Synopsis
Understanding upper limb impairment after stroke is essential to planning therapeutic efforts to restore function. However determining which upper limb impairment to treat and how is complex for two reasons: 1) the impairments are not static, i.e. as motor recovery proceeds, the type and nature of the impairments may change; therefore the treatment needs to evolve to target the impairment contributing to dysfunction at a given point in time. 2) multiple impairments may be present simultaneously, i.e., a patient may present with weakness of the arm and hand immediately after a stroke, which may not have resolved when spasticity sets in a few weeks or months later; hence there may be a layering of impairments over time making it difficult to decide what to treat first. The most useful way to understand how impairments contribute to upper limb dysfunction may be to examine them from the perspective of their functional consequences. There are three main functional consequences of impairments on upper limb function are: (1) learned nonuse, (2) learned bad-use, and (3) forgetting as determined by behavioral analysis of tasks. The impairments that contribute to each of these functional limitations are described.
Keywords: Stroke, Arm, Weakness, Hemiparesis, Motor Control
The nature of upper limb motor impairment
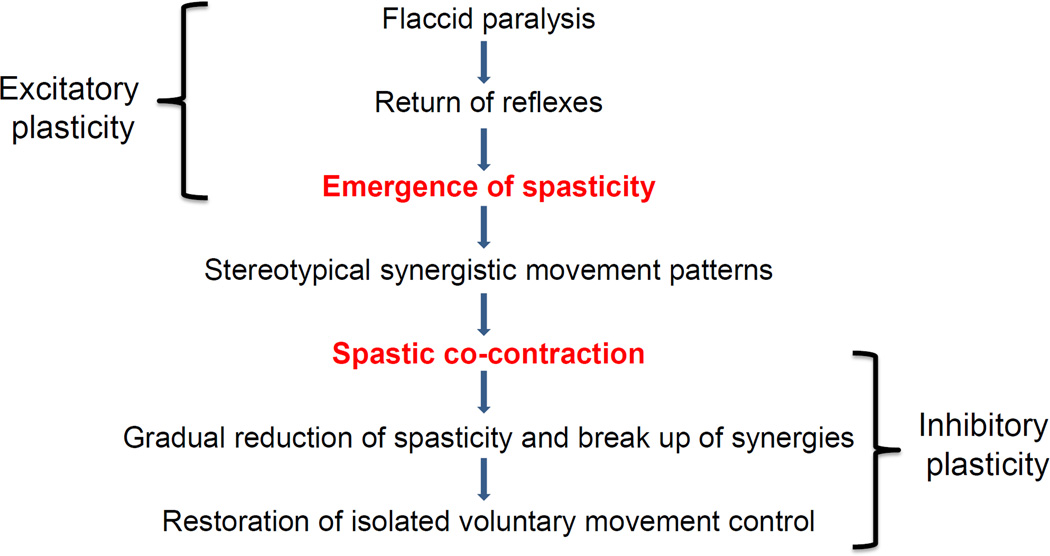
According to the International Classification of Functioning, Disability and Health model (ICF) (Geyh, Cieza et al. 2004), impairments may be described as (1) impairments of body function such as a significant deviation or loss in neuromusculoskeletal and movement related function related to joint mobility, muscle power, muscle tone and/or involuntary movements, or (2) impairment of body structures such as a significant deviation in structure of the nervous system or structures related to movement, for example the arm and/or hand. A stroke may lead to both types of impairments. Upper limb impairments after stroke are the cause of functional limitations with regard to use of the affected upper limb after stroke, so a clear understanding of the underlying impairments is necessary to provide appropriate treatment. However understanding upper limb impairments in any given patient is complex for two reasons: 1) the impairments are not static, i.e. as motor recovery proceeds, the type and nature of the impairments may change; therefore the treatment needs to evolve to target the impairment contributing to dysfunction at a given point in time. 2) multiple impairments may be present simultaneously, i.e., a patient may present with weakness of the arm and hand immediately after a stroke, which may not have resolved when spasticity sets in a few weeks or months later; hence there may be a layering of impairments over time making it difficult to decide what to treat first. It is useful to review the progression of motor recovery as described by Twitchell (Twitchell 1951) and Brunnstrom (Brunnstom 1956) to understand how impairments may be layered over time (Figure 1).
Figure 1.
Sequential progression of motor recovery as described by Twitchell and Brunstrumm. Note that while recovery is proceeding from one stage to the next, residual impairment from preceding stages may still be present leading to the layering of impairment. Also note the underlying physiological processes that may account for progression from one stage to the next.
Understanding motor impairment from a functional perspective
The most useful way to understand how impairments contribute to upper limb dysfunction may be to examine them from the perspective of their functional consequences. There are three main functional consequences of stroke on the upper limb: (1) learned nonuse, (2) learned bad-use, and (3) forgetting as determined by behavioral analysis of a task such as reaching for a food pellet and bringing it to the mouth in animal models of stroke (Whishaw, Alaverdashvili et al. 2008). These are equally valid for human behavior. Each of the functional consequences and the underlying impairments are elaborated below.
Learned nonuse
Initially after a stroke, individuals may not use their affected upper limb suggesting learned nonuse. Nonuse can result from several impairments described below. Initially nonuse may occur due to weakness/ paralysis or sensory loss. However as time progresses, nonuse may become habitual and the limb may not be incorporated into functional activities even though the individual can move it. Now it becomes a learned behavior and is referred to as “learned nonuse”.
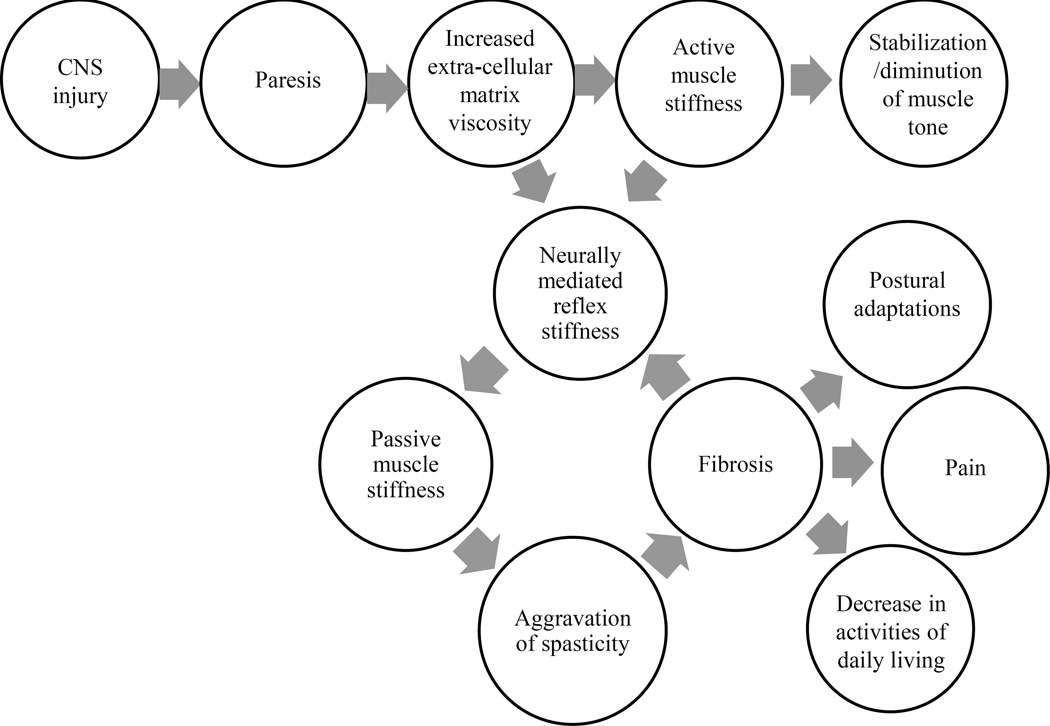
Weakness or paralysis is the predominant impairment that contributes to dysfunction after stroke (Canning, Ada et al. 2004; Wagner, Lang et al. 2007). It is a direct consequence of the lack of signal transmission from the motor cortex, which generates the movement impulse, to the spinal cord which executes the movement via signals to muscles. This results in delayed initiation and termination of muscle contraction (Chae, Yang et al. 2002), and slowness in developing forces (Canning, Ada et al. 1999), manifested as an inability to move or move quickly with negative functional consequences. Abnormally increased EMG-force slopes are seen on the affected side compared to the contralateral side as well as compared to neurologically intact subjects, suggesting that greater EMG activity is necessary to generate a given force in patients with stroke (Suresh, Zhou et al. 2008). This is thought to result from a combination of abnormal firing rate patterns and changes in motor unit control. Weakness may affect all muscle groups of the upper limb, or may be selective, affecting some muscle groups more than others. Large inter-subject differences exist in the pattern of muscle weakness across muscle groups, but research has shown that no consistent proximal-to-distal gradient or greater extensor relative to flexor weakness exists (Mercier and Bourbonnais 2004; Tyson, Chillala et al. 2006). While the absolute strength in any particular muscle group has not shown to predict function, the rate of change of force development in wrist extensor and handgrip strength (Renner, Bungert-Kahl et al. 2009) are good predictors of upper limb function. Isolated motor deficits in a single arm can occur after a stroke without other cranial or sensory dysfunction; these are rare and easily misdiagnosed requiring a high index of suspicion and assessment of risk factors for stroke (Castaldo, Rodgers et al. 2003; Hiraga 2011). In cases where trauma accompanies the vascular lesion, the pattern of weakness may need to be examined carefully to rule out spinal cord injury or peripheral nerve injury. Weakness leads to immobility, which can initiate a cascade of problems that can further contribute to motor impairment (Figure 2), as described below.
Figure 2.
Model of the contribution of paresis and immobility to the evolution of spasticity. (Adapted from Stecco et al., 2014, Current Physical Medicine and Rehabilitation Reports)
Sensory loss across tactile, proprioceptive and/or higher-order sensory modalities such as deficits in two-point discrimination, stereognosis and graphesthesia are common after stroke, and may be associated with the degree of weakness and the degree of stroke severity, as well as mobility, independence in activities of daily living, and recovery (Tyson, Hanley et al. 2008). Sensory impairments without motor weakness may also occur from specific lesions in the parietal cortex (Bassetti, Bogousslavsky et al. 1993). In studies that compared the ability of hemiparetic and healthy subjects to produce symmetrical forces with both upper limbs, it was found that joint maximum voluntary forces and proprioceptive impairments in the affected limb predicted the errors in force matching (Mercier, Bertrand et al. 2004). These results suggest that sensory impairments may lead to inaccurate motor output even though motor capacities were adequate to perform the task. In fact the term learned nonuse was coined from observations in deafferented monkeys who could move but did not do so voluntarily (Taub, Heitmann et al. 1977). Chronic loss of sensation may contribute to motor impairment due to inaccurate internal representations of the task and/or inability to control the motor output appropriately due to lack of feedback about the consequences of the motor action.
Weakness leads to immobility, which can be considered a functional impairment according to the ICF classification (Geyh, Cieza et al. 2004). Immobility in turn can begin a vicious circle of problems including peripheral soft-tissue changes that reduce tissue compliance, potentiation of reflex mechanisms and spasticity, eventually leading to muscle fibrosis and contributing to abnormal limb posturing, pain and decreased function (Figure 2) (Stecco, Stecco et al. 2014). Spasticity is now thought to arise as a consequence of contractures rather than being a cause of contractures (Ward 2012). Hence early interventions to reduce immobility and preserve range of motion either passively or actively despite paresis, and prevent contractures may be critical to prevent spasticity and its ensuing complications. Passive tissue restraint and agonist weakness, rather than antagonist restraint, have been shown to be the most common contributors to decreased active range of motion (Reinkensmeyer, Schmit et al. 1999). Immobility also leads to changes in bone mineral density with increased risk of developing osteoporosis on the paralyzed side and particularly in the upper limbs (Hamdy, Krishnaswamy et al. 1993; Hamdy, Moore et al. 1995). In fact, fractures are common on the paretic side after stroke (Ramnemark, Nyberg et al. 1998). Practitioners need to pay more attention to changes in bone mineral density post stroke and take active measures to prevent problems arising from immobility.
Motor and sensory impairments and immobility are associated with an increased risk for stroke-related pain (Lundstrom, Terent et al. 2008). Stiffness in the connective tissue of the immobilized limb may stimulate free nerve endings and proprioceptors, such as Pacini and Rufini corpuscles (Yahia, Rhalmi et al. 1992; Stecco, Gagey et al. 2007; Tesarz, Hoheisel et al. 2011), in the tissue producing pain (Bell and Holmes 1992; Stecco, Meneghini et al. 2014). Shoulder pain on the paretic side is common after stroke and is strongly associated with abnormal shoulder joint examination, ipsilateral sensory abnormalities and arm weakness (Gamble, Barberan et al. 2002). Deafferentation and sensory loss may also lead to the development of neuronal hypersensitivity and eventually chronic central pain (Boivie, Leijon et al. 1989; Rausell, Cusick et al. 1992; Klit, Finnerup et al. 2009), which is often difficult to treat. Pain can lead to learned nonuse which may persist even after the pain has resolved.
Learned bad-use
When the paretic limb is forced to move, weakness, sensory impairments, and pain can prevent “normal” movement; instead compensatory strategies are used to complete the task(s) (McCrea, Eng et al. 2005). Furthermore, stiffness and contractures resulting from immobility and the development of spasticity and abnormal motor synergies can contribute to compensatory movements. The use of compensatory strategies has been well described for human reaching and grasping after stroke (Levin, Kleim et al. 2009). Patients with stroke use trunk flexion rather than elbow extension to reach for objects (Cirstea and Levin 2000), forearm pronation and wrist flexion rather than neutral forearm position and wrist extension to orient the hand for grasping, and metacarpophalangeal (MCP) joint flexion rather than proximal interphalangeal (PIP) joint flexion to grasp objects (Raghavan, Santello et al. 2010). While the use of compensatory strategies may lead to initial success in completing a task, over time success is reduced due to poor accuracy, which increases the probability of failure. Reinforcement of the abnormal strategy by occasional successes can lead to it becoming a bad habit over time (Skinner 1938), and performance will decline despite extended training because the abnormal behavior is repeated and reinforced at the cost of the correct pattern of behavior (Dickinson 1985). Thus in the absence of appropriate feedback and correction of the abnormal motor behavior “learned bad-use” develops. When the focus of training is to reduce compensatory behaviors, for example, when the trunk is restrained during reach practice, the typical use of a more normal pattern of reaching by extending the elbow is restored along with a reduction in overall impairment (Michaelsen, Luta et al. 2001; Woodbury, Howland et al. 2009). Spasticity and in-coordination due to abnormal motor synergies can lead to the development of learned bad-use.
Spasticity is defined as a motor disorder characterized by a velocity-dependent increase in muscle tone with exaggerated tendon jerks, resulting from hyper excitability of the stretch reflex, as one component of the upper motor neuron syndrome (Lance 1980). The prevalence of spasticity increases with time since stroke (Watkins, Leathley et al. 2002), and is related to the secondary effects of weakness and immobility on skeletal muscles (Hufschmidt and Mauritz 1985; Dietz and Berger 1995; Lundstrom, Terent et al. 2008). Initially spasticity is considered a positive development as it suggests that the nervous system is beginning to initiate repair mechanisms to restore muscle tone and movement. Indeed patients who demonstrate spasticity are further along in their recovery than individuals who are more flaccid (Brunnstrom 1966; Brunnstrom 1970). However, when the threshold for reflex activity continues to reduce due to progressive re-organization of the supraspinal descending drive to the spinal cord, peripheral structures of the muscle, muscle spindles and fascia are further shortened and spasticity evolves into stretch-sensitive forms such as spastic-co-contraction (Gracies 2005). Spastic co-contraction refers to inappropriate antagonist recruitment triggered by volitional command (Gracies 2005). Clinically, spastic co-contraction leads to involuntary movement in the opposite direction of the intended voluntary movement and contributes to impairment in active function. The degree of spastic co-contraction has been shown to be positively related to the Fugl-Meyer Score (Chae, Yang et al. 2002; Aluru, Lu et al. 2014), suggesting that individuals with spastic co-contraction, although significantly impaired, are further along in their recovery process. Spasticity and spastic co-contraction may however lead to learned bad-use. Reduction in spasticity with Botulinum toxin injections has been shown to improve kinematic parameters such as velocity and smoothness, without significant changes in clinical outcomes such as hand function (Bensmail, Robertson et al. 2010). It is possible that weakness and atrophy produced by the injections negates the benefit from improvement in the form of the movement, or that functional improvement requires aggressive concurrent therapy (Canning 2009).
Abnormal motor synergies have been well described post stroke (Brunnstrom 1970). For example, during reaching, the shoulder must flex while the elbow extends. However in patients with stroke attempts at voluntary forward reaching often result in shoulder abduction and elbow flexion due to the constraining effect of abnormal descending motor commands (Beer, Ellis et al. 2007). The abnormal muscle synergies were not related to proximal weakness or abnormalities in the elbow flexor-extensor strength balance. More recently it has been found that the common drive to muscles that are functionally coupled during reaching in healthy individuals, for example, the anterior deltoid and triceps brachii, is weakened after stroke likely due to interruption of information flow in the corticospinal pathway (Kisiel-Sajewicz, Fang et al. 2011). These abnormal muscle synergies have been shown to be independent of weakness, slowness of muscle activation, excessive co-contraction and spasticity and reflects a loss of skill in generating spatial and temporal muscle activation patterns which conform with environmental demands (Canning, Ada et al. 2000). Training strategies that promote movement outside of the abnormal motor patterns may be needed to retrain more normal movement patterns.
Forgetting
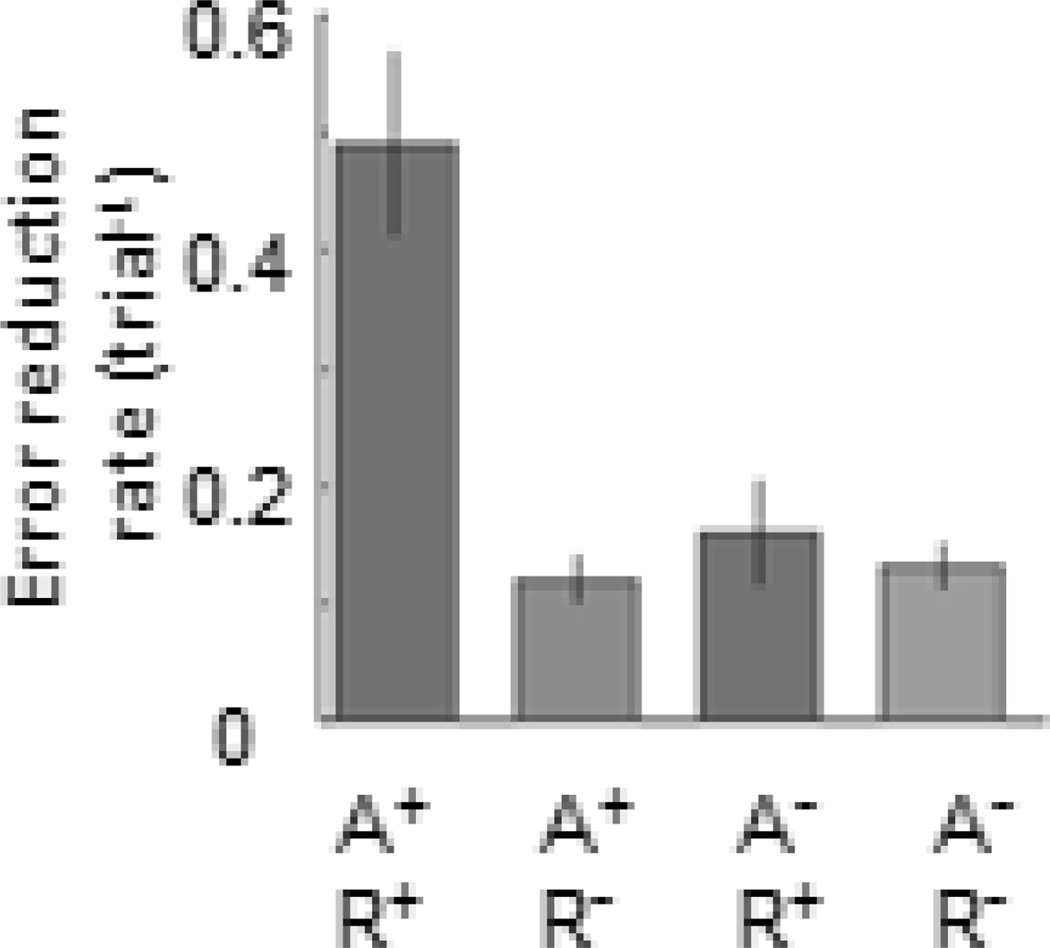
Once a motor skill is attained through training, there is an expectation that it will be retained forever, despite intervals of no training (in the same way that one never forgets how to ride a bicycle). However, rats with motor cortex injury show a decline in performance during intervals of no training, and additional training is required to get performance back to pre-training levels (Whishaw, Alaverdashvili et al. 2008). Breaks in rehabilitation similarly lead to forgetting of upper extremity motor skills in humans post stroke (Takahashi and Reinkensmeyer 2003; Krakauer 2006). Thus new skills, while reasonably stable in healthy individuals, are more transient post stroke. Skill learning requires that at least three independent processes occur across multiple time scales (Huang, Haith et al. 2011). First precise task-specific sensory-motor mappings occur through trial-and-error adaptation during practice with appropriate error sensing. Adaptation is a fast learning process (Joiner and Smith 2008), which leads to a rapid reduction in movement error, and typically takes only a few trials (Gordon, Westling et al. 1993); however it is easily forgotten (Benson, Anguera et al. 2011; Schweighofer, Lee et al. 2011). The second process is repetition, which alters movement biases depending on what is repeated. It leads to a slow tuning of directional biases towards the repeated movement (Galea and Celnik. 2009). A task can be repeated with or without adaptation to error, and does not require error sensing. The third process is reinforcement whereby movements are rewarded intrinsically or extrinsically and reward leads to faster re-learning or savings on subsequent attempts (Haith, Huberdeau et al. 2015). Although these three processes occur independently, it has been shown that learning is most successful when sensorimotor adaptation is combined effectively with repetition (Huang, Haith et al. 2011) (Figure 3). For instance, appropriate sensory-motor mappings learned through adaptation must be repeated over time for sustained and appropriate changes in skill to occur (Bastian 2008). Impaired sensorimotor adaptation and lack of opportunities for long-term practice can lead to unlearning or forgetting after stroke (Kitago, Ryan et al. 2013).
Figure 3.
Error reduction rates, reflecting learning, are greatest when adaptation (A) and repetition (R) combine. (Adapted from Huang et al., 2011, Nature Neuroscience)
Studies from several laboratories have shown that adaptation of reach and grasp are impaired post stroke despite reasonable amounts of repetition with the affected hand (Hermsdorfer, Hagl et al. 2003; Nowak, Hermsdorfer et al. 2003; Raghavan, Krakauer et al. 2006; Raghavan, Santello et al. 2010). This suggests that patients may be unable to effectively sense the error with their affected hand and/or subsequently update their motor behavior. Adaptation requires specific sensory inputs: kinesthetic sense from muscle forces used to lift objects is required to produce fingertip load forces appropriate for object weight (Johansson and Westling 1988); tactile sensation from touch receptors is required to produce grip forces appropriate for object texture, with higher grip forces needed to hold smoother objects (Johansson and Westling 1984); and visual input about object contours determines how the hand is shaped during reach (Sakata, Taira et al. 1997; Santello and Soechting 1998; Marino, Stucchi et al. 2010). In reaching experiments, both vision and proprioception provide information about arm configuration, but faulty integration of visual and proprioceptive signals may introduce errors in motor planning (Gordon, Forssberg et al. 1991; Scheidt, Conditt et al. 2005; Sarlegna, Przybyla et al. 2009); this might explain why we close our eyes when we want to enhance feeling. Thus, although multiple sensory contexts may collaborate to maintain task performance (Holmes and Spence 2005), they can also compete and interfere with the acquisition of accurate sensorimotor associations (Gordon, Forssberg et al. 1991; van Beers, Baraduc et al. 2002; Cole 2008). In the presence of sensory deficits after a stroke, however, one sensory context may substitute for another to improve the accuracy of sensory-motor maps (Quaney, He et al. 2010). Information about how and when sensory substitution should be utilized is key to the development of effective rehabilitation protocols for the recovery of motor skill. Even mild sensory and/or motor deficits can impair error-sensing and affect adaptation of movements and forces with the affected hand post stroke (Raghavan, Krakauer et al. 2006; Raghavan, Santello et al. 2010). Thus, the first step in overcoming learned bad-use and forgetting is to facilitate the formation of sensory-motor mappings or adaptation, which can then be repeated and reinforced for faster re-learning during subsequent encounters.
Therapeutic Considerations
A key consideration to determine treatment may be to first examine which impairment(s) are contributing to the present functional status of the patient. If weakness and immobility are predominant and leading to nonuse, then interventions that potentiate excitatory plasticity (Figure 1) may be warranted. On the other hand, if spasticity, spastic co-contraction and abnormal motor synergies are predominant and lead to the use of abnormal compensatory strategies to accomplish the task, one might consider interventions that potentiate inhibitory plasticity (Aluru, Lu et al. 2014). Since patients with paresis will likely evolve to develop some degree of spasticity, the intervention(s) may need to evolve with the stage of recovery.
Furthermore, in chronic patients with stroke, due to the layering of impairments, it may be important to bear in mind that treatment of one of the impairments might unmask other underlying impairments. For example, spasticity and weakness often co-exist. Therefore treatment of spasticity may unmask underlying weakness which might now need specific intervention. It may be necessary to work on several underlying impairments simultaneously for the best results, and the treatment regimen may need to be individualized for each patient.
Clinical Outcomes
Simple self-report measures used to characterize weakness in the upper limb after stroke, such as the NIH Stroke Scale, and the Stroke Impact Scale provide information about degree of impairment particularly in severely affected individuals, but are not sensitive to mild or moderate weakness of the upper limb after stroke (Bohannon 2004).
The Fugl-Meyer scale is based on the observation of sequential recovery of motor function by Twitchell and Brunnstrom (Twitchell 1951; Brunnstrom 1966; Brunnstrom 1970). It is the most widely-used quantitative measure of motor recovery post stroke (van Wijck, Pandyan et al. 2001; Gladstone, Danells et al. 2002), the scores have been shown to correlate with the extent of corticospinal tract damage (Zhu, Lindenberg et al. 2010). The minimal detectable change on the upper extremity component of the Fugl-Meyer scale is found to be approximately 8% of the maximum score of 66 (5.28 points), supporting its utility in clinical settings (Rabadi and Rabadi 2006; Lin, Hsu et al. 2009). However the Fugl-Meyer Scale was constructed on the assumptions that recovery proceeds in a proximal-to-distal fashion and from synergistic-to-isolated movements (Fugl-Meyer, Jaasko et al. 1975; Gladstone, Danells et al. 2002); however, both these assumptions have been contested recently (Woodbury, Velozo et al. 2007; Beebe and Lang 2008; Crow and Harmeling-van der Wel 2008). Furthermore, the Fugl-Meyer scale may show ceiling effects for fine motor skills in higher functioning patients (Thompson-Butel, Lin et al. 2014).
Grip strength has been found to be a useful objective measure of motor impairment, particularly the rate of increase in grip forces (Renner, Bungert-Kahl et al. 2009). Task-based kinematic measures such as speed and extent of isolated joint range of motion (Raghavan, Santello et al. 2010; Aluru, Lu et al. 2014), might be useful direct, objective and reliable measures of movement ability. In fact it has been shown that active range of motion early on predicts function at later time points (Beebe and Lang 2009). However, longitudinal measurements of active range of motion may not show a linear improvement profile as the Fugl-Meyer Scale does, especially when spasticity and spastic co-contraction set in. Hence more than one type of measurement and frequent assessment of motor impairment to inform a change in strategy to target the critical impairment may be warranted in the clinical setting.
Key Points.
Weakness or paresis is the key impairment early on that leads to learned nonuse. Sensory impairment, immobility and chronic pain may further contribute to learned nonuse.
Spasticity, spastic co-contraction and abnormal motor synergies occur as recovery proceeds and may lead to abnormal compensatory movements, which if repeated and reinforced will lead to learned bad-use.
Impairment in sensorimotor adaptation can lead to transient retention of new skills despite extensive practice; this is referred to as forgetting.
Acknowledgments
Funding
NIH R01HD071978.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluru V, Lu Y, et al. Effect of auditory constraints on motor performance depends on stage of recovery post-stroke. Front Neurol. 2014;5:106. doi: 10.3389/fneur.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti C, Bogousslavsky J, et al. Sensory syndromes in parietal stroke. Neurology. 1993;43(10):1942–1949. doi: 10.1212/wnl.43.10.1942. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Absence of a proximal to distal gradient of motor deficits in the upper extremity early after stroke. Clin Neurophysiol. 2008;119(9):2074–2085. doi: 10.1016/j.clinph.2008.04.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Active range of motion predicts upper extremity function 3 months after stroke. Stroke. 2009;40(5):1772–1779. doi: 10.1161/STROKEAHA.108.536763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RF, Ellis MD, et al. Impact of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve. 2007;36(2):242–250. doi: 10.1002/mus.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Holmes M. Model of the dynamics of receptor potential in a mechanoreceptor. Math Biosci. 1992;110(2):139–174. doi: 10.1016/0025-5564(92)90034-t. [DOI] [PubMed] [Google Scholar]
- Bensmail D, Robertson JV, et al. Botulinum toxin to treat upper-limb spasticity in hemiparetic patients: analysis of function and kinematics of reaching movements. Neurorehabil Neural Repair. 2010;24(3):273–281. doi: 10.1177/1545968309347682. [DOI] [PubMed] [Google Scholar]
- Benson BL, Anguera JA, et al. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol. 2011;105(6):2843–2851. doi: 10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW. Adequacy of simple measures for characterizing impairment in upper limb strength following stroke. Percept Mot Skills. 2004;99(3 Pt 1):813–817. doi: 10.2466/pms.99.3.813-817. [DOI] [PubMed] [Google Scholar]
- Boivie J, Leijon G, et al. Central post-stroke pain--a study of the mechanisms through analyses of the sensory abnormalities. Pain. 1989;37(2):173–185. doi: 10.1016/0304-3959(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Brunnstom S. Associated reactions of the upper extremity in adult patients with hemiplegia: An approach to training. The Physical Therapy Review. 1956;36(4):225–236. doi: 10.1093/ptj/36.4.225. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46(4):357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Movement therapy in hemiplegia. A neurophysiological approach. New York: Harper & Row; 1970. [Google Scholar]
- Canning CG. Constraint-induced movement therapy after injection of Botulinum toxin improves spasticity and motor function in chronic stroke patients. Aust J Physiother. 2009;55(4):286. doi: 10.1016/s0004-9514(09)70012-1. [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, et al. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin Rehabil. 2004;18(3):300–308. doi: 10.1191/0269215504cr715oa. [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, et al. Slowness to develop force contributes to weakness after stroke. Arch Phys Med Rehabil. 1999;80(1):66–70. doi: 10.1016/s0003-9993(99)90309-x. [DOI] [PubMed] [Google Scholar]
- Canning CG, Ada L, et al. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J Neurol Sci. 2000;176(1):45–56. doi: 10.1016/s0022-510x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- Castaldo J, Rodgers J, et al. Diagnosis and neuroimaging of acute stroke producing distal arm monoparesis. J Stroke Cerebrovasc Dis. 2003;12(6):253–258. doi: 10.1016/j.jstrokecerebrovasdis.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Chae J, Yang G, et al. Delay in initiation and termination of muscle contraction, motor impairment, and physical disability in upper limb hemiparesis. Muscle Nerve. 2002;25(4):568–575. doi: 10.1002/mus.10061. [DOI] [PubMed] [Google Scholar]
- Chae J, Yang G, et al. Muscle weakness and cocontraction in upper limb hemiparesis: relationship to motor impairment and physical disability. Neurorehabil Neural Repair. 2002;16(3):241–248. doi: 10.1177/154596830201600303. [DOI] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Cole KJ. Lifting a familiar object: visual size analysis, not memory for object weight, scales lift force. Exp Brain Res. 2008;188(4):551–557. doi: 10.1007/s00221-008-1392-y. [DOI] [PubMed] [Google Scholar]
- Crow JL, Harmeling-van der Wel BC. Hierarchical properties of the motor function sections of the Fugl-Meyer assessment scale for people after stroke: a retrospective study. Phys Ther. 2008;88(12):1554–1567. doi: 10.2522/ptj.20070186. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioral anatomy. Phil Trans R Soc Lond. 1985;(B308):67–78. [Google Scholar]
- Dietz V, Berger W. Cerebral palsy and muscle transformation. Dev Med Child Neurol. 1995;37(2):180–184. doi: 10.1111/j.1469-8749.1995.tb11987.x. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophys. 2009 Jul;102(1):294–301. doi: 10.1152/jn.00184.2009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble GE, Barberan E, et al. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur J Pain. 2002;6(6):467–474. doi: 10.1016/s1090-3801(02)00055-1. [DOI] [PubMed] [Google Scholar]
- Geyh S, Cieza A, et al. ICF Core Sets for stroke. J Rehabil Med. 2004;(44 Suppl):135–141. doi: 10.1080/16501960410016776. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, et al. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Forssberg H, et al. Visual size cues in the programming of manipulative forces during precision grip. Exp Brain Res. 1991;83(3):477–482. doi: 10.1007/BF00229824. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Westling G, et al. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol. 1993;69(6):1789–1796. doi: 10.1152/jn.1993.69.6.1789. [DOI] [PubMed] [Google Scholar]
- Gracies J-M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle & Nerve. 2005;31:535–551. doi: 10.1002/mus.20284. [DOI] [PubMed] [Google Scholar]
- Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve. 2005;31(5):552–571. doi: 10.1002/mus.20285. [DOI] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, et al. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci. 2015;35(13):5109–5117. doi: 10.1523/JNEUROSCI.3869-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy RC, Krishnaswamy G, et al. Changes in bone mineral content and density after stroke. Am J Phys Med Rehabil. 1993;72(4):188–191. doi: 10.1097/00002060-199308000-00003. [DOI] [PubMed] [Google Scholar]
- Hamdy RC, Moore SW, et al. Long-term effects of strokes on bone mass. Am J Phys Med Rehabil. 1995;74(5):351–356. [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, et al. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol. 2003;114(5):915–929. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Hiraga A. Pure motor monoparesis due to ischemic stroke. Neurologist. 2011;17(6):301–308. doi: 10.1097/NRL.0b013e318220c690. [DOI] [PubMed] [Google Scholar]
- Holmes NP, Spence C. Visual bias of unseen hand position with a mirror: spatial and temporal factors. Exp Brain Res. 2005;166(3–4):489–497. doi: 10.1007/s00221-005-2389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, et al. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70(4):787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufschmidt A, Mauritz KH. Chronic transformation of muscle in spasticity: a peripheral contribution to increased tone. J Neurol Neurosurg Psychiatry. 1985;48(7):676–685. doi: 10.1136/jnnp.48.7.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56(3):550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988;71(1):59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol. 2008;100(5):2948–2955. doi: 10.1152/jn.90706.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel-Sajewicz K, Fang Y, et al. Weakening of synergist muscle coupling during reaching movement in stroke patients. Neurorehabil Neural Repair. 2011;25(4):359–368. doi: 10.1177/1545968310388665. [DOI] [PubMed] [Google Scholar]
- Kitago T, Ryan SL, et al. Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time, and removal of errors on motor memory. Front Hum Neurosci. 2013;7:307. doi: 10.3389/fnhum.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klit H, Finnerup NB, et al. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8(9):857–868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30(12):1303–1313. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, et al. What do motor "recovery" and "compensation" mean in patients following stroke? Neurorehabil Neural Repair. 2009;23(4):313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- Lin JH, Hsu MJ, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- Lundstrom E, Terent A, et al. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008;15(6):533–539. doi: 10.1111/j.1468-1331.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- Marino BF, Stucchi N, et al. Distorting the visual size of the hand affects hand pre-shaping during grasping. Exp Brain Res. 2010;202(2):499–505. doi: 10.1007/s00221-009-2143-4. [DOI] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, et al. Saturated muscle activation contributes to compensatory reaching strategies after stroke. J Neurophysiol. 2005;94(5):2999–3008. doi: 10.1152/jn.00732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Bertrand AM, et al. Differences in the magnitude and direction of forces during a submaximal matching task in hemiparetic subjects. Exp Brain Res. 2004;157(1):32–42. doi: 10.1007/s00221-003-1813-x. [DOI] [PubMed] [Google Scholar]
- Mercier C, Bourbonnais D. Relative shoulder flexor and handgrip strength is related to upper limb function after stroke. Clin Rehabil. 2004;18(2):215–221. doi: 10.1191/0269215504cr724oa. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Luta A, et al. Effect of trunk restraint on the recovery of reaching movements in hemiparetic patients. Stroke. 2001;32(8):1875–1883. doi: 10.1161/01.str.32.8.1875. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, et al. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol. 2003;250(7):850–860. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- Quaney BM, He J, et al. Visuomotor training improves stroke-related ipsilesional upper extremity impairments. Neurorehabil Neural Repair. 2010;24(1):52–61. doi: 10.1177/1545968309341646. [DOI] [PubMed] [Google Scholar]
- Rabadi MH, Rabadi FM. Comparison of the action research arm test and the Fugl-Meyer assessment as measures of upper-extremity motor weakness after stroke. Arch Phys Med Rehabil. 2006;87(7):962–966. doi: 10.1016/j.apmr.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Raghavan P, Krakauer JW, et al. Impaired anticipatory control of fingertip forces in patients with a pure motor or sensorimotor lacunar syndrome. Brain. 2006;129(Pt 6):1415–1425. doi: 10.1093/brain/awl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan P, Santello M, et al. Compensatory motor control after stroke: an alternative joint strategy for object-dependent shaping of hand posture. J Neurophysiol. 2010;103(6):3034–3043. doi: 10.1152/jn.00936.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnemark A, Nyberg L, et al. Fractures after stroke. Osteoporos Int. 1998;8(1):92–95. doi: 10.1007/s001980050053. [DOI] [PubMed] [Google Scholar]
- Rausell E, Cusick CG, et al. Chronic deafferentation in monkeys differentially affects nociceptive and nonnociceptive pathways distinguished by specific calcium-binding proteins and down-regulates gamma-aminobutyric acid type A receptors at thalamic levels. Proc Natl Acad Sci U S A. 1992;89(7):2571–2575. doi: 10.1073/pnas.89.7.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Schmit BD, et al. Mechatronic assessment of arm impairment after chronic brain injury. Technol Health Care. 1999;7(6):431–435. [PubMed] [Google Scholar]
- Renner CI, Bungert-Kahl P, et al. Change of strength and rate of rise of tension relate to functional arm recovery after stroke. Arch Phys Med Rehabil. 2009;90(9):1548–1556. doi: 10.1016/j.apmr.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, et al. The TINS Lecture. The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci. 1997;20(8):350–357. doi: 10.1016/s0166-2236(97)01067-9. [DOI] [PubMed] [Google Scholar]
- Santello M, Soechting JF. Gradual molding of the hand to object contours. J Neurophysiol. 1998;79(3):1307–1320. doi: 10.1152/jn.1998.79.3.1307. [DOI] [PubMed] [Google Scholar]
- Sarlegna FR, Przybyla A, et al. The influence of target sensory modality on motor planning may reflect errors in sensori-motor transformations. Neuroscience. 2009;164(2):597–610. doi: 10.1016/j.neuroscience.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Conditt MA, et al. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol. 2005;93(6):3200–3213. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Lee JY, et al. Mechanisms of the contextual interference effect in individuals poststroke. J Neurophysiol. 2011;106(5):2632–2641. doi: 10.1152/jn.00399.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF, editor. The behavior of organisms: an experimental analysis. New York Appleton-Century: 1938. [Google Scholar]
- Stecco A, Meneghini A, et al. Ultrasonography in myofascial neck pain: randomized clinical trial for diagnosis and follow-up. Surg Radiol Anat. 2014;36(3):243–253. doi: 10.1007/s00276-013-1185-2. [DOI] [PubMed] [Google Scholar]
- Stecco A, Stecco C, et al. Peripheral mechanisms of spasticity and treatment implications. Current Physical Medicine and Rehabilitation Reports. 2014 [Google Scholar]
- Stecco C, Gagey O, et al. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie. 2007;91(292):38–43. doi: 10.1016/j.morpho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Suresh NL, Zhou P, et al. Abnormal EMG-force slope estimates in the first dorsal interosseous of hemiparetic stroke survivors. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3562–3565. doi: 10.1109/IEMBS.2008.4649975. [DOI] [PubMed] [Google Scholar]
- Takahashi CD, Reinkensmeyer DJ. Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res. 2003;149(2):131–140. doi: 10.1007/s00221-002-1340-1. [DOI] [PubMed] [Google Scholar]
- Taub E, Heitmann RD, et al. Alertness, level of activity, and purposive movement following somatosensory deafferentation in monkeys. Ann N Y Acad Sci. 1977;290:348–365. doi: 10.1111/j.1749-6632.1977.tb39737.x. [DOI] [PubMed] [Google Scholar]
- Tesarz J, Hoheisel U, et al. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience. 2011;194:302–308. doi: 10.1016/j.neuroscience.2011.07.066. [DOI] [PubMed] [Google Scholar]
- Thompson-Butel AG, Lin G, et al. Comparison of Three Tools to Measure Improvements in Upper-Limb Function With Poststroke Therapy. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314547766. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74(4):443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Tyson SF, Chillala J, et al. Distribution of weakness in the upper and lower limbs post-stroke. Disabil Rehabil. 2006;28(11):715–719. doi: 10.1080/09638280500301584. [DOI] [PubMed] [Google Scholar]
- Tyson SF, Hanley M, et al. Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabil Neural Repair. 2008;22(2):166–172. doi: 10.1177/1545968307305523. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Baraduc P, et al. Role of uncertainty in sensorimotor control. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1137–1145. doi: 10.1098/rstb.2002.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijck FM, Pandyan AD, et al. Assessing motor deficits in neurological rehabilitation: patterns of instrument usage. Neurorehabil Neural Repair. 2001;15(1):23–30. doi: 10.1177/154596830101500104. [DOI] [PubMed] [Google Scholar]
- Wagner JM, Lang CE, et al. Sensorimotor impairments and reaching performance in subjects with poststroke hemiparesis during the first few months of recovery. Phys Ther. 2007;87(6):751–765. doi: 10.2522/ptj.20060135. [DOI] [PubMed] [Google Scholar]
- Ward AB. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur J Neurol. 2012;19(1):21–27. doi: 10.1111/j.1468-1331.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- Watkins CL, Leathley MJ, et al. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16(5):515–522. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Alaverdashvili M, et al. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res. 2008;192(1):124–136. doi: 10.1016/j.bbr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Woodbury ML, Howland DR, et al. Effects of trunk restraint combined with intensive task practice on poststroke upper extremity reach and function: a pilot study. Neurorehabil Neural Repair. 2009;23(1):78–91. doi: 10.1177/1545968308318836. [DOI] [PubMed] [Google Scholar]
- Woodbury ML, Velozo CA, et al. Dimensionality and construct validity of the Fugl-Meyer Assessment of the upper extremity. Arch Phys Med Rehabil. 2007;88(6):715–723. doi: 10.1016/j.apmr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Yahia L, Rhalmi S, et al. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63(2):195–197. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41(5):910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]