndrome. After the complete etiological investigation, including skin biopsies, all six patients with non-specific lymph node biopsies were diagnosed with erythroderma caused by eczema.
Eosinophilia was found to be significantly associated with malignancy-related cases of erythroderma (p=0.03). No other cause of erythroderma was significantly associated with any of the remaining laboratory abnormalities analyzed (including anemia, elevated ESR/CRP or leukocytosis).
Etiology
According to the clinical, laboratory and histological findings, the patients were categorized into four groups [Fig. 2]:
Figure 2.
Etiologies of erythroderma.
Preexisting dermatoses (n=67, 65.0%): psoriasis (n=46, 44.7%), eczema (n=17, 16.5%), subacute cutaneous lupus (n=1, 1.0%), acquired ichthyosis (n=1, 1.0%), pemphigus foliaceus (n=1, 1.0%), and scabies (n=1, 1.0%).
Drug reactions (n=19, 18.4%): carbamazepine (n=4, 3.9%), allopurinol (n=4, 3.9%), amoxicillin (n=2, 1.9%), ciprofloxacin (n=1, 1.0%), clindamycin (n=1, 1.0%), trimethoprim/sulfamethoxazole (TMP/SMX) (n=1, 1.0%), minocycline (n=1, 1.0%), diclofenac (n=1, 1.0%), lamotrigine (n=1, 1.0%), sertraline (n=1, 1.0%), amifostine (n=1, 1.0%), and diltiazem (n=1, 1.0%).
Malignancies (n=13, 12.6%): MF (n=6, 5.8%), Sézary syndrome (n=6, 5.8%), and B-cell chronic lymphocytic leukemia (n=1, 1.0%).
Idiopathic (n=4, 3.9%).
All 46 patients diagnosed with erythroderma caused by psoriasis had previous known history of plaque psoriasis (n=30) or had previous history of silvery scaly erythematous plaques and heavier involvement of body parts where psoriasis is commonly observed (n=16). Most of them also exhibited skin lesions suggestive of psoriasis during the early stages of the erythroderma. In addition, typical nail changes of psoriasis (n=27) and psoriatic arthritis (n=19), when present, were useful clues to psoriasis. In most patients, erythroderma arose on a previous long-standing psoriasis, with a mean duration of 10 years (range: 1-20 years) before hospitalization. During the eleven-year study period, ten other patients suffering from psoriasis were admitted to our department due to other health problems (seven patients suffering from lower extremity skin and soft tissue infections, and three patients with adverse cutaneous drug reactions).
The group of patients diagnosed with eczema (n=17) comprised cases of contact eczema (n=11), atopic eczema (n=4), xerotic eczema (n=1) and seborrheic eczema (n=1). A history of previous contact allergies, as well eczematous lesions and severe oozing patches on body regions exposed to allergens were important in establishing the diagnosis of erythroderma caused by contact eczema. All 11 cases were confirmed by patch testing after the resolution of the erytherythroderma: paraphenylenediamine (n=5), thimerosal (n=4), and nickel (n=2). The group of patients with atopic eczema suffered from allergic rhinitis, asthma, food allergies or allergic conjunctivitis. Typically, during the early stages of the erythroderma, these patients presented with a characteristic flexural distribution of eczema lesions, and in some cases infraorbital folds and palmar hyperlinearity were observed. These findings were important clues in establishing an exacerbation of the atopic eczema as the cause of the erythroderma. The patient diagnosed with erythroderma caused by exacerbation of a xerotic eczema had a ten-year history of relapsing pruriginous and erythematous patches on both anterior shins, associated with dry skin. Before hospitalization, he suffered an abrupt extension of these lesions to the trunk and upper limbs, and the skin biopsy taken was compatible with an acute eczema. Finally, the patient diagnosed with erythroderma associated with seborrheic eczema had a long-standing history of inflammatory scaling patches on the eyebrows, nasolabial folds, ears, chest, and back. He was diagnosed with Parkinson’s disease two years before being admitted. The skin biopsy findings in this case were also consistent with an acute eczema, confirming the clinical diagnosis.
Three cases of erythroderma were considered to be related to other preexisting dermatoses namely, subacute cutaneous lupus (n=1), acquired ichthyosis (n=1) and pemphigus foliaceus (n=1). Specific histopathological features in skin biopsies helped to reach these diagnoses. In addition, one case of severe crusted scabies was diagnosed after the identification of Sarcoptes scabiei mite in microscopy of skin scrapings.
Relationship between a drug and erythroderma was established from the antecedent of intake of the suspected drug in the days preceding the onset of erythroderma, and improvement following the withdrawal of the drug. In fact, the use of the Naranjo adverse drug reaction probability scale[4] indicated a possible/probable relationship between the suspected drugs and erythroderma in all 19 cases (scores ranging from 4 to 8). Incriminated drugs were: allopurinol (n=4), carbamazepine (n=4), amoxicillin (n=2), ciprofloxacin (n=1), clindamycin (n=1), TMP/SMX (n=1), minocycline (n=1), diclofenac (n=1), lamotrigine (n=1), sertraline (n=1), amifostine (n=1), and diltiazem (n=1). Six of these patients developed toxic epidermal necrolysis after treatment with allopurinol (n=1), carbamazepin (n=1), TMP/SMX (n=1), diltiazem (n=1), minocycline (n=1), and amifostine (n=1), and one patient developed DRESS syndrome following the intake of carbamazepine.
The diagnoses of cutaneous T-cell lymphomas (CTCL) were established after skin biopsies and/or cytometric assessment of lymphocyte populations in the peripheral blood. The diagnostic criteria for Sézary syndrome included an absolute Sézary cell count of≥1000 cells/mm3 and/or a CD4/CD8 ratio ≥10. All MF cases were diagnosed from the features found in the histopathological examination of skin biopsies and exclusion of Sézary syndrome. One case of erythroderma was the first manifestation of a B-cell chronic lymphocytic leukemia. After completing the etiological investigation, no other neoplasms were identified in our patients.
The cause of erythroderma could not be determined in four cases and they were classified as suffering from idiopathic erythroderma.
Evolution/Prognosis
Independently of the etiology, the duration of hospitalization for patients with erythroderma (mean 11.8 days, range: 3-73 days) was significantly superior to that of other inpatient cases (mean 20.0 days, range: 1-122 days) (p=0.01). No deaths were recorded during the hospitalization amongst erythrodermic patients. The patients’ records registered that, at discharge, all had achieved some degree of clinical improvement. The group of patients with erythroderma caused by drug consumption showed the shortest hospitalization times (mean 7 days, range: 3-20 days) between all etiological groups (p=0.02). In opposition, patients diagnosed with malignancy-related erythroderma had longer hospital stay (mean 37.3 days, range: 9-73 days) (p=0.03).
We obtained follow-up information from all 103 patients. The mean follow-up period was four years (range 6 months — 10 years). Twenty-seven patients (26.2%) suffered a relapse of the erythroderma: 18 patients with psoriasis (39.1% of all psoriatic patients), 6 with eczema (35.3% of all patients with eczema), and 3 cases with CTCL (21.3% of the malignancy-related group). It was interesting to observe that no patient from the drug-related (n=19) or idiopathic erythroderma (n=4) groups relapsed during follow-up.
Ten patients died. All six patients with CTCL-related erythroderma died from progression of the lymphoproliferative disorder after an average of 5.5 years (range: 6 months-10 years) since the initial diagnosis. One patient diagnosed with eczema and three cases with psoriasis died from unrelated causes.
Discussion
We collected 103 cases of erythroderma in an 11-year period, corresponding to an incidence of 9.4 cases/year. A survey of erythroderma cases conducted in the Netherlands identified an annual incidence of 0.9 cases/100 000 inhabitants,[5] and another Tunisian series reported a hospital incidence of 6.3 cases/year,[6] a figure similar to our own.
Erythroderma usually occurs in the sixth decade.[6-11] A male predominance is also commonly reported.[7-12] Our study is in accordance with the literature concerning the age and gender distribution of erythrodermic patients.
Typically, the onset of erythroderma is gradual and insidious,[6,9-11] except in the drug-induced cases, where it tends to be sudden, and the resolution faster than the other causes.[6,10] In our series we identified similar results, given that drug-induced cases were associated with a shorter duration of erythroderma before admission, as well as shorter hospitalization times, meaning this group experienced a more acute form of erythroderma.
The diagnostic approach of patients with erythroderma depends on their previous dermatological background. Patients with a history of a dermatological disorder may develop erythroderma during a dermatosis flare. In such cases, the etiological diagnosis is relatively straightforward. Otherwise, erythroderma remains a diagnostic challenge. The final diagnosis is a result of the evaluation of the clinical, biochemical, histological findings and the evolution of the erythroderma in each individual patient.
Like many other series,[6-12] the majority of clinical features were non-specific. As in other studies,[9,10,12] pruritus was the most common complaint of our patients and could not be traced to any specific cause of erythroderma. In this respect, only one retrospective study on the subject has found a significant association between pruritus and psoriasis.[6] As was the case of our series, other reports have found that palmoplantar keratoderma[6,7] as well as nail changes[6,7,10] are predictive clinical signs of psoriasis. Thus, in the absence of history of psoriasis, these cutaneous modifications may direct clinicians to psoriasis. We also found a relatively high prevalence (41.3%) of psoriatic arthritis among patients with cutaneous psoriasis, versus rates that are typically lower (11 - 39%) in the general cutaneous psoriasis-alone population.[13-15] This has rarely or never been mentioned in previous reports on erythroderma. Approximately half of our patients had fever, comparable to the incidence reported in other studies.[6,9-12] We also found that fever was more frequently associated with drug reactions, an association previously identified in two other recent series.[6,7]
We found a higher percentage of lymphadenopathy and hepatic/splenic enlargement in comparison with previous series.[6-12] In previous publications, lymphadenopathy has been found to be associated with CTCL, drug reactions and psoriasis.[8] Hepatic or splenic enlargement have been reported in association with erythroderma caused by drug consumption.[11] In our series however, we only found significant associations between both lymphadenopathy and hepatic/splenic enlargement, and the group of patients with CTCL. Though several laboratory abnormalities were identified, nothing remarkable was seen in the laboratory tests. We found a higher incidence of elevated ESR/CRP than most previous series,[7-11] but the only association identified was between eosinophilia and malignancy-related cases of erythroderma. In recent series, not only has eosinophilia been associated with CTCL,[7] but also with cases of drug reactions,[6] psoriasis[7] and eczema.[7] Interestingly, in our series, eosinophilia was not found to be significantly associated with erythroderma caused by drugs (p=0.3).
In previous studies, skin biopsy has been reported to be of variable usefulness in making the correct histological diagnosis. In our study, skin biopsies were useful in establishing a final diagnosis in 63 patients (66.3%) out of the 95 cases in whom cutaneous biopsies were performed. This figure is in line with most previous reports.[6,7,11,12] We therefore regard skin biopsies as a useful tool in making the diagnosis. They are particularly important in patients without a previous history of dermatological diseases and who deny having recently taken any medication. In this and previous studies, lymph node biopsy frequently demonstrates dermatopathic changes in patients with erythroderma.[9-11] In only one of our patients did a lymph node biopsy reveal specific features (lymphomatous infiltration in a patient with Sézary syndrome that had not been previously diagnosed). These results suggest that lymph node biopsy is not indicated in the initial evaluation of most patients with erythroderma and lymphadenopathy.
Comparison of the etiological groups among recent series of erythroderma and our own is given in Table 2. This table reveals a variation of the relative incidence of the different etiological groups of erythroderma. This may be partly related to genetic, geographical and social disparities. As indicated by our survey, exacerbation of preexisting skin diseases is the main cause of erythroderma, with a particular frequency of psoriasis.[6-12] It is important to note that, in our study and some other surveys,[6,7,10] erythroderma occurs more frequently in long-standing psoriasis.
Table 2. Comparison of earlier studies with present study for etiology of erythroderma.
| Author, year, country | Preexisting dermatoses | Drug reactions | Malignancies | Others* | Idiopathic | Total | ||
|---|---|---|---|---|---|---|---|---|
| n (%) | Total | Total | n (%) | Total | Total | Total | ||
| Present study | Psoriasis: 46 (44.7%) Eczema: 17 (16.5%) Subacute cutaneous lupus: 1 (1%) Acquired ichthyosis: 1 (1%) Pemphigus foliaceus: 1 (1%) Scabies: 1 (1%) |
67 (65%) |
19 (18.4%) |
Mycosis fungoides: 6 (5.8%) Sézary syndrome: 6 (5.8%) B-cell chronic lymphocytic leukemia: 1 (1%); |
13 (12.6%) |
4 (3.9%) |
103 | |
| Hulmani M et al., 2014, India11 | Psoriasis: 10 (33.3%) Eczema: 8 (26.6%) Pityriasis rubra pilaris: 1 (3.3%) |
19 (63.3%) |
5 (16.6%) |
Nasopharyngeal carcinoma: 1 (3.3%) | 1 (3.3%) |
5 (16.6%) |
30 | |
| Li J et al., 2012, China10 | Psoriasis: 143 (55%) Eczema: 32 (12.3%) Bullous pemphigoid: 2 (0.8%) Pemphigus foliaceus: 2 (0.8%) Pityriasis rubra pilaris: 1 (0.4%) Dermatomiositis: 1 (0.4%) |
181 (69.6%) |
33 (12.7%) |
Mycosis fungoides: 3 (1.2%) Lung cancer: 1 (0.4%) Tongue cancer: 1 (0.4%) Langerhans cell histiocytosis: 1 (0.4%) |
6 (2.3%) |
3 (1.2%) |
37 (14.2%) |
260 |
| Yuan XY et al., 2010, China9 | Psoriasis: 25 (30.5%) Eczema: 18 (22%) Pityriasis rubra pilaris: 10 (12.2%) Congenital icthyosiform erythroderma: 1 (1.2%) Sarcoidosis: 1 (1.2%) Dermatomyositis: 1 (1.2%) |
56 (68.3%) |
14 (17%) |
Mycosis fungoides: 2 (2.4%) Sézary syndrome: 1 (1.2%) Gastric cancer: 1 (1.2%) |
4 (4.9%) |
3 (3.7%) |
5 (6.1%) |
82 |
| Khaled, 2010, Tunisia6 | Psoriasis: 27 (32.9%) Eczema: 9 (11%) |
36 (43.9%) |
18 (21.9%) |
Mycosis fungoides: 4 (4.9%) | 4 (4.9%) |
3 (3.7%) |
21 (25.6%) |
82 |
| Fernandes et al., 2008, Brasil8 | Psoriasis: 66 (38.8%) Eczema: 28 (16.5%) Congenital icthyosiform erythroderma: 3 (1.8%) Scabies: 1 (0.6%) Pityriasis rubra pilaris: 1 (0.6%) |
99 (58.2%) |
37 (21.8%) |
Mycosis fungoides: 14 (8.2%) Sézary syndrome: 4 (2.4%) |
18 (10.6%) |
16 (9.4%) |
170 | |
| Akhyani M et al., 2005, Iran12 | Psoriasis: 27 (27.8%) Eczema: 18 (18.6%) Pityriasis rubra pilaris: 8 (8.2%) Actinic reticulosis: 1 (1%) Scabies: 1 (1%) Bullous ichthyosiformis: 1 (1%) Pemphigus foliaceus: 1 (1%) Senile xerosis: 1 (1%) |
58 (59.7%) |
21 (21.6%) |
Mycosis fungoides: 8 (8.2%) Sézary syndrome: 2 (2.1%) Lung cancer: 1 (1%) |
11 (11.3%) |
7 (7.2%) |
97 | |
| Rym BM et al., 2005, Tunisia7 | Psoriasis: 41 (51.3%) Eczema: 6 (7.5%) Pemphigus foliaceus: 5 (6.3%) Dermatophytosis: 3 (3.8%) Pityriasis rubra pilaris: 1 (1.3%) Lichen planus: 1 (1.3%) Scabies: 1 (1.3%) |
58 (72.5%) |
9 (11.3%) |
Mycosis fungoides: 5 (6.23%) Sézary syndrome: 2 (2.5%) |
7 (8.8%) |
6 (7.5%) |
80 | |
| * All cases were of Idiopathic Hypereosinophilic Syndrome. | ||||||||
Many drugs can cause erythroderma [Table 3]. Among recent series, including our own, they represent the second most frequent known cause of erythroderma.[6-12] We identified carbamazepine and allopurinol as the agents of greatest erythroderma-inducing potential. Previously Li et al.[10] reported similar findings. Despite the increasing inventory of drugs causing erythroderma, carbamazepine is the most common cause of drug-induced cases in some recent studies.[6,10,12] According to one series, the high frequency of carbamazepine as a cause of erythroderma may be a result of different genetic sensitivities to this drug or the high rate of its prescription.[12] The same may be true in the cases of erythroderma caused by allopurinol. The fact that both carbamazepine and allopurinol are frequently prescribed in our country, possibly explains our findings.
Table 3. Comparison of earlier studies with present study for the drugs casing erythroderma.
| Authors, year, country | Present study | Hulmani M et al., 2014, India11 | Li J et al., 2012, China10 | Yuan XY et al., 2010, China9 | Khaled, 2010, Tunisia 6 | Fernandes et al., 2008, Brasil8 | Akhyani M et al., 2005, Iran12 | Rym BM et al., 2005, Tunisia7 |
|---|---|---|---|---|---|---|---|---|
| Implicated drugs | Allopurinol: 4 (3.9%) Carbamazepine: 4 (3.9%) Amoxicillin: 2 (1.9%) Ciprofloxacin: 1 (1%) Clindamycin: 1 (1%) TMP/SMX: 1 (1%) Minocycline: 1 (1%) Diclofenac: 1 (1%) Lamotrigine: 1 (1%) Sertraline: 1 (1%) Amifostine: 1 (1%) Diltiazem: 1 (1%) |
Phenytoin: 1 (3.3%) Carbamazepine: 1 (3.3%) Nitrazepam: 1 (3.3%) Glipizide: 1 (3.3%) Dapsone: 1 (3.3%) |
Carbamazepine: 11 (33.3%) Allopurinol: 7 (2.69%) Aminopyrine: 3 (1.15%) Chinese traditional herbal medicines: 3 (1.15%) Cephalosporins: 2 (0.77%) Multiple suspected drugs: 7 (2.69%) |
Oral/topical Chinese herbal medicines: 9 (11%) Indomethacin: 2 (2.4%) Penicillin: 1 (1.2%) Sulfanilamide: 1 (1.2%) Amoxicillin: 1 (1.2%) |
Carbamazepine: 5 (6.1%) Penicillin: 4 (4.9%) Trazepam: 2 (2.4%) Allopurinol: 2 (2.4%) TMP/SMX: 1 (1.2%) Rifampicin: 1(1.2%) Multiple suspected drugs: 3 (3.7%) |
Antihypertensive drugs: 6 (3.53%) Sulfonamides: 5 (2.94%) NSAID*: 4 (2.35%) Amoxicillin: 1 (0.6%) Tetracyclin: 1 (0.6%) Multiple suspected drugs: 20 (11.76%) |
Carbamazepine: 12 (12.4%) Phenytoin: 3 (3.1%) Phenobarbital: 2 (2.1%) Lithium: 1 (1%) Penicillin: 1 (1%) Vancomycin: 1 (1%) TMP/SMX: 1 (1%) |
Carbamazepine Phenobarbital Penicillin Acetaminophen (Number of patients not specified) |
| Total % of total patients |
19 (18.4%) |
5 (16.6%) |
33 (12.69%) |
14 (17%) |
18 (21.9%) |
37 (21.77%) |
21 (21.6%) |
9 (11.25%) |
| * NSAID: Nonsteroidal Anti-inflammatory drugs. | ||||||||
The percentage of malignancies as a cause for erythroderma is relatively low in our study as is the case in previous reports.[6-12] MF and Sézary syndrome are usually the most frequent malignant causes of erythroderma but, occasionally, erythroderma was associated with internal malignancies (including cases of lung cancer,[10,12] tongue cancer,[10] nasopharyngeal cancer,[11] gastric cancer,[9] and Langerhans cell histiocytosis[10]). In our study we identified a patient presenting with erythroderma as the first manifestation of a B-cell chronic lymphocytic leukemia, which has never been mentioned in previous series. In this case the hystopathological examination did not show dermal or epidermal infiltration of atypical lymphocytes. Therefore, it is recommended that erythrodermic patients, whose clinicopathological features are inconclusive, should be investigated carefully to rule out any underlying malignant cause.
After an exhaustive etiological screening, the etiology of the erythroderma was not ascertained in four patients. These were classified under idiopathic disorders, also known as the red man syndrome. Our series has recorded the smallest proportion of idiopathic erythroderma cases (3.9%) compared to recent reports (6.1-16.6%).[6-12] Our figure may have been influenced by one of the higher number of skin biopsies reported in recent series (92.2% of all patients), but also due to the pathologist’s knowledge of relevant clinical features. All of our patients with idiopathic erythroderma showed spontaneous resolution, and no relapse was observed during follow-up. We did not find an association between idiopathic cases and older age or palmoplantar keratoderma, as previously reported in other series.[16] Some authors have identified the progression of some patients with idiopathic erythroderma to CTCL.[16] For this reason, and despite our reassuring findings, we consider that the possibility of being a pre-malignant phase justifies a close and longterm follow-up to identify the causes and start earlier treatment.
Conflicting results have been published regarding the prognosis of patients with erythroderma. On follow-up, we found six deaths (5.8%) related to the underlying causes of erythroderma, all of them from progression of the CTCL. In recent series, the death proportion ranges between 1.0 and 3.8%.[6,7,9,10,12] Our numbers can be explained by the longer follow-up period (mean 4 years, range 6 months-10 years) in comparison with all other recent series (with mean follow-up periods ranging from 12 to 30 months).[6,7,10] Our findings support the perception that non-malignant erythroderma, despite often bringing distress to patients, most often does not pose a significant risk to the patient’s life.[2]
Despite its limitations (retrospective design and the missing information in some of the patient’s records), this study provides us with interesting information related to clinical features and prognosis of erythroderma.
Conclusion
In conclusion, erythroderma is a fairly uniform clinical syndrome of generalized erythema and scaling. Most of the clinical features are non-specific, with few cause-orienting clues. Similarly, most laboratory abnormalities were nondiagnostic and probably related to the inflammatory process. The skin biopsy was the most compelling exception, yielding a positive clinical correlation in the majority of cases. Although the causes may be diverse, most cases of erythroderma have a preexisting skin disease. Patients without previous dermatoses often have drug-induced erythroderma or malignancy. In the uncommon cases were no underlying cause is found, close follow-up is recommended.
References
- Botella-Estrada R, Sanmartin O, Oliver V, Febrer I, Aliaga A. Erythroderma. A clinicopathological study of 56 cases. Arch Dermatol. 1994;130:1503–1507. doi: 10.1001/archderm.130.12.1503. [DOI] [PubMed] [Google Scholar]
- Hasan T, Jansén CT. Erythroderma: a follow-up of fifty cases. J Am Acad Dermatol. 1983;8:836–840. doi: 10.1016/s0190-9622(83)80013-9. [DOI] [PubMed] [Google Scholar]
- Sigurdsson V, Toonstra J, Hezemans-Boer M, van Vloten WA. Erythroderma. A clinical and follow-up study of 102 patients, with special emphasis on survival. J Am Acad Dermatol. 1996;35:53–57. doi: 10.1016/s0190-9622(96)90496-x. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- Sigurdsson V, Steegmans PH, van Vloten WA. The incidence of erythroderma: a survey among all dermatologists in The Netherlands. J Am Acad Dermatol. 2001;45:675–678. doi: 10.1067/mjd.2001.116224. [DOI] [PubMed] [Google Scholar]
- Khaled A, Sellami A, Fazaa B, Kharfi M, Zeglaoui F, Kamoun MR. Acquired erythroderma in adults: a clinical and prognostic study. J Eur Acad Dermatol Venereol. 2010;24:781–788. doi: 10.1111/j.1468-3083.2009.03526.x. [DOI] [PubMed] [Google Scholar]
- Rym BM, Mourad M, Bechir Z, Dalenda E, Faika C, Iadh AM, Amel BO. Erythroderma in adults: a report of 80 cases. Int J Dermatol. 2005;44:731–735. doi: 10.1111/j.1365-4632.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Fernandes NC, Pereira FSM, Maceira JP, Cuzzi T, Dresch TFLR, Araújo PP. Eritrodermia: estudo clínico-laboratorial e histopatológico de 170 casos. An Bras Dermatol. 2008;83:532–526. [Google Scholar]
- Yuan XY, Guo JY, Dang YP, Qiao L, Liu W. Erythroderma: A clinical-etiological study of 82 cases. Eur J Dermatol. 2010;20:373–377. doi: 10.1684/ejd.2010.0943. [DOI] [PubMed] [Google Scholar]
- Li J, Zheng HY. Erythroderma: a clinical and prognostic study. Dermatology. 2012;225:154–162. doi: 10.1159/000342365. [DOI] [PubMed] [Google Scholar]
- Hulmani M, Nandakishore B, Bhat MR, Sukumar D, Martis J, Kamath G, Srinath MK. Clinico-etiological study of 30 erythroderma cases from tertiary center in South India. Indian Dermatol Online J. 2014;5:25–29. doi: 10.4103/2229-5178.126024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhyani M, Ghodsi ZS, Toosi S, Dabbaghian H. Erythroderma: a clinical study of 97 cases. BMC Dermatol. 2005;5:5. doi: 10.1186/1471-5945-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, Paul CF, Puig L, Reich K, van de Kerkhof PC. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–881 e1-30. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 Suppl 2:ii14–17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AB, Mease PJ, Mark Jackson J, Eisen D, Amy Xia H, Asare C, Stevens SR. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists' offices. J Dermatolog Treat. 2006;17:279–287. doi: 10.1080/09546630600823369. [DOI] [PubMed] [Google Scholar]
- Pal S, Haroon TS. Erythroderma: a clinico-etiologic study of 90 cases. Int J Dermatol. 1998;37:104–107. doi: 10.1046/j.1365-4362.1998.00228.x. [DOI] [PubMed] [Google Scholar]