Abstract
BACKGROUND AND OBJECTIVE:
Prenatal diagnosis allows improved perioperative outcomes for fetuses with certain forms of congenital heart disease (CHD). Variability in prenatal diagnosis has been demonstrated in other countries, leading to efforts to improve fetal imaging protocols and access to care, but has not been examined across the United States. The objective was to evaluate national variation in prenatal detection across geographic region and defect type in neonates and infants with CHD undergoing heart surgery.
METHODS:
Cardiovascular operations performed in patients ≤6 months of age in the United States and included in the Society of Thoracic Surgeons Congenital Heart Surgery Database (2006–2012) were eligible for inclusion. Centers with >15% missing prenatal diagnosis data were excluded from the study. Prenatal diagnosis rates were compared across geographic location of residence and defect type using the χ2 test.
RESULTS:
Overall, the study included 31 374 patients from 91 Society of Thoracic Surgeons Congenital Heart Surgery Database participating centers across the United States. Prenatal detection occurred in 34% and increased every year, from 26% (2006) to 42% (2012). There was significant geographic variation in rates of prenatal diagnosis across states (range 11.8%–53.4%, P < .0001). Significant variability by defect type was also observed, with higher rates for lesions identifiable on 4-chamber view than for those requiring outflow tract visualization (57% vs 32%, P < .0001).
CONCLUSIONS:
Rates of prenatal CHD detection in the United States remain low for patients undergoing surgical intervention, with significant variability between states and across defect type. Additional studies are needed to identify reasons for this variation and the potential impact on patient outcomes.
What’s Known on This Subject:
Prenatal diagnosis may lead to benefits in outcomes for certain forms of critical congenital heart disease. Despite recognized benefits, single-center studies and focused regional efforts suggest that prenatal detection rates for congenital heart disease remain low in the United States.
What This Study Adds:
We describe prenatal detection rates for a large cohort of neonates and infants undergoing heart surgery across a range of congenital heart defects. Additionally, this study adds new information by demonstrating geographic variability of prenatal detection rates across the United States.
Congenital heart disease (CHD) is the most common class of birth defect, affecting 8 in 1000 live births, with approximately one-quarter of these children needing intervention in the first year of life. 1 – 3 Advances in congenital heart surgery have improved survival rates for neonates and infants with CHD, and efforts are now being made to optimize perinatal and preoperative factors to promote improvements in outcomes. A diagnosis of CHD before birth allows prenatal counseling and coordination of delivery at an experienced cardiac center. Evidence now suggests that a prenatal diagnosis may lead to benefits in early postnatal status and outcomes in certain critical forms of CHD. 4 , 5
Despite recognized benefits of prenatal CHD diagnosis and nearly universal availability of prenatal ultrasound, 6 , 7 recent single-center studies and focused regional efforts suggest that prenatal detection rates (PDRs) for CHD remain low in the United States. 8 – 13 In other countries, large-scale assessments using birth registries have identified significant regional variability of prenatal CHD diagnosis. 14 – 16 Centralized prenatal health care efforts in these countries have led to implementation of protocols to improve access to care and standardization of fetal imaging. In the United States, assessments of national PDRs are lacking, and the extent of variation across regions has not been defined.
The purpose of this study was to provide a baseline assessment of national prenatal CHD detection rates and recent trends for a large cohort of neonates and infants undergoing heart surgery. We used a clinical registry to describe variation in the frequency of prenatal detection across the United States by geographic location and defect type. We also assessed the influence of noncardiac congenital anomalies and chromosomal abnormalities on PDRs.
Methods
Data Source
The Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD) was used for this study. It is estimated that the database represents ∼93% of all US centers that perform congenital heart surgery and >96% of all operations. 17 Coding in the database is performed by clinicians and ancillary support staff using the International Pediatric and Congenital Cardiac Code 18 and is entered into the STS-CHSD data collection form (version 2.5 or 3.0). 19 This study was approved by the STS-CHSD Access and Publications Committee and the Duke University Institutional Review Board.
Study Population
All first cardiovascular operations, including both cardiopulmonary bypass and nonbypass surgeries, in neonates and infants (≤6 months of age) performed in the United States and included in the STS-CHSD (January 2006 to December 2012) were eligible for inclusion. The year 2006 was chosen as the starting point because the variable specifying prenatal diagnosis was added to the STS-CHSD at this time. Patients with a history of previous cardiac surgery and those for whom patent ductus arteriosus ligation or organ procurement was the primary procedure were excluded. From this cohort (n = 44 934 patients, 109 centers), additional exclusions were centers with >15% missing data for prenatal diagnosis (18 centers, 8741 patients), patients from the remaining 91 centers with missing data for the prenatal diagnosis variable (n = 624), and patients with defects for which fetal diagnosis is not routinely made, either because of anatomic challenges or less clinical relevance in the first 6 months of life (n = 4195). These defects included atrial septal defects, coronary artery anomalies, partial anomalous pulmonary venous connection, and vascular rings. For the secondary geographic data analysis we excluded states with <50 potentially eligible patients enrolled in STS-CHSD for the time frame of the study.
Defect Classification
The patient’s fundamental diagnosis, defined previously 20 as the most complex cardiac anomaly or condition, was used to categorize defects. When the fundamental diagnosis was missing, the primary diagnosis of the operation, defined as the most important diagnosis relative to the procedure being performed, was used. Data were compiled overall and for specific diagnostic groups that warrant surgical intervention in the newborn period or early in infancy. Defects were also classified by whether they would probably have abnormalities amenable to detection on a prenatal screening ultrasound 4-chamber view (4CV). For this specific analysis, those with a normal 4CV included transposition of the great arteries (TGA), tetralogy of Fallot (TOF), double-outlet right ventricle, and truncus arteriosus. Defects considered to have an abnormal 4CV included complete atrioventricular septal defect, congenitally corrected transposition of the great arteries, tricuspid valve disease (including Ebstein anomaly), and all single-ventricle defects including hypoplastic left heart syndrome (HLHS). Other defects were not included in this specific analysis because of variability of 4CVs (ie, could be either normal or abnormal depending on severity of defect within a diagnosis category or related to gestational age at time of screening ultrasound).
Patient Region
Patient region was defined by the patient’s permanent residence at the time of admission for surgery as collected in the STS-CHSD. The patient region variable was 99.2% complete in the database. Patient region was chosen as the variable of interest because it more accurately reflected the location where prenatal care took place rather than the geographic location where the surgical intervention was performed. Patient region was classified by both individual state and by the US Department of Health and Human Services (USDHHS) 10 geographic regions. 21 The USDHHS regional classification system assigns a number and a major city to each region. By convention these regions are defined according to major cities as follows: 1, “Boston” (including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont); 2, “New York” (including New Jersey, New York); 3, “Philadelphia” (including Delaware, District of Columbia, Maryland, Pennsylvania, Virginia, West Virginia); 4, “Atlanta” (including Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, Tennessee); 5, “Chicago” (including Illinois, Indiana, Michigan, Minnesota, Ohio, Wisconsin); 6, “Dallas” (including Arkansas, Louisiana, New Mexico, Oklahoma, Texas); 7, “Kansas City” (including Iowa, Kansas, Missouri, Nebraska); 8, “Denver” (including Colorado, Montana, North Dakota, South Dakota, Utah, Wyoming); 9, “San Francisco” (including Arizona, California, Hawaii, Nevada); 10, “Seattle” (including Alaska, Idaho, Oregon, Washington).
Other Data Collection
Other variables collected from the STS-CHSD included patient demographics, presence of noncardiac congenital anatomic abnormalities, chromosomal abnormalities or genetic syndromes, and relative surgical complexity as measured by STAT Mortality category (surgical complexity, scale of 1–5, with a higher number representing higher complexity). 22
Analysis
Patient characteristics and other pertinent variables were summarized overall and stratified by the presence or absence of prenatal detection according to frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. PDRs were assessed overall and by study year. A sensitivity analysis was performed excluding sites that did not submit data during the entire time period (2006–2012) to further examine trends. PDRs were then evaluated across defect type according to the fundamental diagnosis and to whether defects were amenable to detection on 4CV. PDRs were also compared across geographic location of residence and defect type. Finally, subgroup analysis was performed to establish the potential influence of a chromosomal abnormality, syndrome, or noncardiac congenital anatomic abnormality with PDRs. χ2 tests were used in comparisons of PDRs for all analyses. A P value <.05 was considered statistically significant. All analyses were performed in SAS version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Study Population Characteristics and Overall Prenatal Detection Rates
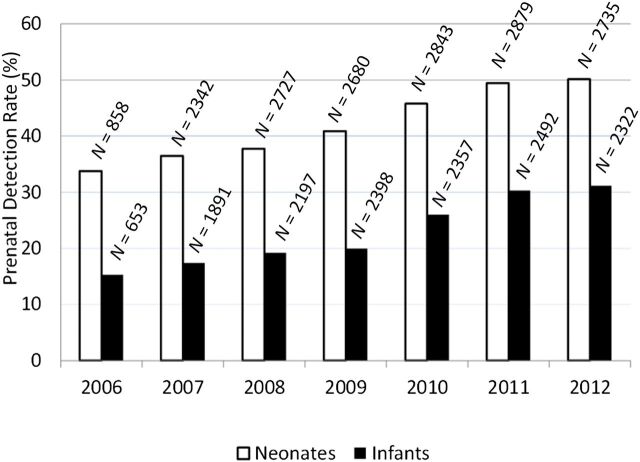
The study included a total of 31 374 patients from 91 STS participating centers across the United States. From this cohort there were 17 064 neonates who underwent surgery at 0 to 30 days of life and 14 310 infants who underwent surgery at 31 days to 6 months of life. Patient characteristics are shown in Table 1, including presence of noncardiac abnormalities and relative surgical complexity (STAT Mortality category). The overall rate of prenatal diagnosis was 34% (10 795/31 374) over the 6-year study period, with higher PDRs in those having surgery as neonates compared with those having surgery as infants (43.2% vs 23.9%, P < .0001). PDRs for both the neonatal and infant groups increased each year, from 26% (2006) to 42% (2012) (Fig 1). Sensitivity analysis including only the centers that participated in the database every year since 2006 confirmed similar trends, P < .0001.
TABLE 1.
Study Population Characteristics
| Variable | Neonates (n = 17 064) | Infants (n = 14 310) | ||||
|---|---|---|---|---|---|---|
| Prenatal Diagnosis Yes (n = 7373) | Prenatal Diagnosis No (n = 9691) | P | Prenatal Diagnosis Yes (n = 3422) | Prenatal Diagnosis No (n = 10 888) | P | |
| Age at surgery, d | 6.0 (4.0, 9.0) | 8.0 (5.0, 13.0) | <.0001 | 101 (63, 137) | 103 (67, 139) | .0068 |
| Wt at surgery, kg | 3.1 (2.7, 3.5) | 3.2 (2.8, 3.6) | <.0001 | 4.6 (3.8, 5.5) | 4.7 (3.9, 5.6) | <.0001 |
| Gender, n (%) male | 4170 (56.6) | 5865 (60.5) | <.0001 | 1761 (51.5) | 5808 (53.3) | .0514 |
| Chromosomal abnormality or syndrome | 1903 (25.8) | 1884 (19.4) | <.0001 | 1784 (52.1) | 3922 (36.0) | <.0001 |
| Noncardiac anomaly | 179 (4.4) | 90 (2.1) | <.0001 | 128 (6.1) | 147 (2.9) | <.0001 |
| STAT category 4 or 5 a | 5422 (73.6) | 5407 (55.8) | <.0001 | 914 (26.7) | 2043 (18.8) | <.0001 |
Data for continuous variables are presented as median and interquartile range and for categorical variables as N (%).
STAT Mortality category indicates relative surgical complexity on a scale of 1–5, with higher numbers representing more complex diagnoses.
FIGURE 1.
PDRs by year for neonatal (white bars) and infant (black bars) cohorts. N indicates the total number of subjects represented by each bar.
Prenatal Detection Rates by Defect Type
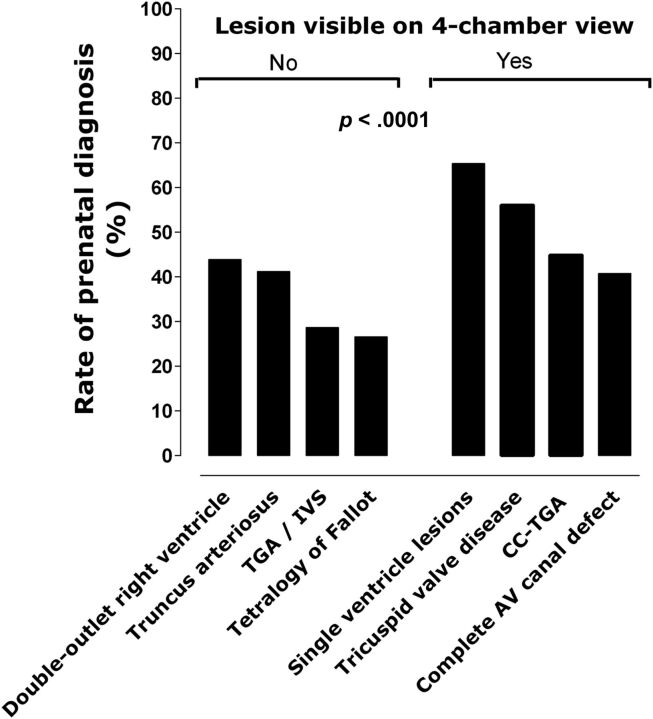
PDRs were examined across several specific diagnoses (Table 2). There was significant variation of PDRs across defect type (P < .0001), with the highest detection rates for HLHS (67%) and the lowest detection rates for total anomalous pulmonary venous connection (9.1%). Defects associated with an abnormal 4CV were more likely to have prenatal identification than those with a normal 4CV (56.7% vs 32.2%, P < .0001; Fig 2). The addition of a ventricular septal defect led to higher PDRs for lesions such as TGA, coarctation of the aorta, and pulmonary atresia. Additional PDRs for various CHD categories are shown in Table 2.
TABLE 2.
PDRs by Fundamental Diagnosis
| Fundamental Diagnosis | Total N | N With Prenatal Detection (%) |
|---|---|---|
| TAPVC | 1359 | 123 (9.1) |
| Ventricular septal defect | 4706 | 577 (12.3) |
| Isolated arch obstruction | 3901 | 841 (21.6) |
| Aortic stenosis | 318 | 81 (25.5) |
| Arch obstruction with VSD | 1174 | 311 (26.5) |
| TOF | 3359 | 895 (26.6) |
| TGA-IVS | 1898 | 530 (27.9) |
| TGA-VSD | 1257 | 463 (36.8) |
| Atrioventricular septal defect | 3172 | 1295 (40.8) |
| Pulmonary stenosis and pulmonary atresia with IVS | 1056 | 433 (41.1) |
| Truncus arteriosus | 761 | 314 (41.2) |
| TOF with APV syndrome | 200 | 82 (41.3) |
| Double-outlet right ventricle | 1227 | 539 (43.9) |
| Congenitally corrected TGA | 127 | 57 (44.9) |
| Pulmonary atresia with VSD | 1082 | 500 (46.2) |
| Tricuspid valve disease a | 262 | 147 (56.1) |
| Single ventricle, other | 2362 | 1482 (62.7) |
| Hypoplastic left heart syndrome | 3153 | 2125 (67.4) |
APV, absent pulmonary valve; IVS, intact ventricular septum; TAPVC, total anomalous pulmonary venous connection; TGA-IVS, transposition of the great arteries with intact ventricular septum; TGA-VSD, transposition of the great arteries with ventricular septal defect; VSD, ventricular septal defect.
Includes Ebstein anomaly and dysplastic tricuspid valve disease.
FIGURE 2.
PDRs are demonstrated by defect visibility on 4CV (No/Yes). AV, atrioventricular; CC-TGA, congenitally corrected transposition of the great arteries; TGA/IVS, transposition of the great arteries with intact ventricular septum. Single-ventricle lesions include HLHS.
Prenatal Detection in Those With Additional Noncardiac Anomalies
Noncardiac anatomic abnormalities, syndromes, or chromosomal abnormalities were found to be present in 10 037 (31.9%) of the overall cohort. These abnormalities were more common in the infant cohort (40.9%) than in the neonatal cohort (23.0%). Patients with additional abnormalities had higher PDRs (39% vs 32%, P ≤ .0001). Similar trends were seen in both the neonate and infant subgroups (Table 3).
TABLE 3.
PDRs by Presence of Additional Noncardiac Anomaly or Genetic Syndrome
| Age Group | Risk Factor Present | PDR | Risk Factor Not Present | PDR | P |
|---|---|---|---|---|---|
| Neonates (n = 16 741) | 3856, 23% | 51% | 12 885, 77% | 41% | <.0001 |
| Infants (n = 14 097) | 5772, 41% | 31% | 8325, 59% | 19% | <.0001 |
| Overall (n = 30 838) | 9628, 31% | 39% | 21 210, 69% | 32% | <.0001 |
Cohorts are grouped by whether a risk factor was present. A risk factor was defined as the presence of a noncardiac anomaly or genetic syndrome. PDRs are provided. A total of 536 patients were excluded from this analysis because of missing data. P represents the difference in PDRs based on presence or absence of a risk factor.
Prenatal Detection by Geographic Location
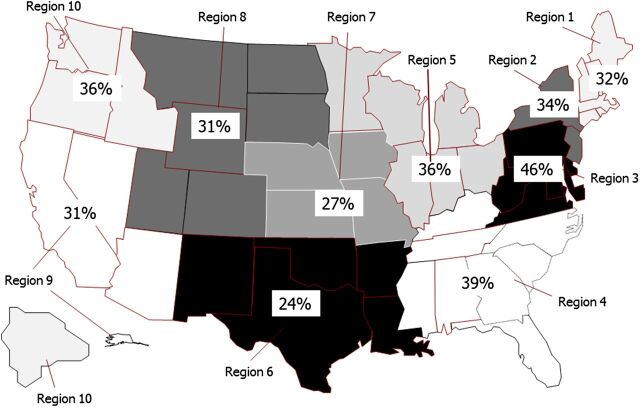
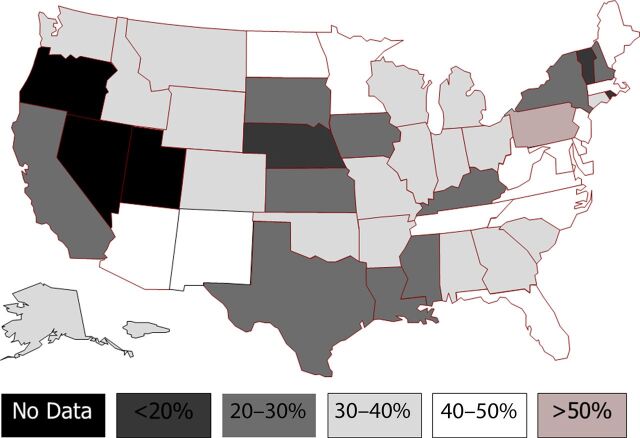
Figure 3 demonstrates PDRs for the 10 USDHHS regions. There was significant variation across regions, with PDRs ranging from 24% (95% confidence interval [CI], 22%–25%) in the “Dallas” region to 46% (95% CI, 44%–47%) in the “Philadelphia” region, P < .0001. There was also significant variation at the state level, with a wide range of PDRs, from a low of 11.8% (95% CI, 4.6%–19.1%) to a high of 53.4% (95% CI, 50.2%–56.5%), P < .0001 (Fig 4).
FIGURE 3.
The 10 USDHHS regions, with PDRs. Oregon, Nevada, and Utah did not have sufficient prenatal diagnosis data during study period to be included in the analysis.
FIGURE 4.
PDRs by state. States in black (Oregon, Nevada, and Utah) did not have sufficient prenatal diagnosis data during study period to be included in the analysis.
Discussion
We assessed PDRs for a large cohort of children with CHD undergoing heart surgery as neonates and infants across the United States. Our analysis of >31 000 patients from 47 states is the largest study to date assessing PDRs. In our cohort, patients with more critical forms of CHD necessitating surgery in the neonatal period had higher PDRs (43%) than those undergoing surgery as infants (24%). Our findings are consistent with recent smaller series in California, Nevada, Ohio, Atlanta, and Utah demonstrating CHD detection rates ranging from 10% to 43%, 8 – 13 despite some differences in methods for selecting study populations across studies. Our current work confirms these findings on a national level with a large cohort of patients with CHD undergoing surgery.
A main goal of the study was to assess potential variability of PDRs across various types of cardiac anomalies. We demonstrated wide variability in PDRs across different anatomic diagnoses, with forms of CHD that have abnormalities amenable to identification on a screening ultrasound 4CV being diagnosed more frequently than those that require outflow tract views (OFTVs) for detection. When compared with recent studies in the United States, our PDRs for specific defect types are similar but with a larger cohort of patients (Table 4).
TABLE 4.
PDRs Across US Studies
| Study, y | Total N | PDRs | TGA | TOF | Atrioventricular Septal Defect | CoA | HLHS |
|---|---|---|---|---|---|---|---|
| Friedberg et al 2009, (%) | 309 | 36% | 6/31 (19) | 14/45 (31) | 8/16 (50) | 9/39 (23) | 11/18 (61) |
| Sklansky et al 2009, (%) | 200 | 33% | 4/24 (17) | 11/34 (32) | 7/16 (44) | 5/26 (19) | 12/20 (60) |
| Acherman et al 2007, (%) | 161 | 36% | 2/6 (33) | 7/23 (30) | 12/28 (43) | 5/27 (19) | 8/13 (62) |
| Pinto et al 2012 a | 1474 | 39% | 15% | 26% | 48.5% | 18.5% | 70% |
| Sekar et al 2013, (%) | 95 | 43% | 4/11 (36) | N/A | 5/9 (56) | 3/12 (25) | 10/13 (77) b |
| Quartermain et al 2015, (%) | 31 074 | 34% | 993/3155 (31) | 873/3359 (27) | 1268/3172 (41) | 841/3901 (22) | 2017/3153 (67) |
Total N, total number of patients included in each study. CoA, coarctation of the aorta; TGA, transposition of the great arteries.
Study reports only the overall percentages for PDRs; specific numbers of patients with each defect type not available.
Data reported in this study represent all forms of single-ventricle heart disease including HLHS.
An additional goal of the study was to examine regional variability of PDRs. To the best of our knowledge this is the first study to examine this subject across the United States. In our cohort, there was significant variation across the country, by both region and state. Previous studies in other countries have assessed variation of PDRs. In Canada, variability by jurisdictions has been reported, and the authors suggested that differences in imaging skills and access to fetal echocardiography were potential causes. 16 Garne et al 14 used data from 20 European registries demonstrating a wide range of PDRs between countries and even between those with similar screening recommendations. Our current findings are similar in that regional variation is significant and probably multifactorial.
Study Implications
Anomalies of the cardiovascular system are the most common form of birth defect, 1 and perinatal death from CHD is the most common form of infant mortality. 23 , 24 Therefore, effective screening for CHD before birth should be a public health priority in the United States. In an era of nearly universal availability of prenatal ultrasound, 8 , 9 , 11 these rates of prenatal CHD diagnosis are suboptimal in this important group of patients who need surgical intervention after birth. From our current data we do not know whether the missed opportunity for prenatal CHD detection results from an unnoticed diagnosis on the screening obstetric ultrasound or possibly a missed diagnosis at the level of a fetal echocardiogram. Previous studies have demonstrated very high sensitivities and specificities for detection of complex CHD by fetal echocardiography. 9 , 11 , 25 Additionally, fetal echocardiography requires an initial referral from the obstetric community. Therefore, the highest-yield strategies to increase detection rates in our current system should involve improvements to the midgestation obstetric screening ultrasound. Multiple factors are probably playing a role in missed CHD identification at the screening ultrasound level, including differences in skill set of the sonographer, experience and training of the physician performing the study, and issues related to access in certain regions to a pediatric cardiologist for fetal echocardiography.
In the United States current recommendations for low-risk pregnancies include a second trimester screening ultrasound to assess for fetal anomalies, 26 with the majority of these studies being performed by community-based physicians. 8 Previous studies have shown that variability in the number and timing of prenatal ultrasounds a patient receives is an important factor, with those lacking a detailed anatomic assessment being more likely to be associated with missed CHD detection. 11 Furthermore, it has been shown that community-based obstetricians and radiologists do not always adhere to the complete components of screening ultrasounds, 27 and when risk factors for CHD are present, referral patterns for fetal echocardiography by a pediatric cardiologist appear to vary significantly. 9 Future efforts to increase prenatal CHD diagnosis rates should include an attempt to identify local barriers to improved adherence to prenatal cardiac screening protocols and referral guidelines for fetal echocardiography.
Another important question is how much improvement in PDRs could be expected by an obstetric screening ultrasound for cardiac anomalies. To this end, comparing PDRs for cardiac defects with rates of other congenital anomalies may help set reasonable goals for the medical community performing fetal imaging. Congenital diaphragmatic hernia is a defect that, similar to CHD, lacks biomarkers to aid in prenatal detection and relies solely on ultrasound for diagnosis. Data from 2 large registry-based studies show that detection rates of 60% to 75% can be attained for this anomaly. 28 , 29 This finding suggests that an improvement in PDRs for cardiac anomalies to a comparable range should be a reasonable goal of fetal screening programs across the United States. Another central element to improving PDRs is related to the role of incorporating OFTVs of the fetal heart on screening ultrasound examination. During the time frame of this study, basic fetal cardiac screening relied mostly on a 4CV, 30 , 31 and this limitation probably contributes to our findings of low detection rates for defects such as TGA, TOF, and double-outlet right ventricle, which require OFTVs for diagnosis. It has been shown that OFTVs are more likely to be abnormal in midgestation screening for fetuses with critical forms of CHD, whereas 4CVs detect a smaller percentage of critical CHD cases. 10 Recently, obstetric guidelines have been updated to recommend OFTVs in addition to the 4CV during the second trimester screening ultrasound. 32 Although this is an important advancement for prenatal screening programs, it remains unclear whether the skill to perform these views is consistently present among doctors at the community level. Efforts in the United States have shown that prenatal screening for CHD can be improved by implementing an educational program for sonographers with specific training in the use of OFTVs. 33 In 1 cohort the addition of OFTVs to the standard 4CV during prenatal screening improved detection rates to 70%. 34 These studies may serve as a model for development of future collaborative fetal centers and provide data on how much improvement may be expected through educational programs aimed at sonographer training and increased use of OFTVs.
Study Limitations
This study was limited to patients eligible for enrollment in a cardiac surgical database. Therefore, we are presenting data on a large cohort of neonates and infants undergoing surgery. We were not able to include information on terminations or fetal demise. Additionally, infants who underwent catheter-based interventions or died with undetected CHD were also not included. Therefore, our PDRs are for a cohort of patients who undergo heart surgery and probably underestimate overall rates for all patients with CHD. In previous reports, fetal demise in fetuses with CHD was uncommon, 15 whereas termination rates in the United States are extremely variable, ranging from as low as 5% in the Southeast for all forms of CHD 35 to as high as 15% for HLHS in the Northeast. 36 A recent study, which included termination cases in their cohort from northern California, 8 demonstrated very similar PDRs for various forms of CHD in comparison with our current study, suggesting that the effect of not including termination data in our cohort, although important, may be minimized by the large size of our study population. Additional study limitations include lack of information on timing of prenatal diagnosis, the type of physician performing the screening ultrasound, or the percentage of patients referred for fetal echocardiography. This information will be important in future efforts as individual states review the status of their current screening programs. Finally, although the STS-CHSD has rigorous data quality control processes and data verification by site audits, the potential for error related to data acquisition at participating institutions remains a possibility.
Conclusions
This is the first study to describe PDRs for a large cohort of neonates and infants undergoing heart surgery across the United States. Our data suggest that opportunities exist to improve prenatal CHD screening because the majority of patients with cardiac defects that require surgery in first 6 months of life go undetected before birth. We found that PDRs vary significantly across defect type, with those having abnormalities on a 4CV being more readily identified. Additionally, significant variability in PDRs by region and state was demonstrated despite national screening guidelines. Future collaborative efforts to improve adherence to prenatal screening programs with development of training opportunities for physicians and sonographers performing fetal imaging may improve the state of prenatal CHD detection in the United States.
Glossary
- 4CV
4 chamber view
- CHD
congenital heart disease
- CI
confidence interval
- HLHS
hypoplastic left heart syndrome
- OFTVs
outflow tract views
- PDRs
prenatal detection rates
- STS-CHSD
Society of Thoracic Surgeons Congenital Heart Surgery Database
- TGA
transposition of the great arteries
- TOF
tetralogy of Fallot
- USDHHS
US Department of Health and Human Services
Footnotes
Drs Quartermain, Pasquali, Hill, Goldberg, Huhta, Jacobs, Jacobs, and Ungerleider conceptualized and designed the study, were involved with data analysis and interpretation, and drafted the initial manuscript; Dr Kim performed the statistical analysis, was involved with study design, and reviewed and revised all versions of the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: Supported by the Society of Thoracic Surgeons.
POTENTIAL CONFLICT OF INTEREST: Dr Jacobs is chair of the Society of Thoracic Surgeons Congenital Heart Surgery Database Task Force. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Ferencz C , Rubin JD , McCarter RJ , et al. Congenital heart disease: prevalence at livebirth. The Baltimore–Washington Infant Study. Am J Epidemiol. 1985;121(1):31–36 [DOI] [PubMed] [Google Scholar]
- 2. Hoffman JI . Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16(4):155–165 [DOI] [PubMed] [Google Scholar]
- 3. Talner CN . Report of the New England Regional Infant Cardiac Program, by Donald C. Fyler, MD, Pediatrics, 1980;65(suppl):375–461. Pediatrics. 1998;102(1 pt 2):258–259 [PubMed] [Google Scholar]
- 4. Tworetzky W , McElhinney DB , Reddy VM , Brook MM , Hanley FL , Silverman NH . Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103(9):1269–1273 [DOI] [PubMed] [Google Scholar]
- 5. Bonnet D , Coltri A , Butera G , et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99(7):916–918 [DOI] [PubMed] [Google Scholar]
- 6. Allan LD . Evolution of echocardiographic findings in the fetus. Circulation. 1997;96(2):391–392 [PubMed] [Google Scholar]
- 7. Fernandez CO , Ramaciotti C , Martin LB , Twickler DM . The four-chamber view and its sensitivity in detecting congenital heart defects. Cardiology. 1998;90(3):202–206 [DOI] [PubMed] [Google Scholar]
- 8. Friedberg MK , Silverman NH , Moon-Grady AJ , et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;155(1):26–31, e1 [DOI] [PubMed] [Google Scholar]
- 9. Pinto NM , Keenan HT , Minich LL , Puchalski MD , Heywood M , Botto LD . Barriers to prenatal detection of congenital heart disease: a population based study. Ultrasound Obstet Gynecol. 2012;40:418–425 [DOI] [PubMed] [Google Scholar]
- 10. Sklansky MS , Berman DP , Pruetz JD , Chang RK . Prenatal screening for major congenital heart disease: superiority of outflow tracts over the 4-chamber view. J Ultrasound Med. 2009;28(7):889–899 [DOI] [PubMed] [Google Scholar]
- 11. Sekar P , Heydarian HC , Cnota JF , Hornberger LK , Michelfelder EC . Diagnosis of congenital heart disease in an era of universal prenatal ultrasound screening in southwest Ohio. Cardiol Young. 2015;25(1):35–41 [DOI] [PubMed] [Google Scholar]
- 12. Acherman RJ , Evans WN , Luna CF , et al. Prenatal detection of congenital heart disease in southern Nevada: the need for universal fetal cardiac evaluation. J Ultrasound Med. 2007;26(12):1715–1719, quiz 1720–1721 [DOI] [PubMed] [Google Scholar]
- 13. Oster ME , Kim CH , Kusano AS , et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113(6):1036–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garne E , Stoll C , Clementi M . Evaluation of prenatal diagnosis of congenital heart disease by ultrasound: experience from 20 European registries. Ultrasound Obstet Gynecol. 2001;17(5):386–391 [DOI] [PubMed] [Google Scholar]
- 15. Marek J , Tomek V , Skovránek J , Povysilová V , Samánek M . Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart. 2011;97(2):124–130 [DOI] [PubMed] [Google Scholar]
- 16. Trines J , Fruitman D , Zuo KJ , Smallhorn JF , Hornberger LK , Mackie AS . Effectiveness of prenatal screening for congenital heart disease: assessment in a jurisdiction with universal access to health care. Can J Cardiol. 2013;29(7):879–885 [DOI] [PubMed] [Google Scholar]
- 17. Jacobs ML , Daniel M , Mavroudis C , et al. Report of the 2010 Society of Thoracic Surgeons Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2011;92(2):762–768, discussion 768–769 [DOI] [PubMed] [Google Scholar]
- 18. Franklin RC , Jacobs JP , Krogmann ON , et al. Nomenclature for congenital and paediatric cardiac disease: historical perspectives and the International Pediatric and Congenital Cardiac Code. Cardiol Young. 2008;18(suppl 2):70–80 [DOI] [PubMed] [Google Scholar]
- 19.Society of Thoracic Surgeons. STS congenital heart surgery database v3.0. 2012. Available at: www.sts.org/node/518
- 20.www.sts.org/sites/default/files/documents/pdf/CongenitalDataSpecificationsV3020090904.pdf
- 21.US Department of Health & Human Services. Regional offices. 2014. Available at: www.hhs.gov/about/regionmap.html
- 22. O’Brien SM , Clarke DR , Jacobs JP , et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138(5):1139–1153 [DOI] [PubMed] [Google Scholar]
- 23. Rosano A , Botto LD , Botting B , Mastroiacovo P . Infant mortality and congenital anomalies from 1950 to 1994: an international perspective. J Epidemiol Community Health. 2000;54(9):660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee K , Khoshnood B , Chen L , Wall SN , Cromie WJ , Mittendorf RL . Infant mortality from congenital malformations in the United States, 1970–1997. Obstet Gynecol. 2001;98(4):620–627 [DOI] [PubMed] [Google Scholar]
- 25. Allan LD , Chita SK , Sharland GK , Fagg NL , Anderson RH , Crawford DC . The accuracy of fetal echocardiography in the diagnosis of congenital heart disease. Int J Cardiol. 1989;25(3):279–288 [DOI] [PubMed] [Google Scholar]
- 26. AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2013;32:1083–1101 [DOI] [PubMed] [Google Scholar]
- 27. Smulian JC , Vintzileos AM , Rodis JF , Campbell WA . Community-based obstetrical ultrasound reports: documentation of compliance with suggested minimum standards. J Clin Ultrasound. 1996;24(3):123–127 [DOI] [PubMed] [Google Scholar]
- 28. Garne E , Haeusler M , Barisic I , Gjergja R , Stoll C , Clementi M Euroscan Study Group . Congenital diaphragmatic hernia: evaluation of prenatal diagnosis in 20 European regions. Ultrasound Obstet Gynecol. 2002;19(4):329–333 [DOI] [PubMed] [Google Scholar]
- 29. Boyd PA , Tonks AM , Rankin J , Rounding C , Wellesley D , Draper ES BINOCAR Working Group . Monitoring the prenatal detection of structural fetal congenital anomalies in England and Wales: register-based study. J Med Screen. 2011;18(1):2–7 [DOI] [PubMed] [Google Scholar]
- 30. International Society of Ultrasound in Obstetrics & Gynecology . Cardiac screening examination of the fetus: guidelines for performing the “basic” and “extended basic” cardiac scan. Ultrasound Obstet Gynecol. 2006;27(1):107–113 [DOI] [PubMed] [Google Scholar]
- 31. Lee W . Performance of the basic fetal cardiac ultrasound examination. J Ultrasound Med. 1998;17(9):601–607 [DOI] [PubMed] [Google Scholar]
- 32. Carvalho JS , Allan LD , Chaoui R , et al. International Society of Ultrasound in Obstetrics and Gynecology . ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41(3):348–359 [DOI] [PubMed] [Google Scholar]
- 33. Levy DJ , Pretorius DH , Rothman A , et al. Improved prenatal detection of congenital heart disease in an integrated health care system. Pediatr Cardiol. 2013;34(3):670–679 [DOI] [PubMed] [Google Scholar]
- 34. Carvalho JS , Mavrides E , Shinebourne EA , Campbell S , Thilaganathan B . Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart. 2002;88(4):387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zyblewski SC , Hill EG , Shirali G , et al. Chromosomal anomalies influence parental treatment decisions in relation to prenatally diagnosed congenital heart disease. Pediatr Cardiol. 2009;30(8):1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rychik J , Szwast A , Natarajan S , et al. Perinatal and early surgical outcome for the fetus with hypoplastic left heart syndrome: a 5-year single institutional experience. Ultrasound Obstet Gynecol. 2010;36(4):465–470 [DOI] [PubMed] [Google Scholar]